Abstract
The poor therapy response and poor prognosis of esophageal cancer has made it one of the most malignant carcinoma, and the complicated multidisciplinary treatment failed to achieve a long-term disease-free survival. To diagnose esophageal cancer at an earlier stage, and to improve the effect of anticancer therapy would improve the therapeutic efficacy. After retrospective analysis of the cancer samples of patients who received esophagectomy, we found the relevance between ratio of either ALDH1 or CD133-positive cancer stem cells and 2-year recurrence. Higher ratios of cancer stem cells indicated later clinical stages, and Wnt signaling activation was more frequent in later esophageal carcinoma. Further in bench studies, we explored the suppressive roles and the mechanisms involved in Let-7 on self-renewal in ECA-109 and ECA-9706 esophageal cancer stem cells. Isolated cancer stem cells naturally divide symmetrically and are therapy resistant. Therapy of fluorouracil and docetaxel both enriched the stem cells, proving the resistant characteristics of cancer stem cells. Wnt activation stimulated more symmetric division of stem cells, resulting in self-renewal promotion, which could be blocked by Let-7 overexpression. Furthermore, enforced Let-7 sensitized the stem cells to chemotherapies in a Wnt pathway inhibition-dependent manner, contributing to Let-7 sensitization of chemotherapeutic response. Wnt activation weakened the suppressive Let-7b through the sponge functions of CCAT-1, forming the negative feedback loop of Let-7b/Wnt/CCAT1. These results identified the crucial participation of stem cells in esophageal cancer occurrence and progression as the potent indicator, and also indicate the potential powerful agent of Let-7 nano-particles in treatment of cancer.
Keywords: esophageal cancer, Wnt signaling, Let-7b, cancer stem-like cells, division manner
Introduction
Higher recurrence rate and poorer survival prognosis have made esophageal cancer one of the most lethal malignancies, ranked the fourth in cancer-related mortality (1–3). The overall 5-year survival rate varies from 15 to 25%, and still no efficient treatments are available (2). Non-coding Let-7 could target and degrade its downstream CCND1, HMGA2, RAS and other oncogenic factors to function as one of the strongest suppressors in both cancer cells and cancer stem cells (4,5). The novel regulative mechanisms of miRNAs were defined as 'Sponge action' (6,7). The miRNA sponge was accepted as an innovative concept to regulate miRNAs (8), which could produce segments of RNA, containing repeats of tandem-binding sites, which were complementary to seed regions of certain miRNAs (9). Through base-pair-dependent interaction to the seed region, the sponge leads to a reduction of active miRNAs. Adenoviral and lentiviral constructs of miRNA sponges utilize RNA polymerase III for transcription (10), and in detail, H19, CCAT1 of Lnc-RNAs and other circular-RNAs were the molecular sponge corresponding to Let-7 expression (11,12).
Tumorigenic transformation occurs in the immortal or repeatedly dividing cells more commonly, and cancer stem-like cells (CSCs) were blamed for tumor recurrence and resistance (5,13), whereas, how these CSCs emerge is still unclear. Cancer stem cells could be identified and isolated by FACS sorting in cell lines, and be identified in cancer tissues by immunofluorescence or immunohistochemical staining (14–16). Esophageal cancer stem cells could be identified with surface markers of CD133 (17,18) and ALDH1 (19–23). The treatments aiming to eliminate the stem cells will help in cancer treatment, yielding diagnostic and therapeutic approaches (4,5). The way stem cells divide affects greatly the stem cell numbers, but how the division influence the cell renewal capacity is still in debate. Carcinogenesis may arise as a consequence of adult stem-cell dysfunction, which fails to undergo asymmetric cell division (ACD) (24,25). The fine regulations of stem cells allow themselves to self-renew and generate the differentiated cells, forming and maintaining mature tissues and organs. The uncontrolled symmetric cell division (SCD) will expand the stem cell pool, resulting in numerous stem-like cells in carcinoma (26,27).
The aim of ACD is to create two different daughter cells; one is to sustain the stem cell group, and another is to differentiate into certain type. The way to achieve this is the asymmetric segregation of cell fate determinants, such as Numb, PKC, and p53 (28–31), which could instruct the cell that inherits it to adopt a certain identity (27). The asymmetric distribution of cell fate determinants makes the cells segregate in a polarized way, with the mitotic spindle enriched asymmetrically. The influences on ACD decrease the stem cell number, determining the stem cells fate. The division manner in esophageal cancer cells, and the relationship between the stem-like cells and cancer biology are barely known in esophageal cancer. In the present study, we explored the mechanistic phenotypes of division of stem-like cells in esophageal cancer, and the application of tentative usage of nanoliposomal non-coding RNA in cancer treatment.
Materials and methods
Enrollment of patients
From July 2008 to November 2014, 317 Chinese patients consecutively underwent radical esophagectomy and reconstruction for esophageal tract at the First Affiliated Hospital of Xi'an Jiaotong University, were evaluated and enrolled. The pathological examinations were confirmed and filed in order. The clinicopathological characteristics, and results of pathological sections were collected and shown in detail in Table I. Patients diagnosed with phase IV carcinoma preoperatively were not enrolled, and grouped patients with phase IV were all diagnosed postoperatively. Histopathologic evaluation was confirmed by two separate pathologic professors, and patients were diagnosed with no other system malignancies. Written informed consent was obtained from each patient, in accordance with the Declaration of Helsinki before sample collection. The study protocol and patients' informed consent statements were approved and supervised by the Ethics committee of the First Affiliated Hospital and the Second Affiliated Hospital of Xi'an Jiaotong University.
Table I.
The clinicopathological characteristics of patients enrolled (N=317).
| Category | RE |
|---|---|
| Sex | |
| Male/Female | 255/62 |
| Age (median) | 53.2 |
| <65/≥65 | 204/113 |
| Location of tumor | |
| Upper/middle/lower | 44/151/122 |
| Histological type | |
| SC/AC/AS | 260/39/18 |
| Neoadjuvant or adjuvant therapy | |
| Yes/No | 270/47 |
| Neoadjuvant therapy/adjuvant therapy | |
| Yes/No | 29/241 |
| Recurrence within 2 years | |
| Yes/No | 74/243 |
| Pathological tumor stage | |
| I/II/III/IV | 14/156/135/12 |
| Operation time | |
| <4/≥4 hours | 90/227 |
SC, squamous carcinoma; AC, adenocarcinoma; AS, adenosquamous; RE, radical esophagectomy.
Isolation and culturing of stem cells
Esophageal cancer cells were maintained at 37°C, 5% CO2 in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclone, Salt Lake City, UT, USA) and 1% penicillin/streptomycin (Cellgro, Lowell, MA, USA). fluorouracil and docetaxel were used at a concentration of 0.2 mM. The stem-like cells were cultured as we previously described. Briefly, cells were grown in ultra-low attachment dishes (Corning Inc., Lowell, MA, USA), supplemented with serum-free medium (DMEM/F12), 1:50 B27 (Invitrogen), 20 ng/ml recombinant human basic FGF (Invitrogen), 5 μg/ml insulin, 0.5 mg/ml hydrocortisone and 20 ng/ml epidermal growth factor (Invitrogen). The sphere forming efficiency was calculated as the ratio of obtained spheres verses number of plated cells (spheres/1,000 cells). All sphere formation experiments were performed in triplicate independently.
RNA and protein detection
Total RNA was extracted from cells with TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. The reverse-transcription was conducted by using Prime Script™ RT reagent kit (Takara, Dalian, China). The qRT-PCR was performed on a CFX96™ Real-Time PCR Detection system (Bio-Rad, USA) with SYBR® Premix Ex-Taq™ II (Takara). The primers for qRT-PCR detection were synthesized by Invitrogen (Shanghai, China). RNU6B (for mature miRNA) or 18S was taken as internal control. Fold change was determined as 2−ΔΔCt. All experiments were performed in triplicate, independently. Cell lysates were prepared with RIPA buffer containing Protease Inhibitor Cocktail Tablets (Roche) for 15 min on ice. The total protein concentrations were determined using Protein BCA assay kit (Bio-Rad). Protein samples were denatured with 5X loading buffer at 100°C for 5 min. Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto NC membranes (Bio-Rad). The membrane was blocked with 5% non-fat milk for 2 h at room temperature, and subsequently incubated with primary antibody overnight at 4°C. The primary antibodies used were as follows: HMGA2 (1:1,000, ab52039; Abcam, Cambridge, MA, USA), CCND1 (1:1,000; #2922; Cell Signaling Technology, Inc., USA), TCF-4 (1:1,000, ab60727; Abcam), β-catenin (1:2,000, ab78483; Abcam). Then the membranes were incubated with HRP-conjugated secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.) for 2 h. An anti-vinculin antibody (1:5,000, #4650; Cell Signaling Technology, Inc.) was used as internal control.
Construction of CCAT-1 and Let-7b deregulated cells
For constructing cells with enforced CCAT1, a genomic region encoding CCAT1 was PCR-amplified using PrimeSTAR® HS DNA Polymerase (Takara) and subcloned into the pcDNA3.1 vector (Invitrogen), named pcDNA3.1-CCAT1. The pcDNA3.1 vector was used as a negative control. The primers were as follows: 5′-CTAGCTAGCACAACATCGACTTTGAAGTT-3′ (sense) and 5′-CCCAAGCTTAAGACTTAATATACTTATATTTA-3′ (antisense). To obtain cell lines stably expressing CCAT1, ECA-109 and ECA-9706 cells were transfected with the plasmid pcDNA3.1-CCAT1 or pcDNA3.1 vector by using Lipofectamine 2000 according to the manufacturer's instructions. Cells were selected with neomycin (800 μg/ml) for four weeks. Nanoliposomes containing Let-7b mimics were prepared as previously described (32–34). Briefly, let-7b mimics were mixed with 1, 2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) (Avanti Polar Lipids, Alabaster, AL, USA) in the presence of excess tertiary butanol at a ratio of 1:10 (w/w) let-7b mimics/DOPC. The mixture was vortexed and lyophilized. Before experiments, this mixture was hydrated with normal 0.9% saline and purified by separating free mimics from liposomes with 30,000 nominal molecular weight limit filter units (Millipore, Billerica, MA, USA).
Immunochemistry and immunofluorescence staining
The cells were fixed in 4% formaldehyde, washed with PBS for 15 min, and permeabilized with 0.2% Triton X-100 for 20 min. After permeabilization, the cells were blocked with bovine serum albumin (BSA) at 37°C for 30 min. Fixed cells were incubated with the antibodies against H3K9me2 (1:200, ab1220; Abcam) at 4°C overnight, followed by Alexa Fluor 594 goat anti-rabbit IgG (H+L) secondary antibody (1:1,000, A-11012; Life Technologies, Gaithersburg, MD, USA) for 1 h at room temperature. The nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI, 1:10,000; 4084; Cell Signaling Technology, Inc.). The fluorescence images were obtained using an Olympus microscope.
For immunochemistry test of clinical samples, briefly, formalin-fixed paraffin-embedded samples were prepared as 4-μm-thick sections as previously described (35). The sections were incubated with primary antibodies against CD133 (ab19898; Abcam) and ALDH1 (sc-166362, Santa Cruz Biotechnology, Inc.) at 4°C overnight, and subjected to be incubated with the appropriate secondary antibody for 30 min at room temperature. IHC staining results in each sample was scored using semiquantitative scoring system, taking into consideration the staining intensity obtained and the proportion of positive cells observed.
Statistical analysis
The association between the postoperative complications, recurrence ratio, the ratio of stem-like cell markers and the clinicopathological factors was assessed using the Chi-square two-tailed test, ANOVA analysis or Fisher's exact test. The independent factor associated with clinicopathological factors and the ratio of stem-like cell markers were evaluated using a logistic regression analysis and Cox regression analysis. All statistical analyses were performed using the GraphPad Prism 5.01 or Microsoft Excel 2011, and a P-value <0.05 was considered to indicate a statistically significant difference.
Results
The ratio of stem-like cells was responsible for prognosis of postoperative survivors
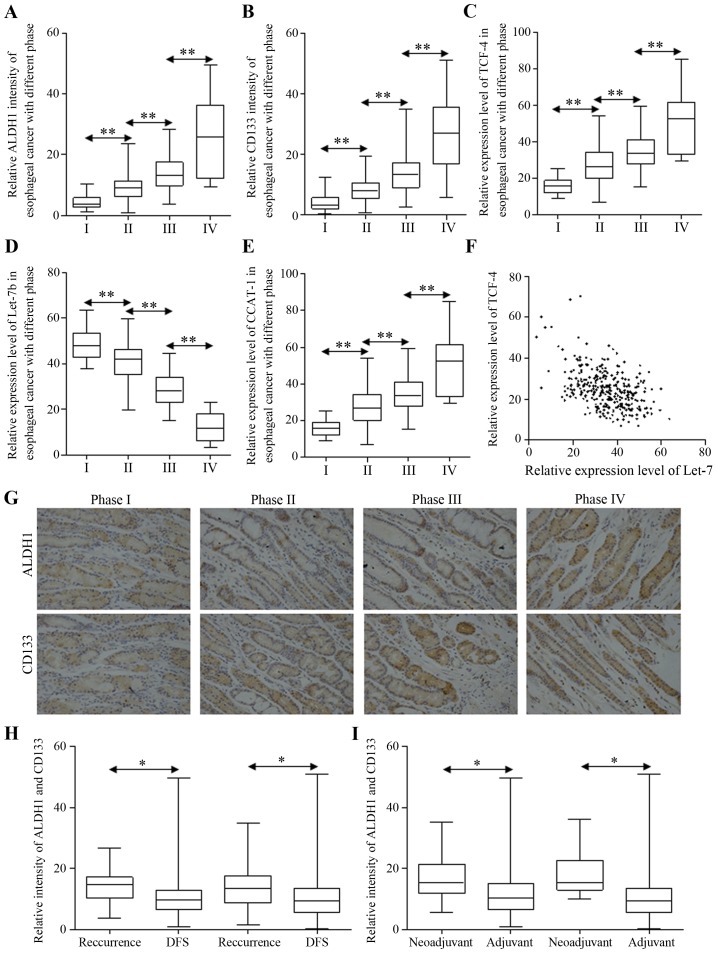
Specimens were tested for the ratio of cancer stem-like cells from each patient, and for the correlation between certain stem cell marker and the ratio of patients who were diagnosed with recurrent esophageal carcinoma after receiving radical esophagectomy. Stem-like cells were more likely to be enriched in carcinoma of later stages (Fig. 1A and B). Furthermore, esophageal carcinoma with more stem-like cancer cells tends to relapse more often in two years postoperatively (Table II). Wnt signaling activation and Let-7b repression were both involved in carcinoma progression, strengthened in esophageal cancer of later stages (Fig. 1C and D). The potential miRNA sponge of CCAT1 was increased as Wnt signaling did (Fig. 1E). The correlation was also observed between Let-7b and TCF-4, indicating the indirect regulation (Fig. 1F). The staining of ALDH1 and CD133 are shown in Fig. 1G. Last but not least, patients diagnosed with recurrent esophageal carcinoma presented different patterns of stem cell numbers, and carcinoma harbored less stem-like cells indicated longer disease-free survival time, while more cancer stem-like cells were correlated to poorer survival (Fig. 1H). Additionally, we identified the enriched stem-like cells in patients who received neo-adjuvant chemotherapy, compared to patients undergoing radical esophagectomy with none preoperative treatment (Fig. 1I).
Figure 1.
The correlation between clinicopathological patterns and signatures of stem cell enrichment in esophageal cancer. The expression levels of ALDH1 (A) and CD133 (B) in esophageal cancer of different stages were detected by immunohistochemistry, and the differences between ratios of stem cells are significant. The relative expression levels of TCF4 (C), Let-7b (D) and CCAT1 (E) in esophageal cancer of different stages were detected by qRT-PCR, and the difference between the expression of each stage is significant. (F) The correlation between levels of TCF-4 and Let-7b was analyzed with Pearson correlation coefficient, results showing R=−0.4621, P<0.0001, 95% confidence interval: −0.5445 to −0.3708. (G) The representative immunohistochemistry images for ALDH1 and CD133 intensity in esophageal cancer are shown. The expression levels of ALDH1 and CD133 in patients with 2-year recurrence or disease-free survival are presented (H), and the different expression levels of ALDH1 and CD133 in patients who underwent adjuvant or neoadjuvant chemotherapy were identified (I). *P<0.05, **P<0.01.
Table II.
The phenotypes and signatures of stem cell potency involving in recurrence after patients receiving radical esophagectomy.
| Category | Recurrence
|
P-value | Chi-square | |
|---|---|---|---|---|
| Yes | No | |||
| Relative ALDH1 intensity | ||||
| +/++/+++/++++ | 6/16/26/26 | 20/97/108/18 | <0.0001 | 37.91 |
| Relative CD133 intensity | ||||
| +/++/+++/++++ | 4/10/38/22 | 25/85/124/22 | 0.0005 | 17.63 |
| Relative β-catenin intensity | ||||
| +/++/+++/++++ | 6/16/30/22 | 47/156/39/1 | <0.0001 | 106.1 |
| Relative Wnt1 intensity | ||||
| +/++/+++/++++ | 8/12/26/28 | 62/70/60/51 | 0.0005 | 17.77 |
| Relative TCF-4 intensity | ||||
| +/++/+++/++++ | 2/10/28/34 | 49/86/70/38 | <0.0001 | 44.15 |
| Relative Let-7 expression | ||||
| +/++/+++/++++ | 16/46/8/4 | 23/31/104/85 | <0.0001 | 97.91 |
| Relative CCAT1 expression | ||||
| +/++/+++/++++ | 4/12/34/24 | 12/58/81/92 | 0.2147 | 4.473 |
SC, squamous carcinoma; AC, adenocarcinoma; AS, adenosquamous; RE, TCF-4, T-cell factor.
Harbored stem-like cells could revive from therapeutic procedure
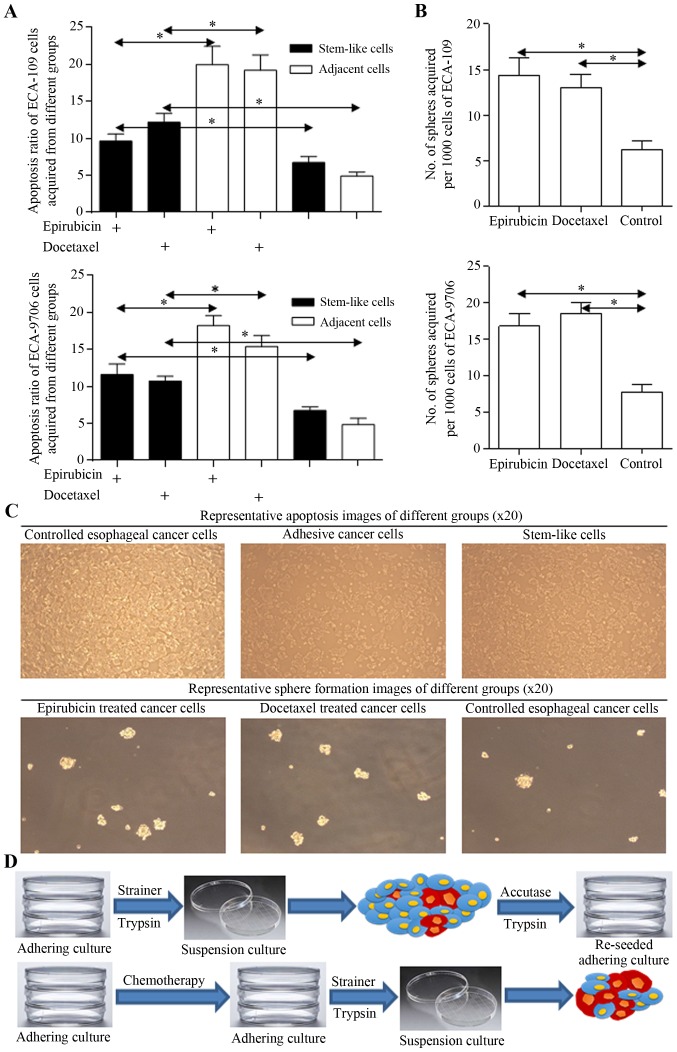
Stem cells harboring in cancer cells group could survive through multidisciplinary treatment, for their less proliferative signatures and native drug resistant nature. Stem cells acquired from spheres were found to be naturally resistant to chemotherapy (Fig. 2A), and more spheres could be enriched from cancer cells treated with fluorouracil or docetaxel (Fig. 2B), supporting the hypothesis we concluded. Representative images and the scheme are illustrated in Fig. 2C and D.
Figure 2.
Harbored stem-like cells could be revived from therapeutic procedure. (A) Apoptosis ratio of adjacent cells (normal cancer cells) and stem cells (spheres) derived from ECA-109 (upper) and ECA-9706 (lower) cells with epirubicin or docetaxel was analyzed, and the significant differences were identified. (B) The number of spheres acquired from ECA-109 and ECA-9706 cells treated with epirubicin or docetaxel was counted, and the adjuvant treatment enriched the stem-like cells. (C) Representative images of apoptosis (upper) and sphere formation images (lower) of different groups are shown. Original magnification, ×20. (D) A schematic diagram was illustrated to show the experimental procedure. *P<0.05, **P<0.01.
Wnt signaling activation drives stem-like cells to divide symmetrically to form more spheres
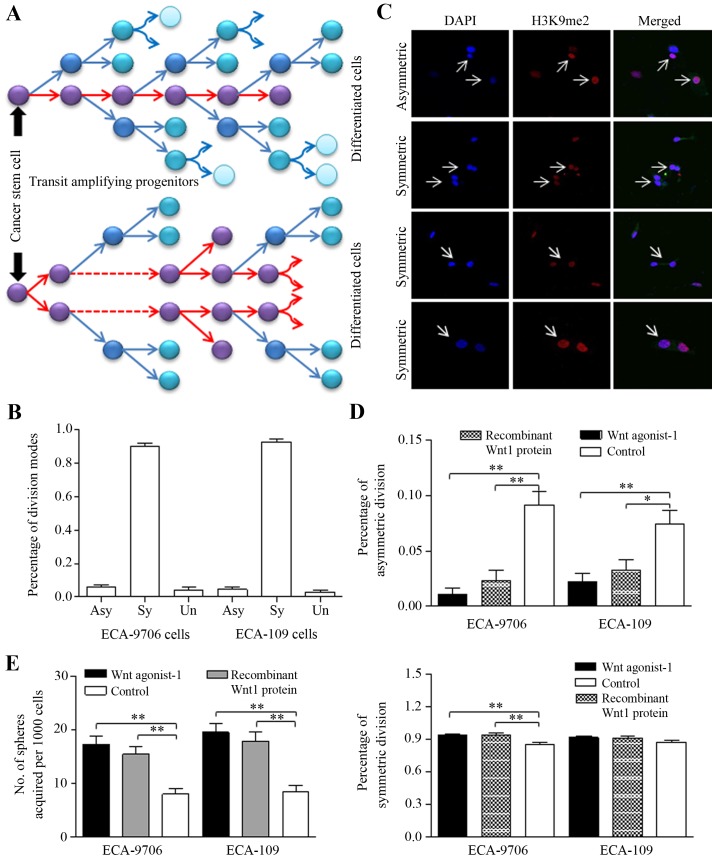
Stem cells and cancer stem-like cells divide asymmetrically to generate two daughter cells with diverse phenotype, as illustrated in Fig. 3A. Cancer stem-like cells tend to divide symmetrically generating two unique stem cells to expand the stem cell pool (36,37). In stem cells enriched from spheres of esophageal cancer cells, we identified the frequent occurrence of symmetric division through H3K9me2 staining (24,25,38–40), as presented in Fig. 3B and C. Both Wnt pathway agonist-1 (50 nM, S8178, Selleckchem, USA) and recombinant Wnt1 protein (50 ng/ml; Gibco, Life Technologies, USA) decreased ratio of asymmetric division than that of controlled group significantly (Fig. 3D, upper), and to the contrary, increased the ratio of symmetric division (Fig. 3D, lower). The manner of deregulated division contributed to sphere number increasing (Fig. 3E).
Figure 3.
Wnt signaling activation drives esophageal cancer stem-like cells to divide symmetrically and to form more spheres. (A) The schematic diagram showed that cancer stem cells prefer to divide symmetrically to expand the stem cells pool, rather than to differentiate. (B) The patterns of symmetric division and asymmetric division in ECA-9706 or ECA-109 cells was identified, and the percentage evaluated. (C) A set of immunofluorescence images showed symmetric division and asymmetric division of esophageal cancer stem cells through H3K9me2 staining. (D) ECA-9706 and ECA-109 cells were treated with Wnt pathway agonist-1 (50 nM) or recombinant Wnt1 protein (50 ng/ml) respectively, and Wnt pathway activation inhibits the asymmetric division (upper), but promotes the symmetric division (lower). (E) Treatment with Wnt pathway agonist-1 or recombinant Wnt1 protein promoted sphere formation efficiency of stem-like cells from ECA-9706 or ECA-109 cells. *P<0.05, **P<0.01.
Delivering nanoliposome of Let-7b promotes asymmetric division of cancer stem-like cells
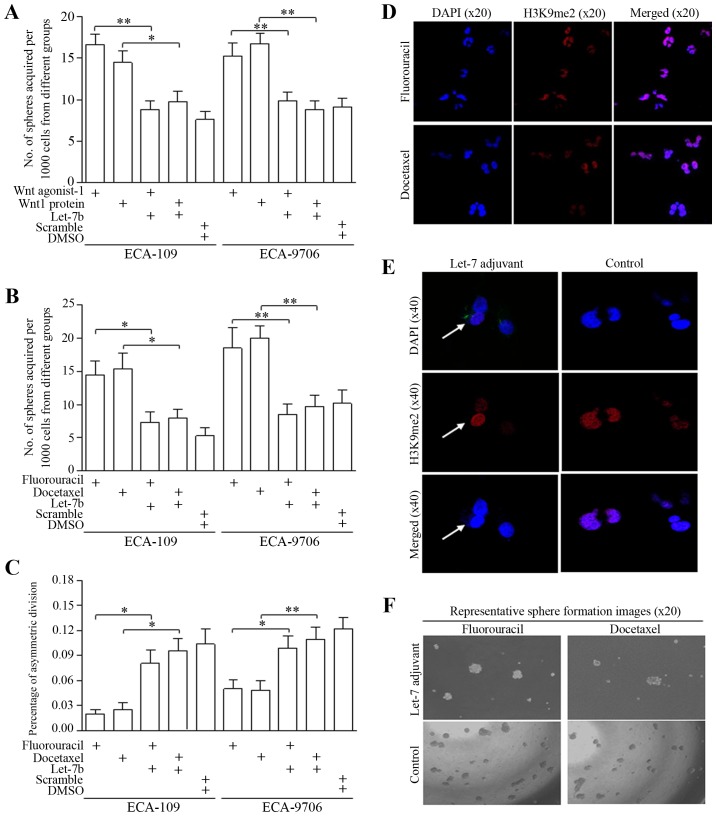
Let-7 family of miRNAs includes of Let-7a/b/c/d/e/f/g/I and miRNA-98, and Let-7b was selected as the candidate for its stably inhibitive function after we deeply explored their roles in multiple malignancies. For the first time, we tentatively used the nanoliposome based Let-7 which is closer to the clinical application and has not been explored. The delivery of nanoliposomal Let-7b attenuated the Wnt activator induction of self-renewal (Fig. 4A). In groups treated with either fluorouracil or docetaxel, Let-7b counteracted the stemness enrichment of chemotherapeutic agents, as was shown in Fig. 4B (ECA-109) and Fig. 4C (ECA-9706). Representative images are shown in Fig. 4D–F.
Figure 4.
Nanoliposome of Let-7b promotes asymmetric division of esophageal cancer stem-like cells. (A) Nanoliposome of Let-7b attenuated the Wnt activator induction of sphere formation of ECA-9706 or ECA-109 stem-like cells. (B) ECA-9706 and ECA-109 cells were treated with fluorouracil or docetaxel, which increased the number of spheres, proving the therapeutic enrichment of stem-like cells. Let-7b counteracted the stemness enrichment of chemotherapeutic agents. (C) Let-7b attenuated the fluorouracil or docetaxel inhibition of asymmetric division of ECA-9706 or ECA-109 stem cells. (D) Representative immunofluorescence images showed the division modes of esophageal cancer stem-like cells treated with fluorouracil or docetaxel. (E) Representative immunofluorescence images showed that Let-7b promoted asymmetric division of esophageal cancer stem cells (signified with the white arrow). (F) A set of representative images showing that Let-7b attenuated the induction of sphere formation by fluorouracil or docetaxel. *P<0.05, **P<0.01.
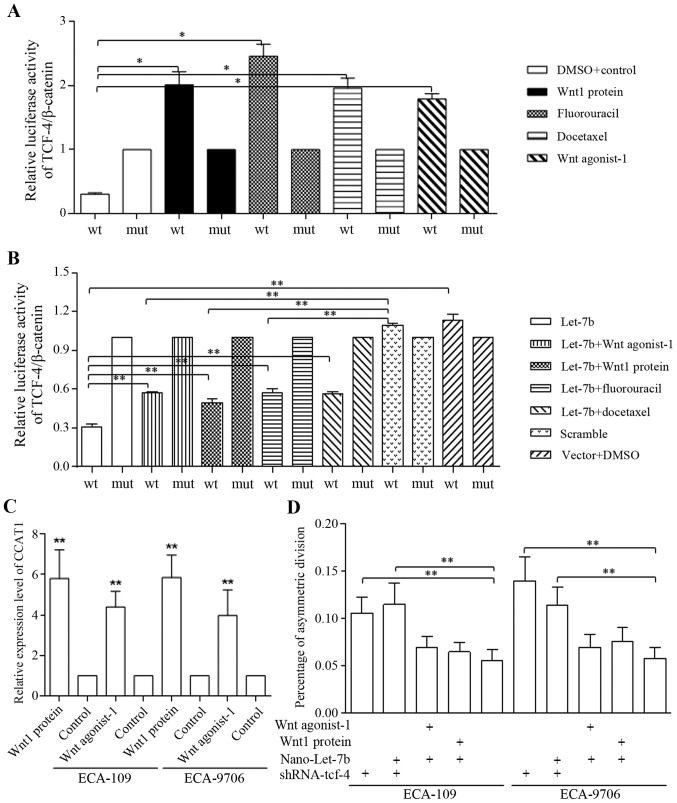
Regulatory feedback loop of Let-7 and Wnt signaling was connected through CCAT1
Either Wnt activation or therapeutic agents stimulated the TCF-4/Wnt activation greatly, detected by luciferase assay (Fig. 5A). Let-7 decreased self-renewal of esophageal cancer stem-cells via direct inhibition on TCF-4/β-catenin complex activity, and only the TCF-4 promoter activity of wild-type (compared with mutant group) was inhibited (Fig. 5B). Enforced Let-7b via Nano-delivery also blocked the Wnt signaling activators (Fig. 5B). Wnt stimulators accounted for CCAT1 overexpression, formed the Let-7/Wnt/CCAT1 signaling (Fig. 5C). We further identified that the TCF-4 inhibition stimulated cells to divide asymmetrically, and Let-7b functioned through TCF-4 repression (Fig. 5D). Wnt activators of inhibited asymmetrically division could be reversed by Let-7b delivery (Fig. 5D).
Figure 5.
Nano-Let-7b promotes asymmetric division via direct inhibition on TCF-4. (A) The addition of Wnt agonist-1, Wnt1 protein, fluorouracil and docetaxel alone stimulated the activation of TCF-4 promoter significantly in H293-T cells. (B) We identified the direct repression of TCF-4 promoter caused by nano-Let-7. Both Wnt sinaling activators of agonist and recombinant protein, and therapeutic agents of fluorouracil and docetaxel stimulated TCF-4 promoter functions of wild-type (wt) could be blocked by Nano-Let-7b, compared with negative control group of mutant-type (mut). In detail, Let-7b alone affected the TCF-4 promoter activity effectively, and wnt signaling activators of either Wnt agonist-1 or Wnt protein attenuated the Let-7b functions significantly. Combined usage of therapeutic agent of either fluorouracil or docetaxel with Let-7b increased the TCF-4 activity when compared to groups treated with Let-7b alone, however, the TCF-4 activity when applying combination was still significantly lower than that of controlled group (C) Wnt signaling activation increased CCAT1 level effectively, forming the Let-7/Wnt/CCAT1 cascade. (D) Let-7b sustained the ratio of asymmetric division as shRNA of TCF-4 did, and counteracted the functions of Wnt signaling activator through a TCF-4 inhibition-dependent maner. *P<0.05, **P<0.01.
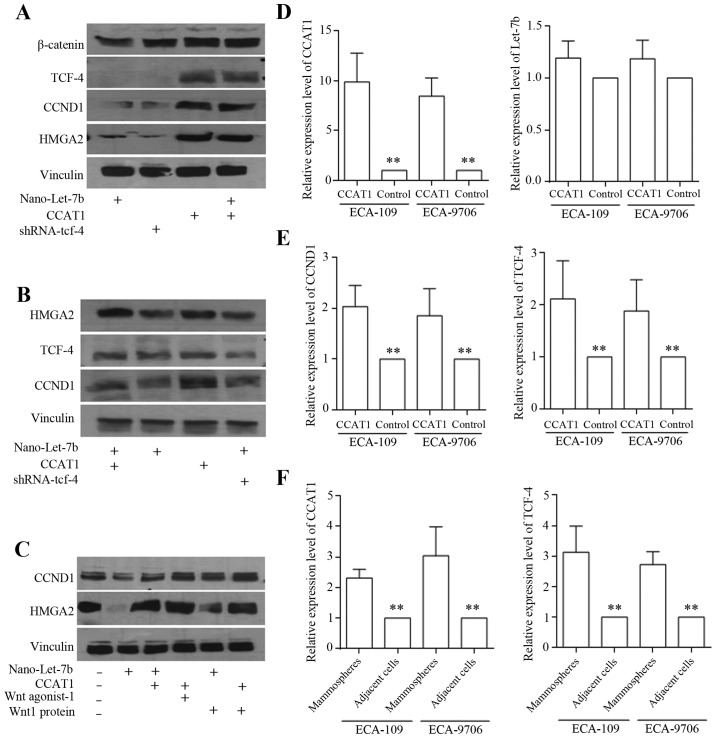
Let-7b decreased HMGA2/Wnt signaling factors through repressing TCF-4 activity in ECA-9706 (Fig. 6A) and ECA-109 (Fig. 6B) cells. CCAT1 overexpression released the downstream oncogenes of CCND1 and HMGA2, which were directly targeted and degraded by Let-7b in ECA-9706 (Fig. 6A) and ECA-109 (Fig. 6B) cells. In spheres of ECA-109 cells, Wnt signaling activators sustained HMGA2 and CCND1 level could be equaled by the overexpression of CCAT1 (Fig. 6C). Enforced CCAT1 expression released the downstream oncogenes of Let-7b effectively, with Let-7b level staying stable (Fig. 6D), and further, Let-7b downstream genes were identified to be increased with CCAT1 enforcement (Fig. 6E), which further proving the hypothesis of CCAT1 interaction with Let-7/Wnt regulatory feedback loop. We found higher level of miRNA sponge of CCAT1 and TCF-4 in esophageal cancer stem-like cells (Fig. 6F), indicating the crucial oncogenic roles in tumor formation and progression.
Figure 6.
Regulatory feedback loop of Let-7 and Wnt signaling was connected through CCAT1. Wnt signaling factors including β-catenin, HMGA2, CCND1 and TCF-4, and Let-7b decreased HMGA2/Wnt signaling factors through repressing TCF-4 activity in ECA-9706 (A) and ECA-109 (B) cells. CCAT1 overexpression released the downstream oncogenes inhibited by Let-7b, counteracted Let-7b functions effectively in ECA-9706 (A) and ECA-109 (B) cells. (C) In spheres of ECA-109 cells, Wnt signaling activators sustained HMGA2 and CCND1 level equally by the overexpression of CCAT1. The expression of CCAT1, Let-7b, CCND1 and TCF-4 was detected by qRT-PCR. CCAT1 overexpression (D, left) did not alternate Let-7b level (D, right). Enforced CCAT1 increased CCND1 (E, left) and TCF-4 (E, right) expression significantly. (F) The cancer stem-like cells derived from spheres exhibited higher expression levels of CCAT1 and TCF-4, compared to that of adjacent cells. *P<0.05, **P<0.01.
Discussion
Esophageal carcinoma is one of the lethal malignancies, especially in East Asia and China. Due to its specific biological location and inevitable surgery wound, patients diagnosed suffered greatly from preclinical malaise, dysphagia, malnutrition and slowing postoperative recovery. Apart from the above, poorer prognosis and recurrence are the main obstacles in treatment of esophageal cancer. Cancer possesses mutations that impair the capacity of normal cells responding to the signals that regulate proliferation. However, the theory of CSCs reversed this opinion, meaning that cancer could arise from a few cells that have the capacity to generate the numerous different cells types in a tumor. We previously studied the mechanisms through which the CSCs may emerge, and paid close attention to the formation of different cell types through ACD and SCD, which are crucial to understand carcinogenesis from the viewpoint of stem cells (4). ACD will decrease the stem cell population through inhibiting the self-renewal and then blocking the proliferating rates of cancer cells. We were determined to find new strategies and reagents to induce more ACD of cancer stem cells, and thought that the decreased stem cell population will inhibit malignancy and prevent tumor recurrence.
Stem cell signatures could be influenced by multiple non-coding RNAs, which are also known as fates' determinations. Let-7 and other suppressive miRNAs could decrease the stem cell numbers via inhibition on self-renewal, which were confirmed in multiple systemic malignancies. In the present study, we found that the higher ratio of ALDH1 or CD133-positive cancer stem cells, which was identified with higher intensity of protein level by IHC or IF, is associated with later clinical stages and 2-year recurrence, as was expected. Besides, Wnt signaling activation was more frequent in later esophageal cancer. Cancer stem cells derived from spheres naturally divided symmetrically and are therapy resistant with lower apoptosis ratio consequently. Furthermore, we found that Let-7b directly inhibited TCF-4/β-catenin complex activity in a promoter alternation manner, functioning as negative regulator of self-renewal. Wnt activation released CCAT1 overexpression and rescued the downstream oncogenes of Let-7b effectively, with Let-7b level staying stable. We also demonstrated that a regulatory feedback loop of Let-7 and Wnt signaling was connected through CCAT1, indicated as Let-7/Wnt/CCAT1.
The ratio of stem cells harbored in esophageal carcinoma indicates the prognosis of patients undergoing esophagectomy, and neoadjuvant chemotherapy could enlarge the stem cells pool. Let-7 promotes asymmetric division of esophageal cancer stem cells, which resulted in stem cell renewal repression. Wnt activation of self-renewal could be blocked by Let-7 overexpression, and Let-7 sensitization of adjuvant therapy of fluorouracil and docetaxel was achieved through Wnt signaling inhibition. Moreover, the interaction between non-coding genes greatly expanded the non-coding RNAs controlling the stem cell fate. Traditionally, invisible miRNA and lncRNA functioned through alternating downstream effectors, however, importance of their mutual effect was noted. The basic findings of Let-7 inhibited Wnt signaling, the feedback loop of Let-7b/Wnt was linked via lncRNA of CCAT-1, proving the cascade of Let-7b/Wnt/CCAT-1 signaling. The clear focus of mechanistic regulation of Let-7 and its downstream oncogenic signaling will help to define the prospect application of Nano-Let-7b. Based on the novel roles of stem cell ratio and crucial suppressive functions of Let-7, the detection and targeted therapy of cancer stem cells will pave the way for improving prognosis and the response of comprehensive treatment.
Acknowledgments
The authors acknowledge assistants in the Center for Translational Medicine of The First Affiliated Hospital of Xi'an Jiaotong University, for their technical assistance. The team appreciate Prof. Peijun Liu for help in experiments and technique guidance. This study was mainly supported by National Science Foundation for Young Scientists of China, grant no. 81602597 (to Xin Sun). This study was also supported in part by National Natural Science Foundation of China, grant no. 81272418 (to Hong Ren), Natural Science Foundation of Shaanxi Province, grant no. 2016JM8007 (to Jing Zhang), and National Science Foundation for Young Scientists of China, grant no. 81402506 (to Sida Qin).
References
- 1.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Liu J, Xu C, Tang SC, Ren H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J Cell Mol Med. 2016;20:1779–1788. doi: 10.1111/jcmm.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E, Ren H, Pestell RG. MicroRNAs and cancer stem cells: The sword and the shield. Oncogene. 2014;33:4967–4977. doi: 10.1038/onc.2013.492. [DOI] [PubMed] [Google Scholar]
- 6.Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Circular RNA-ITCH suppresses lung cancer proliferation via inhibiting the Wnt/β-catenin pathway. BioMed Res Int. 2016;2016:1579490. doi: 10.1155/2016/1579490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blagodatski A, Poteryaev D, Katanaev VL. Targeting the Wnt pathways for therapies. Mol Cell Ther. 2014;2:28. doi: 10.1186/2052-8426-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011;71:6684–6695. doi: 10.1158/0008-5472.CAN-11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110:972–977. doi: 10.1073/pnas.1221055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Printz C. Radiation treatment generates therapy-resistant cancer stem cells from less aggressive breast cancer cells. Cancer. 2012;118:3225–3225. doi: 10.1002/cncr.27701. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Yang S-B, Xu F-F, Zhang J-H. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34:18. doi: 10.1186/s13046-015-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Xu C, Tang SC, Wang J, Wang H, Wang P, Du N, Qin S, Li G, Xu S, et al. Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells. Cancer Gene Ther. 2016;23:83–89. doi: 10.1038/cgt.2016.3. [DOI] [PubMed] [Google Scholar]
- 14.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson RL, Yang WT, Rosen DG, Landis MD, Wong H, Lewis MT, Creighton CJ, Sexton KR, Hilsenbeck SG, Sahin AA, et al. Cancer stem cell markers are enriched in normal tissue adjacent to triple negative breast cancer and inversely correlated with DNA repair deficiency. Breast Cancer Res. 2013;15:R77. doi: 10.1186/bcr3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hang D, Dong HC, Ning T, Dong B, Hou DL, Xu WG. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:638–644. doi: 10.1111/j.1442-2050.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Luo M, Brooks M, Clouthier SG, Wicha MS. Biological and clinical significance of cancer stem cell plasticity. Clin Transl Med. 2014;3:32. doi: 10.1186/s40169-014-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Molavi O, Su M, Lai R. The clinical and biological significance of STAT1 in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:791. doi: 10.1186/1471-2407-14-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Ma L, Xie YK, Miao XB, Jin C. Esophageal cancer tumorspheres involve cancer stem-like populations with elevated aldehyde dehydrogenase enzymatic activity. Mol Med Rep. 2012;6:519–524. doi: 10.3892/mmr.2012.939. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao HL, Wang WG, Xu SL, Yang J, Cui W, et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod Pathol. 2014;27:775–783. doi: 10.1038/modpathol.2013.189. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Torres M, Allan AL. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin Exp Metastasis. 2016;33:97–113. doi: 10.1007/s10585-015-9755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang C-C, Nieh S, Lai C-H, Tsai CS, Chang LC, Hua CC, Chi WY, Chien HP, Wang CW, Chan SC, et al. A retrospective review of the prognostic value of ALDH-1, Bmi-1 and Nanog stem cell markers in esophageal squamous cell carcinoma. PLoS One. 2014;9:e105676. doi: 10.1371/journal.pone.0105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey-Guha I, Wolfer A, Yeh AC, G Albeck J, Darp R, Leon E, Wulfkuhle J, Petricoin EF, III, Wittner BS, Ramaswamy S. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci USA. 2011;108:12845–12850. doi: 10.1073/pnas.1109632108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-López S, Lerner RG, Petritsch C. Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell Mol Life Sci. 2014;71:575–597. doi: 10.1007/s00018-013-1386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fürthauer M, González-Gaitán M. Endocytosis, asymmetric cell division, stem cells and cancer: Unus pro omnibus, omnes pro uno. Mol Oncol. 2009;3:339–353. doi: 10.1016/j.molonc.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Hashash AHK, Warburton D. Numb expression and asymmetric versus symmetric cell division in distal embryonic lung epithelium. J Histochem Cytochem. 2012;60:675–682. doi: 10.1369/0022155412451582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kechad A, Jolicoeur C, Tufford A, Mattar P, Chow RWY, Harris WA, Cayouette M. Numb is required for the production of terminal asymmetric cell divisions in the developing mouse retina. J Neurosci. 2012;32:17197–17210. doi: 10.1523/JNEUROSCI.4127-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Blanco NM, Checquolo S, Del Gaudio F, Palermo R, Franciosa G, Di Marcotullio L, Gulino A, Canelles M, Screpanti I. Numb-dependent integration of pre-TCR and p53 function in T-cell precursor development. Cell Death Dis. 2014;5:e1472. doi: 10.1038/cddis.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand AH. A new dawn for Aurora? Nat Cell Biol. 2008;10:1253–1254. doi: 10.1038/ncb1108-1253. [DOI] [PubMed] [Google Scholar]
- 32.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 33.Seviour EG, Sehgal V, Mishra D, Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Lee J-S, Sood AK, Kim MP, Mills GB, et al. Targeting KRas-dependent tumour growth, circulating tumour cells and metastasis in vivo by clinically significant miR-193a-3p. Oncogene. 2017;36:1339–1350. doi: 10.1038/onc.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rupaimoole R, Ivan C, Yang D, Gharpure KM, Wu SY, Pecot CV, Previs RA, Nagaraja AS, Armaiz-Pena GN, McGuire M, et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene. 2016;35:4312–4320. doi: 10.1038/onc.2015.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X, Jiang S, Liu J, Wang H, Zhang Y, Tang SC, Wang J, Du N, Xu C, Wang C, et al. MiR-208a stimulates the cocktail of SOX2 and β-catenin to inhibit the let-7 induction of self-renewal repression of breast cancer stem cells and formed miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget. 2015;6:32944–32954. doi: 10.18632/oncotarget.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang W-L, Jiang J-K, Yang S-H, Huang TS, Lan HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW, et al. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat Cell Biol. 2014;16:268–280. doi: 10.1038/ncb2910. [DOI] [PubMed] [Google Scholar]
- 37.Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011;2:e200. doi: 10.1038/cddis.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: Promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 39.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 40.Berika M, Elgayyar ME, El-Hashash AHK. Asymmetric cell division of stem cells in the lung and other systems. Front Cell Dev Biol. 2014;2:33. doi: 10.3389/fcell.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]