Abstract
Purpose
Charcot Marie Tooth disease (CMT) describes a group of hereditary neuropathies that present with distal weakness, wasting and sensory loss. Small studies indicate that people with CMT have reduced daily activity levels. This raises concerns as physical inactivity increases the risk of a range of co- morbidities, an important consideration in the long-term management of this disease. This study aimed to compare physical activity, patterns of sedentary behavior and overall energy expenditure of people with CMT and healthy matched controls.
Methods
We compared 20 people with CMT and 20 matched controls in a comparison of physical activity measurement over seven days, using an activity monitor. Patterns of sedentary behavior were explored through a power law analysis.
Results
Results showed a decrease in daily steps taken in the CMT group, but somewhat paradoxically, they demonstrate shorter bouts of sedentary activity and more frequent transitions from sedentary to active behaviors. No differences were seen in energy expenditure or time spent in sedentary, moderate or vigorous activity.
Conclusion
The discrepancy between energy expenditure and number of steps could be due to higher energy requirements for walking, but also may be due to an over-estimation of energy expenditure by the activity monitor in the presence of muscle wasting. Alternatively, this finding may indicate that people with CMT engage more in activities or movement not related to walking.
Keywords: Accelerometer, physical activity monitoring, energy expenditure, step count, peripheral neuropathy
Introduction
Charcot Marie Tooth disease (CMT) describes a group of hereditary neuropathies that present with distal weakness, wasting and sensory loss. These symptoms impact on physical activity [1] and functions such as walking.[2] The primary impairments lead to altered gait patterns, requiring compensatory strategies to achieve functional mobility.[3] It has been shown that walking is more effortful for people with CMT [4,5] and this could contribute to reductions in physical activity.
Physical inactivity is implicated as a risk factor for developing a range of morbidities including obesity, cardiovascular disease, stroke, type-2 diabetes and osteoporosis.[6] Most forms of CMT do not alter life expectancy, so the prevention of co-morbidities is an important consideration in the long-term management of this disease.[7]
Small studies indicate that people with CMT have reduced daily activity levels. Accelerometers were used to quantify activities of daily living for eight people with CMT1A and eight matched controls over 24 h.[7] No difference in step count was observed between the groups, but there were lower counts for stair climbing and sit to stand transitions. Knee extensor strength correlated positively with both activities. The aerobic capacity of people with CMT is reduced, with V02 max during exercise testing lower than normal values.[8,9] This is an indicator of cardiovascular de-conditioning secondary to reduced physical activity.
The reasons for inactivity have had some exploration. A recent study used a mixed methods approach to explore facilitators and barriers to physical activity [10] using the Physical Activity Disability Survey. In addition, open ended questions were asked to identify barriers and facilitators in 44 people with CMT. The most active people tended to be younger, have greater self-efficacy, lower fatigue and greater balance confidence. Self-efficacy for physical activity and fatigue predicted 31% of the variance in physical activity on regression modeling. Key barriers related to impairments of physical functioning, including poor balance, muscle weakness and pain.
Study purpose
It is suggested that optimizing physical activity should be part of a multi-factorial approach to improve societal participation in people with neuromuscular disease.[11] In order to embark on this, there must be a greater understanding of the patterns of activity observed in people with CMT. This study aimed to compare physical activity, patterns of sedentary behavior and overall energy expenditure of people with CMT and healthy matched controls.
Materials and methods
Subjects
Twenty individuals with CMT were recruited from genetic peripheral neuropathy clinics at the National Hospital for Neurology and Neurosurgery (NHNN, London, UK) as part of another trial of a home exercise intervention.[12] Physical activity data collection took place during the control phase of the exercise trial, where participants were asked to continue with their normal routine and activities. Participants were included if they were aged 18–70 years, had a clinical diagnosis of CMT, or related condition, and were able to walk 50 meters with or without an aid. Participants were excluded if they had another significant neurological disorder, major co-morbidities, severe congenital hip dysplasia, or had undergone lower limb surgery in the six months prior to recruitment.
Data for 20 healthy control participants was extracted from a normative database, held at the MRC Muscle Performance and Training Laboratory (Movelab), Newcastle-upon-Tyne, UK and were matched for age and sex. Subjects included in the database were recruited from the Inland Revenue, Newcastle Council, Newcastle University employees and from an advert on a website. They were included if they had the ability to undertake physical activity monitoring for 7 days, were aged 18–70 years, had no heart complaints, or any other conditions that may affect day-to-day activity such as diabetes, chronic pain, heart, liver or neuromuscular disease.
All participants gave written informed consent, and a local research ethics committee gave the study approval before data collection began.
Physical activity measures
Physical activity was objectively measured using the SenseWear activity monitor (SenseWear Pro3, Bodymedia Inc, PA, USA), which is a validated multi-sensor device that incorporates skin temperature, galvanic skin response and bi-axial accelerometry with demographic information such as age, sex and body mass index (BMI).[13] From this, the SenseWear activity monitor derives measures of physical activity and energy expenditure. The SenseWear activity monitor’s output is represented as calorie expenditure (kcal), energy expenditure (METs; 1 MET is a person’s resting metabolic activity: 1 kcal kg−1 h−1), steps taken, as well as time spent in sedentary (<3 METs), moderate (3–6 METs) and vigorous activities (≥6 METs). The SenseWear activity monitor has been shown to correlate strongly (r = 0.85, p < 0.001) with indirect calorimetry when measuring energy expenditure in healthy people and chronic respiratory diseases, but it may underestimate step count at high walking speeds.[14–16] The SenseWear armband was placed in direct contact with the skin, over the triceps muscle of the right arm. The monitor was worn for seven days during waking hours but removed if there was to be contact with water.
Clinical measures
Control participants and people with CMT underwent height and weight measurement by physiotherapists at the MRC Muscle Performance and Training Laboratory and NHNN, respectively. BMI was calculated from these data.
People with CMT underwent additional assessments, including a full medical history, physical status, gait performance and self-reported measures. Disease severity was scored by a neurologist using the CMT Examination Score.[17] Lower limb muscle strength was evaluated using the CITEC hand held dynamometer (C.I.T. Technics, Haren, Netherlands). The following muscle groups were tested: hip extensors, hip abductors, hip adductors, hip flexors, knee extensors, knee flexors, plantarflexors, dorsiflexors. Walking endurance was measured using the six minute walk test.[18] Self-reported fatigue, walking ability and reported activity levels were measured in people with CMT using the Fatigue Severity Scale (FSS), Walk-12 and Phone-FITT questionnaires, respectively.[19,20]
Analysis
Each control participant’s SenseWear results were synchronized, by time period worn, to their matched subject with CMT. Hence, between group results were compared for the same time periods. Variables were averaged across the seven day period of wear. The following measures were recorded using the SenseWear activity monitor in both groups, and are expressed as an individual’s average day: calorie expenditure (kcals); energy expenditure (METs); step count; minutes spent performing sedentary activities (<3 METs); minutes spent in moderately vigorous activities (3–6 METs); minutes spent in vigorous activities (≥6 METs).
A further analysis of sedentary behavior patterns was performed, by a method used to evaluate activity in people with mitochondrial disease.[21,22] Data from the SenseWear activity monitor were used for a power law analysis. The density of bouts of sedentary behavior, for a specific time bin width, was plotted against the length of the bout on a logarithmic scale to calculate power distribution. Lorentz curves were calculated from the power distributions as a fraction of the total sedentary time that is accumulated in bouts longer than any sedentary period of length.
Pairs of curves for patient and control groups were then plotted as the fraction of sedentary time/density of sedentary bouts. In addition, the patterns of activity were explored through identifying transitions from inactive to active behavior. These sedentary to active transitions were normalized to the length of the recorded activity.
Unpaired two-tailed t-tests were performed to explore differences in activity variables and BMI between people with CMT and control participants. A secondary analysis using linear regression was used to explore predictors of activity variables, measured by the SenseWear activity monitor, from the clinical measures. All analyses were performed using STATA statistical software, version 13. All values are expressed as mean ± standard deviation (SD) unless otherwise stated.
Results
Group Differences
Twenty people with CMT (Table 1) and 20 healthy matched controls were compared in this study (Table 2). Seven of the people with CMT wore ankle foot orthoses (AFOs) and one of those people also used an elbow crutch unilaterally. The CMT group were heterogeneous and their diagnoses and presentation are outlined in Table 1. No difference was seen in age and BMI between groups.
Table 1.
Diagnosis of study participants with CMT.
| Type of CMT | Number of participants | CMTES (median score) | FSS (median score) | Walk-12 (median score) | 6MTW (m) | Hip Ext MVC (N/Kg) | Hip Flex MVC (N/Kg) | Hip Abd MVC (N/Kg) | Hip Add MVC (N/Kg) | Knee Ext MVC (N/Kg) | Knee Flex MVC (N/Kg) | PF MVC (N/Kg) | DF MVC (N/Kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMT type 1A | 11 (10 genetically confirmed PMP22 duplication) | 10 | 30 | 38 | 357 ± 70 | 3.2 ± 0.8 | 2.6 ± 0.8 | 2.3 ± 1.2 | 1.9 ± 0.8 | 3.5 ± 1.5 | 1.8 ± 0.9 | 3.6 ± 1.6 | 1.1 ± 1.0 |
| CMT type 2 | 5 (1 MPZ mutation, 1 MFN2 mutation, 3 clinical diagnoses) | 10 | 20 | 39 | 350 ± 45 | 3.3 ± 0.4 | 2.7 ± 0.5 | 1.9 ± 0.5 | 2.7 ± 1.0 | 5.0 ± 0.6 | 2.1 ± 0.6 | 3.6 ± 2.4 | 0.9 ± 1.0 |
| Intermediate CMT | 2 (clinical diagnoses) | 11.5 | 26 | 33 | 331 ± 81 | 4.0 ± 2.0 | 2.9 ± 1.9 | 2.8 ± 1.9 | 2.3 ± 1.0 | 3.5 ± 3.5 | 2.3 ± 2.1 | 3.1 ± 3.8 | 0.6 ± 0.3 |
| CMTX | 1 (genetically confirmed Cx32 mutation) | 10 | 47 | 40 | 322 | 3.4 | 2.2 | 1.7 | 1.8 | 4.4 | 1.3 | 4.6 | 1.2 |
| Hereditary sensory neuropathy type 1 | 1 (SPTLC1 mutation) | 14 | 30 | 35 | 360 | 3.1 | 2.3 | 1.9 | 1.9 | 4.6 | 2.1 | 2.7 | 0.1 |
| Overall | 20 | 10 | 30 | 38.5 | 351 ± 59 | 3.3 ± 0.8 | 2.7 ± 0.8 | 2.2 ± 1.0 | 2.1 ± 0.8 | 4.0 ± 1.6 | 1.9 ± 0.9 | 3.5 ± 1.8 | 1.0 ± 0.9 |
PMP22: peripheral myelin protein 22 gene; MPZ: myelin protein zero gene; MFN2: mitofusin 2 gene; Cx32: connexin 32 gene; SPTLC1: serine palmitoyltransferase, long chain base subunit 1 gene. CMTES: CMT examination score; FSS: fatigue severity score; MVC: maximum voluntary contraction; ext: extensor; flex: flexor; abd: abductor; add: adductor; PF: plantar flexor; DF: dorsiflexor. Values are mean ± standard deviation, unless specified as median.
Table 2.
Comparison of the demographics and activity variables for CMT and control participants.
| Variable | People with CMT | Controls |
|---|---|---|
| Demographics | ||
| Age (years) | 46 ± 15 | 46 ± 15 |
| Sex | 12 men, 8 women | 12 men, 8 women |
| Body mass index (kg/m2) | 24.5 ± 3.4 | 24.7 ± 3.4 |
| Activity variables | ||
| Calories per day | 1916 ± 366.4 | 1946 ± 451.7 |
| Average Mets (kcal kg−1 h−1) | 1.86 ± 0.4 | 1.86 ± 0.4 |
| Sedentary time (min) per day | 720.1 ± 111.2 | 718.5 ± 115.6 |
| Moderately active time (min) per day | 133.0 ± 76.6 | 140.0 ± 94.1 |
| Vigorously active time (min) per day | 7.4 ± 10.1 | 6.6 ± 7.4 |
| Number of steps per day | 6814.3 ± 2587.0* | 11217.6 ± 2846.0* |
p < 0.0001, no significant difference observed in other measures.
There were no significant group differences between calorie expenditure, energy expenditure (METs), or time in sedentary, moderate or vigorous activities (Table 2). However, as shown in Figure 1(A), people with CMT took significantly fewer steps each day than the control group (difference between groups: −4403.25 steps, p < 0.00001, 95% Confidence Intervals CI: −6144.25 to 2662.25).
Figure 1.
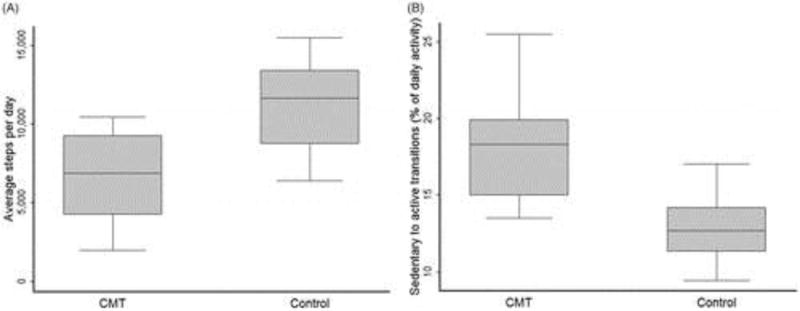
(A) Box plot of mean (± standard deviation) average daily step rate over seven days for 20 people with CMT and 20 matched controls. The difference in means was significant, p < 0.0001. (B) Box plot of mean (± standard deviation) sedentary to active transitions for 14 people with CMT and 18 matched controls. The difference in means was significant, p < 0.001.
For the power law analysis, some errors in raw data export led to some missing data. Six records were lost from the CMT group and two from the control group. The remaining data were analyzed and showed that transitions from being sedentary to active, relative to daily activity, were significantly higher in patients with CMT compared with controls (Figure 1(B), mean difference 5.54; 95% CI: 3.23–7.86; p < 0.001). Sedentary time in people with CMT tends to be made up of shorter bouts of sedentary activity compared to controls (Figure 2)
Figure 2.
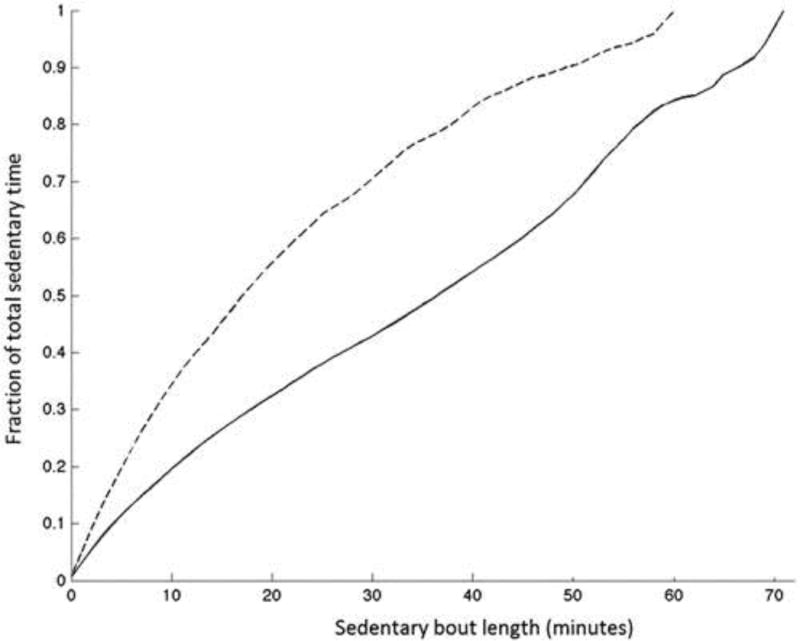
Power law analyses of the lengths of sedentary bouts of patients 14 people with CMT [dashed line] and 18 controls [solid line]. The sample of people with CMT had shorter bouts of sedentary time within the total sedentary time compared to controls.
Predictors of physical activity for people with CMT
Linear regression showed significant predictors of some of the physical activity variables: calorie expenditure, METs, time spent sedentary and in moderate activity (Table 3). No predictors from the data collected in this study were identified for step count, time spent in vigorous activity or sedentary to active transitions. Calorie expenditure had significant positive relationships with the Walk-12 score and hip abductor strength; METs related negatively to BMI; sedentary time related positively to BMI; time spent in moderate activity related to disease severity (CMTES), the walk-12 score and hip abductor strength (Table 3).
Table 3.
Summary of significant predictors of physical activity parameters following linear regression analysis.
| Variable | Regression outcome | p Values |
|---|---|---|
| Calorie expenditure (kcal) | Walk-12 score: R2 = 0.26, a = 1185, b = 20 | 0.021 |
| Hip abductor MVC: R2 = 0.3, a = 1471, b = 200 | 0.012 | |
| METs | BMI: R2 = 0.25, a = 3.3, b = −0.06 | 0.026 |
| Steps per day | No significant predictors | – |
| Sedentary time (min) | BMI: R2 = 0.25, a = 312, b = 16.7 | 0.024 |
| Moderate activity (min) | CMTES score: R2 =0.21, a = 32.9, b = 9.61 | 0.043 |
| Hip abductor MVC: R2 =0.48, a = 15.7, b = 52.9 | 0.001 | |
| Walk-12 score: R2 =0.22, a =−7.02, b = 3.86 | 0.037 | |
| Vigorous activity (min) | No significant predictors | – |
| Sedentary to active transitions | No significant predictors | – |
MVC: maximum voluntary contraction (Newtons); a =intercept constant, b =slope coefficient.
Discussion
The results of this study indicate a number of paradoxical findings. People with CMT expend the same amount of energy (calories and METs) as the matched controls, but take fewer steps per day. An earlier study of activity monitoring [7] found no difference in step count, but this was only for eight people monitored over 24 h. There will be a greater risk of bias due to the short duration and a small sample of a heterogeneous group. There is a suggestion, however, that greater effort and energy is expended when people with CMT walk.[4,5] It is possible that this could account for the higher levels of energy expenditure, if we assume that walking is the main mode of activity. This is difficult to verify, however, plus there were no differences in the time spent in moderate activity.
An alternative explanation is that the algorithms used to calculate energy expenditure rely on anthropomorphic assumptions according to normal body composition. There is an estimation of the amount of muscle tissue, which is key for energy generation. People with CMT have significant levels of muscle wasting, so there may be an over-estimation of energy expenditure. It is possible that they do expend less energy, but the SenseWear activity monitor is not yet validated in people with CMT. That would require comparison with indirect calorimetry or doubly labeled water measurements to explore and quantify the shortfall.
The second paradoxical finding is that people with CMT have shorter bouts of sedentary activity and make more transitions from sedentary to active behaviors. This is despite fewer steps per day. This may be an indication that people with CMT move in other ways than just accumulating steps when walking, for example time spent in standing, however it is generally reported that people with CMT are a less physically active group. Frequent falls and poor balance are commonly reported problems and could be associated with limited walking.[22] People with CMT may break up sedentary periods with more frequent transitions into standing, without accumulating a larger number of steps, however this would require more detailed exploration with accelerometers that can distinguish between lying, sitting and standing. The sample for this study was taken from participants who volunteered for a trial of exercise training. It is possible that this was a more motivated and active group than the general CMT population in that they put themselves forward for the main study. This analysis would need repeating on another sample of people with CMT without the participation bias in order to see if this finding re-occurs.
The secondary analysis sought to observe whether clinical measures could predict levels of physical activity in the CMT sample. A higher BMI predicted lower MET expenditure and greater time spent sedentary. It is difficult to know at present which factor may have come first. Larger people may move less and be more sedentary, but it could also be these behaviors that contribute to higher BMI. If BMI is addressed through non exercise interventions, it would be interesting to see if changes in physical activity follow suit.
People with stronger hip abductor muscles expended more energy and spent more time in moderate activities. People with greater proximal strength will be less severely affected by CMT, so may find it easier to move more often. The CMTES measure of disease severity supports this in the prediction of moderate activity levels. A larger sample may demonstrate additional relationships with other muscle groups and may also reveal predictors of higher functioning individuals, of which there were few in this study.
Self-reported walking ability, measured by the walk-12 score, predicted greater calorie expenditure and time spent in moderate activity. This may indicate that the people who are more moderately active perceive a better ability to mobilize in general. There were no relationships identified for step count, vigorous activity levels and sedentary to active transitions. This may have been due to the relatively small sample, but it could also be due to the limited parameters measured. Exploration of barriers to physical activity for people with CMT found that self-efficacy and balance confidence were significant factors.[10] These factors were not explored in this presented work and may have accounted for these variables. In particular, identifying the predictor of step count would have value in guiding targeting of physical activity interventions to improve overall mobility.
The sample for this study were heterogeneous genetically (Table 1), which introduces variability into the sample, but common patterns of impairment were noted. There were insufficient numbers in the rarer genetic groups to make meaningful comparisons, but the general presentation of distal muscle weakness was consistent in this sample.
Conclusion
Physical activity monitoring of 20 people with CMT and 20 matched controls revealed that significantly fewer steps are taken by the CMT group, though clinical predictors were not identified. Paradoxically, the participants with CMT took shorter sedentary bouts and had more sedentary to active transitions than the control group that may have been non-walking activity. There may have been a selection bias in this group as they volunteered for a study of exercise. Further work needs to be done to validate the use of activity monitors in key activities due to assumptions that may not account for muscle wasting. In addition, a greater range of clinical measures need to be used to explore predictions of activity behaviors.
Implications for Rehabilitation.
Charcot-Marie-Tooth disease
People with Charcot-Marie-Tooth disease did not show a difference in energy expenditure over seven days compared to healthy controls, but this may be due to higher energy costs of walking, and/or an over estimation of energy expenditure by the activity monitor in a population where there is muscle wasting. This needs to be considered when interpreting activity monitor data in people with neuromuscular diseases.
Compared to healthy controls, people with Charcot-Marie-Tooth disease had a lower step count over seven days, but exhibited more frequent transitions from sedentary to active behaviors
High Body Mass Index and increased time spent sedentary were related factors that have implications for general health status.
Understanding the profile of physical activity and behavior can allow targeting of rehabilitation interventions to address mobility and fitness.
Acknowledgments
Funding
This work was funded by a research grant from the Muscular Dystrophy Campaign (RA2/782/1). MMR is grateful to the Medical Research Council (MRC), MRC Center grant (G0601943), and the National Institutes of Neurological Diseases and Stroke and office of Rare Diseases (U54NS065712) for their support. This research was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Center.
Footnotes
Disclosure statement
The authors report no declarations of interest.
References
- 1.Kalkman JS, Schillings ML, Zwarts MJ, et al. The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J Psychosom Res. 2007;62:571–579. doi: 10.1016/j.jpsychores.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Newman CJ, Walsh M, O’Sullivan R, et al. The characteristics of gait in Charcot-Marie-Tooth disease types I and II. Gait Posture. 2007;26:120–127. doi: 10.1016/j.gaitpost.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Don R, Serrao M, Vinci P, et al. Foot drop and plantar flexion failure determine different gait strategies in Charcot-Marie-Tooth patients. Clin Biomech (Bristol, Avon) 2007;22:905–916. doi: 10.1016/j.clinbiomech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Menotti F, Felici F, Damiani A, et al. Charcot-Marie-Tooth 1A patients with low level of impairment have a higher energy cost of walking than healthy individuals. Neuromuscul Disord. 2011;21:52–57. doi: 10.1016/j.nmd.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Ramdharry GM, Day BL, Reilly MM, et al. Hip flexor fatigue limits walking in Charcot-Marie-Tooth disease. Muscle Nerve. 2009;40:103–111. doi: 10.1002/mus.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GOV.UK. Everybody active, every day: a framework to embed physical activity into daily life [Internet] [cited 2016 Jul 20]. Available from: https://www.gov.uk/government/publications/everybody-active-every-day-a-framework-to-embed-physical-activity-into-daily-life.
- 7.Menotti F, Laudani L, Damiani A, et al. Amount and intensity of daily living activities in Charcot–Marie–Tooth 1A patients. Brain Behav. 2014;4:14–20. doi: 10.1002/brb3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter GT, Abresch RT, Fowler WM, Jr, et al. Profiles of neuromuscular diseases. Hereditary motor and sensory neuropathy, types I and II. Am J Phys Med Rehabil Assoc Acad Physiatr. 1995;74:S140–S149. doi: 10.1097/00002060-199509001-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wallace A, Dewar L, Sterr A, et al. Normative aerobic exercise values in CMT. Neuromuscul Disord. 2015;25:S285–S286. [Google Scholar]
- 10.Anens E, Emtner M, Hellström K. An exploratory study of physical activity in persons with Charcot-Marie-Tooth disease. Arch Phys Med Rehabil. 2015;96:260–268. doi: 10.1016/j.apmr.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Voet NBM, Bleijenberg G, Padberg GW, et al. Effect of aerobic exercise training and cognitive behavioural therapy on reduction of chronic fatigue in patients with facioscapulohumeral dystrophy: protocol of the FACTS-2-FSHD trial. BMC Neurol. 2010;10:56. doi: 10.1186/1471-2377-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramdharry GM, Pollard A, Anderson C, et al. A pilot study of proximal strength training in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2014;19:328–332. doi: 10.1111/jns.12100. [DOI] [PubMed] [Google Scholar]
- 13.St-Onge M, Mignault D, Allison DB, et al. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–749. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- 14.Moore SA, Hallsworth K, Bluck LJC, et al. Measuring energy expenditure after stroke: validation of a portable device. Stroke. 2012;43:1660–1662. doi: 10.1161/STROKEAHA.111.646257. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer TJ, Alison JA, McKeough ZJ, et al. Evaluation of the SenseWear activity monitor during exercise in cystic fibrosis and in health. Respir Med. 2009;103:1511–1517. doi: 10.1016/j.rmed.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Furlanetto KC, Bisca GW, Oldemberg N, et al. Step counting and energy expenditure estimation in patients with chronic obstructive pulmonary disease and healthy elderly: accuracy of 2 motion sensors. Arch Phys Med Rehabil. 2010;91:261–267. doi: 10.1016/j.apmr.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Shy ME, Blake J, Krajewski K, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64:1209–1214. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]
- 18.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 19.Gill DP, Jones GR, Zou GY, et al. The Phone-FITT: a brief physical activity interview for older adults. J Aging Phys Act. 2008;16:292–315. doi: 10.1123/japa.16.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Graham RC, Hughes RAC. Clinimetric properties of a walking scale in peripheral neuropathy. J Neurol Neurosurg Psychiatry. 2006;77:977–979. doi: 10.1136/jnnp.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apabhai S, Gorman GS, Sutton L, et al. Habitual physical activity in mitochondrial disease. PLoS One. 2011;6:e22294. doi: 10.1371/journal.pone.0022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chastin SFM, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31:82–86. doi: 10.1016/j.gaitpost.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Ramdharry GM, Entwistle L, Reilly MM. Frequency and circumstances of falls for adults with Charcot Marie Tooth diseases. Neuromuscular Disorders. 2011;21:S18. [Google Scholar]


