Abstract
Curcumin, a natural polyphenolic compound, has commonly been used as a food additive or in many traditional medicine remedies for over 2,000 years in many Asian countries. Melatonin is a hormone secreted from pineal glands of mammals and possesses diverse physiological functions. Both curcumin and melatonin have the effective potential to inhibit proliferation of various types of cancers, but there is no report on their combination for bladder cancer treatment, and the underlying mechanism remains poorly understood. In the present study, we investigated whether the combination of curcumin and melatonin leads to an enhanced inhibition of cell proliferation in bladder cancer cells. Our results showed that the combinational treatment enhanced the repression of nuclear translocation of NF-κB and their binding on COX-2 promoter via inhibiting IKKβ activity, resulting in inhibition of COX-2 expression. In addition, combined treatment with curcumin and melatonin induced cell apoptosis in bladder cancer through enhancing the release of cytochrome c from the mitochondrial intermembrane space into the cytosol. These results, therefore, indicated that melatonin synergized the inhibitory effect of curcumin against the growth of bladder cancer by enhancing the anti-proliferation, anti-migration, and pro-apoptotic activities, and provide strong evidence that combined treatment with curcumin and melatonin might exhibit an effective therapeutic option in bladder cancer therapy.
Keywords: curcumin, melatonin, bladder cancer, COX-2, NF-κB/IKKβ
Introduction
Bladder cancer is one of the most malignant types of cancer, which ranks ninth among the overall cancers worldwide with an estimated 430,000 new cases diagnosed per year and caused 165,000 deaths in 2012 (1). Although radical cystectomy is introduced as the standard treatment for bladder cancer with neoadjuvant chemotherapy (2), the 5-year survival rate is still very low in patients, who suffer from invasive and metastatic bladder cancer (3,4). Thus, it is urgent to find novel therapeutic strategies and more effective agents to prolong and improve patients' survival and life quality, with no damage on normal cells.
Curcumin is a natural polyphenolic compound isolated from the rhizome of Curcuma longa (turmeric) (5), and has commonly been used as a food additive or in many traditional medicine remedies for over 2,000 years in many Asian countries (6). Previous studies have demonstrated that curcumin possesses various physiological and pharmacological properties as shown by in vitro and in vivo studies, including anti-oxidant, anti-bacterial, anti-inflammatory, immunomodulatory, free radical scavenging and antidiabetic activities (7–10). In particular, curcumin could potentially inhibit cell proliferation, induce cell apoptosis and cell cycle arrest and suppress angiogenesis in a huge amount of cancers through modulating all kinds of molecular targets and signaling pathways (11–15). Furthermore, curcumin has been shown to induce apoptosis and cell cycle arrest and proliferation inhibition in bladder cancer cells (16,17). Although curcumin presents itself as a pharmacologically safe and effective potential candidate for anticancer therapy, its effectiveness is not powerful enough due to its side effects in high doses and other properties, such as poor absorption, rapid metabolism, and rapid systemic elimination (18). Therefore, increasing attention should be paid on combinational treatment of curcumin with other anti-tumor agents, especially natural antitumor compound, and the detailed molecular mechanisms of such combination deserve better investigation.
Melatonin is a major secretory product of pineal gland in vertebrates (19,20), modulating circadian rhythms, sleep, mood, reproduction and other biological processes (21,22). In the last few decades, many studies in vitro and in vivo have illustrated that melatonin had various physiological and pharmacological activities including anti-proliferation, anti-angiogenesis, anti-inflammatory, suppressing tumor metastasis and inducing cell apoptosis activities (23–26), by affecting multiple signaling pathways, including NF-κB (27). Based on its multiple physiological actions and low side-effects, more attempts deserve to be made to develop melatonin as an alternative chemopreventive or chemotherapeutic agent partner to form a better and novel strategy for cancer treatment, moreover, reducing their side effects.
Many reports have demonstrated cyclooxygenase-2 (COX-2), involved in inflammatory progression, and that it is can be inducible in response to certain stimuli such as growth factors and cytokines, thus, is causally associated with progression of many human tumors (28–30). Previous studies have indicated that COX-2 protein is highly expressed in a broad range of human tumors, including bladder cancer (31,32), and has been associated with high tumor aggressiveness and poor prognosis of patients (33,34). COX-2 expression is strictly and transcriptionally regulated by the recruitment of transactivators such as nuclear factor κB (NF-κB) to the corresponding sites of its promoters (35,36). Therefore, inhibition of COX-2 expression might be an effective way to inhibit the development of human tumors. However, whether curcumin could downregulate COX-2 expression and whether curcumin and melatonin combination could enhance this inhibition to further suppress bladder cancer cell growth remains poorly understood.
In the present study, we hypothesized that melatonin might play a role in potentiating or enhancing curcumin's antitumor effect in human bladder cancer cells. To test this hypothesis, we analyzed the effects of this combinational mode on cell proliferation, migration, and apoptosis in bladder cancer cells, and detected some key changes in proteins to uncover the underlying molecular mechanisms. Our study showed that melatonin could be used as a potential combinational agent to sensitize the antitumor effect of curcumin. Such sensitization was mediated through IKKβ/NF-κB/COX-2 signaling pathways, implying that this combinational treatment might become an effective alternative approach in bladder cancer therapy.
Materials and methods
Chemicals and reagents
Curcumin and melatonin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents were dissolved in dimethyl sulphoxide (DMSO) as the initial concentrate and diluted with medium before use, and the final concentration of DMSO was <0.1%. Control cultures received the carrier solvent (0.1% DMSO).
Antibodies and other materials
Antibodies specific to cleaved caspase-3, COX-2, p-IKKβ, IKKβ, p-IκBα, IκBα, p65, β-actin and all the secondary antibodies were purchased from Cell Signaling Technology (Cell Signaling Technology, Inc., USA). Antibodies specific to cytochrome c, and p50 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies specific to Bcl-2, BAX, and Lamin B1 were purchased from Proteintech Group (Proteintech, Inc., USA). Antibodies specific to MMP-2/9 and TIMP-2 were purchased from Abcam. RPMI-1640 media, fetal bovine serum (FBS), and trypsin were purchased from Gibco. All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise specified.
Cell culture
Human bladder cancer cell lines T24, UMUC3 and 5637 were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 media, supplemented with 10% fetal bovine serum (FBS), and grown at 37°C in a humidified atmosphere with 5% CO2The authenticity of all these cell lines was verified through the genomic short tandem repeat (STR) profile by Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd., and the cell lines have been confirmed to be free of mycoplasma by Mycoplasma Detection Kit-Quick Test (Biotool).
Cell viability assay
Cell viability was measured using MTT assay. Briefly, 6×103 cells were counted and seeded into 96-well culture plates followed by adhesion overnight, and then treated with appropriate concentrations of curcumin along with and without melatonin. The quintuplicate was set for each concentration group. Followed by incubation for 48 h, 10 µl of MTT (5 mg/ml) was added to each well. For 4-h incubation at 37°C, the medium containing MTT was replaced by 0.15 ml DMSO. The absorbance was measured at 490 nm by EnSpire® Multimode Plate Reader (Perkin-Elmer, USA). Each experiment was repeated at least three times.
Colony formation assay
Bladder cancer cells were treated with curcumin or melatonin for 24 h, and then trypsinized into single cells and seeded into a 6-well plate at 1,500 cells/well. After being incubated at 37°C with 5% CO2 for 10 days until colonies were large enough to be visualized, the cells were washed with PBS and fixed with the mixture (methanol: glacial acetic: ddH2O =1:1:8) for 10 min, and stained with 0.1% crystal violet for 30 min.
Wound healing assay
Wound healing assay (scratch assay) was performed to detect cell migration. Briefly, UMUC3 and T24 cells were grown to full confluence in 6-well culture plates. After 6 h of serum starvation, the confluent cell mono-layer was scraped with a sterile 100-µl pipette tip and treated with appropriate dose of curcumin or melatonin.
Confocal immunofluorescence analysis
For immunofluorescence analysis, T24 cells were seeded on coverslips, and treated with different concentrations of curcumin or melatonin for 48 h. After that, the cells were fixed with 4% paraformaldehyde at room temperature, and permeabilized with 0.2% Triton X-100, and then blocked in PBS containing 5% BSA. Subsequently, the cells were incubated with diluted primary antibodies against cytochrome c and p65 overnight at 4°C. Following this, the cells were incubated with fluorescein isothiocyanate or rhodamineisothiocyanate-conjugated secondary antibodies for 60 min at room temperature in the dark. Finally, DAPI was added to each sample for nuclear counterstaining and fluorescent images were examined using a Leica DM 14000B confocal microscope.
Western blot analysis
Proteins from cell lysates or streptavidin-agarose pulldown assay were subjected to SDS-PAGE and then transferred to a PVDF membrane. Protein bands were visualized by enhanced chemiluminescence (ECL) and integrated optical density of bands was quantitated by the ImageQuant software (GE Healthcare). The concentration of proteins was determined in the cell lysates using BCA method. Similar experiments were performed at least three times.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent according to the kit protocol (Takara Bio, Dalian, China). cDNA was reverse-transcribed using the PrimeScript RT reagent kit (Takara Bio) according to the manufacturer's instructions. PCR analysis was performed on aliquots of the cDNA preparations to detect gene expression. Primer pairs were: COX-2, 5′-TCACAGGCTTCCATTGACCAG-3 and 5′-CCGAGGCTT TTCTACCAGA-3′; β-actin, 5′-GGCACCCAGCACAAT GAA-3′ and 5′-TAGAAGCATTTGCGGTGG -3′. Amplification products were analyzed on 1.5% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
Streptavidin-agarose pulldown assay
Nuclear extract proteins (400 µg) were incubated in a 400-µl mixture containing biotinylated DNA probe (4 µg), streptavidin-conjugated agarose beads (40 µl), and supplemented with PBSi (PBS buffer with 1 mM EDTA, 1 mM DTT, and protease inhibitor cocktail Complete) buffer at RT for 5 h in a rotating shaker. Thereafter, the beads were pelleted by centrifugation, and dissociated in 50 µl of 2X Laemmli sample buffer by boiling at 100°C for 10 min. The supernatant samples were analyzed by western blotting.
Flow cytometry analysis
To determine the distribution of the cells in the cell cycle and the proportion of apoptotic cells, flow cytometry analysis was performed using a flow cytometer (BD FACS Accuri C6; BD Biosciences, CA, USA). Briefly, the treated cells were collected and fixed with ice-cold 70% ethanol at 4°C for 4 h, and then stained with propidium iodide (PI) staining buffer (0.2% Triton X-100, 100 µg/ml DNase-free RNase A, and 50 µg/ml propidium iodide in PBS) in the dark for 30 min. For apoptosis examination, the treated cells were stained with Annexin V-FITC Apoptosis Detection kit in the dark at RT for 15 min. The cell cycle distribution and the fraction of apoptotic cells were determined using a FACS analysis system.
DNA ladder
T24 cells were cultured as described above using the different treatments. After the treatments, DNA was extracted and purifed with an Apoptotic DNA Ladder kit (Beyotime, Shanghai, China) according to the manufacturer's instructions. An equal amount of purified apoptotic DNA was applied to electrophoresis on a 1.5% agarose gel, and the DNA bands were visualized by UV light and photographed.
Animal studies
Male nude mice (BALB/c nu/nu, 4 weeks old, 18–19 g) were purchased from SPF Laboratory Animal Center of Dalian Medical University (Dalian, China). Briefly, human T24 cells (5×106 in 100 µl PBS) were injected subcutaneously near the axillary fossa of the nude mice. The tumor-bearing mice were randomly divided into four treatment groups with five mice in each group. Up to three weeks, when the tumor diameters reached 4×5 mm, group A was treated with propylene glycol; group B with 10 mg/kg melatonin; group C with 30 mg/kg curcumin; group D with 10 mg/kg melatonin and 30 mg/kg curcumin by intraperitoneal injection each day. Tumors were measured with a caliper every 2 days, and the tumor volume was calculated using the formula: V = 1/2 (width2 × length). Body weights were also recorded. After treatment for 11 days, all experimental mice were terminated with general anesthetic, ether and the total weight of the tumors in each mouse was measured.
To determine COX-2 expression, the tumor tissues were harvested and freshly fixed with 10% neutral formalin and desiccated and embedded in paraffin. Sections (4 µm) were stained with hematoxylin and eosin, COX-2 antibody (1:150). The images were captured under a Leica DM 4000B fluorescence microscope equipped with a digital camera.
All the animals were given free access to sterilized food and water and were under habituation for 7 days before experiments. All procedures were in accordance with the recommendations established by Animal Care and Ethics Committee of Dalian Medical University as well as the guidelines by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Animal Care and Ethics Committee of Dalian Medical University.
Statistical analysis
The data are presented as the mean ± SD for at least three independent experiments. Statistical analysis was performed with SPSS 17.0 software. One-way analysis of variance (ANOVA) or Student's t-tests was used to evaluate the statistical significance between controls with treated groups. Results were considered statistically significant at the level of P<0.05.
Results
Melatonin and curcumin combination enhances the inhibition of cell proliferation
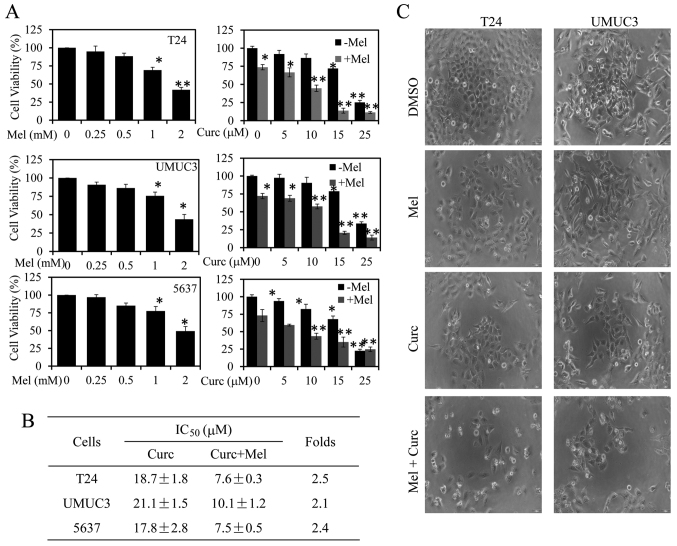
To determine whether melatonin could potentiate the inhibition of curcumin on bladder cancer cell proliferation, we first quantitatively analyzed the effect of melatonin or curcumin alone or their combination on cell proliferation and cell morphological change in human bladder cancer T24, UMUC3 and 5637 cells. As shown in Fig. 1A, treatment with melatonin or curcumin alone dose-dependently suppressed bladder cancer cell viability from 0.25 to 2 mM or 5 to 25 µM. However, combined with melatonin (1.0 mM) it could significantly enhance curcumin-mediated inhibition on cell viability as compared with curcumin alone in T24, UMUC3 and 5637 cells. The IC50 values of curcumin alone or their combination for cell proliferation inhibition in the three cell lines were next calculated. As shown in Fig. 1B, the combined treatment with melatonin (1.0 mM) resulted in a marked reduction of the IC50 values when compared to the cells treated with curcumin alone. The T24 cells were more sensitive to combinational treatment than the other two cells (Fig. 1B).
Figure 1.
Effect of melatonin and curcumin combination on cell proliferation and cell morphology changes in bladder cancer cells. (A) Human T24, UMUC3 and 5637 cells were treated with melatonin or curcumin alone or their combination at the indicated doses. At 48 h after treatment, the cell viability was determined by an MTT assay. The cells treated with vehicle control DMSO were used as the referent group with cell viability set at 100%. (B) The IC50 values of curcumin for cell viability inhibition in cells treated with or without melatonin (Mel) were determined. (C) The changes in cell morphology and spreading of T24 and UMUC3 cells treated with curcumin (10 µM) or melatonin (Mel) (1 mM) or their combination for 48 h were observed, and the cells were photographed using a microscope fitted with digital camera. Data are represented as the mean ± SD of three independent experiments. The level of significance is indicated by *P<0.05, **P<0.01.
Next, we detected the changes in cell morphology and spreading of T24 and UMUC3 cells with the combined treatment of melatonin and curcumin. As shown in Fig. 1C, the combined treatment exhibited highly reduced cell-to-cell contact and had lower spreading with fewer filopodia as compared with treatment with curcumin or melatonin alone. These results demonstrate that combination with melatonin enhanced the antitumor activity of curcumin in bladder cancer.
Melatonin and curcumin combination enhanced colony formation inhibition and cell cycle arrest
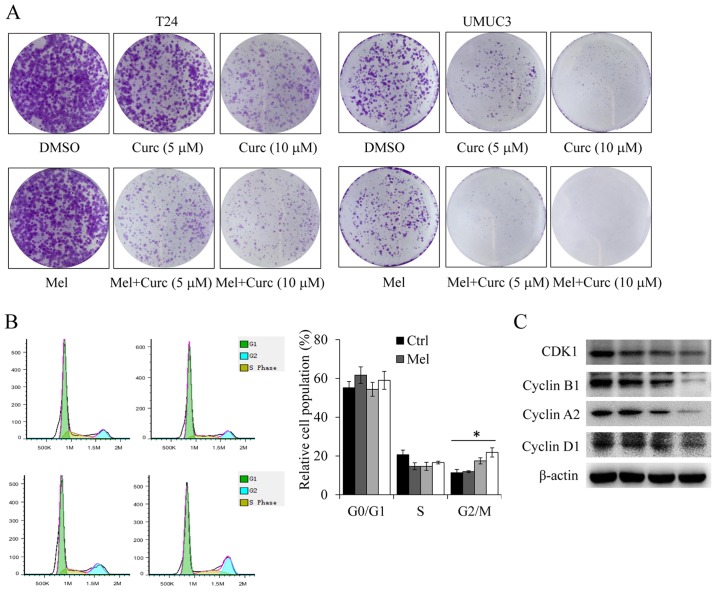
We next investigated the effect of curcumin in combination with melatonin on colony formation in bladder cancer cells by clonogenic cell survival assay. As shown in Fig. 2A, combinational treatment with melatonin and curcumin also significantly potentiated the inhibition of colony formation when compared with the single agent treatments in both T24 and UMUC3 cell lines. We next evaluated the degree of melatonin or curcumin alone or their combination on cell cycle arrest. As shown in Fig. 2B, treatment with curcumin alone induced cell cycle arrest at G2/M phase, whereas the combined treatment with melatonin exhibited a high percentage of G2/M phase.
Figure 2.
Effect of melatonin and curcumin combination on cell colony formation and cell cycle. (A) Human T24 and UMUC3 cells were treated with curcumin (10 µM) or melatonin (Mel) (1 mM) alone or their combination. The colony formation of colon cancer cells was photographed. (B) The T24 cell cycle analysis was performed after 48 h of treatment with curcumin (10 µM) or melatonin (Mel) (1 mM) alone or their combination by BD Accuri C6 Flow Cytometer. (C) The expression of the CDK1, cyclin A2, cyclin B1, and cyclin D1 proteins were analyzed by western blotting. Data are represented as the mean ± SD of three independent experiments. The level of significance was indicated by *P<0.05.
Furthermore, to ascertain detailed mechanisms, the expression of key proteins involved in cell cycle (CDK1, cyclin A2, cyclin B1 and cyclin D1) were evaluated by western blotting in T24 cells at 48 h after treatment. We found that co-treatment with melatonin (1 mM) and curcumin (10 µM) resulted in dramatic reduction of the CDK1, cyclin A, cyclin B and cyclin D1 proteins (Fig. 2C). These data provide evidence that melatonin could sensitize curcumin-mediated cell proliferation inhibition, at least in part, by inducing cell cycle arrest at G2/M phase.
Melatonin and curcumin combination enhances the inhibition of cell migration and invasion
Wound healing assays and Transwell assays were employed to detect the combinational effect of curcumin with melatonin on cell migration and invasion in human bladder cancer cells. Treatment with curcumin or melatonin (1 mM) alone suppressed cell migration and invasion; however, the combined treatment significantly enhanced this inhibition (Fig. 3A and C). Quantitative analysis for cell migration and invasion were calculated (Fig. 3B and D). Further, to ascertain detailed underlying mechanisms the combinational mode on cell migration and invasion, the key protein markers including matrix metalloproteinases (MMP-2/9) and TIMP-2 were evaluated (Fig. 3E). These results confirmed that melatonin enhanced the curcumin-mediated inhibitions of cell migration and invasion in bladder cancer cells.
Figure 3.
Effect of melatonin and curcumin combination on cell migration and invasion and the related signaling pathways in bladder cancer cells. (A) Cell migration was analyzed by a scratch assay in T24 and UMUC3 cells. After 48 h of treatment with curcumin or melatonin (Mel) alone or their combination, the wound gap was observed and photographed. (B) The percentage of migration cells were calculated relative to the original gap. (C) Cell invasion was analyzed in T24 cells treated with indicated doses of curcumin or melatonin (Mel) alone or their combination for 24 h. Cell invasion was observed and photographed (C), and the percentage of invasion cells (D) were calculated. (E) The expression of TIMP-2 and MMP-2/9 proteins were analyzed by western blotting in different treatment group. Data are represented as the mean ± SD of three independent experiments. The level of significance is indicated by *P<0.05, **P<0.01.
Melatonin and curcumin combination promotes cell apoptosis induction
We next determined whether the synergistic inhibition of cell proliferation induced by curcumin and melatonin combination is associated with the enhanced activation of the apoptotic pathway in bladder cancer cells. As shown in Fig. 4A, treatment with curcumin (10 µM) and melatonin (1 mM) alone for 48 h induced 33.8 and 29.4% of the cells to become apoptotic in T24 cells, respectively. However, the combination markedly enhanced cell apoptosis, resulting in 45.7% apoptotic cells (Fig. 4A). The apoptosis levels from DNA ladder results were consistent with those from flow cytometry analysis (Fig. 4C). We also detected the levels of apoptosis-relative proteins (cleaved caspase-3, BAX and Bcl-2) in 48 h-treated cells by western blotting. As shown in Fig. 4C, the co-treatment with melatonin (1 mM) and curcumin (10 µM) resulted in an increased level of the protein, the cleaved caspase-3 and the ratio of Bax/Bcl-2.
Figure 4.
Effect of melatonin and curcumin combination on caspase-dependent apoptosis in bladder cancer cells. T24 and UMUC3 cells were treated with melatonin (Mel, 1 mM) or curcumin (10 µM) alone or their combination. (A) At 48 h, apoptosis was determined by a FACS analysis, and the percentage of apoptotic cells was calculated relative to that in DMSO-treated cells. (B) T24 cell apoptosis in different groups was analyzed by DNA ladder respectively. (C) The expression of the cleaved caspase-3 and Bcl-2/Bax proteins in bladder cancer cells were analyzed by western blotting. (D) The releasing process of cytochrome c from mitochondria to cytoplasm was observed by immunofluorescence imaging analysis in U87 cells. (E) The protein expression of cytochrome c in the cytoplasm of these cells was detected by western blot assay.
Moreover, studies have shown that the release of cytochrome c (cyt c) from mitochondria into cytosol could induce apoptosis. We next performed immunofluorescence imaging (IFI) analysis to confirm the co-localization of cyt c and mitochondria to determine whether melatonin could sensitize curcumin-mediated cyt c release. The results showed that treatment with curcumin alone could effectively induce the release of cyt c from the intermitochondrial space into the cytosol in T24 cells, whereas co-treatment with melatonin greatly triggered the release of cyt c (Fig. 4D). Moreover, the protein level of cyt c was also detected in cytoplasm, where the mitochondrial fraction was isolated (Fig. 4E). These results indicate melatonin and curcumin combination promoted cell apoptosis induction by triggering cyt c release facilitating the caspase activation in the cytosol.
Melatonin and curcumin combination enhances COX-2 signaling inhibition
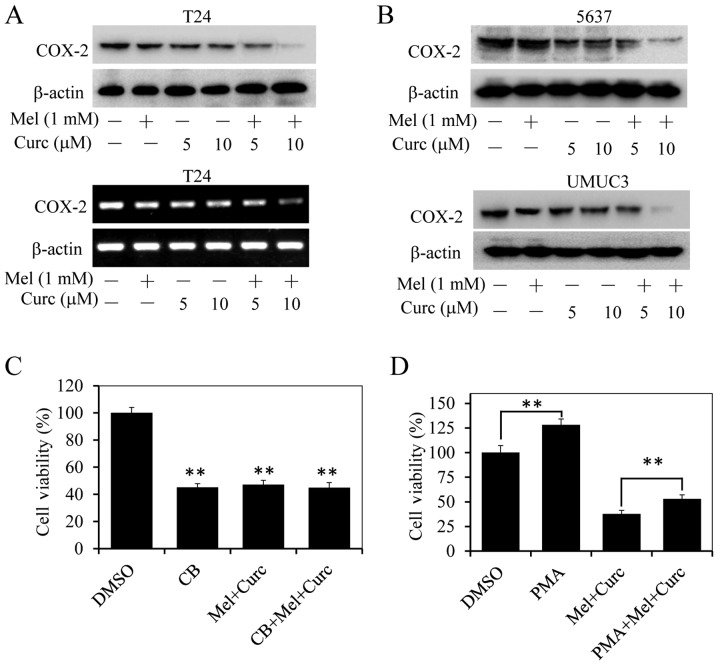
High expression of COX-2 is associated with cell proliferation, migration and invasion in cancer cells (31,32,37,38). We next evaluated the activities of crucumin and melatonin combination on COX-2 expression in bladder cancer cells at protein and mRNA levels by western blotting and RT-PCR. As shown in Fig. 5A and B, treatment with curcumin alone at the dose of 5 or 10 µM slightly inhibited COX-2 protein expression, while the combinational treatment with melatonin (1.0 mM) significantly decreased the expression of COX-2 in T24, UMUC3 and 5637 cells. Next, we pretreated T24 cells with a COX-2-selective inhibitor CB (50 µM) for 8 h, and followed with curcumin (10 µM) and melatonin (1.0 mM) co-treatment. After continuous incubation of 48 h, the cell viability was analyzed by MTT assay. As shown in Fig. 5C, treatment with CB or curcumin and melatonin alone significantly inhibited cell proliferation compared with control group, whereas CB pretreatment followed by curcumin and melatonin co-treatment did not significantly alter cell viability inhibition compared with treatment alone. Furthermore, T24 cells were also incubated with melatonin (1.0 mM) and curcumin (10 µM) co-treatment after COX-2 inducer PMA (phorbol 12-myristate 13-acetate) pretreatment for 8 h, then cell mortality was evaluated. Our results showed that inducing exogenous COX-2 overexpression by PMA could restore the cell viability of melatonin and curcumin co-treatment (Fig. 5D). The above results implied that the enhanced proliferation inhibition of this combined mode might partially be mediated through suppressing COX-2 signaling.
Figure 5.
Effect of melatonin and curcumin combination on COX-2 expression inhibition in bladder cancer cells. The expression level of COX-2 protein was analyzed by western blotting in human bladder cancer T24 (A), UMUC3 (B), and 5637 cells (C) treated with the indicated doses of curcumin and melatonin (MT) (1 mM or 1 mM) for 48 h, and its mRNA level was also analyzed in T24 cells (A). (C) T24 cells were treated with curcumin (10 µM) in combination with MT (1.0 mM) for 48 h after pretreatment with the COX-2 selective inhibitor celecoxib (CB) (25 µM) for 8 h, and the cell viability was determined by MTT analysis. (D) T24 cells were treated with curcumin (10 µM) in combination with MT (1.0 mM) for 48 h after pretreatment with COX-2 inducer PMA (200 nM) for 8 h, and the cell viability was determined by MTT analysis. Data are represented as the mean ± SD of three independent experiments. The level of significance is indicated by **P<0.01.
Melatonin and curcumin combination enhanced the inhibition of NF-κB translocation and binding to COX-2 promoter
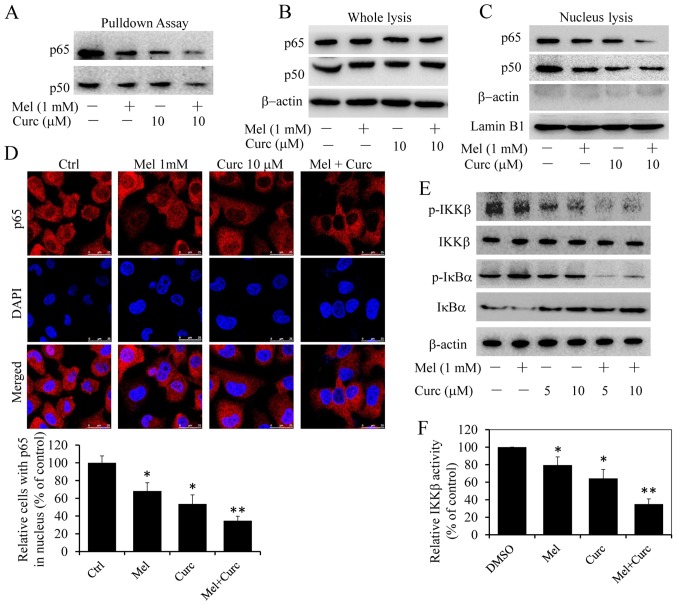
The above-mentioned results showed melatonin synergized the effect of curcumin to inhibit COX-2 expression, however COX-2 expression was regulated by several transcription factors and transcriptional coactivator on its promoter region, such as NF-κB (39). Then we carried out streptavdin-agarose pulldown assay to evaluate the enhancement of curcumin and melatonin co-treatment on NF-κB binding activities to COX-2 promoter by a DNA probe, which is a 478-bp biotin-labeled double-stranded oligonucleotide probe corresponding to the 5′-flanking sequence of the COX-2 gene from -30 to -508 (40). The results showed that combinational treatment with melatonin significantly suppressed NF-κB p65/p50 binding to COX-2 promoter DNA probe (Fig. 6A) as compared with curcumin treatment alone. We also detected the protein levels of NF-κB p50/p65 subunits in whole-cell lysates and nuclear lysates. As shown in Fig. 6B, p65/p50 protein levels in nuclear lysates decreased significantly in combinational treatment cells than in those treated with melatonin and curcumin alone, and different treatment has no obvious effects on the protein expression (Fig. 6C). To exclude the possibility of contamination between cytoplasmic and nuclear fractions, we also checked the expression of β-actin in the nuclear fraction. No obvious contamination was found (Fig. 6C). From these results, we hypothesized that the combinational treatment markedly enhanced the inhibition of the translocation of NF-κB p65/p50 dimer proteins from cell cytoplasm to nucleus. To verify this hypothesis, immunofluorescence assays were carried out. As expected, treatment with curcumin alone suppressed NF-κB p65 translocation from cell cytoplasm to nucleus, while the co-treatment enhanced the suppression (Fig. 6D).
Figure 6.
Effect of melatonin and curcumin combination on IKKβ/NF-κB signaling. T24 cells were treated with melatonin (Mel, 1 mM) or curcumin (10 µM) alone or their combination. (A) After 48-h treatment, the binding of p65 and p50 to COX-2 promoter probe was detected via streptavidin-agarose pulldown assays in T24 cells. The protein levels of p65 and p50 in cytoplasm (B) and nucleus (C) were detected by western blot analysis. (D) At 48 h after treatment, we also observed the subcellular localization of p65 by confocal microscopy analysis. The percentage of NF-κB (p65) in cell nucleus was also quantified by counting the cell number of random five fields (control group was set as 100%). (E) At 48 h after treatment, the IKKβ, p-IKKβ, IκBα and p-IκBα proteins were analyzed by western blotting. (F) At 48 h after treatment, the IKKβ kinase activity was also detected in vitro using a cell IKKβ kinase activity spectrophotometry quantitative detection kit. The activity value and percentage was calculated by bringing into the formula. Results represent the mean ± SD of three experiments. *P<0.05, **P<0.01.
Previous studies have shown that only activated NF-κB p50/p65 dimers were able to translocate to the nucleus that then promoted gene transcription (41). Furthermore, NF-κB activation depends on IκB kinase (IKK) complex which is a major upstream kinase of IκBα in canonical NF-κB signaling pathway. Next, we investigated whether the combinational treatment exerted inhibition on IKK activity with an aim to explore the potential molecular mechanisms. As shown in Fig. 6E, combinational treatment with melatonin significantly decreased the protein levels of p-IκBα and p-IKKβ when compared to treatment with curcumin alone, while had little influence on overall IκBα and IKKβ expression. Moreover, we also detected the inhibition of curcumin and melatonin on IKKβ kinase activity in vitro using a cell IKKβ kinase activity spectrophotometry quantitative detection kit. As shown in Fig. 6F, the IKKβ kinase activity inhibition was similar to the above results. All of these results supported that melatonin and curcumin combination potentiated the suppression of NF-κB/COX-2 signaling through inhibition of IKKβ kinase activity.
Melatonin and curcumin combination inhibits tumor xenografts growth in nude mouse model
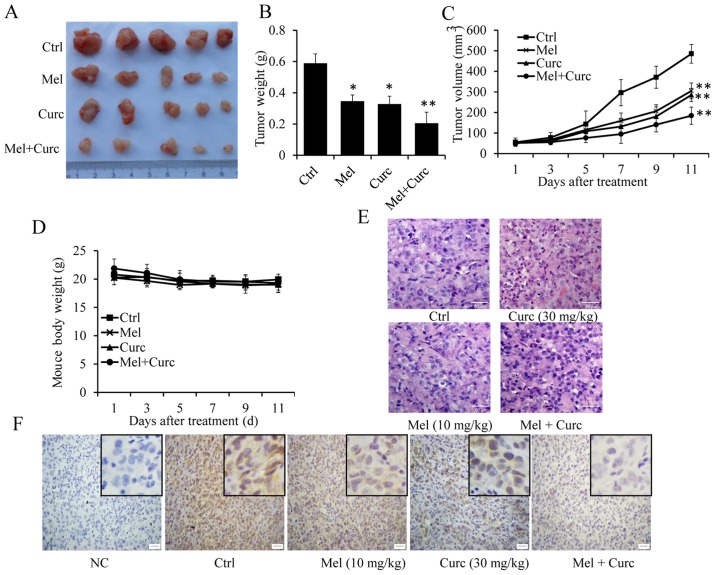
Based on the above results, we further investigated the potential of melatonin and curcumin co-treatment for tumor growth inhibition in a human bladder cancer xenograft mouse model. As shown in Fig. 7, both the tumor volume (Fig. 7A and C) and tumor weight (Fig. 7B) were inhibited by melatonin or curcumin alone. However, co-treatment with melatonin and curcumin together dramatically enhanced the growth inhibition of xenografts as compared with treatment with curcumin alone. In addition, the combined treatment did not affect significantly the body weight of the mice (Fig. 7D), and no other signs of acute or delayed toxicity were observed in the mice during treatment.
Figure 7.
Effect of melatonin and curcumin combination on tumor growth in a xenograft mouse model of human bladder cancer. An orthotopic mouse model of human T24 cells was used to evaluate the effect of melatonin and curcumin combination. The tumor images (A), tumor volume (B), tumor weight (C) and mouse body weights (D) were calculated. (E) H&E staining. (F) Immunohistochemical analysis of COX-2 protein expression in tumor samples. Neutral formalin-fixed tumor samples were prepared from animals and analyzed by immunohistochemical staining with rabbit anti-rabbit second antibody using the Vectastain Elite ABC kit, and examined under a microscope. Data were represented as the mean ± SD of three independent experiments. The level of significance is indicated by *P<0.05, **P<0.01.
In addition, HE staining also showed that the tumor cells in PBS-treated control mice were of irregular shape, and had abundant cytoplasm and large and deformed nuclei (Fig. 7E). However, in the tumor cells co-treated with melatonin and curcumin group, the nuclei were smaller and more regular in shape than those in curcumin-treated group alone (Fig. 7E). Moreover, immunohistochemical staining was also performed to examine COX-2 expression in vivoAs shown in Fig. 7F, COX-2 expression was markedly suppressed by the combined treatment with melatonin and curcumin as compared with the control group. From the above results, it was confirmed that melatonin could synergize the effect of curucmin to suppress the growth of xenografted T24 cells by downregulating the expression of COX-2.
Discussion
Curcumin, as a therapeutic or preventive agent, is involved in a variety of antitumor cellular processes, especially used for human cancers treatment (16,17,42,43). A recent phase I clinical trial showed that curcumin could produce significant biological improvements and clinical response in patients when combining with other standard chemotherapy, such as docetaxel (44). Melatonin is also known to have antitumor properties in various types of cancer with low toxicity. However, curcumin or melatonin alone is not powerful enough to damage cancer, and adjunct therapy might be meaningful way to improve their efficacy in cancer therapy. In this study, we investigated the response of human bladder cancer cells to the combined treatment of melatonin and curcumin. Our results illustrated that melatonin could enhance curcumin mediated antitumor activity through triggering cytochrome c/caspase-dependent-apoptotic pathway, and inhibiting NF-κB/COX-2 signaling pathway via suppressing IKKβ activity. Here, to best of our knowledge, this is the first report that melatonin synergized the antitumor effect of curcumin in human bladder cancer cells and to demonstrate the underlying mechanisms of action.
COX-2 signaling is implicated in regulating physiological responses in many kinds of cancer cells. It is well-known that activation of nuclear factor-κB (NF-κB) p65/p50 contributes to COX-2 overexpression through binding to sites of its promoter (45,46). Our study demonstrated that the increased COX-2 expression inhibition by curcumin and melatonin co-treatment was very possibly mediated by repressing NF-κB dimer translocation from cytosol to nucleus and further resulting in COX-2 promoter binding inhibition, thereby abrogating COX-2 transcriptional activation in bladder cancer cells.
In canonical NF-κB signaling, IκB kinase (IKK) complex is required for its activation, which could rapidly lead to the inducible phosphorylation and degradation of IκB proteins. This complex is composed of two catalytic subunits (IKKα and IKKβ) and one regulatory subunit [IKKγ/NEMO (NF-κB essential modulator)] (47). However, IKKβ kinase plays key roles for phosphorylation of IκB proteins. Phosphorylated IκB proteins subsequently undergo proteasome-mediated degradation, thereby liberating free NF-κB p65/p50 dimers to translocate to the nucleus that can then modulate gene transcription (41). Therefore, it is essential to evaluate a therapy strategy selectively targeting IKKβ, and explore its underlying molecular mechanisms regulating NF-κB activation. The present study detected the enhanced inhibition of IKKβ activity after co-treatment with curcumin and melatonin in comparison with single agent treatment, suggesting that inhibition of COX-2/NF-κB/IKKβ signaling at least partially contributed to melatonin enhanced curcumin mediated cell proliferation inhibition in bladder cancer cells.
The infiltrative growth is another major reason for tumor refractory tumor. Migration and invasion are two important prerequisites for infiltrative growth, and degradation of extracellular matrix is a key step. MMP-2 and MMP-9 as the gelatinases, are important members of MMPs family, which play a significant role in breaking through the extracellular matrix of cells (48). Our results in this study demonstrated that the combined treatment significantly inhibited MMP-2/9 expression but promoted TIMP-2 expression as compared to the treatment with curcumin or melatonin alone, implying that enhancement of cell proliferation inhibition induced by curcumin and melatonin combination is associated with increased inhibition of cell migration and invasion in bladder cancer cells.
In our study, considering that T24 cells overexpress COX-2, and have a higher ability to form xenografts in nude mice and are more sensitive to combinational treatment than the other two cells (Fig. 1B), we performed in vitro experiments in T24 cells to study the molecular mechanism. However, from the nude mouse study, we found that the folds of increasing sensitivity on tumor growth inhibition in vivo study were not greater than that in vitro study by the two chemicals. It is probably because there are other factors in vivo that could affect the combined effects of the chemicals, such as metabolism by liver or excretion by kidney.
In conclusion, the present study demonstrated that melatonin synergized the ability of curcumin to inhibit bladder cancer growth, both in vivo and in vitroFurthermore, we identified the underlying mechanisms, that is, the combi-national treatment of curcumin and melatonin on bladder cancer cells is achieved through simultaneously targeting cyto c/caspase and IKKβ/NF-κB/COX-2 signaling. These findings provide new insights into the molecular mechanisms of the combinational treatment with curcumin and melatonin on bladder cancer cell growth inhibition and provide strong evidence that combined treatment with curcumin and melatonin might exhibit an effective therapeutic option in bladder cancer therapy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 81271603 and 11472074).
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA. Bladder cancer in 2015: Improving indication, technique and outcome of radical cystectomy. Nat Rev Urol. 2016;13:74–76. doi: 10.1038/nrurol.2015.272. [DOI] [PubMed] [Google Scholar]
- 3.Bellmunt J, Guix M. New agents for bladder cancer. Ann Oncol. 2010;21(Suppl 7):vii56–vii58. doi: 10.1093/annonc/mdq367. [DOI] [PubMed] [Google Scholar]
- 4.Anghel RM, Gales LN, Trifanescu OG. Outcome of urinary bladder cancer after combined therapies. J Med Life. 2016;9:95–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 7.Shehzad A, Lee YS. Curcumin: Multiple molecular targets mediate multiple pharmacological actions - A review. Drugs Future. 2010;35:113–119. doi: 10.1358/dof.2010.035.02.1426640. [DOI] [Google Scholar]
- 8.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 9.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemo-prevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, He ZM, Wang FL, Zhang ZS, Liu XZ, Zhai DD, Chen WD. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol. 2016;772:33–42. doi: 10.1016/j.ejphar.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Kasi PD, Tamilselvam R, Skalicka-Wozniak K, Nabavi SF, Daglia M, Bishayee A, Pazoki-Toroudi H, Nabavi SM. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016;37:13017–13028. doi: 10.1007/s13277-016-5183-y. [DOI] [PubMed] [Google Scholar]
- 13.Rahmani AH, Al Zohairy MA, Aly SM, Khan MA. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. BioMed Res Int. 2014;2014:761608. doi: 10.1155/2014/761608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346:197–205. doi: 10.1016/j.canlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Tuorkey MJ. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv Med Appl Sci. 2014;6:139–146. doi: 10.1556/IMAS.6.2014.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang M, Ho JN, Kook HR, Lee S, Oh JJ, Hong SK, Lee SE, Byun SS. Theracurmin® efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol Rep. 2016;35:1463–1472. doi: 10.3892/or.2015.4537. [DOI] [PubMed] [Google Scholar]
- 17.Liu HS, Ke CS, Cheng HC, Huang CY, Su CL. Curcumin-induced mitotic spindle defect and cell cycle arrest in human bladder cancer cells occurs partly through inhibition of aurora A. Mol Pharmacol. 2011;80:638–646. doi: 10.1124/mol.111.072512. [DOI] [PubMed] [Google Scholar]
- 18.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 19.McIsaac WM, Farrell G, Taborsky RG, Taylor AN. Indole compounds: Isolation from pineal tissue. Science. 1965;148:102–103. doi: 10.1126/science.148.3666.102. [DOI] [PubMed] [Google Scholar]
- 20.Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- 21.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh M, Jadhav HR. Melatonin: Functions and ligands. Drug Discov Today. 2014;19:1410–1418. doi: 10.1016/j.drudis.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Rondanelli M, Faliva MA, Perna S, Antoniello N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: Review and remarks. Aging Clin Exp Res. 2013;25:499–510. doi: 10.1007/s40520-013-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J Pineal Res. 2014;56:225–237. doi: 10.1111/jpi.12128. [DOI] [PubMed] [Google Scholar]
- 25.Xin Z, Jiang S, Jiang P, Yan X, Fan C, Di S, Wu G, Yang Y, Reiter RJ, Ji G. Melatonin as a treatment for gastrointestinal cancer: A review. J Pineal Res. 2015;58:375–387. doi: 10.1111/jpi.12227. [DOI] [PubMed] [Google Scholar]
- 26.Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al. Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer. 2015;22:R183–R204. doi: 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ordoñez R, Carbajo-Pescador S, Prieto-Dominguez N, García-Palomo A, González-Gallego J, Mauriz JL. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 28.Marks F, Müller-Decker K Fürstenberger G. A causal relationship between unscheduled eicosanoid signaling and tumor development: Cancer chemoprevention by inhibitors of arachidonic acid metabolism. Toxicology. 2000;153:11–26. doi: 10.1016/S0300-483X(00)00301-2. [DOI] [PubMed] [Google Scholar]
- 29.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta. 2006;1761:1335–1343. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan GX, Chen P, Yu XJ, Di QS, Yu YD, Lei JH, Tai YY, Cao FJ. Cyclooxygenase-2 polymorphisms and bladder cancer risk: A meta-analysis based on case-control studies. Int J Clin Exp Med. 2015;8:3935–3945. [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuo T, Miyata Y, Mitsunari K, Yasuda T, Ohba K, Sakai H. Pathological significance and prognostic implications of heme oxygenase 1 expression in non-muscle-invasive bladder cancer: Correlation with cell proliferation, angiogenesis, lymphangiogenesis and expression of VEGFs and COX-2. Oncol Lett. 2017;13:275–280. doi: 10.3892/ol.2016.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Festa-Vasconcellos JS, Piranda DN, Amaral LM, Indio-do-Brasil V, Koifman S, Vianna-Jorge R. Polymorphisms in cycloxygenase-2 gene and breast cancer prognosis: Association between PTGS2 haplotypes and histopathological features. Breast Cancer Res Treat. 2012;132:251–258. doi: 10.1007/s10549-011-1828-0. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Li X, Yang J, Li J, Wang X, He J, Huang Z. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: A meta-analysis. Arch Med Sci. 2016;12:1110–1117. doi: 10.5114/aoms.2016.61916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park E-J, Cheenpracha S, Chang LC, Kondratyuk TP, Pezzuto JM. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[ (2′-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera. Nutr Cancer. 2011;63:971–982. doi: 10.1080/01635581.2011.589960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asting AG, Carén H, Andersson M, Lönnroth C, Lagerstedt K, Lundholm K. COX-2 gene expression in colon cancer tissue related to regulating factors and promoter methylation status. BMC Cancer. 2011;11:238. doi: 10.1186/1471-2407-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther. 2013;344:360–367. doi: 10.1124/jpet.112.200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34:413–423. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Su Z-Y, Wang L, Shu L, Yang Y, Guo Y, Pung D, Bountra C, Kong AN. Epigenetic blockade of neoplastic transformation by bromodomain and extra-terminal (BET) domain protein inhibitor JQ-1. Biochem Pharmacol. 2016;117:35–45. doi: 10.1016/j.bcp.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu ZL, Guo W, Ma XC, Zhang B, Dong P, Huang L, Wang X, Wang C, Huo X, Yu W, et al. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol Cancer. 2014;13:203. doi: 10.1186/1476-4598-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 42.Bimonte S, Barbieri A, Leongito M, Piccirillo M, Giudice A, Pivonello C, de Angelis C, Granata V, Palaia R, Izzo F. Curcumin anticancer studies in pancreatic cancer. Nutrients. 2016;8:8. doi: 10.3390/nu8070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charpentier MS, Whipple RA, Vitolo MI, Boggs AE, Slovic J, Thompson KN, Bhandary L, Martin SS. Curcumin targets breast cancer stem-like cells with microtentacles that persist in mammospheres and promote reattachment. Cancer Res. 2014;74:1250–1260. doi: 10.1158/0008-5472.CAN-13-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 45.García-Rivera D, Delgado R, Bougarne N, Haegeman G, Berghe WV. Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cells. Cancer Lett. 2011;305:21–31. doi: 10.1016/j.canlet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Kim H-N, Kim D-H, Kim E-H, Lee MH, Kundu JK, Na HK, Cha YN, Surh YJ. Sulforaphane inhibits phorbol ester-stimulated IKK-NF-κB signaling and COX-2 expression in human mammary epithelial cells by targeting NF-κB activating kinase and ERK. Cancer Lett. 2014;351:41–49. doi: 10.1016/j.canlet.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]