Abstract
Several previous studies have revealed that the expression of zinc finger E-box binding homeobox 1 (ZEB1) in solid malignancies has an important significance on the clinical outcome of patients. However, the association between ZEB1 expression and survival in patients with epithelial ovarian carcinoma (EOC) remains unclear. The objective of the present study was to examine the extent of ZEB1 expression in EOC using immunohistochemical staining and investigate its association with patient outcome. A total of 40 patients with EOC initially treated with cytoreductive surgery and systematic chemotherapy were enrolled. ZEB1 expression was immunohistochemically categorized as negative, weak, moderate and strong according to the size of the staining area, and intensity. Subsequently, the associations between ZEB1 expression and recurrence/progression-free survival (RFS) rate were examined. The median age of patients in the current study was 54 years old (range, 22–72 years old). Among these patients, 15 (37.5%) exhibited International Federation of Gynecology and Obstetrics stage I disease, and 10 (25.0%), 13 (32.5%), and 2 (5%) had stage II, III, and IV disease, respectively. No patients with negative expression of ZEB1 experienced recurrence. In addition, ZEB1 expression was identified to be a significant predictor of a poorer RFS rate compared with negative expression (negative vs. weak, moderate and strong, P=0.0126). Furthermore, multivariate analyses revealed that moderate and strong ZEB1 expression levels were significant prognostic indicators of a poorer RFS rate in patients with EOC (hazard ratio, 2.265; 95% confidence interval, 1.072–8.021; P=0.0349). Confining analysis to patients with the clear-cell/mucinous histological type, those with moderate/strong ZEB1 expression demonstrated a significantly poorer RFS rate (P=0.0025). Positive ZEB1 expression may be an indicator to predict unfavorable RFS in patients with EOC.
Keywords: epithelial ovarian carcinoma, zinc finger E-box binding homeobox 1, epithelial-mesenchymal transition, recurrence/progression-free survival, immunohistochemical staining
Introduction
Epithelial-mesenchymal transition (EMT) is a cornerstone phenomenon in which epithelial cells lose cell polarity and their cell-to-cell adhesion and acquire increased motility and invasive hallmarks to become mesenchymal-like cells (1). Cells undergoing EMT degrade the neighboring microenvironment, and migrate from the primary site to new frontier organs. Several families of transcriptional repressors, including zinc finger E-box binding homeobox 1 (ZEB1), Twist, SNAIL, and basic helix-loop-helix factors, have been identified as direct downregulators of E-cadherin transcription and representative inducers of EMT (2,3). Among these molecules, ZEB1 is known as a member of the zinc-finger E-box binding homeobox (ZFH) family, and this molecule suppresses the expression of certain microRNAs, such as miR-183, miR-203, and miR-200 family members, which function as inhibitors of stem-like hallmarks as well as positive inducers of epithelial differentiation (4). Also, it has been reported to be a major transcriptional factor in cancer progression/metastasis (5). In fact, earlier studies reported that ZEB1 promotes tumor invasiveness and metastasis and is correlated with a poorer clinical prognosis in patients with several solid cancers (6–8). According to the prior report from Siebzehnrubl et al (9), ZEB1 is an important marker of glioblastoma recurrence, including the capability of evading chemotherapy, suggesting that this molecule acts in both glioblastoma invasion and chemoresistance. In addition, silencing ZEB1 expression could significantly restore the chemosensitivity of docetaxel-resistant human lung adenocarcinoma cells as well as inhibit their migratory ability through reversing the mesenchymal phenotype (10).
Epithelial ovarian cancer (EOC) is the one of the most lethal cancers among the gynecologic malignancies worldwide, with more than 238,700 newly diagnosed cases and 151,900 reported deaths per year (11). In general, epithelial ovarian carcinoma (EOC) is a neoplasm originating from surface epithelial cells of the ovary, and it is called a silent killer because most patients with this disease are less symptomatic until the tumor has widely formed metastases in the peritoneal cavity, systematic lymph nodes, and distant parenchymal organs. Therefore, a number of EOC patients are frequently diagnosed when they enter an advanced stage (12). The majority of patients with EOC are categorized into four histological subtypes: High/low-grade serous, clear-cell, endometrioid, and mucinous carcinoma (13). In this context, EOC is biologically heterogeneous in nature, with different epidemiological and genetic backgrounds, molecular profiles, and behavioral responses toward chemotherapy and other treatments (13,14), resulting in the difficulties in establishing unified, satisfactory treatments. Moreover, approximately three in four EOC patients show a favorable initial response to cytotoxic chemotherapy; however they gradually become chemoresistant, leading to recurrence and death. Correctively, intrinsic and/or acquired resistance to chemotherapeutic agents is the primary obstacle in the actual treatment of patients with EOC. The lack of strategies to cope with the biological complexity and change to being treatment-refractory is one of the main causes preventing improvement of the patient prognosis. Thus, it is crucial to develop more precise and effective therapies from a biological point of view.
These clinical and molecular backgrounds led us to hypothesize that ZEB1 plays a central role in cancer progression, and that positive ZEB1 expression may be a helpful indicator to predict an unfavorable clinical outcome in patients with EOC. In the present study, the need for a novel investigation of the possible correlation between immunostaining expression of ZEB1 and other clinicopathologic indicators and the oncologic outcome of EOC patients was proposed.
Patients and methods
Patients and immunohistochemical staining
The total of 40 ovarian carcinomas were categorized into the following pathological types: 11 serous, 18 clear cell, 8 endometrioid, and 3 mucinous carcinomas. As the histological types, we adopted the World Health Organization (WHO) classification criteria. The clinical stage was assigned according to the International Federation of Gynecology and Obstetrics (FIGO) staging system (15,16).
Tissue samples of EOC were collected after obtaining informed consent from EOC patients who had been surgically treated at Nagoya University Hospital between 2001 and 2006. The present study was approved by the Ethics committee of Nagoya University (Approval no. 1234). Formalin-fixed, paraffin-embedded tissue sections were cut at a thickness of 4 µm. For heat-induced epitope retrieval, deparaffinized sections in 0.01 M citrate buffer (Target Retrieval Solution pH 6.1; Dako Japan Co., Ltd., Tokyo, Japan) were heated three times at 90°C for 5 min using a microwave oven. Immunohistochemical staining was performed using the avidin-biotin immunoperoxidase technique with the Histofine SAB-PO kit (Nichirei, Tokyo, Japan) according to the manufacturer's protocol, and the experimental procedure was comprehensively described previously (3). Sections were incubated at 4°C for 12 h with primary antibody (anti-rabbit-ZEB1 polyclonal, at a 1:1,000 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA). The sections were rinsed and incubated for 30 min with biotinylated anti-rabbit IgG antibody (second antibody). As a negative control, the primary antibody was replaced with normal rabbit IgG at an appropriate dilution.
Evaluation of immunohistochemical staining
For the evaluation of the results of immunohistochemical staining, 10 fields of each specimen were selected, and evaluated with both low- (×100) and high-power (×400) microscopy. Two investigators assessed the slides without knowledge of the clinicopathologic features and were blinded to each other's evaluation. The two investigators were in agreement on all the slides examined. Based on the ZEB1 immunostaining activity, a four-tiered semiquantitative score was assigned according to the intensity and area of stained cells as follows: For the evaluation of ZEB1 expression, the staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), or 3 (strong). Percentage of staining the area was scored as 0 (0%), 1 (1–10%), 2 (10–50%), and 3 (51% <) relative to the total tumor area. The sum of the staining intensity and area scores was calculated as the final score (0–6) for ZEB1. Tumors with a final score of 0, 1–2, 3–4, or 5–6 were classified as showing negative, weakly, moderately, and strongly positive expression, respectively.
Statistics
The distributions of clinicopathologic factors were statistically assessed using the Chi-square test or Fisher's exact test. The Cochran-Armitage test for trend was used to examine whether the frequency of recurrence was significantly different with each staining intensity. The recurrence/progression-free survival (RFS) was defined as the time interval between the date of surgery and date of the last follow-up or recurrence/progression. The survival curves were compared employing the Log-rank test. Survival analysis was conducted using the Kaplan-Meier method. The prognostic significance of ZEB1 expression concerning other clinicopathologic variables was assessed using the multivariate Cox's proportional hazard's analysis. All statistical analyses were performed with JMP Pro Ver.10.0 (SAS Institute Japan). P<0.05 was considered to indicate a statistically significant difference.
Results
Patients' characteristics
Patients' characteristics are detailed in Table I. The median (range) age was 52 (22–72) years. The distributions of the FIGO stage were 37.5% (15/40) stage I, 25.0% (10/40) stage II, 32.5% (13/40) stage III, and 5.0% (2/40) stage IV, respectively. Of all patients, 38 (95%) were postoperatively administered more than 3 cycles of chemotherapy. Two patients (5.0%) did not undergo postoperative chemotherapy owing to their strong wishes or severe complications. A total of 6 patients had residual tumor at the initial surgery. The ZEB1 immunoreactivity was classified into the four scoring types as described in ‘Patients and methods’ (negative, weakly, moderately, and strongly positive expressions). Representative images of each histological feature are shown in Fig. 1.
Table I.
Patients' characteristics.
| Characteristics | No. | % |
|---|---|---|
| Total | 40 | |
| Age | ||
| Median (range) | 54 (22–72) | |
| ≤50 | 13 | 32.5 |
| >50 | 27 | 32.5 |
| FIGO stage | ||
| I | 15 | 37.5 |
| II | 10 | 25.0 |
| III | 13 | 32.5 |
| IV | 2 | 5.0 |
| Histological type | ||
| Serous | 11 | 27.5 |
| Mucinous | 3 | 7.5 |
| Endometrioid | 8 | 20.0 |
| Clear-cell | 18 | 45.0 |
| Surgery | ||
| Standard surgery + RPN | 19 | 47.5 |
| Standard surgery + intestine resection | 4 | 10.0 |
| Standard surgerya | 13 | 32.5 |
| Exploratory laparotomy | 4 | 10.0 |
| Chemotherapy | ||
| Taxane plus platinum | 37 | 92.5 |
| Conventinal platinum-based | 1 | 2.5 |
| None | 2 | 5.0 |
| Recurrence/progression | ||
| Yes | 19 | 47.5 |
| No | 21 | 52.5 |
| ZEB1 immunostaining classification | ||
| Negative | 7 | 17.5 |
| Weakly positive | 14 | 35.0 |
| Moderately positive | 11 | 27.5 |
| Strongly positive | 8 | 20.0 |
FIGO, International Federation of Gynecology and Obstetrics; RPN, retroperitoneal lymphadenectomy
Standard surgery, including hysterectomy, bilateral salpingo-oophorectomy, with or without omentectomy.
Figure 1.
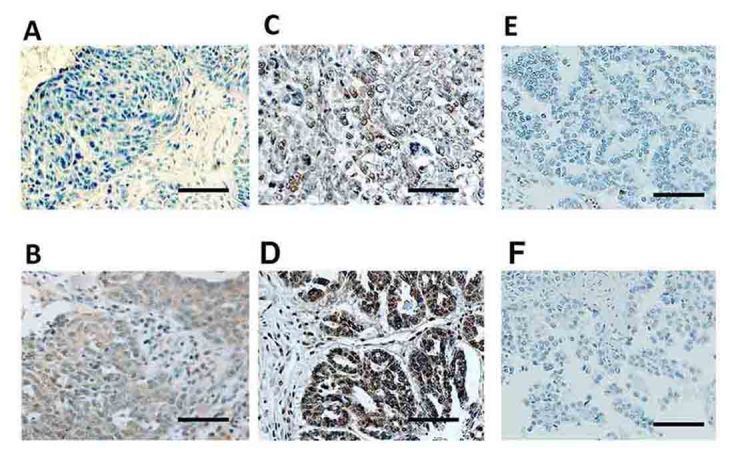
Survival impact of ZEB1 expression in EOC tissues. Immunoreactivity of ZEB1 observed in surgical EOC samples (paraffin sections), positive or negative expression of ZEB1 in EOCs. (A) Negative (serous carcinoma), (B) weakly positive (clear-cell carcinoma), (C) moderately positive (serous carcinoma), (D) strongly positive (mucinous carcinoma); magnification ×100. (E and F) Negative controls (the primary antibody was replaced with normal rabbit IgG: Clear-cell carcinoma), Bars: 100 µm.
In several cases, the immunoexpression of ZEB1 was found in the stroma as well as carcinoma tissues. Of the 40 carcinomas, negative, weakly, moderately, and strongly positive ZEB1 immunoexpressions were observed in 7 (17.5%), 14 (35.0%), 11 (27.5%), and 8 (20.0%) patients, respectively.
Table II shows the association between ZEB1 expression and clinicopathologic parameters of primary EOC. Two categorized ZEB1 expressions (negative + weakly, moderately + strongly positive) were not correlated with any of the clinicopathologic parameters examined: Age, histological type, FIGO stage, and surgical procedure.
Table II.
Relationship between the expression of ZEB1 and clinicopathologic parameters of primary EOC.
| ZEB1 | |||||
|---|---|---|---|---|---|
| Negative-Weak | Moderate-Strong | ||||
| Parameters | N | % | N | % | P-value |
| Total | 21 | 52.5 | 19 | 47.5 | |
| Age | |||||
| ≤50 | 6 | 15.0 | 7 | 17.5 | 0.737 |
| >50 | 15 | 37.5 | 12 | 30 | |
| FIGO | |||||
| I+II | 15 | 37.5 | 10 | 25.0 | 0.328 |
| III+IV | 6 | 15.0 | 9 | 22.5 | |
| Histological type | |||||
| Serous | 4 | 10.0 | 7 | 17.5 | 0.163 |
| Mucinous | 3 | 7.5 | 0 | 0 | |
| Endometrioid | 3 | 7.5 | 5 | 12.5 | |
| Clear-cell | 11 | 27.5 | 7 | 17.5 | |
| Surgery | |||||
| Standard surgery + RPN | 11 | 27.5 | 8 | 20.0 | 0.692 |
| Standard surgery + intestine resection | 2 | 5 | 2 | 5.0 | |
| Standard surgerya | 7 | 17.5 | 6 | 15.0 | |
| Exploratory laparotomy | 1 | 2.5 | 3 | 7.5 | |
FIGO, International Federation of Gynecology and Obstetrics; RPN, retroperitoneal lymphadenectomy
Standard surgery, including hysterectomy, bilateral salpingo-oophorectomy, with or without omentectomy.
Oncologic outcome and the extent of ZEB1 positivity
The median follow-up duration was 94.8, ranging from 3.8–202.0 months in the surviving patients. During this period, 19 patients (47.5%) developed recurrence. The median time to recurrence was 10.8 months. The five-year RFS rate of all EOC patients was 52.5%.
Regarding the frequency of recurrence according to the extent of ZEB1 positivity, there was no patient with negative expression of ZEB1 who experienced recurrence. Patients with higher ZEB1 positivity showed a higher rate of recurrence (Cochran-Armitage test for trend, P=0.042) (Fig. 2). Confining analysis to patients with the mucinous/clear-cell histological type, the frequency of an unfavorable oncologic outcome (death) was higher in patients with higher ZEB1 positivity (Moderate + Strong expression) (P=0.0086) (Fig. 3). Furthermore, compared with negative expression, two-scale positive ZEB1 expression predicted a significantly poorer RFS {Negative vs. weak, moderate, and strong (P=0.002), Negative plus weak vs. moderate plus strong (P=0.001)} (Fig. 4). Confining analysis to patients with the mucinous/clear-cell histological type, patients with lower ZEB1 expression (negative/weak) showed poorer RFS than those with higher ZEB1 expression (moderate/strong) (P=0.0025) (Fig. 5). In the current survival analyses, the post-hoc powers we calculated were ranging from 0.565 to 0.786.
Figure 2.
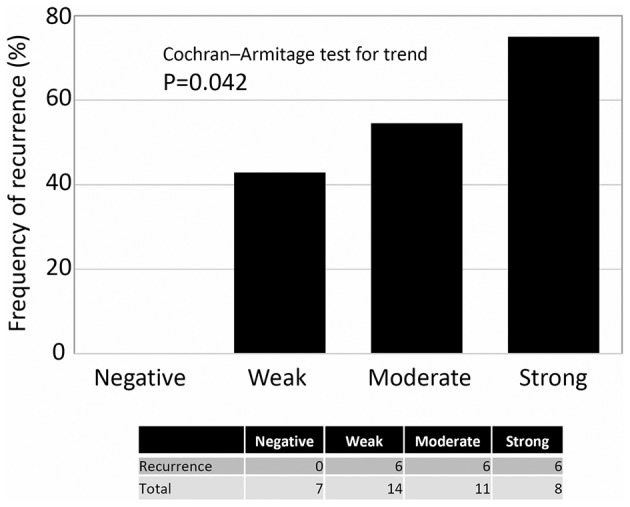
The frequency of recurrence according to the extent of ZEB1 positivity. There was no patient with negative expression of ZEB1 who experienced recurrence. Patients with higher ZEB1 positivity showed a higher rate of recurrence (Cochran-Armitage test for trend, P=0.042).
Figure 3.
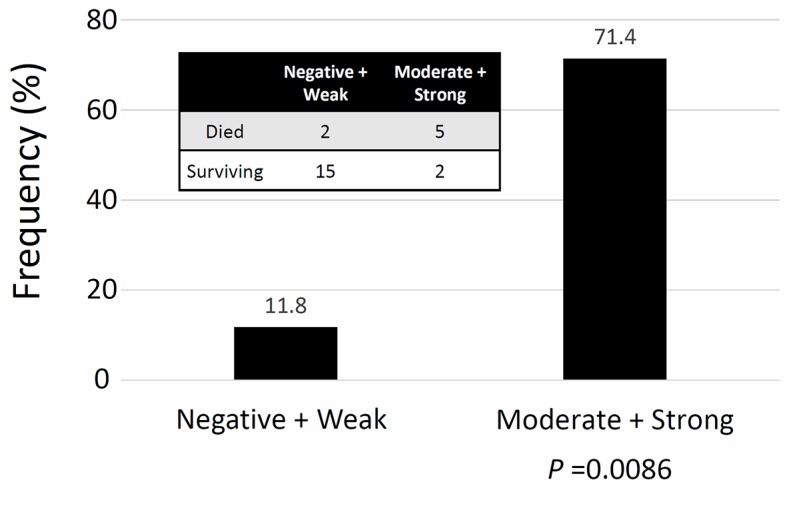
The frequency of recurrence according to the extent of ZEB1 positivity in patients with the clear-cell/mucinous histological type. Confining analysis to patients with the clear-cell/mucinous histological type, the frequency of an unfavorable oncologic outcome (death) was higher in patients with a higher ZEB1 positivity (Moderate + strong expression) (Fisher's exact test: P=0.0086).
Figure 4.
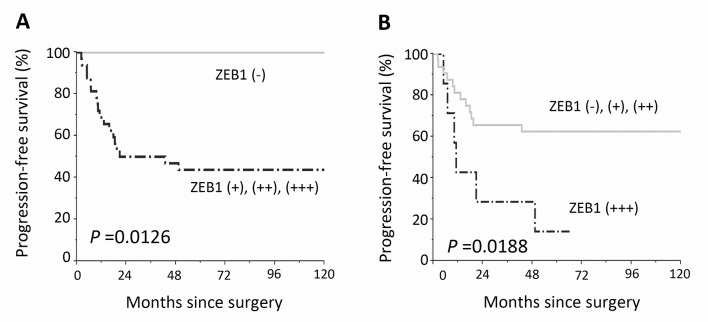
Kaplan-Meier progression-free survival curves for primary EOC patients according to the immunoreactivity of ZEB1. (A) Solid line indicates negative ZEB1 expression (negative: N=7). The discontinuous line represents positive ZEB1 immunoexpression (weakly, moderately, and strongly positive: N=33). Patients with positive ZEB1 expression showed a significantly poorer recurrence/progression-free survival (P=0.0126). (B) The solid line represents negative, weakly, and moderately positive ZEB1 expression (N=32). The discontinuous line represents strongly positive ZEB1 immunoexpression (N=8). Patients with strongly positive ZEB1 expression showed a significantly poorer recurrence/progression-free survival (P=0.0188).
Figure 5.
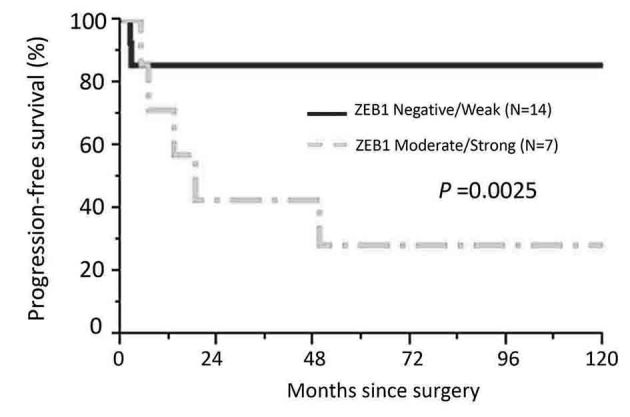
Kaplan-Meier progression-free survival curves in patients with the mucinous/clear-cell histological type according to the immunoexpression of ZEB1. The solid line represents negative-weakly positive ZEB1 expression (negative: N=14). The discontinuous line represents moderately-strongly positive ZEB1 immunoexpression (N=7). Patients with moderate-strong ZEB1 expression showed a significantly poorer recurrence/progression-free survival (P=0.0025).
Multivariate analysis
In multivariate RFS analyses, age (≤50 vs. >50), FIGO stage (I+II vs. III+IV), histological type (serous/endometrioid vs. mucinous/clear-cell), and ZEB1 immunoreactivity (negative/weak vs. moderate/strong) were included in the Cox proportional hazard analysis. The age, histological type, and ZEB1 expression were significant independent prognostic indicators of a poor RFS. The hazard ratio (HR) for moderately/strongly positive ZEB1 expression was as follows: HR: 2.2265, 95% CI: 1.102–8.021; P=0.0349) (Table III).
Table III.
Uni- and multivariable analyses of clinicopathologic parameters in relation to recurrence/progression-free survival of patients
| Recurrence/progression-free survival | ||||
|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | |||
| Parameters | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| Age | ||||
| ≤50 | 1 | 1 | ||
| >50 | 0.469 (0.188–1.183) | 0.106 | 0.323 (0.114–0.908) | 0.0325 |
| FIGO | ||||
| I–II | 1 | 1 | ||
| III–IV | 3.450 (1.373–9.057) | 0.0087 | 5.013 (1.679–15.952) | 0.0038 |
| Histological type | ||||
| S/E | 1 | 1 | ||
| M/C | 0.529 (0.202–1.320) | 0.172 | 0.738 (0.422–3.211) | 0.2966 |
| ZEB1 expression | ||||
| Negative/weak | 1 | 1 | ||
| Moderate/strong | 3.415 (1.329–9.865) | 0.010 | 2.265 (1.072–8.021) | 0.0349 |
FIGO, International Federation of Gynecology and Obstetrics; S/E, serous/endometrioid carcinoma; M/C, mucinous/clear-cell carcinoma.
Recurrence site in relapsed patients
Table IV shows the clinical features in the 18 relapsed patients. ZEB1 was expressed in 17 of the 18 (94.4%) patients. In the majority of relapsed patients, the most frequent site of recurrence was the peritoneal cavity (16/18:88.9%).
Table IV.
Clinical features and the ZEB1 immunostaining intensity in the relapsed patients.
| Case | Age | Histological type | FIGO stage | Rec. site | Time to Rec. (Mo) | Follow-up (Mo) | ZEB1 expression score |
|---|---|---|---|---|---|---|---|
| 1 | 70 | S | IIIC | PC | 15.9 | 34.0 | 2 |
| 2 | 50 | S | IIC | PC, Distant | 43.4 | 107.3 | 3 |
| 3 | 57 | S | IIIC | PC | 21.0 | 55.0 | 4 |
| 4 | 61 | S | IVB | PC | 10.2 | 52.5 | 4 |
| 5 | 69 | S | IIIC | PC | 10.4 | 38.8 | 3 |
| 6 | 47 | S | IVB | PC | 17.9 | 32.4 | 2 |
| 7 | 48 | S | IIIC | PC | 9.6 | 31.4 | 2 |
| 8 | 22 | M | IIB | PC | 2.2 | 3.8 | 2 |
| 9 | 49 | E | IIIC | PC | 11.2 | 31.3 | 4 |
| 10 | 53 | E | IIIC | RPM, Distant | 19.6 | 38.6 | 2 |
| 11 | 53 | E | IIC | RPM, Distant | 4.9 | 84.2 | 4 |
| 12 | 58 | E | IIIC | PC | 6.9 | 14.4 | 4 |
| 13 | 53 | C | IC | PC | 6.8 | 10.8 | 3 |
| 14 | 38 | C | IIB | PC | 13.3 | 24.1 | 3 |
| 15 | 39 | C | IA | PC | 18.6 | 49.7 | 3 |
| 16 | 57 | C | IIB | PC, Distant | 2.4 | 4.3 | 2 |
| 17 | 57 | C | IIIC | PC | 49.9 | 74.8 | 4 |
| 18 | 48 | C | IIIC | PC | 4.9 | 88.2 | 3 |
FIGO (2014), International Federation of Gynecology and Obstetrics; S, serous carcinoma; M, mucinous carcinoma; E, endometrioid carcinoma; C, clear-cell carcinoma; PC, peritoneal cavity; RTN, retroperitoneal lymph node; Distant, distant parenchymal organs; Rec, recurrence.
Discussion
In general, patients with EOC show an unfavorable prognosis, principally owing to its asymptomatic hallmark until the late stage, and it is frequently linked with disseminated intraperitoneal and/or distant metastases (17–19). Especially, peritoneal dissemination is the most common presentation, consisting of multi steps: First, tumor cells are released from the original tumor, and then they migrate in the abdominal cavity. When the tumor cells attach to the peritoneum, they start to invade tissues through the mesothelium (20,21). In the current investigation of 40 EOC patients, various levels of ZEB1 expression were identified in 82.5% (33 of 40), and patients with higher ZEB1 expressions showed a significantly poorer prognosis. Furthermore, multivariate analyses showed that a higher expression of ZEB1 was an independent prognostic indicator of a poorer RFS of EOC patients. Currently, studies demonstrated the important association between ZEB1 expression and aggressive phenotypes in several solid malignancies. Spoelstra et al (22) revealed that ZEB1 was aberrantly expressed in carcinoma cells of aggressive poorly-differentiated endometrioid carcinomas and other kinds of aggressive endometrial cancers, including uterine serous carcinomas. In addition, Hashiguchi et al (8) reported the clinical effect of ZEB1 and E-cadherin expression in 108 patients with primary hepatocellular carcinoma. They demonstrated that positive immunohistochemical activity of ZEB1 was significantly correlated with a reduced expression of E-cadherin, and those with positive ZEB1/reduced E-cadherin expression particularly showed a poorer overall survival. These previous findings are consistent with our current results. If ZEB1 is directly linked with EMT of EOC, a ZEB1-positive clone may easily spread into the peritoneal cavity and have a greater opportunity to adhere to the mesothelium, resulting in the increasing formation of micro-and/or macroscopic peritoneal disseminations. According to earlier study from Chen et al (23), silencing of ZEB1 expression induced the colony-forming, wound-healing, and cellular migration abilities were downregulated with enhanced the expression of miR-200c to inhibit the epithelial-mesenchymal transition in ovarian cancer cells. In our next study, we aim to perform functional analysis of ZEB1 in EOC. At least, the current findings indicate that the immunoreactive identification of ZEB1 expression might be a crucial predictor of patients who will show a poor oncologic outcome and its identification may lead to the selection of better treatment strategies.
As well as metastasis to the peritoneal cavity and distant parenchymal organs, intrinsic or acquired chemoresistance remains a major challenge to improve the prognosis of patients with EOC. Recently, several studies suggested that the EMT shows therapy resistance, resulting in tumor recurrence (24–26). Also, the EMT-inducer ZEB1 was revealed to be involved in tumor stemness and treatment resistance. ZEB1 represses miR-200 as well as miR-203, which can also suppress stemness hallmarks (27). The level of ZEB1 is also upregulated in melanoma cells with acquired resistance and in biopsies from relapsed patients during treatment (28). Additionally, patients with a mucinous and clear-cell histology generally showed a very low response rate to platinum-based chemotherapy, leading to intrinsic chemoresistance (29,30). Indeed, in patients with the mucinous/clear-cell histological type, the frequency of an unfavorable oncologic outcome was higher in those with higher ZEB1 positivity. ZEB1 expression may be involved in inheriting or the acquisition of EOC chemoresistance. The mechanism of patients with ZEB1 expression showing an unfavorable clinical outcome may be due to both the chemoresistant hallmark and metastasis-promoting effect of ZEB1 in the peritoneum via EMT. Indeed, in our study, the majority of patients with positive ZEB1 expression experienced recurrence {17/18 (94.4%)}. The remaining chemoresistant clone, which is linked with ZEB1 expression, may be a cause of the high rate of recurrence. However, a functional analysis of ZEB1-expressing EOC cells was not carried out. Therefore, we can only hypothesize regarding the possibility of close linkage between chemoresistance and ZEB1 expression in EOC at present. We hope to test this hypothesis in the next study in order to clarify the EOC-specific biological hallmarks.
In the present study, there were several limitations, including a non-prospective, exploratory study, limited patient number, heterogeneous treatment modalities, and different follow-up periods. Especially, reflecting the small-scale patient number, our study did not have sufficient power. Nevertheless, we observed statistically significant difference at least in the two group comparison regarding ZEB1 expression (negative/weak vs. moderate/strong). The result indicates our patients were not necessary insufficient to withdraw the conclusion that the ZEB1 expression was significant prognostic indicator of EOC. In addition, the heterogeneity of EOC is now the biggest challenge in all relevant studies. To investigate the effect of the ZEB1 expression in each histological type, we had categorized patients into the mucinous/clear-cell and other histological type. Nevertheless, we sincerely felt that the number of patients was so limited. Therefore, our finding that there was an association between ZEB1 expression and the unfavorable oncologic outcome of EOC patients is only weakly supported. We need to reanalyze and confirm the expression of ZEB1 in EOC samples in a larger patient population.
In conclusion, to our knowledge, this is the first study showing that the expression of ZEB1 was closely associated with a poor oncologic outcome of patients with EOC. The current findings may be based on the metastasis- and/or chemoresistant-promoting effects of ZEB1, although further investigation is needed to clarify the molecular mechanisms of ZEB1. In addition, at present, there are a number of problems, including the histological heterogeneity and lack of power. Nevertheless, our evidence provided sheds some light on the clinical and biological behavior of this malignancy. Although the current findings must be confirmed by other future studies, the expression of ZEB1 can be a helpful predictor factor for metastasis and/or relapse of EOC. We believe that this will help improve EOC treatment by adding criteria for the administration of systematic therapy in the future.
Acknowledgements
We sincerely thank Dr K. Tamakoshi (Nagoya University, School of Health Science), for his advice on statistical analyses as an expert statistician.
References
- 1.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosono S, Kajiyama H, Terauchi M, Shibata K, Ino K, Nawa A, Kikkawa F. Expression of Twist increases the risk for recurrence and for poor survival in epithelial ovarian carcinoma patients. Br J Cancer. 2007;96:314–320. doi: 10.1038/sj.bjc.6603533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Ahn YH, Chen Y, Tan X, Guo L, Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, et al. ZEB1 sensitizes lung adenocarcinoma to metastasis suppression by PI3K antagonism. J Clin Invest. 2014;124:2696–2708. doi: 10.1172/JCI72171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–3456. doi: 10.1007/s00018-012-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashiguchi M, Ueno S, Sakoda M, Iino S, Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et al. Clinical implication of ZEB-1 and E-cadherin expression in hepatocellular carcinoma (HCC) BMC Cancer. 2013;13:572. doi: 10.1186/1471-2407-13-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren J, Chen Y, Song H, Chen L, Wang R. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem. 2013;114:1395–1403. doi: 10.1002/jcb.24481. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 12.Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::AID-SSU2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23:x111–x117. doi: 10.1093/annonc/mds300. (Suppl 10) [DOI] [PubMed] [Google Scholar]
- 14.Groen RS, Gershenson DM, Fader AN. Updates and emerging therapies for rare epithelial ovarian cancers: One size no longer fits all. Gynecol Oncol. 2015;136:373–383. doi: 10.1016/j.ygyno.2014.11.078. [DOI] [PubMed] [Google Scholar]
- 15.Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839–842. doi: 10.1007/s00404-014-3364-8. [DOI] [PubMed] [Google Scholar]
- 16.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:S2631–S2642. doi: 10.1002/cncr.11345. (10 Suppl) [DOI] [PubMed] [Google Scholar]
- 17.Kajiyama H, Shibata K, Mizuno M, Umezu T, Suzuki S, Yamamoto E, Fujiwara S, Kawai M, Nagasaka T, Kikkawa F. Long-term clinical outcome of patients with recurrent epithelial ovarian carcinoma: Is it the same for each histological type? Int J Gynecol Cancer. 2012;22:394–399. doi: 10.1097/IGC.0b013e31823eed2c. [DOI] [PubMed] [Google Scholar]
- 18.Kikkawa F, Nawa A, Ino K, Shibata K, Kajiyama H, Nomura S. Advances in treatment of epithelial ovarian cancer. Nagoya J Med Sci. 2006;68:19–26. [PubMed] [Google Scholar]
- 19.Yoshikawa N, Kajiyama H, Mizuno M, Shibata K, Kawai M, Nagasaka T, Kikkawa F. Clinicopathologic features of epithelial ovarian carcinoma in younger vs. older patients: Analysis in Japanese women. J Gynecol Oncol. 2014;25:118–123. doi: 10.3802/jgo.2014.25.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 21.Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K, et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24:329–339. doi: 10.1007/s10585-007-9070-1. [DOI] [PubMed] [Google Scholar]
- 22.Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893–3902. doi: 10.1158/0008-5472.CAN-05-2881. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Wang J, Zhang Y, Chen J, Yang C, Cao W, Zhang H, Liu Y, Dou J. Effect of down-regulated transcriptional repressor ZEB1 on the epithelial-mesenchymal transition of ovarian cancer cells. Int J Gynecol Cancer. 2013;23:1357–1366. doi: 10.1097/IGC.0b013e3182a5e760. [DOI] [PubMed] [Google Scholar]
- 24.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 25.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 26.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 27.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 28.Richard G, Dalle S, Monet MA, Ligier M, Boespflug A, Pommier RM, de la Fouchardière A, Perier-Muzet M, Depaepe L, Barnault R, et al. ZEB1-mediated melanoma cell plasticity enhances resistance to MAPK inhibitors. EMBO Mol Med. 2016;8:1143–1161. doi: 10.15252/emmm.201505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I, Taguchi K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, Nishimura R, Tabata T, Sugiyama T, Kaku T. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113:331–334. doi: 10.1016/j.ygyno.2009.02.010. [DOI] [PubMed] [Google Scholar]