Abstract
The present study aimed to reveal the molecular mechanisms of multiple myeloma (MM) and monoclonal gammopathy of undetermined significance (MGUS). This was a secondary study on microarray dataset GSE80608, downloaded from the Gene Expression Omnibus database, which included 10 control samples, 10 MGUS samples and 10 MM samples. Differentially expressed genes (DEGs) were identified between control and MGUS samples, and between control and MM samples. A protein-protein interaction (PPI) network was built for studying the interactions between the DEGs. Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis was performed for the genes in a gene co-expression network. A microRNA (miRNA/miR)-gene network was built to the evaluate possible the miRNAs and genes involved in the diseases. The present study identified 136 common upregulated DEGs and 165 common downregulated DEGs between MM and MGUS. Pathway enrichment analysis of the genes in the gene co-expression network revealed that the complement and coagulation cascades pathway was significantly enriched for certain complement and coagulation-associated genes. Endothelin-1 (EDN1) was significantly enriched in the hypoxia inducible factor-1 (HIF-1) and tumor necrosis factor signaling pathways. EDN1 was an important node in the PPI network, and a target gene of let-7e, let-7b and miR-19a in the miRNA-gene network. The results of the present study indicate that complement and coagulation-associated genes, the complement and coagulation cascades pathway, EDN1, let-7e, let-7b-5p, miR-19a, and the tumor necrosis factor and HIF-1 signaling pathways may all be implicated in MM and MGUS.
Keywords: multiple myeloma, monoclonal gammopathy of undetermined significance, pathway, gene co-expression network, microRNA
Introduction
Multiple myeloma (MM) is a cancer of the plasma cells within the bone marrow (BM), which caused ~79,000 mortalities in 2013 (1). It has been widely accepted that MM arises from monoclonal gammopathy of undetermined significance (MGUS), which is a pre-malignant condition that can be present for several years prior to the development of MM (2). As MM is incurable in the vast majority of patients (3), there is an urgent requirement for novel therapies. Intervening at the MGUS stage may aid to prevent the progression of MGUS to MM (4,5). Therefore, a thorough understanding of the molecular pathogenesis of MM and MGUS is urgently required to guide interventions that target the precursor state (MGUS) and MM itself.
Kubiczkova et al (6) revealed the identity of five microRNAs (miRNAs/miRs) that were deregulated in the circulating serum of patients with MM and MGUS, compared with that of healthy subjects. Shvartsur et al (7) suggested that Raf-1 kinase inhibitor protein-associated genes could be implicated in MM and MGUS using a bioinformatics approach. Amend et al (8) provided in vivo evidence that the absence of SAM domain, SH3 domain and nuclear localization signals 1 may be associated with genetic susceptibility to MGUS in mice. Furthermore, there is evidence that a dysregulated cyclin D/retinoblastoma signaling pathway is involved in the molecular pathogenesis of MM and MGUS (9,10). The nuclear factor-κB (NF-κB) signaling pathway serves an important role in the mechanism of MM pathogenesis (11). Despite these advances, the molecular mechanisms behind the transformation of MUGS into MM have not been fully elucidated.
McNee et al (12) observed that the citrullination of histone H3 promotes interleukin-6 (IL-6) production by BM mesenchymal stem cells, resulting in pro-malignancy signaling in patients with MGUS and MM. However, the genes and pathways involved in MM and MGUS have not been fully delineated. Therefore, the present study reports a secondary analysis of the microarray dataset GSE80608 (12). Differentially expressed genes (DEGs) in MM, MGUS and control subjects were identified, and a protein-protein interaction (PPI) network was built to analyze the interactions between genes. A gene co-expression network was then built to analyze the co-expression of these DEGs in MGUS and MM. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway-enrichment analysis was performed to reveal the signaling pathways that may be involved in MGUS and MM. The data may provide indications of the genes and pathways that determine the progression of MGUS to MM.
Materials and methods
Microarray dataset preprocessing
The present study was a secondary study on the GSE80608 microarray dataset, obtained from the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) (13) and based on the Affymetrix Human Exon 1.0 ST Array platform (Affymetrix; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The dataset consisted of 10 control BM samples, 10 BM samples from the iliac crest of 10 patients with MGUS and 10 BM samples from the iliac crest of 10 patients with MM.
With the aid of oligo software (version 5.1; National Biosciences, Inc., Plymouth, Minn., USA), the raw data were subjected to data preprocessing, including background correction, data normalization and calculation of probe expression values. By using huex10sttranscriptcluster.db (14) and annotate (15) software in R (16), each probe was mapped to its corresponding gene symbol.
DEGs screening
DEGs were screened between MGUS and control, and between MM and control samples, using the LIMMA package in R (17). A strict cutoff was set at a false discovery rate <0.05 and fold change (|log2FC|) ≥1.5.
Venn diagram analysis and KEGG pathway enrichment analysis
DEGs in MGUS or MM were analyzed using a Venn diagram (18), which revealed the number of the common upregulated DEGs, common downregulated DEGs and contra-regulated DEGs between MGUS and MM in a Venn diagram.
KEGG pathway enrichment (19) analysis was performed for the identified DEGs using DAVID software (20). KEGG pathways with a gene count ≥2 and P<0.05 were considered to indicate a statistically significant difference.
Analysis of a PPI network
A PPI network (PPI score=0.7) was constructed with the identified DEGs using the STRING online tool (21) to analyze the interactions between the DEGs. In the PPI network, a node denoted a gene and a link between two nodes denoted the interaction between the two genes. The number of interactions between one gene and other genes in the network was represented by the ‘degree’ value. The topological parameters of the nodes in the PPI network were analyzed using the Cytoscape CytoNCA application (22), and included degree, betweenness (23), closeness (24) and subgraph (25).
Gene co-expression analysis
A gene co-expression network was built to analyze the co-expression of the DEGs in MGUS and MM. The co-expression coefficient between two genes was calculated based on the Pearson's correlation coefficient. The P-value was calculated using the Z-score (26). Significant co-expressed gene pairs were determined to have a co-expression coefficient >0.85 and P<0.05. KEGG pathway enrichment analysis was then conducted for the co-expressed genes.
Analysis of disease-related miRNA and target genes
MM-associated miRNAs were downloaded from the miR2Disease database (27), which included 17 miRNAs that were associated with MM. On the basis of these miRNAs, target genes were predicted using miRwalk2.0 (28). The miRNA-target gene interaction pairs were obtained from the validated target module in miRwalk2.0, which had been validated previously (28). Finally, the differentially expressed target genes were selected.
Results
Identification and Venn diagram analysis of DEGs
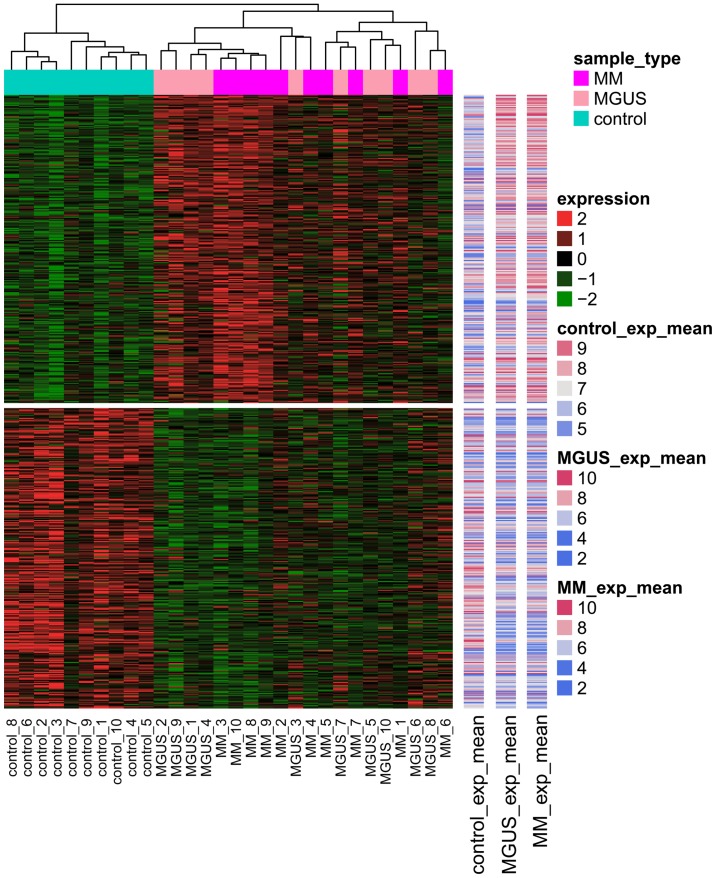
The present study identified 387 DEGs between MGUS and control samples, and 463 DEGs between MM and control samples. Venn diagram analysis revealed that there were 136 common upregulated DEGs, 165 common downregulated DEGs and no contra-regulated DEGs between MGUS and MM (Fig. 1). MGUS had 34 specific upregulated DEGs and 52 specific downregulated DEGs, whereas MM had 108 specific upregulated DEGs and 54 specific downregulated DEGs. A heat map revealed that DEG expression in the control samples was markedly different from that in MM and MGUS samples (Fig. 2).
Figure 1.
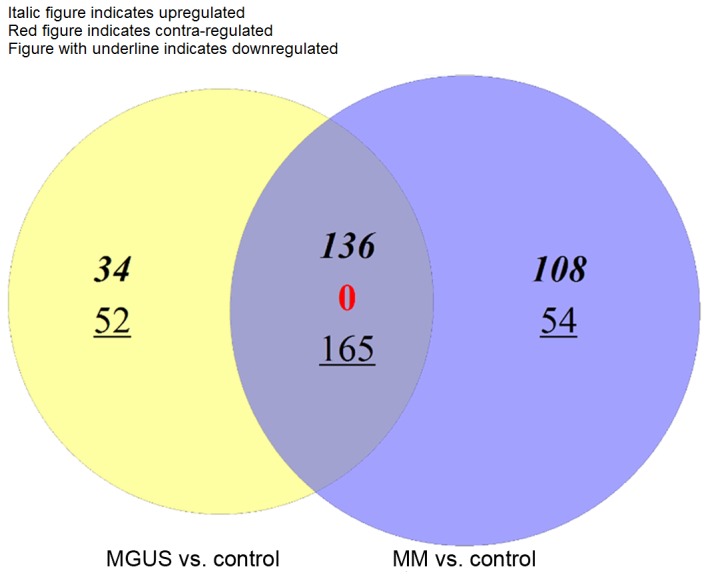
Venn diagram of differently expressed genes in MM and MGUS. MM, multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance.
Figure 2.
Heat map of differently expressed genes. MM, multiple myeloma; MGUS, monoclonal gammopathy of undetermined significance; exp, expression.
KEGG pathway enrichment analysis
KEGG pathway enrichment analysis (Table I) revealed that the common upregulated DEGs were significantly enriched in five pathways, including the Rap1 signaling pathway and the regulation of actin cytoskeleton pathway. The common downregulated DEGs were significantly associated with the complement and coagulation cascades, including cluster of differentiation 55 (CD55), thrombomodulin (THBD), mannan-binding lectin serine protease 1 (MASP1), complement factor B (CFB), coagulation factor III (F3), complement component 1 (C1)R, C1S and coagulation factor II receptor (F2R). Furthermore, common downregulated DEGs were significantly associated with axon guidance, ATP-binding cassette transporters, Staphylococcus aureus infection response and steroid hormone biosynthesis pathways. MM-specific upregulated DEGs were significantly associated with the regulation of actin cytoskeleton pathway.
Table I.
Significant Kyoto Encyclopedia of Genes and Genomes pathways of DEGs.
| DEGs | Pathway | P-value | Genes |
|---|---|---|---|
| Common upregulated DEGs | hsa04360: Axon guidance | 8.43×10−4 | PLXNA3, SEMA7A, MET, NTN4, SEMA3A, CXCL12, EPHA3 |
| hsa04514: Cell adhesion molecules | 8.17×10−3 | NRXN3, ICAM2, CD4, ITGB2, NECTIN3, PDCD1LG2 | |
| hsa05200: Pathways in cancer | 2.16×10−2 | FGF5, PLCB4, PGF, SLC2A1, MET, BDKRB1, ITGA3, FGF1, CXCL12 | |
| hsa04015: Rap1 signaling pathway | 3.75×10−2 | FGF5, PLCB4, PGF, MET, ITGB2, FGF1 | |
| hsa04810: Regulation of actin cytoskeleton | 3.82×10−2 | FGF5, SCIN, BDKRB1, ITGB2, ITGA3, FGF1 | |
| Common downregulated DEGs | hsa04610: Complement and coagulation cascades | 7.15×10−6 | CD55, THBD, MASP1, CFB, F3, C1R, C1S, F2R |
| hsa04360: Axon guidance | 1.09×10−2 | EPHA4, SEMA6D, NTNG1, EFNA5, UNC5D, SLIT3 | |
| hsa02010: ABC transporters | 1.11×10−2 | ABCA8, ABCC9, ABCA9, ABCA6 | |
| hsa05150: Staphylococcus aureus infection | 1.65×10−2 | MASP1, CFB, C1R, C1S | |
| hsa00140: Steroid hormone biosynthesis | 2.32×10−2 | AKR1C3, AKR1C2, HSD11B1, AKR1C1 | |
| MM-specific upregulated DEGs | hsa04810: Regulation of actin cytoskeleton | 4.79×10−2 | RAC2, MRAS, ITGA8, DIAPH3, ITGA7 |
| MGUS-specific downregulated DEGs | hsa05410: Hypertrophic cardiomyopathy | 1.91×10−2 | SGCD, IGF1, CACNA2D3 |
| hsa05414: Dilated cardiomyopathy | 2.20×10−2 | SGCD, IGF1, CACNA2D3 |
DEG, differently expressed gene; hsa, Homo sapiens; ABC, ATP-binding cassette.
PPI network
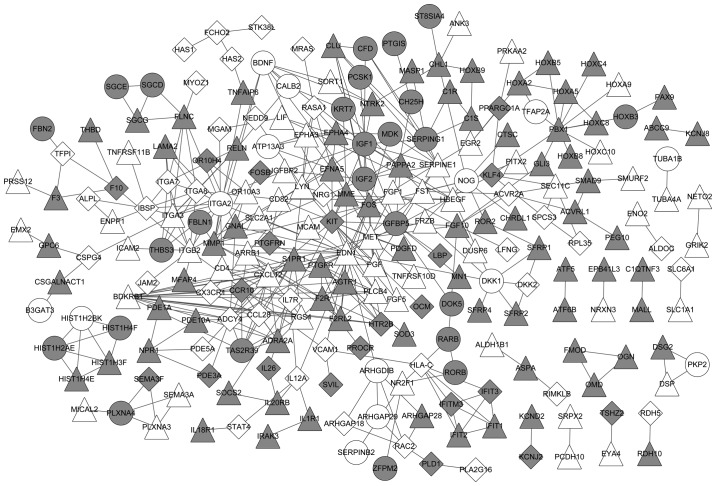
A PPI network was built with the DEGs in MM and MGUS (Fig. 3). The network included 233 nodes and 435 gene pairs. Of these genes, 39 were MGUS-specific, 59 were MM-specific and 125 were common between MGUS and MM.
Figure 3.
Protein-protein interaction network. Grey nodes, downregulated genes; white nodes, upregulated genes; round nodes, MGUS-specific genes; diamond nodes, MM-specific genes; triangular nodes, common genes between MGUS and MM. A link between two nodes represents an interaction between two genes. MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma.
In the PPI network, the top 20 genes (nodes) were selected, based on their values of degree, betweenness, closeness and subgraph, to be the important nodes in the PPI network (Table II). Of the top 20 genes, endothelin 1 (EDN1), C-X-C motif chemokine ligand 12, adenylate cyclase 4, matrix metallopeptidase 1, insulin like growth factor 1 and Finkel-Biskis-Jinkins osteosarcoma oncogene were common between MGUS and MM.
Table II.
Degree, betweenness, closeness and subgraph of the top 20 nodes in the protein-protein interaction network.
| Node, gene | Subgraph | Degree | Betweenness | Closeness |
|---|---|---|---|---|
| BDKRB1 | 1,515.014801 | 15 | 2.18×10−2 | |
| EDN1 | 1,337.5355 | 24 | 14,298.21 | 2.20×10−2 |
| CXCL12 | 1,014.57958 | 14 | 1,799.90 | 2.18×10−2 |
| ADCY4 | 691.12317 | |||
| F2R | 687.05615 | 11 | 2.18×10−2 | |
| CCR10 | 641.9886599 | 9 | ||
| S1PR1 | 633.8678 | 10 | ||
| CCL28 | 597.2848528 | 9 | ||
| AGTR1 | 580.683 | 10 | 2.18×10−2 | |
| ADRA2A | 559.71972 | |||
| TAS2R39 | 559.718772 | |||
| RGS4 | 542.425323 | |||
| F2RL2 | 511.2666327 | |||
| PLCB4 | 510.37097 | 9 | 2.18×10−2 | |
| PTGFR | 486.30338 | |||
| HTR2B | 432.8887689 | |||
| ARRB1 | 363.97263 | |||
| MMP1 | 248.22731 | 11 | 4,609.06 | 2.19×10−2 |
| IGF1 | 233.1976220 | 17 | 4,473.53 | |
| FOS | 213.7347773 | 13 | 6,338.76 | 2.19×10−2 |
| ADCY4 | 13 | 2,133.74 | 2.18×10−2 | |
| KIT | 13 | 4,026.67 | 2.18×10−2 | |
| ITGA2 | 12 | 5,403.44 | 2.19×10−2 | |
| MET | 12 | 3,152.53 | 2.18×10−2 | |
| PBX1 | 11 | 3,496.13 | ||
| ITGA8 | 10 | |||
| LYN | 10 | 3,282.67 | 2.18×10−2 | |
| ITGA3 | 10 | 2,372.76 | ||
| KLF4 | 4,272.19 | |||
| ITGB2 | 3,974.00 | |||
| FGF10 | 3,904.25 | |||
| VCAM1 | 2,679.60 | |||
| NOG | 2,237.87 | |||
| ARHGDIB | 2,199.00 | |||
| IL12A | 1,979.33 | |||
| F10 | 1,848.00 | |||
| FST | 2.17×10−2 | |||
| BDNF | 2.18×10−2 | |||
| ARRB1 | 2.18×10−2 | |||
| MME | 2.18×10−2 | |||
| DKK1 | 2.18×10−2 | |||
| PGF | 2.19×10−2 | |||
| IGF1 | 2.19×10−2 |
Analysis of a gene co-expression network
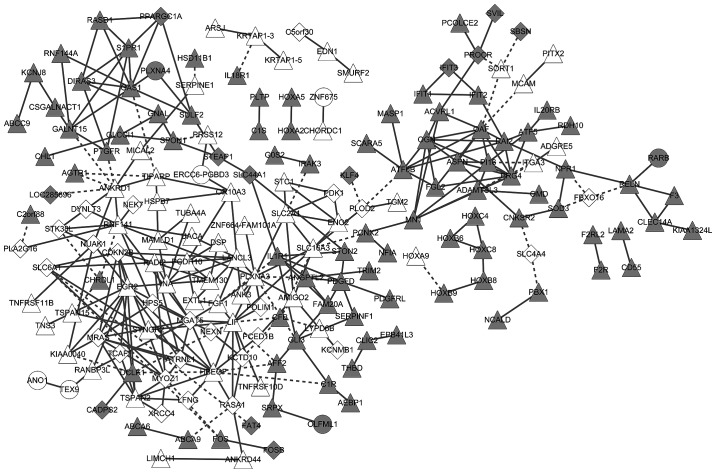
The gene co-expression network included 312 co-expressed gene pairs, 186 MM- or MGUS-specific genes, and 141 genes common to MM and MGUS. The co-expressed genes exhibited consistent changes in MM and MGUS (Fig. 4). These co-expressed genes were significantly associated with the complement and coagulation cascades pathway, tumor necrosis factor (TNF) signaling pathway, S. aureus infection pathway, hypoxia-inducible factor-1 (HIF-1) signaling pathway and pathways in cancer (Table III).
Figure 4.
Gene co-expression network. Grey nodes, downregulated genes; white nodes, upregulated genes; round nodes, MGUS-specific genes; diamond nodes, MM-specific genes; triangular nodes, common genes between MGUS and MM. Solid lines indicate positive correlations, dotted lines indicate negative correlations. MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma.
Table III.
Significantly enriched pathways of genes in the gene co-expression network.
| Pathway | P-value | Genes |
|---|---|---|
| hsa04610: Complement and coagulation cascades | 8.11×10−7 | CD55, THBD, MASP1, CFB, F3, SERPINE1, C1R, C1S, F2R |
| hsa05150: Staphylococcus aureus infection | 0.019029 | MASP1, CFB, C1R, C1S |
| hsa04066: HIF-1 signaling pathway | 0.023618 | PDK1, EDN1, SLC2A1, SERPINE1, ENO2 |
| hsa04668: TNF signaling pathway | 0.029493 | LIF, IL18R1, FOS, EDN1, ATF6B |
| hsa05200: Pathways in cancer | 0.030231 | LAMA2, AGTR1, FOS, CDKN2B, SLC2A1, ITGA3, RARB, FGF1, GLI3, F2R |
hsa, Homo sapiens; HIF, hypoxia inducible factor; TNF, tumor necrosis factor.
Analysis of a miRNAs-gene network
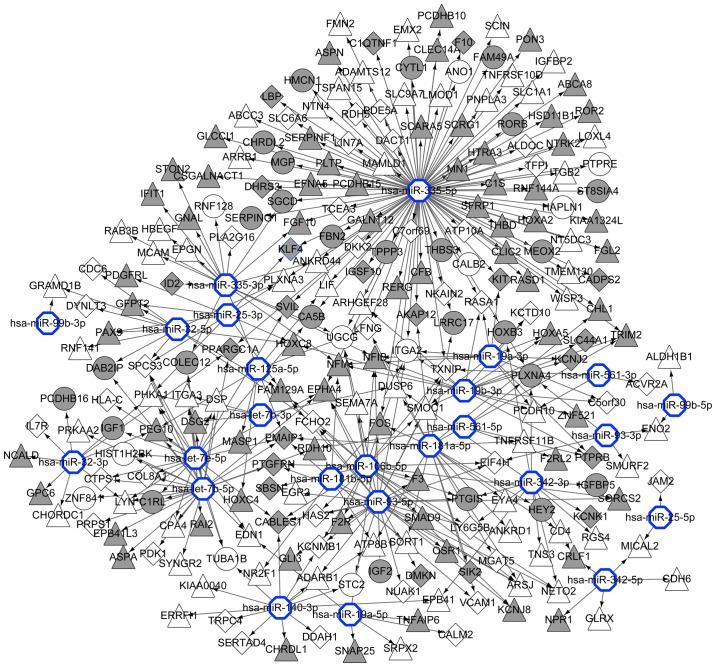
A total of 16 MM-associated miRNAs were obtained from the miR2Disease database: miR-99b, miR-93, miR-561, miR-342, miR-335, miR-32, miR-25, miR-19b, miR-19a, miR-181b, miR-181a, miR-140-3p, miR-125a-5p, miR-106b, let-7e and let-7b. Fig. 5 shows a miRNA-gene network, featuring the associations between the 16 miRNAs and their target genes.
Figure 5.
miRNA-gene network. Blue hexagonal nodes represent miRNAs; grey nodes, downregulated genes; white nodes, upregulated genes; round nodes, MGUS-specific genes; diamond nodes, MM-specific genes; triangular nodes, common genes between MGUS and MM. Connecting arrows indicate regulatory interactions between miRNAs and target genes. MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; miRNA, microRNA.
Discussion
MGUS is premalignant plasma-cell proliferative condition that consistently precedes MM (29). The current study identified that common downregulated DEGs, including CD55, THBD, MASP1, CFB, F3, C1R, C1S and F2R, were significantly enriched in complement and coagulation cascades pathway. CD55 encodes complement decay-accelerating factor, which regulates the complement system on the cell surface (30). THBD encodes thrombomodulin, which participates in the anticoagulant pathway. MASP1 is an enzyme involved in the lectin pathway of the complement system (31). CFB, F3, C1R, C1S and F2R are components of the complement or coagulation system (32). Crowely et al (33) suggested that patients with MGUS have an intermediate coagulation profile, between that of patients with myeloma and that of healthy controls. There is evidence that MM is associated with an increased risk of venous thromboembolism (34). These findings indicate that these downregulated complement and coagulation genes may be involved in the development of MM and MGUS through the regulation of the complement and coagulation cascade pathways.
HIFs are transcription factors that respond to hypoxia; the HIF signaling pathway mediates the effects of hypoxia on the cell (35). It has been suggested that a hypoxic BM environment serves a role in the pathogenesis of MM (36,37). Azab et al (38) provided evidence that hypoxia promotes the dissemination of MM. In concordance with these findings, the current study noticed that the HIF-1 signaling pathway was significantly enriched for co-expressed genes. This enrichment indicates that the HIF-1 signaling pathway may have a role in the development of MM and MGUS. Furthermore, suppression of HIF-1 decreases the expression of the anti-apoptotic protein survivin, strengthening the sensitivity of MM cells to melphalan (39). HIF-1 inhibition may be a promising therapeutic strategy for treating MM and premalignant MGUS.
Lee et al (40) reported that TNF expression is significantly increased in patients with active MM and regulates IL-6 production. Previous studies have demonstrated that the NF-κB pathway, which is downstream of TNF, is involved in MM (11,41). In the present study, the TNF signaling pathway was significantly enriched by co-expressed genes. EDN1, a common DEG between MGUS and MM and an important node in the PPI network, was significantly enriched in the TNF and HIF-1 signaling pathways. EDN1 is a potent vasoconstrictor produced by vascular endothelial cells, whose expression is stimulated by hypoxia (42). These findings indicate that END1 could serve an important role in premalignant MGUS and MM by influencing the TNF and HIF-1 signaling pathways.
The present study additionally investigated a miRNA-gene regulatory network. In the miRNA-gene regulatory network, END1 was regulated by let-7e-5p, let-7b-5p, and miR-19a-5p, suggesting that the role of EDN1 in premalignant MGUS and MM may be affected by these miRNAs. Similarly, Kubiczkova et al (6) revealed that circulating serum levels of let-7e are deregulated in MM and MGUS compared with those in healthy subjects.
The present study has certain limitations. Firstly, the study samples are limited. It is a secondary study on the microarray dataset GSE80608, meaning that the number of samples in the study cannot be increased. Secondly, the study only used bioinformatics approaches, meaning that experimental data are necessary to validate these findings in further studies.
The present study suggests that the complement and coagulation-associated genes EDN1, let-7e, let-7b, miR-19a, along with the complement and coagulation, and the TNF and HIF-1 signaling pathways, may be involved in the molecular mechanism of MM and MGUS pathogenesis. Further experiments are warranted to verify the findings of the current study.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 81400168); the Guangzhou Health Care and Cooperative Innovation Major Project (no. 201400000003-1); the Science and Technology Planning Project of Guangdong Province, China (no. 2014A020209047 and 2015A020210068); the Foundation of Guangdong Traditional Chinese Medicine (no. 20131104); the Foundation of Guangdong Medicine (no. A2013128 and A2017266); the Foundation of Technological Support of Xinjiang Uygur Autonomous Region (no. 201491185); and the Foundation of Guangdong Second Provincial General Hospital (grant nos. YY2014-002, YQ2015-004/005/012/016, 2016-011/013 and 2017001).
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators: Global, regional, and national levels of age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007;13:1762–1768. doi: 10.1158/1078-0432.CCR-06-1812. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Ghobrial IM. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: A review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin Cancer Res. 2013;19:985–994. doi: 10.1158/1078-0432.CCR-12-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: Biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:478–487. doi: 10.1182/asheducation-2013.1.478. [DOI] [PubMed] [Google Scholar]
- 6.Kubiczkova L, Kryukov F, Slaby O, Dementyeva E, Jarkovsky J, Nekvindova J, Radova L, Greslikova H, Kuglik P, Vetesnikova E, et al. Circulating serum microRNAs as novel diagnostic and prognostic biomarkers for multiple myeloma and monoclonal gammopathy of undetermined significance. Haematologica. 2014;99:511–518. doi: 10.3324/haematol.2013.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shvartsur A, Chatterjee D, Chen H, Berenson J, Garban H, Bonavida B. A bioinformatics approach revealed a differential expression of RKIP-related genes in pre-multiple myeloma and multiple myeloma: Clinical implications. Blood. 2015;126:5309. [Google Scholar]
- 8.Amend S, Liang C, Serie D, Vachon CM, Lu L, Vij R, Colditz GA, Weilbaecher KN, Tomasson MH. Deletion of samsn1 underlies genetic susceptibility to Monoclonal Gammopathy of undetermined significance (MGUS) in mice. Blood. 2013;122:397. [Google Scholar]
- 9.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122:3456–3463. doi: 10.1172/JCI61188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Jr SJ. Cyclin D dysregulation: An early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNee G, Eales KL, Wei W, Williams DS, Barkhuizen A, Bartlett DB, Essex S, Anandram S, Filer A, Moss PA, et al. Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma. Leukemia. 2017;31:373–381. doi: 10.1038/leu.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110. doi: 10.1007/978-1-4939-3578-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald JW. Huex10sttranscriptcluster. db: Affymetrix huex10 annotation data (chip huex10sttranscriptcluster) R package. 2017 version 8.2.0. [Google Scholar]
- 15.Gentleman R. Annotate: Annotation for microarrays. R package. 2017 version 1.54.0. [Google Scholar]
- 16.R Foundation for Statistical Computing. Vienna, Austria: 2017. R Development Core Team: R: A language and environment for statistical computing. [Google Scholar]
- 17.Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; New York: 2005. pp. 397–420. [DOI] [Google Scholar]
- 18.Chen H, Boutros PC. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. Bmc Bioinformatics. 2011;12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for Integration and Interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 21.Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et al. STRING 8-a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y, Li M, Wang J, Pan Y, Wu FX. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems. 2015;127:67–72. doi: 10.1016/j.biosystems.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Goh KI, Oh E, Kahng B, Kim D. Betweenness centrality correlation in social networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67:017101. doi: 10.1103/PhysRevE.67.017101. [DOI] [PubMed] [Google Scholar]
- 24.Du Y, Gao C, Chen X, Hu Y, Sadiq R, Deng Y. A new closeness centrality measure via effective distance in complex networks. Chaos. 2015;25:033112. doi: 10.1063/1.4916215. [DOI] [PubMed] [Google Scholar]
- 25.Estrada E, Rodríguezvelázquez JA. Subgraph centrality in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:056103. doi: 10.1103/PhysRevE.71.056103. [DOI] [PubMed] [Google Scholar]
- 26.Liang M, Zhang F, Jin G, Zhu J. FastGCN: A GPU accelerated tool for fast gene co-expression networks. PloS One. 2015;10:e0116776. doi: 10.1371/journal.pone.0116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–D104. doi: 10.1093/nar/gkn714. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dweep H, Gretz N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12:697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 29.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spendlove I, Ramage JM, Bradley R, Harris C, Durrant LG. Complement decay accelerating factor (DAF)/CD55 in cancer. Cancer Immunol Immunother. 2006;55:987–995. doi: 10.1007/s00262-006-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000;165:2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 32.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: Strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Crowely MP, Quinn S, Coleman E, Eustace JA, Gilligan OM, O'Shea SI. Differing coagulation profiles of patients with monoclonal gammopathy of undetermined significance and multiple myeloma. J Thromb Thrombolysis. 2015;39:245–249. doi: 10.1007/s11239-014-1140-z. [DOI] [PubMed] [Google Scholar]
- 34.De Stefano V, Za T, Rossi E. Venous thromboembolism in multiple myeloma. Semin Thromb Hemost. 2014;40:338–347. doi: 10.1055/s-0034-1370793. [DOI] [PubMed] [Google Scholar]
- 35.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia. 2011;25:1533–1542. doi: 10.1038/leu.2011.122. [DOI] [PubMed] [Google Scholar]
- 37.Asosingh K, De Raeve H, de Ridder M, Storme GA, Willems A, Van Riet I, Van Camp B, Vanderkerken K. Role of the hypoxic bone marrow microenvironment in 5T2 MM murine myeloma tumor progression. Haematologica. 2005;90:810–817. [PubMed] [Google Scholar]
- 38.Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–5794. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Kirito K, Yoshida K, Mitsumori T, Nakajima K, Nozaki Y, Hamanaka S, Nagashima T, Kunitama M, Sakoe K, Komatsu N. Inhibition of hypoxia-inducible factor-1 function enhances the sensitivity of multiple myeloma cells to melphalan. Mol Cancer Ther. 2009;8:2329–2338. doi: 10.1158/1535-7163.MCT-09-0150. [DOI] [PubMed] [Google Scholar]
- 40.Lee C, Oh JI, Park J, Choi JH, Bae EK, Lee HJ, Jung WJ, Lee DS, Ahn KS, Yoon SS. TNF α mediated IL-6 secretion is regulated by JAK/STAT pathway but Not by MEK phosphorylation and AKT phosphorylation in U266 multiple myeloma cells. Biomed Res Int. 2013;2013:580135. doi: 10.1155/2013/580135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: Therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 42.Elton TS, Oparil S, Taylor GR, Hicks PH, Yang RH, Jin H, Chen YF. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am J Physiol. 1992;263:R1260–1264. doi: 10.1152/ajpregu.1992.263.6.R1260. [DOI] [PubMed] [Google Scholar]