Abstract
Background
The long-term effects of combined antiretroviral therapy (cART) on CD4 percentage in HIV-infected children are incompletely understood, with evidence from resource-deprived areas particularly scarce even though most children with HIV live in such settings. We sought to describe this relationship.
Methods
Observational longitudinal data from cART-naive children enrolled between December 2004 and May 2010 into an HIV care and treatment program in Kinshasa, Democratic Republic of Congo were analyzed. To estimate the effect of cART on CD4 percentage while accounting for time-dependent confounders affected by prior exposure to cART, a marginal structural linear mean model was used.
Results
Seven hundred ninety children were active for 2090 person-years and a median of 31 months; 619 (78%) initiated cART. At baseline, 405 children (51%) were in HIV clinical stage 3 or 4; 528 (67%) had advanced or severe immunodeficiency. Compared with no cART, the estimated absolute rise in CD4 percentage was 6.8% [95% confidence interval (CI), 4.7% to 8.9%] after 6 months of cART, 8.6% (95% CI, 7.0% to 10.2%) after 12 months, and 20.5% (95% CI, 16.1% to 24.9%) after 60 months. cART-mediated CD4 percentage gains were slowest but greatest among children with baseline CD4 percentage <15. The cumulative incidence of recovery to “not significant” World Health Organization age-specific immunodeficiency was lower if cART was started when immunodeficiency was severe rather than mild or advanced.
Conclusions
cART increased CD4 percentages among HIV-infected children in a resource-deprived setting, as previously noted among children in the United States. More gradual and protracted recovery in children with lower baseline CD4 percentages supports earlier initiation of pediatric cART.
Keywords: HIV, antiretroviral therapy, paediatrics, CD4, Democratic Republic of the Congo, models, statistical
INTRODUCTION
The CD4 cell count and percentage are key markers of HIV disease progression, since throughout the course of infection CD4 cells are generally depleted in the absence of combined antiretroviral therapy (cART).1,2 In children, initiation of cART at low CD4 levels is associated with a reduced likelihood of immune reconstitution3–6 and greater likelihoods of growth failure,3,7 clinical progression,7,8 and mortality.9–12 HIV treatment guidelines therefore recommend cART initiation at higher CD4 cell counts and percentages.13,14 Multiple randomized controlled trials15,16 and prospective cohort studies17–19 have demonstrated that cART effectively suppresses viral load in children. However, the effect of cART on immune reconstitution in pediatric populations, particularly in resource-deprived settings, remains incompletely understood.
While randomized trials in the United States20 and Europe15 demonstrated that children receiving more potent cART had greater CD4 percentage increases than those receiving cART comprised of fewer drugs, the profound survival and clinical progression benefits of cART21,22 restricted subsequent investigations of immunological responses to therapy to observational contexts. Prior pediatric studies have generally been of short duration3,6,7,9–11,18,19,23–32 with limited available sample sizes3,4,7,10,11,19,23–26,28–36 and were unable to contrast the effect of cART in comparison to no cART. One study estimated the effect of cART versus no cART on CD4 percentage, finding that the mean CD4 percentage of 1236 US children rose by 2.3% after 1 year of cART compared with no cART, with the difference increasing to 4.4% by 5 years.37 Of the studies in low resource areas,3,9–11,18,19,23,25–28,38 just 223,38 appraised CD4 responses at more than 2 years after cART initiation with none quantifying the effect of cART on immune reconstitution relative to no cART.
Global access to cART is expanding,39 including the more than 90%40 of children living with HIV infection in areas of the world where poverty and undernutrition are common and may affect cART outcomes.41,42 Because cART reduces morbidity and mortality among HIV-infected children in resource-deprived settings,43–45 these children may be on cART for decades. Hence, understanding immune reconstitution over longer periods of time in this population is essential. Further work on responses to cART in this group may also inform the optimal timing of cART initiation in children older than 2 years.14,46–48 Therefore, we estimated the effect of cART on mean CD4 percentage in an observational clinical cohort of HIV-infected children in the Democratic Republic of Congo (DRC), using marginal structural models (MSMs) to account for confounders affected by prior exposure while adjusting for time-dependent confounding by indication.
METHODS
Study Population, Measurements, and Follow-up
We used data from a comprehensive family-centered HIV care and treatment program at 2 sites implementing the same protocol in Kinshasa, DRC: Bomoi Healthcare Center and Kalembe Lembe Pediatric Hospital.43,44 The study population was restricted to cART-naive children younger than 18 years at baseline with HIV infection confirmed by serology if at least 18 months of age and by DNA polymerase chain reaction or HIV viral load otherwise. Children initiated HIV care between December 2004 and May 2010. Baseline was defined as the date of first CD4 percentage result; as in previous studies,49,50 this allowed children to be included despite missing data at program enrollment. Follow-up continued until either the date of death or the clinic visit preceding loss to follow-up, transfer of care, or August 2010. Children were classified as lost to follow-up if they withdrew from care or were not located by 3 tracking attempts in the subsequent months after a missed visit. All children who initiated cART contributed no cART person-time before cART initiation.
Laboratory monitoring, diagnosis and treatment of opportunistic infections, and prescription of cotrimoxazole prophylaxis and cART were based on national51 and World Health Organization (WHO)14,52–54 guidelines. The first-line cART regimen consisted of zidovudine or stavudine, lamivudine, and nevirapine or efavirenz and could be initiated at any visit after enrollment. Infants younger than 2 years were not always started on therapy immediately, as recommended by WHO in 2009,14 because virological diagnostics were only intermittently available. Physicians documented clinical data using standardized forms during routine visits (scheduled monthly for children receiving cART and quarterly for children not receiving cART) and unscheduled visits (for children needing acute care). Children not receiving cART were not eligible for treatment according to guidelines. If a child died, program personnel collected the date and suspected cause of death. CD4 cell count and percentage were assessed biannually at the DRC National AIDS Reference Laboratory. HIV viral load was only occasionally available.
Parental written informed consent for the HIV care program was obtained for all children, with minors at least 12 years of age additionally providing their written assent. The University of North Carolina at Chapel Hill Institutional Review Board and the Ethics Committee of the Kinshasa School of Public Health approved the study.
Statistical Analysis
Estimation of the total effect of cART on change in mean CD4 percentage at time t, the primary aim of analysis, entails adjustment for factors that are common causes, or confounders, of both exposure and outcome. Whereas some confounders are measured at baseline only, others, known as time-varying confounders affected by prior exposure, are measured throughout follow-up and are causal intermediates between cART and CD4 percentage at time t while concurrently common causes of subsequent cART status and CD4 percentage at time t. Specifying time-varying confounders affected by prior exposure, for example CD4 percentage at times prior to t, as time-varying covariates in a standard regression model will fail to yield the total effect of cART on change in mean CD4 percentage at time t because the effects of cART are mediated in part through the time-varying factors. Therefore, to appropriately estimate the immunological effect of cART in the presence of such factors, we used inverse probability weighting.55
Our data set was configured into a one row per person-day structure, with missing covariate data carried forward from last measurement. We used 6 logistic regression models to predict the outcomes of child and time-specific probabilities of treatment, censoring, and visit attendance based on covariate histories (ie, both current and past covariate data). These were then multiplied to calculate composite stabilized inverse probability weights. Specifically, time and baseline confounders were predictors in the models for the treatment weight numerator and denominator, with time-varying confounders affected by prior exposure also included in the latter. The censoring weight numerator and denominator models, as well as those for the visit attendance weight numerator and denominator, were identical to the treatment weight models except for the additional inclusion of time-varying cART as a predictor in the 2 censoring models, and both time-varying cART and time since last visit in the 2 visit attendance models. The combined weights were then applied in a linear regression model with cumulative cART exposure and baseline confounders as independent variables and CD4 percentage as the outcome, using only observations with a measured CD4 percentage result, to estimate the parameters of a linear repeated-measures MSM. To account for within-subject correlation induced by weighting and resulting from repeated CD4 percentage measurements in individuals, and to yield 95% confidence intervals (CIs) based on robust variance, the model was fitted using generalized estimating equations with an independent working covariance matrix.49 If children received uninterrupted cART during follow-up, plausible in our program because physicians routinely assessed adherence and never discontinued treatment for active patients, and assuming correct model specification, positivity, and no unmeasured confounding or informative censoring, the MSM yields the difference in mean CD4 percentage had all children in the population started cART immediately at baseline compared with had they never started cART during follow-up. The MSM therefore mimics a randomized controlled trial.
A posited directed acyclic graph56 (see Appendix, Supplemental Digital Content, http://links.lww.com/QAI/A332) and previous studies informed confounder selection. Age, gender, and WHO HIV clinical stage and severity of immunodeficiency were baseline confounders, whereas time-varying confounders affected by prior exposure were cotrimoxazole prophylaxis, HIV-related symptoms or conditions, and CD4 percentage. Age and CD4 levels were used to calculate age-specific severity of immunodeficiency (not significant, mild, advanced, or severe), based on WHO guidelines.53 For all children, including those for whom follow-up started at first CD4 result rather than HIV care initiation, HIV clinical stage (1–4) was that at enrollment because it was assessed only at first visit. A child had HIV-related symptoms or conditions if diagnosed with one or more of the following: Kaposi sarcoma, oral or esophageal candidiasis, severe weight loss, tuberculosis, fever or diarrhea of 1 month or more, lymphocytic interstitial or Pneumocystis jirovecii pneumonia, chronic herpes simplex, oral hairy leukoplakia, cryptococcal meningitis, toxoplasma or HIV encephalopathy, or HIV-associated nephropathy. Gender, cotrimoxazole, and symptoms or conditions were coded dichotomously, as was cART in logistic models. In linear models, cumulative cART exposure was coded into indicators for 6-month categories, that is, no cART, >0 to 6 months, >6 to 12 months, and so on through a maximum of 66 months. Age and time were modeled as restricted cubic splines with 4 knots at the fifth, 35th, 65th and 95th percentiles, as was CD4 percentage when a time-varying confounder affected by prior exposure. CD4 percentage, which was examined because it is a more stable metric than absolute CD4 count in children,57 allowed young children to be included, and enabled comparison with a similar US study,37 was expressed as a whole number when an outcome in linear models.
Results from unadjusted and adjusted but unweighted linear repeated measures models were examined for comparison to elucidate reductions in bias by use of weighted models. To estimate the effect of cART on CD4 percentage by baseline categories (CD4 percentage <15%, 15%–24%, and ≥25%), models were fitted within those strata. We used the log-rank test after constructing Kaplan–Meier plots and Cox proportional hazards regression to assess whether time to reaching “not significant” WHO age-specific immunodeficiency (CD4 percentage >35% for age <1 year, >30% for age ≥1 to <3 years, >25% for age ≥3 to <5 years, and >500 cells/mm3 for age ≥5 years) differed by degree of immunodeficiency (mild, advanced, and severe) at cART initiation. The proportionality of hazards was verified by visual inspection of log-negative-log survival estimates. All analyses, including a macro58 to generate cumulative incidence curves59 to visually depict time to immunological recovery, were completed in SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the 790 children at the start of and during follow-up are presented in Table 1. At baseline, which corresponded with HIV care initiation for 749 children (94.8%), the median age was 5.9 years [interquartile range (IQR), 2.7–9.8] and more than half (405, 51.3%) had clinically evident stage 3 or 4 HIV disease. As the median CD4 percentage was 15 (IQR, 9–22), immunodeficiency was either advanced (76, 9.6%) or severe (452, 57.2%) in most patients. Cotrimoxazole was initiated by almost all children (726, 91.9%) at first visit.
TABLE 1.
Characteristics of 790 Children Initiating HIV Care Between December 2004 and May 2010 in Kinshasa, DRC
| Baseline* | |
|---|---|
| Median age, years (IQR) | 5.9 (2.7–9.8) |
| Age [n (%)] | |
| <1 | 63 (8.0) |
| 1–4 | 277 (35.1) |
| 5–9 | 265 (33.5) |
| 10–17 | 185 (23.4) |
| Female sex [n (%)] | 415 (52.5) |
| WHO HIV clinical stage [n (%)] | |
| 1 | 153 (19.4) |
| 2 | 232 (29.4) |
| 3 | 369 (46.7) |
| 4 | 36 (4.6) |
| Median CD4 percentage (IQR) | 15 (9–22) |
| WHO age-specific severity of immunodeficiency [n (%)] | |
| Not significant | 174 (22.0) |
| Mild | 88 (11.1) |
| Advanced | 76 (9.6) |
| Severe | 452 (57.2) |
| Started cotrimoxazole at first visit [n (%)] | 726 (91.9) |
| Follow-up (all children) | |
| Total person-years accrued | 2089.8 |
| Median months of follow-up (IQR) | 31.2 (10.3–53.6) |
| Median number of program visits (IQR) | 30 (11–57) |
| Died [n (%)] | 80 (10.1) |
| Lost to follow-up [n (%)] | 76 (9.6) |
| Transferred care [n (%)] | 6 (0.8) |
| Initiated cART | 619 (78.4) |
| Number of CD4 percentage results | 3137 |
| Follow-up (children initiating cART) | |
| Median weeks of follow-up prior to cART initiation (IQR) | 4.0 (2.2–15.3) |
| Severe immunodeficiency at cART initiation [n (%)] | 454 (73.3) |
| Median CD4 percentage at cART initiation (IQR) | 12 (7–18) |
| cART person-years accrued | 1620.9 |
| Median months on cART (IQR) | 31.3 (11.4–52.0) |
| Switched cART regimen [n (%)] | 110 (17.8) |
| Number of CD4 percentage results during cART | 2485 |
Baseline (first CD4 percentage result) was not at program enrollment for 41 of 790 children (5.2%). For these 41 children, the median number of months between enrollment and first CD4 percentage result was 2.3 (IQR, 1.1–5.3).
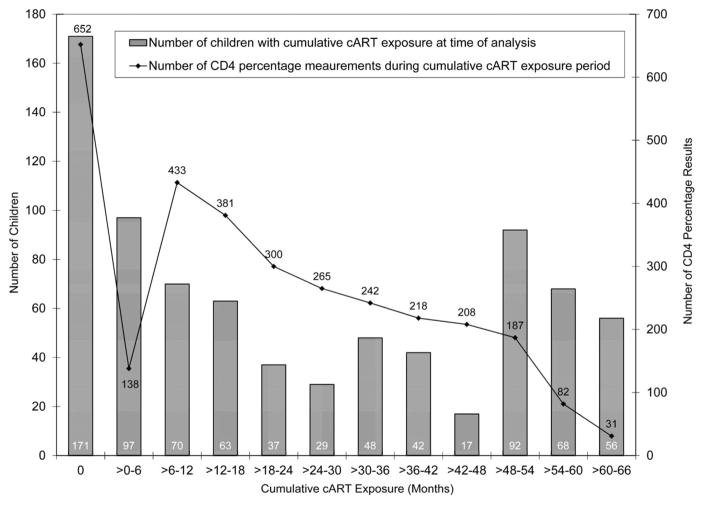
During the 2089.8 person-years accrued, the median duration of follow-up was 31.2 months (IQR, 10.3–53.6), and the median number of clinic visits was 30 (IQR, 11–57). The number and proportion of patients active in HIV care for at least 12, 24, 36, 48, and 60 months were 573 (72.5%), 440 (55.7%), 352 (44.6%), 279 (35.3%), and 127 (16.1%), respectively. Eighty children (10.1%) died, 76 (9.6%) were lost to follow-up, and 6 (0.8%) transferred to another care facility. cART was started by 619 children (78.4%) at a median of 4.0 weeks (IQR, 2.2–15.3) after baseline. At initiation, the median CD4 percentage was 12 (IQR, 7–18) and 454 children (73.3%) were severely immunosuppressed. Of the children who initiated cART, 62 (10.0%) met the WHO definition of immunological failure (after 24 weeks of cART, developing or returning to a CD4 count ≤200 cells/mm3 or percentage ≤10% if older than 2 to younger than 5 years, or a CD4 count ≤100 cells/mm3 if age older than or equal to 5 years).14 Children’s durations of cART (including no cART) during follow-up, and the number of CD4 percentage measurements during no cART and each of the 6-month cumulative cART exposure periods, are depicted in Figure 1. cART was received by 171 children (21.6%) for zero months, 167 children (21.1%) for >0–12 months, 177 (22.4%) for >12–36 months, and 275 (34.8%) for >36 months. Those receiving cART, 110 (17.8%) of whom changed regimens because of treatment failure or an adverse event, were followed for a median of 31.3 months (IQR, 11.4–52.0) and contributed 1620.9 person-years (77.6% of the total) while on cART. Of the 3137 total CD4 percentage measurements, 2485 (79.2%) were obtained during receipt of cART.
FIGURE 1.
Duration of cART (bars) and number of CD4 percentage measurements during each of the 6-month periods (lines) in 790 children initiating HIV care between December 2004 and May 2010 in Kinshasa, DRC.
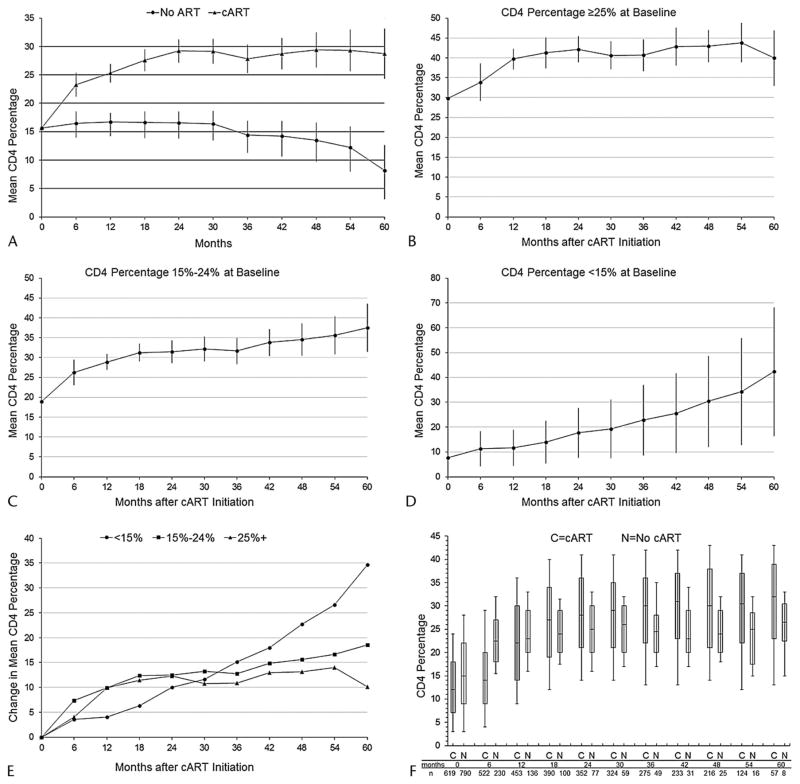
According to the MSM, cART resulted in a 6.8% (95% CI, 4.7% to 8.9%) absolute rise in mean CD4 percentage in the first 6 months and an 8.6% (95% CI, 7.0% to 10.2%) gain at 12 months, relative to no cART (Table 2, model 1; Fig. 2, panel A). The rate of increase was less in subsequent months and years. Not properly accounting for time-varying confounders affected by prior exposure yielded attenuated effects (Table 2, models 2–4). For comparison to the appropriately adjusted model-based results, we also plotted the observed, unadjusted CD4 percentage evolutions for children receiving cART and children not receiving cART (Fig. 2, panel F).
TABLE 2.
Estimated Effect of cART on CD4 Percentage,* 790 Children Initiating HIV Care Between December 2004 and May 2010 in Kinshasa, DRC
| Model | Absolute Change in CD4 Percentage From Baseline (95% CI)
|
|||||
|---|---|---|---|---|---|---|
| <0–6 mo of cART | <6–12 mo of cART | <18–24 mo of cART | <30–36 mo of cART | <42–48 mo of cART | <54–60 mo of cART | |
| 1. Weighted, adjusted† (baseline confounders) | 6.8 (4.7–8.9) | 8.6 (7.0–10.2) | 12.7 (10.7–14.7) | 13.4 (10.9–15.9) | 15.9 (12.8–19.0) | 20.5 (16.1–24.9) |
| 2. Unweighted, unadjusted (no confounders) | 0.3 (−1.5–2.2) | 1.2 (−0.1–2.6) | 4.0 (2.3–5.6) | 3.4 (1.2–5.6) | 3.6 (0.7–6.5) | 3.7 (−0.5–7.8) |
| 3. Unweighted, adjusted (baseline confounders) | 4.2 (2.2–6.1) | 5.7 (4.1–7.3) | 9.9 (7.9–11.9) | 10.8 (8.2–13.4) | 12.6 (9.6–15.7) | 16.3 (12.0–20.5) |
| 4. Unweighted, adjusted (baseline and time-varying confounders) | 8.4 (6.7–10.2) | 8.4 (7.3–9.4) | 5.0 (3.8–6.1) | 3.6 (2.2–5.1) | 4.2 (5.8–4.2) | 4.8 (2.6–6.9) |
All estimates are derived from repeated measures linear models, fit with generalized estimating equations, that include time modeled as a restricted cubic spline with 4 knots.
MSM: Weighting appropriately accounts for time-varying confounders affected by prior exposure.
FIGURE 2.
Estimated effect of cART on CD4 percentage from marginal structural model,a overall (Panel A) and by category of CD4 percentage at baseline (Panels B–E), and observed unadjusted CD4 percentage evolutions among children receiving cART and children not receiving cART (Panel F),b 790 children initiating HIV care between December 2004 and May 2010 in Kinshasa, DRC. aEstimates are from repeated measures linear models, fit with generalized estimating equations, that include time modeled as a restricted cubic spline with 4 knots. To plot CD4 percentages among children not receiving cART, time was coded categorically (6-month periods). As there were no discernible upward or downward trends in CD4 percentage over time among children not receiving cART when spline terms were included as predictors in the stratum-specific models, it is assumed that the cumulative cART exposure parameters represent change from baseline. Error bars represent 95% CIs. bThe time scale for children not receiving cART is time since the start of follow-up, whereas the time scale for children receiving cART is time since cART initiation. Each box plot depicts the median and IQR, with the error bars marking the 10th and 90th percentiles. The most recent CD4 percentage was carried forward to each 6-month cut point.
The effect of cART on CD4 percentage differed by category of CD4 percentage at baseline (Fig. 2, panels B–E). In children with a baseline CD4 percentage ≥25%, CD4 percentage rose by almost 10% (95% CI, 7.4% to 12.4%) in the first 12 months, to a mean of 40%, and stabilized. In children with a baseline CD4 percentage of 15% to 24%, CD4 percentage increased rapidly in the first 12 months, to a mean of 29%, with more gradual increases thereafter. Gains were slower in children with a baseline CD4 percentage <15%.
Only 83 of 619 children (13.4%) had “not significant” WHO age-specific immunodeficiency at time of cART initiation. There was a reduced hazard of recovery to “not significant” immunodeficiency (unadjusted hazard ratio, 0.62; 95% CI, 0.40 to 0.96) and a corresponding longer time to recovery (log-rank P = 0.03) if cART was initiated when immunodeficiency was severe rather than mild or advanced. In the 82 children who initiated cART with either mild or advanced immunodeficiency, the cumulative incidences of recovery to “not significant” immunodeficiency at 12, 24, and 36 months were respectively 28.5%, 36.3%, and 42.7%, whereas the proportions in the 454 children who started cART with severe immunodeficiency were 17.5% by 12 months, 26.0% by 24 months, 27.7% by 36 months, and 29.9% by 48 months (Fig. 3).
FIGURE 3.
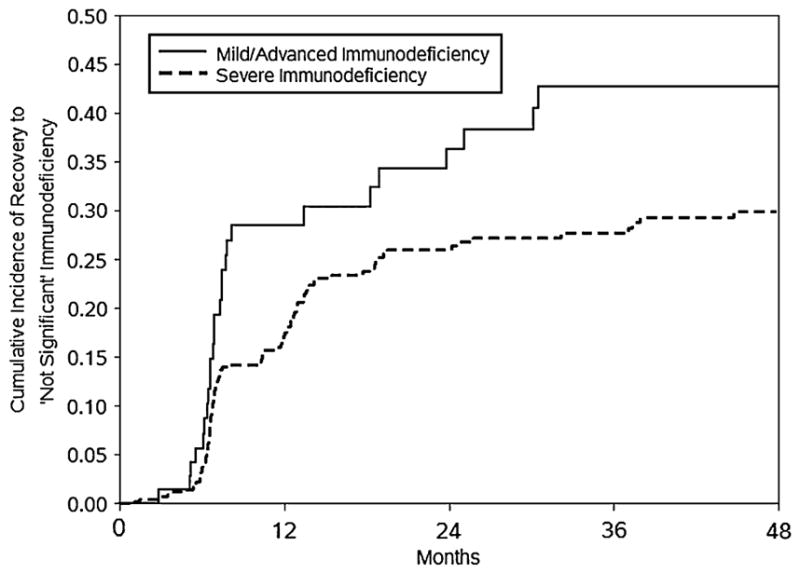
Cumulative incidence curves of recovery to “not significant” WHO age-specific immunodeficiency, by category of suppression at the start of cART, 536 children initiating cART with mild, advanced, or severe immunodeficiency between December 2004 and May 2010 in Kinshasa, DRC.
DISCUSSION
cART markedly increased the mean CD4 percentage in a cohort of HIV-infected children in Kinshasa, DRC compared with no cART, by 8.6% (absolute) at 12 months and 20.5% at 60 months of cART. Our study provides the first estimate of the effect of cART on CD4 percentage relative to no cART among antiretroviral-naive children in a resource-deprived setting and just the second estimate of this informative contrast overall.37 This was achieved by using inverse probability weighting to account for evident biases. Additionally, our study is one of the few providing evidence of sustained CD4 percentage improvements following cART initiation in children.44,50
Our findings that cART increased CD4 percentages in children over the short and long term, both overall and within baseline CD4 percentage categories, were generally concordant with those from the methodologically similar US study.37 That study reported lesser gains throughout follow-up (eg, 2.3% and 4.4% at 12 and 60 months, in contrast to 8.6% and 20.5%). This could be because of the large difference in the baseline CD4 percentage distributions of the study populations, as the median baseline CD4 percentage was approximately 25% in the US cohort compared with 15% in the Kinshasa cohort. A higher baseline CD4 percentage results in lower potentials for gains. The difference was also present within strata, and may contribute to the greater and more rapid change we noted among children with a baseline CD4 percentage ≥25%.
Among children with a baseline CD4 percentage <15%, the CD4 percentage increases at 12 and 24 months of cART (4.0% and 10.0%) were close to those noted in the US study (4.4% and 8.0%). In these children, the mean CD4 percentage recovered by 42 months to “normal” levels (>25%).8,27,37 Although this indicates that recovery is possible for children initiating cART with advanced immunodeficiency, CD4 percentages remained below 20% even after 24 months. The prolonged period of immunodeficiency likely contributes to prolonged morbidity and mortality during cART. The decreased probability of immunological treatment success following initiation at low CD4 levels revealed in our and prior studies,3–6,37 and high mortality rates in children initiating cART at low CD4 percentages,9–12 provide compelling evidence that pediatric cART should not be delayed until CD4 percentages drop below 15%. The newly revised WHO guidelines are in accordance with this evidence.14
Not being able to include HIV viral load as a confounder was a limitation (although a prior study suggests that not accounting for this factor may have only resulted in nomimal bias50), as was the lack of diagnostic capacity in Kinshasa to identify all HIV-related symptoms and conditions. The appropriateness of the methodological approach, including accounting for the dynamic visit schedule and its potential to influence result validity,60 was supported by the dissimilar results from weighted and standard unweighted models. Exposure misclassification was minimized through accurate recordkeeping of initiation dates and frequent monitoring of adherence. However, because virological monitoring was infrequent and drug options were limited in our setting, the possibility exists that some children recorded as receiving cART were actually failing therapy, which may have biased our estimates downward. Bias resulting from carrying forward CD4 percentage when a result was missing, while possible, was likely minimal—assuming that children were on average halfway to their next scheduled test when follow-up concluded, 83% of the expected number of results was available. The study population probably included a number of HIV slow progressors given that some children, whose median CD4 percentage remained stable at approximately 25%, were ineligible for cART even after several years (Fig. 2F). Our estimates would likely be attenuated relative to those from an otherwise similar population without any slow progressors. Conclusions about immunological responses to cART in very young children should be guarded given that just 8% of the population was younger than 1 year at baseline, although it should be noted that 43% of children, compared with 29% in the US study,37 were younger than 5 years at baseline. A strength of our study is that we examined immunological recovery by severity of immunodeficiency and not just CD4 percentage categories.
Our results build upon prior evidence showing the positive immunological impact of pediatric cART across CD4 levels and supporting therapy initiation before immune system degradation. By contributing unique relevant information on long-term responses in a resource-deprived setting, this study widens the breadth of understanding on a relationship that is central to the morbidity and mortality outcomes of HIV-infected children. Future efforts should focus on pooling data from similar cohorts to more precisely estimate effects within immunological subgroups and even longer after cART begins.
Acknowledgments
The HIV care and treatment program at Kalembe Lembe Pediatric Hospital and Bomoi Healthcare Center, conducted in collaboration with the Kinshasa School of Public Health, the National AIDS Control Program, and the Salvation Army (Bomoi), was funded by the Centers for Disease Control and Prevention Global AIDS Program Grant U62/CCU422422 and the President’s Emergency Plan for AIDS Relief Grant 5U2GPS001179-01, with additional support from the Elizabeth Glaser Pediatric AIDS Foundation, the Belgian Development Cooperation, the William J. Clinton Foundation, the United Nations Children’s Fund, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication. Drs Edmonds, Yotebieng, Napravnik, Cole, Van Rie, and Behets conceived and designed the study. Drs Lusiama, Matumona, Kitetele, and Nku directed implementation of clinical protocols (including patient enrollment and management) and were responsible for data collection and quality. Dr Edmonds analyzed the data, with contributions and input from Drs Yotebieng, Napravnik, Cole, Van Rie, and Behets, and wrote the first draft of the manuscript. All authors contributed to final review and editing, including interpretation of results, and read and approved the text as submitted to JAIDS. We are grateful for the patient care, program administration and coordination, and data entry contributions of Drs Catherine Akele, Marie Louise Batumbula, Steven Callens, Jean Lambert Chalachala, Luca Flamigni, Vicky Ilunga Kambaji, François Kitenge, Patricia Lelo, Bina Mboma, Léon Motingia, Aimee Mupuala, Papy Ndjibu, Emile Okitolonda, Nicole Shabani, Tomi Tshikandu, and Landry Wenzi; Ms Therese Bapampa, Ms Clarisse Bokwala, Ms Odette Daiku, Ms Karen Hawkins Reed (CDC Nigeria), Mr Alphonse Itshieki, Mr Jacques Kafulu, Mr John Kalombo, Ms Nene Kilese, Ms Delphine Kizungu, Ms Mamie Lema, Ms Jeanne Luvuma, Ms Christine Mbombo, Ms Françoise Mbuyulu, Ms Hortense Miandabu, Mr Steve Mpuate, Mr Gabin Mukalakala, Ms Adele Mumpasi, Mr Kashamuka Mwandagalirwa, Mr Jean Pierre Ndaye, Ms Bibole Ngalamulume, Mr Jose Ngamboli, Ms Jeane Ngwele, Mr Guy Nkoba, Mr Roger Pongi, Mr Sammy Siwadio, Ms Alice Tabala, Ms Martine Tabala, Ms Deidre Thompson, and Ms Sandrine Usaku.
Footnotes
Presented as abstracts at the XVIV International AIDS Conference, July 17–20, 2011, Rome, Italy and the 3rd International Workshop on HIV Pediatrics, July 15–16, 2011, Rome, Italy.
Supplemental digital content is available for this article. Direct URL citations seem in the printed text and are provided in the HTML and PDF versions of this article on the Web site of the journal (www.jaids.com).
The authors have no conflicts of interest to disclose. However, Drs Edmonds, Yotebieng, Lusiama, Matumona, Kitetele, Van Rie, and Behets declared that money was paid to their institution, in grants, for the HIV care and treatment program that provided the data for this study (grants were not for this specific secondary data analysis; rather, they were for the “parent” program). Dr Napravnik has stated that her institution has received grant support from Pfizer, Bristol-Myers Squibb, and Merck (these financial activities are outside of the submitted work).
References
- 1.Fahey JL, Prince H, Weaver M, et al. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984;76:95–100. doi: 10.1016/0002-9343(84)90756-3. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Macher AM, Longo DL, et al. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 3.Musoke PM, Mudiope P, Barlow-Mosha LN, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. doi: 10.1186/1471-2431-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolic-Djokic D, Essajee S, Rigaud M, et al. Immunoreconstitution in children receiving highly active antiretroviral therapy depends on the CD4 cell percentage at baseline. J Infect Dis. 2002;185:290–298. doi: 10.1086/338567. [DOI] [PubMed] [Google Scholar]
- 5.Soh CH, Oleske JM, Brady MT, et al. Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003;362:2045–2051. doi: 10.1016/s0140-6736(03)15098-2. [DOI] [PubMed] [Google Scholar]
- 6.Walker AS, Doerholt K, Sharland M, et al. Response to highly active antiretroviral therapy varies with age: the UK and Ireland Collaborative HIV Paediatric Study. AIDS. 2004;18:1915–1924. doi: 10.1097/00002030-200409240-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ghaffari G, Passalacqua DJ, Caicedo JL, et al. Two-year clinical and immune outcomes in human immunodeficiency virus-infected children who reconstitute CD4 T cells without control of viral replication after combination antiretroviral therapy. Pediatrics. 2004;114:e604–e611. doi: 10.1542/peds.2004-0274. [DOI] [PubMed] [Google Scholar]
- 8.Ylitalo N, Brogly S, Hughes MD, et al. Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med. 2006;160:778–787. doi: 10.1001/archpedi.160.8.778. [DOI] [PubMed] [Google Scholar]
- 9.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 10.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d’Ivoire. AIDS. 2004;18:1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 11.George E, Noel F, Bois G, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis. 2007;195:1411–1418. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 12.KIDS-ART-LINC Collaboration. Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 13.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. [Accessed August 16, 2010];Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 14.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Recommendations for a Public Health Approach—2010 Revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 15.A randomized double-blind trial of the addition of lamivudine or matching placebo to current nucleoside analogue reverse transcriptase inhibitor therapy in HIV-infected children: the PENTA-4 trial. Paediatric European Network for Treatment of AIDS. AIDS. 1998;12:F151–F160. [PubMed] [Google Scholar]
- 16.Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet. 2002;359:733–740. doi: 10.1016/S0140-6736(02)07874-1. [DOI] [PubMed] [Google Scholar]
- 17.Eley B, Davies MA, Apolles P, et al. Antiretroviral treatment for children. S Afr Med J. 2006;96(9 pt 2):988–993. [PubMed] [Google Scholar]
- 18.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–e1140. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 19.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachman SA, Stanley K, Yogev R, et al. Nucleoside analogs plus ritonavir in stable antiretroviral therapy-experienced HIV-infected children: a randomized controlled trial. Pediatric AIDS Clinical Trials Group 338 Study Team. JAMA. 2000;283:492–498. doi: 10.1001/jama.283.4.492. [DOI] [PubMed] [Google Scholar]
- 21.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 22.Henry K, Erice A, Tierney C, et al. A randomized, controlled, double-blind study comparing the survival benefit of four different reverse transcriptase inhibitor therapies (three-drug, two-drug, and alternating drug) for the treatment of advanced AIDS. AIDS Clinical Trial Group 193A Study Team. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:339–349. doi: 10.1097/00042560-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 23.De Beaudrap P, Rouet F, Fassinou P, et al. CD4 cell response before and after HAART initiation according to viral load and growth indicators in HIV-1-infected children in Abidjan, Cote d’Ivoire. J Acquir Immune Defic Syndr. 2008;49:70–76. doi: 10.1097/QAI.0b013e3181831847. [DOI] [PubMed] [Google Scholar]
- 24.Jankelevich S, Mueller BU, Mackall CL, et al. Long-term virologic and immunologic responses in human immunodeficiency virus type 1-infected children treated with indinavir, zidovudine, and lamivudine. J Infect Dis. 2001;183:1116–1120. doi: 10.1086/319274. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien DP, Sauvageot D, Olson D, et al. Treatment outcomes stratified by baseline immunological status among young children receiving nonnucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in resource-limited settings. Clin Infect Dis. 2007;44:1245–1248. doi: 10.1086/513433. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien DP, Sauvageot D, Zachariah R, et al. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20:1955–1960. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- 27.Puthanakit T, Kerr S, Ananworanich J, et al. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. Pediatr Infect Dis J. 2009;28:488–492. doi: 10.1097/inf.0b013e318194eea6. [DOI] [PubMed] [Google Scholar]
- 28.Puthanakit T, Oberdorfer A, Akarathum N, et al. Efficacy of highly active antiretroviral therapy in HIV-infected children participating in Thailand’s National Access to Antiretroviral Program. Clin Infect Dis. 2005;41:100–107. doi: 10.1086/430714. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblatt HM, Stanley KE, Song LY, et al. Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis. 2005;192:445–455. doi: 10.1086/431597. [DOI] [PubMed] [Google Scholar]
- 30.Sleasman JW, Nelson RP, Goodenow MM, et al. Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. J Pediatr. 1999;134:597–606. doi: 10.1016/s0022-3476(99)70247-7. [DOI] [PubMed] [Google Scholar]
- 31.Starr SE, Fletcher CV, Spector SA, et al. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N Engl J Med. 1999;341:1874–1881. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 32.van Rossum AM, Scherpbier HJ, van Lochem EG, et al. Therapeutic immune reconstitution in HIV-1-infected children is independent of their age and pretreatment immune status. AIDS. 2001;15:2267–2275. doi: 10.1097/00002030-200111230-00008. [DOI] [PubMed] [Google Scholar]
- 33.Chiappini E, Galli L, Tovo PA, et al. Five-year follow-up of children with perinatal HIV-1 infection receiving early highly active antiretroviral therapy. BMC Infect Dis. 2009;9:140. doi: 10.1186/1471-2334-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newell ML, Patel D, Goetghebuer T, et al. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: is it associated with age at initiation? J Infect Dis. 2006;193:954–962. doi: 10.1086/500842. [DOI] [PubMed] [Google Scholar]
- 35.Resino S, Resino R, Micheloud D, et al. Long-term effects of highly active antiretroviral therapy in pretreated, vertically HIV type 1-infected children: 6 years of follow-up. Clin Infect Dis. 2006;42:862–869. doi: 10.1086/500412. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg A, Dickover R, Britto P, et al. Continuous improvement in the immune system of HIV-infected children on prolonged antiretroviral therapy. AIDS. 2008;22:2267–2277. doi: 10.1097/QAD.0b013e3283189bb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis. 2008;46:1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr. 2010;55:503–509. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO, UNAIDS, and UNICEF. Towards Universal Access: Scaling up Priority HIV/AIDS Interventions in the Health Sector: Progress Report 2010. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 40.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2010. [Google Scholar]
- 41.De Baets AJ, Bulterys M, Abrams EJ, et al. Care and treatment of HIV-infected children in Africa: issues and challenges at the district hospital level. Pediatr Infect Dis J. 2007;26:163–173. doi: 10.1097/01.inf.0000253040.82669.22. [DOI] [PubMed] [Google Scholar]
- 42.De Baets AJ, Ramet J, Msellati P, et al. The unique features of pediatric HIV-1 in sub-Saharan Africa. Curr HIV Res. 2008;6:351–362. doi: 10.2174/157016208785132491. [DOI] [PubMed] [Google Scholar]
- 43.Edmonds A, Lusiama J, Napravnik S, et al. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38:1612–1621. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med. 2011;8:e1001044. doi: 10.1371/journal.pmed.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ananworanich J, Puthanakit T, Saphonn V, et al. Lessons from a multicentre paediatric HIV trial. Lancet. 2008;372:356–357. doi: 10.1016/S0140-6736(08)61139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verweel G, Saavedra-Lozano J, van Rossum AM, et al. Initiating highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children in Europe and the United States: comparing clinical practice to guidelines and literature evidence. Pediatr Infect Dis J. 2006;25:987–994. doi: 10.1097/01.inf.0000242670.11693.56. [DOI] [PubMed] [Google Scholar]
- 48.Welch SB, Gibb D. When should children with HIV infection be started on antiretroviral therapy? PLoS Med. 2008;5:e73. doi: 10.1371/journal.pmed.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole SR, Hernan MA, Margolick JB, et al. Marginal structural models for estimating the effect of highly active antiretroviral therapy initiation on CD4 cell count. Am J Epidemiol. 2005;162:471–478. doi: 10.1093/aje/kwi216. [DOI] [PubMed] [Google Scholar]
- 50.Patel K, Hernan MA, Williams PL, et al. Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 51.National Program for the Fight against AIDS. National Guide for Antiretroviral Treatment of HIV Infection. Kinshasa, Democratic Republic of the Congo: Ministry of Health; 2005. [Google Scholar]
- 52.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach—2006 Rev. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 53.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 54.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach—2010 Rev. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 55.Petersen ML, Wang Y, van der Laan MJ, et al. Assessing the effectiveness of antiretroviral adherence interventions. Using marginal structural models to replicate the findings of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S96–S103. doi: 10.1097/01.qai.0000248344.95135.8d. [DOI] [PubMed] [Google Scholar]
- 56.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 57.Raszka WV, Jr, Meyer GA, Waecker NJ, et al. Variability of serial absolute and percent CD4+ lymphocyte counts in healthy children born to human immunodeficiency virus 1-infected parents. Military Pediatric HIV Consortium. Pediatr Infect Dis J. 1994;13:70–72. [PubMed] [Google Scholar]
- 58.Pintilie M. Competing risks: A Practical Perspective. Chichester, England: John Wiley & Sons; 2006. [Google Scholar]
- 59.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 60.Hernan MA, McAdams M, McGrath N, et al. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18:27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]