Abstract
Nausea and vomiting (NV) are the most prevalent adverse effects of chemotherapy (CT). This study was conducted to evaluate adherence of the health care team to standard guidelines for antiemetics usage to prevent acute chemotherapy-induced nausea and vomiting (CINV) in a large CT center. A prospective study was performed during an 11-month period on patients receiving CT. A form was designed to collect patients’ demographic information and their chemotherapeutic and antiemetic regimen data. The Likert scale was used to measure the effectiveness of the antiemetics in patients. In this study, the effect of patient-related risk factors on the incidence rate of CINV was examined. Based on the results, CINV events were reported by 74.4% of patients. The antiemetic regimen of 71.2% of the patients complied with the guidelines. The complete response, complete protection, and complete control end points did not differ significantly between patients undergoing guidelines-consistent prophylaxis or guidelines-inconsistent prophylaxis. The females clearly showed a higher incidence rate of CINV (P=0.001) during the first course of CT (P=0.006). A history of motion sickness did not affect the incidence of NV. The maximum compliance error occurred for the use of aprepitant, as 16.16% of the patients who were receiving aprepitant did not comply with its instructions. The results of this study highlight how CINV was controlled in this center, which was significantly lower than that of the global standard. Perhaps, factors such as noncompliance to antiemetic regimens with standard guidelines and the failure to adhere to the administration instructions of the antiemetics were involved in the incomplete control of CINV.
Keywords: antiemetics, nausea, vomiting, chemotherapy
Introduction
Despite considerable advances in antiemetic treatments, patients find chemotherapy-induced nausea and vomiting (CINV) as the most distressing adverse effect of chemotherapy (CT).1,2 CINV is the most adverse effect reported after CT.3 In addition, vomiting can cause complications such as weakness, weight loss, dehydration, water and electrolyte imbalance, malnutrition, aspiration, and pneumonia.4 Nausea and vomiting (NV) can also reduce absorption of nutrients and the ability for self-care.5 Patients’ quality of life with CINV is significantly reduced compared to that of healthy people. Physicians can reduce the severity and adverse effects of NV through appropriate health care practices and by prescribing antiemetic regimens for patients at risk. An important practice to reduce the CINV is by prescription of antiemetics, appropriate to the chemotherapeutic regimen.4
Numerous guidelines have been published and are updated to prevent CINV.6 However, clinical results of these guidelines are not optimal, and CINV persists as a sustainable complication in CT.2,7–9
This study was conducted to examine the consistency of antiemetics with the CT regimens, as well as to measure the compliance of the antiemetics administration in those patients who received chemotherapeutic regimens with standard guidelines in the largest CT center in southern Iran. Various risk factors of nausea were examined in this study. To the researcher knowledge, this is the first study of this type in Iran.
Methods
Patient population
A prospective study was performed during an 11-month period from September 2014 to August 2015 on patients admitted to the ambulatory CT ward of Nemazee Hospital, the largest CT center in southern Iran, affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. The local ethics committee of Shiraz University of Medical Sciences reviewed and approved this study. The patients completed written informed consent before participating in this study. The study included all adult patients (>18 years of age) who were receiving chemotherapeutic and antiemetic regimens at the same time and had agreed to participate in this study. The patients who were undergoing radiotherapy with CT were excluded from the study.
Data collection
A data collection form was designed and used to record the patients’ demographic information, their previous CINV experience, and risk factors of CINV. The information on the antiemetic regimens, chemotherapeutic regimen, chemotherapeutic dose, and antiemetic drugs were collected from patients’ medical records. The severity, onset, and duration of acute emesis were recorded through face-to-face interview with patients. The frequency of CINV within 24 hours of CT and during 5 days after the start of CT treatment was recorded. The researcher directly observed and recorded the method of antiemetic drug administration and the necessary variables, including type of drugs, dose, and the interval between them, in order to compare them with standard methods. In this study, the Likert scale was used, and patients selected a score of 0–10 to show the severity of nausea. The scores of 0, 1–2, 3–6, and 7–9 determined no nausea, mild nausea, moderate nausea, and severe nausea, respectively. The score 10 was assigned to vomiting. The major end points of this study included the complete response (no emetic episode and no rescue antiemetics), complete protection (no vomiting, no significant nausea [scores ≤2], and no rescue antiemetics), and complete control (no emetic episode and no nausea, no vomiting and no rescue antiemetics).
Guideline design
The therapeutic protocols and guidelines used in this study were extracted from the American Society of Clinical Oncology (ASCO)10 and the Multinational Association of Supportive Care in Cancer (MASCC).11,12 The protocols were used to compare the therapies performed in the patients’ chemotherapeutic regimens to control the adverse effects of CT. The studied criteria were determined accordingly.
Statistical analysis
Continuous variables are presented as mean ± SD. Categorical data are shown as percentage. Chi-square was used to compare association between categorical variables. Univariate regression was used to investigate the incidence of end points (complete response, complete protection, and complete control) and guideline consistency. Multivariate logistic regressions were used to compare proportions of patients achieving complete response, complete protection, and complete control. The models included guideline-consistent CINV prophylaxis (GCCP) or guideline-inconsistent CINV prophylaxis (GICP), whether aprepitant was prescribed as a binary variable, and other relevant demographic and clinical variables. In all statistical tests, P-value <0.05 was considered as significant. All analyses were performed using SPPS, version 18.0, statistical software (SPSS Inc., Chicago, IL, USA).
Results
Patients and their demographic specifications
Of the 150 outpatients who had undergone CT, 125 patients agreed to participate in this study. Table 1 provides demographic specifications, and Table 2 lists the frequency of different cancers diagnosed.
Table 1.
Demographic specifications of the patients undergoing CT in Nemazee Hospital in Shiraz (n=125)
| Characteristics | CINV
|
||||
|---|---|---|---|---|---|
| No sign of NV | Mild nausea | Moderate nausea | Severe nausea | Vomiting | |
| Age (mean ± SD) | 57.34±14.53 | 53.38±11.89 | 50.36±11.53 | 46.75±10.46 | 46.83±11.07 |
| Sex, n (%) | |||||
| Female | 17 (13.6) | 20 (16.0) | 26 (20.8) | 21 (16.8) | 2 (1.6) |
| Male | 15 (12.0) | 9 (7.2) | 2 (1.6) | 3 (2.4) | 10 (8.0) |
| History of previous CT, n (%) | 7 (5.6) | 3 (2.4) | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| History of motion sickness, n (%) | 1 (0.8) | 1 (0.8) | 2 (1.6) | 3 (2.4) | 1 (0.8) |
| History of CINV, n (%) | 1 (0.8) | 3 (2.4) | 2 (1.6) | 4 (3.2) | 3 (2.4) |
Abbreviations: CT, chemotherapy; CINV, chemotherapy-induced nausea and vomiting; NV, nausea and vomiting.
Table 2.
The frequency of different cancers diagnosed in the patients undergoing CT in Nemazee Hospital in Shiraz (n=125)
| Diagnosis | (n=125) n (%) |
|---|---|
| Breast cancer | 51.2 (64) |
| Intestinal and colorectal cancer | 11.2 (14) |
| Prostate cancer | 5.6 (7) |
| Gastric cancer | 4.8 (6) |
| Ovary cancer | 4 (5) |
| Malignant lymphoma | 4 (5) |
| Cerebral tumor | 4 (5) |
| Pancreatic tumor | 3.2 (4) |
| Renal cancer | 2.4 (3) |
| Liver cancer | 2.4 (3) |
| Lung cancer | 2.4 (3) |
| Esophagus cancer | 2.4 (3) |
| Skin tumor | 1.6 (2) |
| Pancreatic cancer | 0.8 (1) |
Abbreviation: CT, chemotherapy.
Frequency of different chemotherapeutic regimens and the incidence of CINV
The patients receiving chemotherapeutic regimens causing high, moderate, mild, and minimal nausea comprised 46.4%, 20.8%, 31.2%, and 1.6%, respectively. CINV was reported by 74.4% of patients (acute, 44.8%; delayed, 29.6%). Figure 1 shows the severity of nausea in patients based on the Likert scales. The comparison of the group with and without CINV showed a significant difference in terms of emetogenic risk level of the anticancer drugs (P=0.03).
Figure 1.
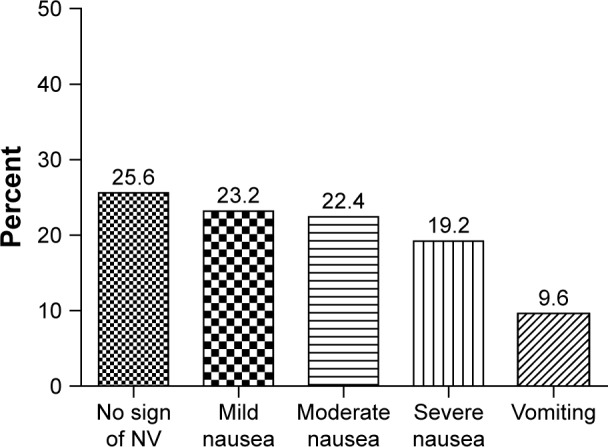
The percentage of patients on the basis of the severity of the CINV in Nemazee Hospital in Shiraz (n=125).
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; NV, nausea and vomiting.
Antiemetic regimens
According to the results, the antiemetic regimens were not prescribed in compliance with the guidelines in 28.8% of the patients. The 5-hydroxytryptamine-3 (5-HT3) receptor antagonists (RAs) including granisetron and ondansetron, and dexamethasone were prescribed in almost all the patients (97.6% and 98.4% of all the patients, respectively). Aprepitant was prescribed in 75.2% of the patients. The minimum compliance error occurred with the parenteral drugs (5-HT3 RA, 1.6%, and dexamethasone, 0.8%). The maximum compliance error occurred in the use of aprepitant, as 16.16% of the patients receiving aprepitant did not comply with its instructions.
Impact of patient-related risk factors on the incidence of CINV
Females suffered from CINV more than males (P=0.001). As shown in Figure 2, the incidence of mild, moderate, and severe CINV was higher in women in comparison with men, which was statistically significant (P=0.003).
Figure 2.
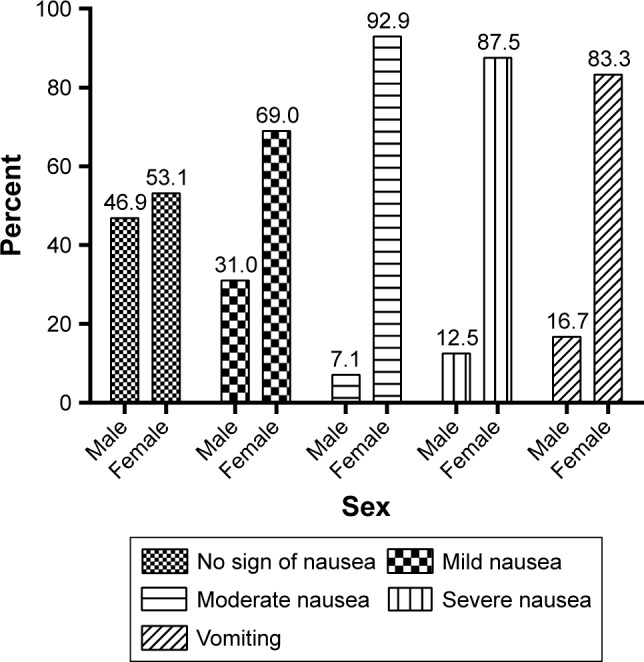
The percentage of patients’ sex in terms of the severity of CINV in Nemazee Hospital in Shiraz (n=125).
Abbreviation: CINV, chemotherapy-induced nausea and vomiting.
The frequency of patients in terms of previous CT experience significantly varied with the severity of CINV (P=0.025). The incidence of CINV in patients was significantly correlated with their CT prior experience in general (P=0.006; Figure 3).
Figure 3.
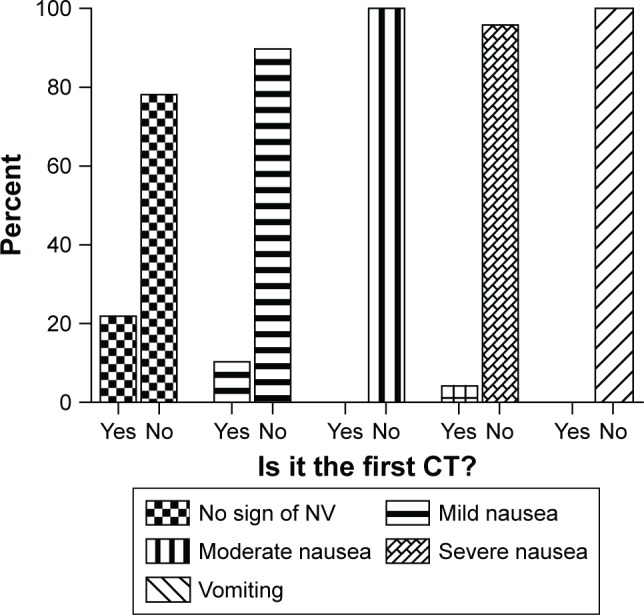
The percentage of patients with and without a history of CT in terms of the incidence of CINV (n=125).
Abbreviations: CT, chemotherapy; CINV, chemotherapy-induced nausea and vomiting; NV, nausea and vomiting.
The history of motion sickness was not significantly correlated with the severity of CINV (P=0.59) and the incidence of CINV in general (P=0.68).
Comparing the CINV degree in terms of the administration and nonadministration of aprepitant
Administration of aprepitant (Abitant®; Abidi, Tehran, Iran) increased the percentage of patients with CINV in all groups with mild, moderate, and severe CINV, contrary to our expectation (Figure 4). However, the statistical comparison of CINV severity in patients who received and did not receive aprepitant did not show any significant difference (P=0.1).
Figure 4.
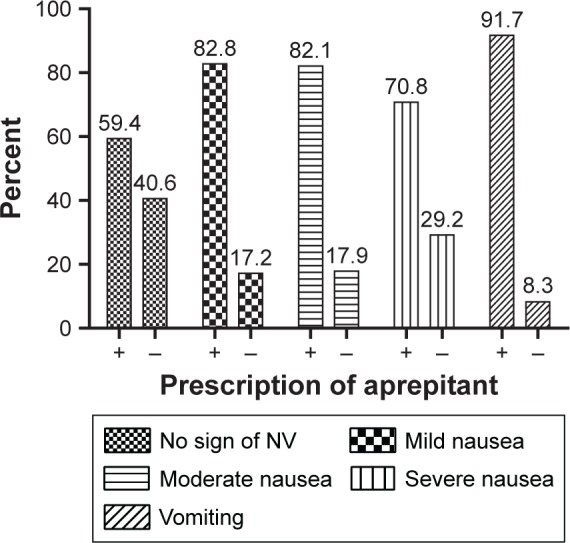
The effect of the administration of aprepitant on the degrees of CINV in patients undergoing CT in Nemazee Hospital in Shiraz (n=125).
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; CT, chemotherapy; NV, nausea and vomiting.
In this study, the frequency of acute and delayed nausea in patients who received or did not receive aprepitant was examined. There were more patients with acute and delayed nausea in the group who were receiving aprepitant than those in the other group (83.9% vs 16.1% and 75.7% vs 24.3%, respectively, for the acute and delayed nausea; P=0.03).
The overall incidence of CINV in patients who were receiving or did not receive aprepitant showed that CINV occurred more frequently in the aprepitant receiving group (P=0.02).
Comparisons of complete response, complete protection, and complete control end points in terms of GCCP and GICP
The complete response, complete protection, and complete control end points were examined in the two groups of patients with GCCP and GICP. Based on the results, 113 patients (90.4%), 52 patients (41.6%), and 32 patients (25.6%) showed complete response, complete protection, and complete control, respectively (Table 3). The statistical comparison of the patients with GCCP and GICP in terms of these end points did not show any significant difference. The modeling was performed on the basis of age and sex factors in two ways: adjusted and unadjusted (based on the multivariate logistic regression modeling). However, the number patients whose antiemetic regimens complied with the guidelines was greater than the patients in the other group in all end points. A similar comparison was carried out between the group receiving aprepitant and the group that did not receive aprepitant in terms of the end points. In this comparison, the two groups were significantly different from each other only in terms of the complete control end point in an unadjusted manner (Table 4).
Table 3.
The comparison of the end points in terms of the compliance and noncompliance of the antiemetic regimen with the guidelines in patients undergoing CT in Nemazee Hospital in Shiraz (n=125)
| Outcome | Guideline consistency
|
P-value | Multivariate model
|
||
|---|---|---|---|---|---|
| Consistent (n=89) | Inconsistent (n=36) | OR (95% CI) | P-value | ||
| Complete response,a n (%) | 82 (65.6) | 31 (24.8) | 0.32 | 1.70 (0.47–6.09) | 0.41 |
| Complete protection,b n (%) | 39 (31.2) | 13 (10.4) | 0.55 | 0.81 (0.25–2.58) | 0.72 |
| Complete control,c n (%) | 24 (19.2) | 8 (6.4) | 0.66 | 1.03 (0.28–3.84) | 0.96 |
Notes:
No vomiting and no rescue antiemetics.
No vomiting, no significant nausea (scores 0 and 2), and no rescue antiemetics.
No vomiting, no nausea (score 0), and no rescue antiemetics.
Abbreviations: CT, chemotherapy; OR, odds ratio; CI, confidence interval.
Table 4.
The comparison of the group receiving aprepitant and the group not receiving aprepitant in terms of the end points in patients undergoing CT in Nemazee Hospital in Shiraz (n=125)
| Outcome | Aprepitant administration
|
P-value | Multivariate model
|
||
|---|---|---|---|---|---|
| Yes (n=94) | No (n=31) | OR (95% CI) | P-value | ||
| Complete response,a n (%) | 83 (66.4) | 30 (24.0) | 0.29 | 0.36 (0.04–3.09) | 0.35 |
| Complete protection,b n (%) | 35 (28.0) | 17 (13.6) | 0.10 | 0.86 (0.24–3.08) | 0.81 |
| Complete control,c n (%) | 19 (15.2) | 13 (10.4) | 0.02 | 2.53 (0.66–9.67) | 0.17 |
Notes:
No vomiting and no rescue antiemetics.
No vomiting, no significant nausea (scores 0 and 2), and no rescue antiemetics.
No vomiting, no nausea (score 0), and no rescue antiemetics.
Abbreviations: CT, chemotherapy; OR, odds ratio; CI, confidence interval.
Discussion
According to the results of this study, more than half of the patients who received CT experienced overall CINV. The antiemetic regimens of 71.2% of the patients complied with the guidelines. Thus, this was a significant problem in Nemazee Hospital, which is a referral center for CT in Iran. In this study, the incidence rates of CINV did not differ significantly between patients who were receiving either GCCP or GICP.
Patients receiving chemotherapeutic regimens with high, moderate, mild, and minimal nausea comprised 46.4%, 20.8%, 31.2%, and 1.6%, respectively. In the study of Craver et al13 on 11,496 patients undergoing CT in the US, patients receiving chemotherapeutic regimens with high, moderate, mild, and minimal nausea comprised 26%, 38.4%, 26.4%, and 3.2%, respectively. Moreover, the most frequent cancers diagnosed in the patients in the abovementioned study were lung cancer (19.8%) and breast cancer (15.9%). In the present study, the most frequent types of cancers were breast cancer (51.2%) and intestinal and colorectal cancer (11.2%). The differences between our study and the study of Craver et al are justifiable, due to the different type of cancers studied.13
Based on the Likert scale for the severity of CINV, 64.8% of the patients experienced nausea (at least mild and greater) and 9.6% experienced vomiting. In the study of Ballatori et al14 on 152 patients undergoing CT, 62% and 34% of the patients suffered from nausea and vomiting, respectively. Similar to this study, our study showed that delayed CINV was more common than acute CINV. The different rate of vomiting in the study of Ballatori et al versus ours was because less than one half of the patients had received appropriate prophylaxis for delayed NV.
As it was stated in the “Results” section, 25.6% of the patients had reported lack of NV. However, other studies reported complete control of CINV (70%–80%).15,16 This difference could be due to emetogenic risk level of anticancer drugs, since a significant number of patients (46.4%) received high emetogenic risk-level anticancer regimens. Other factors were the GICP and improper use of antiemetics by the patients.
Similar to our study, breast cancer was the most frequent (59%) among diagnosed patients in the study of Glaus et al.17 The visual analog scale (VAS) was used to measure CINV in the abovementioned study. In this regard, 42%–52% of the patients experienced nausea (VAS >5 mm) and 14%–22% of the patients had a significant degree of nausea (VAS >25 mm). Moreover, 13% and 38% of the patients experienced acute and delayed nausea.17
According to the results of this study, females (P=0.001) and those with a previous history of CT (P=0.006) were more prone to CINV. However, a history of motion sickness did not affect the CINV incidence rate. This result can be due to fewer patients with motion sickness. Numerous studies have shown the effect of patient risk factors, such as female sex, young age, a history of alcohol use, impaired quality of life, and a previous history of CT, on the incidence of CINV.18,19
Unexpectedly, when patients with GCCP and GICP were compared in terms of complete response, complete protection, and complete control end points, they did not show any significant difference. However, the number of patients at all end points was higher in the GCCP.
Poli-Bigelli et al16 performed a study on patients receiving CT regimens that caused high degrees of nausea. In this study, 44%, 41%, and 39% of the patients in the group with a standard regimen showed complete response, complete protection, and complete control, respectively.16 Based on the results of the present study, 90.4%, 41.6%, and 25.6% of all the patients showed complete response, complete protection, and complete control, respectively. In this study, the antiemetic regimens of 71.2% of the patients complied with the standard guidelines.
In the study of Aapro et al20 on 991 CT patients, the proportion of patients with complete response was significantly higher in the GCCP cohort in comparison with the GICP cohort. The reason why the CINV end points were not different between GCCP and GICP cohorts in our study is unclear, although definitions of consistency, follow-up periods, and limited patient populations might have influenced our findings.
In the present study, surprisingly, the overall incidence of CINV was higher in patients receiving regimen containing aprepitant compared to those who did not receive regimen containing aprepitant. Aprepitant was not prescribed in compliance with the standard guidelines in 28.8% of the patients. Furthermore, 16.16% of the patients did not use aprepitant in accordance with its instructions. In this regard, the influential factors might be the patients’ compliance error with the instructions and/or prescription error by the physicians.
On the other hand, some studies have clearly shown the effect of triple regimen containing aprepitant (Emend®; Merck & Co., Inc., Whitehouse Station, NJ, USA) in comparison with dual regimen containing dexamethasone plus 5-HT3 RA regimens to reduce acute and delayed emesis in patients receiving high and moderate emetogenic CT.15,16,21 Hence, further controlled studies should be conducted to confirm the results of this study and compare the aprepitant Abitant® with Emend®.
There are a few limitations in this study. A limited number of patients might have led to reduced power to determine the differences between the groups. Since Nemazee Hospital is a major referral center for CT in southern Iran, the trends observed in this study can still represent the pattern of antiemetics prescription for CINV. Another limitation was heterogeneous groups of cancers with different CT regimens who were enrolled in this study. It is recommended to assess the pattern of antiemetic prescription following antiemetic prophylaxis for patients undergoing high emetic risk CT in homogeneous cancer patients in Iran.
This prospective study has enabled us to evaluate antiemetic prescription patterns in a large CT center in Iran. According to the results, the control of CINV was significantly lower than that of the global standard, where more than half of the patients showed overall CINV control. The antiemetic regimen for 28.8% of the patients did not comply with the guidelines of ASCO and MASCC, and 16.16% of the patients did not adhere to the proper usage of aprepitant. Most likely, other influential factors such as the GICP and the compliance errors were involved in the incomplete control of CINV in addition to other factors. In this regard, clinicians can contribute to better control of CINV by being more acquainted with CINV risk factors and by prescribing appropriate antiemetic regimens. Besides, pharmacists should thoroughly explain the correct instruction on how to use antiemetics.
Acknowledgments
This research was conducted by Davood Eslami in partial fulfillment of the requirements for certification as a pharmacist. The present paper was adopted from the proposal number 92-01-05-7040 approved by the vice-chancellor of research affairs at Shiraz University of Medical Sciences. The authors would like to thank the Research Consultation Center (RCC) at Shiraz University of Medical Sciences for their invaluable assistance in editing this manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jordan K, Kinitz I, Voigt W, Behlendorf T, Wolf H, Schmoll H. Safety and efficacy of a triple antiemetic combination with the NK-1 antagonist aprepitant in highly and moderately emetogenic multiple-day CT. Eur J Cancer. 2009;45(7):1184–1187. doi: 10.1016/j.ejca.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Naeim A, Dy S, Lorenz K, Sanati H, Walling A, Asch S. Evidence-based recommendations for cancer nausea and vomiting. J Clin Oncol. 2008;26(23):3903–3910. doi: 10.1200/JCO.2007.15.9533. [DOI] [PubMed] [Google Scholar]
- 3.Wiser W, Berger A. Practical management of CT-induced nausea and vomiting. Oncology. 2005;19(5):637–645. [PubMed] [Google Scholar]
- 4.Morran C, Smith D, Anderson D, McArdle C. Incidence of nausea and vomiting with cytotoxic CT: a prospective randomised trial of antiemetics. BMJ. 1979;1(6174):1323. doi: 10.1136/bmj.1.6174.1323-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14(2):85–93. doi: 10.1097/PPO.0b013e31816a0f07. [DOI] [PubMed] [Google Scholar]
- 6.Zaidan M, Soufi L, Hafeez M, Abdelwahid M, Rasul K. Assessing prescribing patterns for the prevention of CT-induced nausea and vomiting in the national center for cancer care and research. Saudi Pharm J. 2015;23(4):381–387. doi: 10.1016/j.jsps.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell E. Gastrointestinal toxicity of chemotherapeutic agents. Semin Oncol. 2006;33(1):106–120. doi: 10.1053/j.seminoncol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Chan A, Tan S, Low X, Yap K. Antiemetic effectiveness and nausea and vomiting incidence during capecitabine and oxaliplatin CT. Nurs Res. 2012;61(6):405–412. doi: 10.1097/NNR.0b013e3182691438. [DOI] [PubMed] [Google Scholar]
- 9.Lindley CM, Hirsch JD, O’Neill CV, Transau MC, Gilbert CS, Osterhaus JT. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1(5):331–340. doi: 10.1007/BF00434947. [DOI] [PubMed] [Google Scholar]
- 10.Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol. 1999;17(9):2971–2994. doi: 10.1200/JCO.1999.17.9.2971. [DOI] [PubMed] [Google Scholar]
- 11.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 12.Basch E, Prestrud AA, Hesketh PJ, et al. American Society of Clinical Oncology Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craver C, Gayle J, Balu S, Buchner D. PCN48 impact on hospital outpatient visit costs by initiating palonosetron versus other 5-hydroxytryptamine 3 receptor antagonists for prevention of chemotherapy induced nausea and vomiting (CINV) among patients with cancer. Value Health. 2011;14(3):A163. [Google Scholar]
- 14.Ballatori E, Roila F, Ruggeri B, et al. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15(2):179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 15.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 16.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer. 2003;97(12):3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 17.Glaus A, Knipping C, Morant R, et al. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer. 2004;12(10):708–715. doi: 10.1007/s00520-004-0662-x. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 19.Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer. 2010;18(9):1171–1177. doi: 10.1007/s00520-009-0737-9. [DOI] [PubMed] [Google Scholar]
- 20.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER) Ann Oncol. 2012;23(8):1986–1992. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 21.de Wit R, Herrstedt J, Rapoport B, et al. The oral NK 1 antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomised, placebo-controlled Phase III clinical trials. Eur J Cancer. 2004;40(3):403–410. [PubMed] [Google Scholar]


