Abstract
Background
Altered coagulation function after trauma may contribute to venous thromboembolism (VTE) development. Severe trauma impairs coagulation function, but the trajectory for recovery is not known. We hypothesized that enhanced, early recovery of coagulation function increases VTE risk in severely-injured trauma patients.
Study Design
Secondary analysis was performed on data from The Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial, excluding patients who died within 24 hours and/or were on pre-injury anticoagulants. Patient characteristics, adverse outcomes, and parameters of platelet function (PF) and coagulation (thromboelastography; TEG) were compared from admission to 72 hours between VTE (n=83) and non-VTE (n=475) patients. p<0.05 indicated significance.
Results
Despite similar patient demographics, VTE patients exhibited hypercoagulable TEG parameters and enhanced PF at admission (p<0.05). Both groups exhibited hypocoagulable TEG parameters, platelet dysfunction and suppressed clot lysis (low LY30) 2HR following admission (p<0.05). VTE patients exhibited delayed coagulation recovery (a significant change compared to 2HR) of K (48 vs 24HR), α-angle (no recovery), MA (24 vs 12HR) and LY30 (48hrs vs 12HR). PF recovery mediated by arachidonic acid (72 vs 4HR), adensine-5’-diphosphate (72 vs 12HR), and collagen (48 vs 12HR) were delayed in VTE patients. VTE patients had lower mortality (4% vs 13%, p<0.05), but less hospital free days (0 (0–8) vs 10 (0–20), p<0.05) and higher complication rates (p<0.05).
Conclusion
Recovery from platelet dysfunction and coagulopathy following severe trauma were delayed in VTE patients. Suppressed clot lysis and compensatory mechanisms associated with altered coagulation that may potentiate VTE formation require further investigation.
Keywords: trauma, venous thromboembolism, platelet function, coagulopathy
INTRODUCTION
The prevention of venous thromboembolism (VTE) following traumatic injury is an ongoing challenge. VTE occurs in as many as 25% of trauma patients with pharmacological prophylaxis,1 and 58% in non-prophophylaxed patients,2 and is associated with an increased risk of morbidity and mortality.3, 4 As defined by Virchow’s triad, the core factors that contribute to thrombosis are static blood flow, endothelial injury and hypercoagulability.5 Therefore, indices of hypercoagulability, such as enhanced thrombin formation, hypercoagulable thromboelastography (TEG) parameters,1, 6, 7 platelet levels,8,10 enhanced platelet function,9 and fibrinogen activity10 have all been identified as key clinical risk factors for VTE formation following trauma.
The rationale for targeting enhanced coagulation and platelet formation pathways to prevent VTE formation appears to be contradictory during severe traumatic hemorrhage, for the combined effect of injury and hemorrhage induces the development of acute traumatic coagulopathy (ATC).11 ATC is primarily mediated by the hypocoagulable effects of the activated protein C (aPC) pathway, although enhanced fibrinolysis,12 platelet dysfunction,13–15 and other hypocoagulable pathways independent of aPC12 also contribute to this coagulopathic state. Despite this phenomenon, VTE development is still prevalent in this patient population. Recent findings from The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial, which enrolled 680 severely injured hemorrhaging patients predicted to receive a massive transfusion, showed that 15% of patients had a thrombotic event (deep vein thrombosis (DVT) or pulmonary embolism (PE)) during the duration of the study.16
While the mediators of VTE development following severe hemorrhage are not known, the recovery process from ATC may be a contributing factor. Using platelet aggregometry, Kutcher et al. 13 showed that multiple platelet function pathways are initially inhibited by trauma, but then are slowly restored over time. However, platelet function restoration has not been evaluated specifically following severe traumatic hemorrhage, or in patients who developed a VTE. Since hypercoagulability enhances VTE development, we hypothesize that an early onset of recovery from coagulopathy and/or platelet dysfunction following traumatic hemorrhage enhances coagulation, serving as a precursor for VTE development. By utilizing the PROPPR database to target a population of patients who would exhibit ATC and impaired platelet function, we performed a secondary analysis of prospectively collected data to characterize the trajectory of coagulation and platelet function over time in severely injured trauma patients with and without VTE.
METHODS
The PROPPR trial randomized 680 severely injured trauma patients from 12 level 1 trauma centers to receive either 1:1:1 or 1:1:2 ratios of plasma: platelets: red blood cells (RBCs). The eligibility criteria for the PROPPR trial are previously described. 16 For this analysis, patients were dichotomized into either the non-VTE or VTE group. The VTE group was defined as patients who developed a DVT or PE (asymptomatic or symptomatic). DVT was diagnosed with duplex ultrasound. PE was diagnosed by CT angiogram, pulmonary angiogram, or ventilation perfusion scan. However, screening and diagnosis of thromboembolic events was not standardized in PROPPR. To remove patients who did not live long enough to have the chance to develop a VTE, patients who died within 24 hours were excluded from the analysis. To remove the bias for VTE prevention, patients prescribed anticoagulants prior to admission (warfarin, Plavix, aspirin, thrombin inhibitors, or other) were also excluded.
Admission patient characteristics including age, body mass index, and sex (% male), were compared between VTE and non-VTE patients. Injury profile characteristics, such as mechanism of injury (blunt vs penetrating), Injury Severity Score (ISS), Glasgow Coma Scale score (GCS), and injury types associated with VTE including chest trauma, long bone fracture, pelvic fracture, pulmonary contusion, spinal cord injury, spine fracture, and venous injury were also compared between groups. Clinical outcomes (hospital free days, ICU free days, ventilator free days, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), systemic inflammatory response syndrome (SIRS), infection, sepsis, multiple organ failure, death), total blood products over 24 hours (plasma, RBCs and platelets), procoagulant use (Aminocarproic Acid, Tranexamic Acid, Prothrombic Complex Concentrate, Quickclot, Gelform, Evicel, Thrombin, Vitamin K, or Factors VIIa, VII, & IX), and transfusion ratios (1:1:1 versus 1:1:2) were also compared between groups.
During the PROPPR trial, blood samples were drawn at admission, and 2, 4, 6, 12, 24, 48, and 72 hours following admission. Samples were assessed for whole blood coagulation using TEG, and platelet function using Multiplate® aggregometry. Parameters assessed by TEG included time to clot initiation (R), time to 20mm clot displacement (K), rate of clot formation (α-angle), clot strength (MA), and clot lysis at 30 minutes (LY30). Parameters of platelet function aggregation included the contribution of arachidonic acid (ASPI), adensine-5’-diphosphate (ADP), collagen (COL), von Willebrand Factor (RISTO), and glycoprotein IIb/IIIc (TRAP). Plasma samples were also assessed for fibrinogen levels and coagulation factor activity.
Data were analyzed using SPSS statistical software, version 22.0 (SPSS, Inc, Chicago, IL). Baseline patient demographics and clinical outcomes between VTE and non-VTE patients were compared using χ2 tests or Mann-Whitney U test. A multivariate logistical regression analysis was also conducted to identify risk factors for VTE in this patient population. Factors used for the regression analysis included age, BMI, mechanism of injury, sex, ISS, GCS, GCS≤8, hospital free days, ICU free days, ventilator free days, ARDS, AKI, SIRS, infection, sepsis, transfusion group, total blood products over 24 hours (plasma, RBCs and platelets), long bone fracture, venous injury, spinal fracture, spinal cord injury, pulmonary contusion, OR procedure, and systolic blood pressure of <90mmHg at admission. Differences in TEG and platelet function between the non-VTE and VTE groups at each time point were compared with a Kruskal Wallis test. Overall differences in TEG and platelet function within groups over time were compared using a Friedman test, followed by a Wilcoxon Signed Rank Test with Bonferroni correction to compare specific differences between time points within each group. p<0.05 indicated statistical significance.
RESULTS
Patient Characteristics and Complication Incidence
After excluding for 24-hour mortality and anticoagulant use, 558 patients were included in the analysis and dichotomized into the non-VTE (n=475) or VTE (n=83) group. Age, BMI, mechanism of injury, sex, ISS, and GCS, or injury patterns that are associated with VTE development (chest trauma, long bone fracture, pulmonary contusion, venous injury, spine fracture, spinal cord injury and pelvic fracture) did not differ between non-VTE and VTE patients (Table 1). There were no differences in EMS transport time, or the administration of prehospital normal saline, hypertonic saline, crystalloid, plasmalite, albumin, hextend, colloid, RBCs, plasma, platelets, prothrombin complex concentrate (PCC), or tranexamic acid (TXA). The only difference in prehospital treatment was an increase in lactated ringers administration in the VTE group (700 (0, 1200) ml) versus non-VTE group (0 (0,0) ml); p<0.01). The median time to VTE occurrence was 2 (1,4) days. Patients who developed VTE received more units of RBCs (p<0.05) and plasma (p<0.05) over 24 hours, but similar amount of platelets (Table 2). The percent of patients randomized to 1:1:1 versus 1:1:2 resuscitation was also similar between groups. VTE patients also had fewer hospital free days, ICU free days, and ventilator free days (p<0.05 for all). Complications (Table 3) were prevalent in the VTE group, with higher rates of SIRS, AKI, infection, sepsis, and ventilator associated pneumonia (p<0.05 for all). Despite excluding patients for 24-hour mortality, mortality was higher in the non-VTE group (p<0.05). Regional (head, neck, face chest, abdomen, extremity, external) AIS values did not differ between groups. Multivariate regression analysis identified sepsis (OR: 2.10 (1.14–3.87), infection (1.86 (1.03–3.38) and ventilator free days (1.04 (1.01–1.07) as risk factors for VTE. Hospital free days (0.95 (0.91–0.99) was a negative risk factor for VTE.
Table 1.
Patient Characteristics
| Patient Profile | No VTE (n=475) | VTE (n=83) | P-value |
|---|---|---|---|
| Age | 32 (24, 47) | 38 (27, 47) | 0.92 |
| Sex (% male) | 82% | 81% | 0.87 |
| Body Mass Index | 26 (23, 30) | 27 (24, 30) | 0.38 |
| Injury Profile | |||
|
| |||
| Mechanism of Injury (% blunt vs penetrating) | 49% | 57% | 0.28 |
| Injury Severity Score | 26 (16, 36) | 27 (19, 41) | 0.17 |
| Glasgow Coma Score | 14 (3, 15) | 14 (3, 15) | 0.38 |
| Chest Trauma | 50% | 50% | 0.90 |
| Long Bone Fracture | 41% | 49% | 0.15 |
| Pelvic Fracture | 24% | 22% | 0.68 |
| Pulmonary Contusion | 27% | 23% | 0.50 |
| Spinal Cord Injury | 2% | 1% | 0.99 |
| Spine Fracture | 27% | 24% | 0.60 |
| Venous Injury | 25% | 22% | 0.58 |
Data are presented as median (IQR) or percent occurrence.
Table 2.
Transfusion Units and Procoagulants Received
| No VTE (n=475) | VTE (n=83) | P-value | |
|---|---|---|---|
| Total Red Blood Cells 24hrs | 8 (7, 15) | 9 (7, 15) | <0.05 |
| Total Plasma 24hrs | 5 (2, 10) | 7 (4, 12) | <0.05 |
| Total Platelet 24hrs | 6 (6, 12) | 6 (6, 18) | 0.34 |
| % Patients Randomized to 1:1:1 group | 50% | 47% | 0.36 |
| % Patients Given Procoagulants* | 24% | 30% | 0.16 |
Data are presented as median (IQR) or percent occurrence.
Procoagulants administered during the PROPPR trial include Aminocarproic Acid, Tranexamic Acid, Prothrombic Complex Concentrate, Quickclot, Gelform, Evicel, Thrombin, Vitamin K, or Factors VIIa, VII, & IX.
Table 3.
Clinical Outcomes
| Length of Stay | No VTE (n=475) | VTE (n=83) | P-value |
|---|---|---|---|
| Total Hospital Days | 16 (8, 29) | 30 (22, 30) | <0.001 |
| Hospital Free Days | 10 (0, 20) | 0 (0, 8) | <0.01 |
| ICU Free Days | 6 (1, 11) | 10 (3, 16) | <0.001 |
| Ventilator Free Days | 8 (4, 15) | 15 (8, 24) | <0.001 |
| Complications | |||
|
| |||
| Acute Lung Injury | 17% | 15% | 0.59 |
| SIRS | 72% | 83% | <0.05 |
| Acute Kidney Injury | 26% | 36% | <0.05 |
| Infection | 31% | 61% | <0.001 |
| Sepsis | 28% | 60% | <0.001 |
| Multiple Organ Failure | 6% | 8% | 0.33 |
| ARDS | 14% | 22% | 0.09 |
| Ventilator Associated Pneumonia | 19% | 34% | <0.01 |
| Death | 13% | 4% | <0.05 |
Data are presented as median (IQR) or percent occurrence.
Within the VTE group, 7% of patients presented with only DVT, 6% of patients presented with only PE, and 3% of patients presented with both DVT and PE. There was no association between injury patterns and the development of DVT and/or PE, and there were no differences in GCS or AIS for any region between patients who had no VTE, DVT or PE only, or both DVT and PE. Although previous studies identify chest trauma as a predictor for the development of PE,17–19 the occurrence of chest trauma was similar between patients without VTE (49%), DVT only (49%), PE only (57%), or with both DVT and PE (36%).
TEG parameters in non-VTE and VTE patients
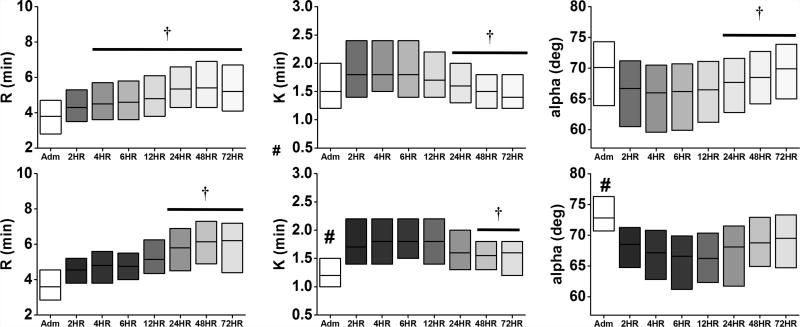
Relative to non-VTE patients, VTE patients also exhibited hypercoagulable TEG values at admission, specifically a lower K-time, higher α-angle, and higher MA (Figures 1 & 2, all p<0.05). Similar to Cotton et al.,6 there was a higher percentage of patients with admission MA>65 in the VTE group (43%) compared to the non-VTE group (26%; p<0.001). At the 2-hour time point, TEG parameters trended towards hypocoagulability in both non-VTE and VTE patients relative to admission levels, characterized by elevated R and K, and lower α-angle and MA (p<0.05 vs admission). In both groups, the K, α-angle, and MA slowly recovered towards admission levels, while the R elongated over time.
Figure 1.
Time course of TEG parameters (R, K, α-angle) relative to clot formation in non-VTE (top) and VTE (bottom) patients. The change in shade refers to each time point, ranging from admission to 72 hours. #p<0.05 vs No VTE at admission; †p<0.05 vs 2HR within group. Data are presented as median (IQR).
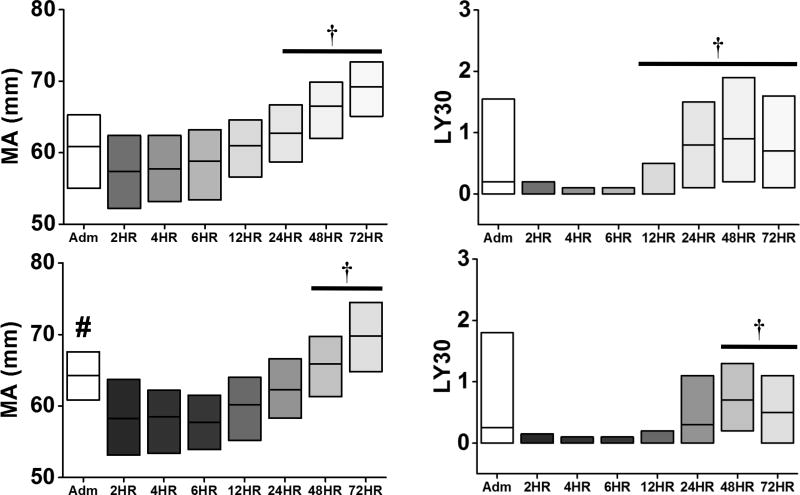
Figure 2.
Time course of TEG parameters (MA, and LY30) relative to clot strength and lysis in non-VTE (top) and VTE (bottom) patients. The change in shade refers to each time point, ranging from admission to 72 hours. #p<0.05 vs No VTE at admission; †p<0.05 vs 2HR within group. Data are presented as median (IQR).
Contrary to hypocoagulable TEG parameters, both groups showed inhibition of clot lysis (LY30) at the 2-hour time point. Furthermore, the occurrence of complete fibrinolytic shutdown (LY30=0%) was similar between non-VTE (32%) and VTE (30%; p=0.46) patients. In contrast to the non-VTE patients, the onset of recovery (defined as the first time point when there was a significant rise in the TEG parameter compared to the 2-hour time point) for K (48 vs 24 hours), MA (24 vs 12 hours), and LY30 (48 vs 12 hours) was delayed in VTE patients (Figure 2). The α-angle remained attenuated at the 72-hour time point in VTE patients. (Figure 2).
Platelet function activity in non-VTE and VTE patients
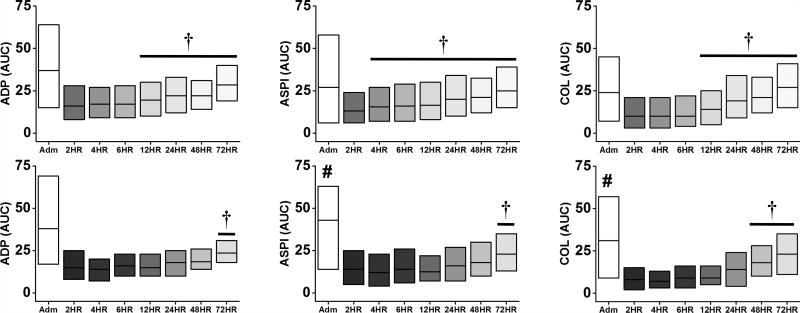
Relative to the non-VTE group, the VTE group also exhibited enhanced platelet function at admission, specifically for ASPI, COL, RISTO, and TRAP aggregation (p<0.05, Figures 3 & 4). Similar to Kutcher et al.,17 both non-VTE and VTE patients exhibited a profound attenuation of all platelet function parameters at the 2-hour time point (p<0.05 vs admission, Figures 3 & 4), which slowly recovered over time (p<0.05 vs 2-hour). Similar to TEG, the onset of platelet function recovery was delayed in VTE patients, specifically for ADP (72 vs 12 hours), ASPI (72 vs 4 hours), and COL (48 vs 12 hours) (Figure 3). The onset of recovery for RISTO and TRAP occurred at the same time point in both non-VTE and VTE patients (Figure 4).
Figure 3.
Time course of ADP, ASPI, and COL-mediated platelet aggregation in non-VTE (top) and VTE (bottom) patients. The change in shade refers to each time point, ranging from admission to 72 hours. #p<0.05 vs No VTE at admission; †p<0.05 vs 2HR within group. Data are presented as median (IQR).
Figure 4.
Time course of RISTO and TRAP-mediated platelet aggregation in non-VTE (top) and VTE (bottom) patients. The change in shade refers to each time point, ranging from admission to 72 hours. #p<0.05 vs No VTE at admission; †p<0.05 vs 2HR within group. Data are presented as median (IQR).
Plasma markers of hypercoagulability in non-VTE and VTE patients
In agreement with the TEG and platelet function data, patients with VTE exhibited higher procoagulant factor activity at admission (Table 4), particularly for Factors V, VII, VIII, IX, X, XI, XII (all p<0.05 versus non-VTE patients). Fibrinogen levels were also higher in VTE patients at admission (p<0.001). There was no difference in these plasma markers between groups at any other time point.
Table 4.
Prothrombotic Markers at Admission
| No VTE | VTE | P-value | |
|---|---|---|---|
| Factor II (% activity) | 65 (49,78) | 70 (62, 83) | <0.01 |
| Factor V (% activity) | 55 (35, 77) | 68 (49,86) | <0.01 |
| Factor VII (% activity) | 77 (60 ,94) | 85 (66, 107) | <0.05 |
| Factor VIII (% activity) | 230 (149, 301) | 287 (224, 410) | <0.01 |
| Factor IX (% activity) | 106 (76, 136) | 127 (108, 171) | <0.001 |
| Factor X (% activity) | 72 (56, 88) | 80 (67, 99) | <0.01 |
| Factor XI (% activity) | 83 (57, 118) | 111 (81, 129) | <0.001 |
| Factor XII (% activity) | 82 (58, 113) | 95 (72, 124) | <0.05 |
| Fibrinogen (ml/dL) | 163 (123, 215) | 202 (155, 251) | <0.001 |
Data are presented as median (IQR).
DISCUSSION
In agreement with previous studies, this study confirms that severely injured trauma patients exhibit acute platelet dysfunction and coagulopathy relative to admission values that recovers over time. Furthermore, patients who develop VTE following trauma exhibited hypercoagulable TEG parameters and enhanced platelet function at admission relative to non-VTE patients, and were at higher risk for complications. However, this is the first study to test the hypothesis that an earlier onset of platelet function and/or coagulation recovery is associated with VTE. Contrary to our hypothesis, the onset of recovery for platelet dysfunction and coagulopathy was delayed in VTE patients, suggesting that an alternative mechanism contributes to VTE development in this patient population.
Hypercoagulable TEG parameters predict the development of VTE in trauma, surgical and ICU patients.1, 6, 7 In agreement, our study also showed that VTE patients exhibited several TEG and platelet function parameters that trended towards hypercoagulability at admission. These patients also exhibited higher plasma levels of several coagulation factors that enhance coagulation and promote thrombosis. Furthermore, VTE patients were given more RBCs and plasma within the first 24 hours, and were more susceptible to complications throughout the duration of the study. Despite these characteristics, it was surprising that over time the VTE patients showed a subsequent inhibition of coagulation and platelet function similar to non-VTE patients, and that the onset of platelet function and coagulation recovery was delayed, especially since the median time to VTE occurrence was 2 days. The delayed onset of recovery for the K, α-angle, MA and Multiplate® platelet aggregation pathways would indicate that clot formation and stability would prevent, rather than enhance, VTE development. Taken together, this may suggest that the coagulation state at admission and the amount of blood product given in the first 24 hours may have important roles in determining whether a patient is prone to VTE development. Alternatively, the delayed recovery of platelet function and coagulation may have placed patients at higher risk for complications and longer hospital stay, resulting in subsequent VTE development. In support of this hypothesis, both length of stay and sepsis are preceded by VTE, and thus are a result of rather than a risk factor for VTE.
It is also possible that an alternative mediator of VTE development may be upregulated to compensate for the prolonged attenuation of coagulation and platelet function in the VTE group. Several studies have indicated that fibrinogen acts as a compensatory mechanism for platelet dysfunction and coagulopathy following severe injury.13, 20–22 In turn, this could also enhance VTE risk in our patient population. Fibrinogen significantly contributes to clot strength, ranging from 23% in healthy individuals 9, 10 to over 40% in trauma patients. 20 Moore et al. 21 showed that compared to healthy controls, the overall contribution of functional fibrinogen to clot strength was higher in trauma patients while ADP-mediated clot strength was suppressed. Impairment of functional fibrinogen was rare, occurring in only 10% of trauma patients compared to 78% of patients who exhibited attenuated ADP-mediated platelet function.
As an index of fibrinogen contribution to clot strength, a novel finding from this study shows that unlike AA, ADP and collagen (Figure 3), the onset of recovery for von-Willebrand factor (activated by RISTO) and glycoprotein IIb/IIc-mediated platelet aggregation (activated by TRAP, Figure 4) were similar between VTE and non-VTE patients. Since fibrinogen binds to the glycoprotein IIb/IIc receptor on platelets,23 this shows that fibrinogen is an early mediator of platelet activation during the recovery process. Given that fibrinogen is more resilient in maintaining clot strength following trauma compared to ADP, 24 the contribution of fibrinogen to clot strength and platelet activation in patients who develop VTE following severe trauma merits further investigation.
An alternative interpretation of the platelet function data is that the platelets are activated in response to trauma. Therefore, platelet aggregation studies are interpreted to show platelet inhibition using the Multiplate® assay because the receptors for each agonist are already saturated due to the effects of trauma. If this is the case, then the delayed onset of recovery that we observe with the platelet function test indicates that the platelets are active for longer in the VTE group, potentially contributing to enhanced platelet-mediated clot strength for a longer period of time. However, future studies are required to determine if severe trauma maximally stimulates, rather than inhibits, platelet aggregation. Studies are also needed to determine the functionality of the platelets that are transfused into the patient.
The prolonged inhibition of clot lysis may also contribute to VTE development in this patient cohort. Hypofibrinolyis is a strong predictor for VTE,25–27 and despite overall signs of prolonged coagulopathy and platelet function, the VTE group exhibited a delayed restoration of LY30. This suggests that while the restoration of clot formation and absolute clot strength are compromised, patients who developed a VTE were more susceptible to maintaining formed clots for a longer period of time. The mechanisms of the suppressed fibrinolysis are unclear, but similar to fibrinogen this could also be a compensatory response to impaired coagulation and platelet function following trauma. Lack of fibrinolysis observed following trauma has been described as fibrinolysis shutdown and has been associated with increased mortality.24 Future assessment of plasma markers that promote and inhibit fibrinolysis, such as tissue plasminogen activator (tPA) plasminogen activated inhibitor 1 (PAI-1), and thrombin activatable fibrinolysis inhibitor (TAFI), is needed to further determine the mechanisms that delay the onset of fibrinolysis in this patient cohort.
Limitations to this study must be considered. First, this is a secondary analysis of prospectively collected data, which limits the parameters that can be assessed. For example, it would have been ideal to know which patients received VTE prophylaxis and when it was administered during their hospital stay, but these data were not recorded. Furthermore, not all patients were hypocoagulable at admission. This may be explained by the findings from the primary PROPPR paper, where 46% (313/680) patients received a massive transfusion.16 Although massively bleeding patients are known to be hypocoagulable, the other 64% were moderately injured, which can lead to hypercoagulability. In addition, coagulation will correct towards a normal state over time and with the administration of additional blood products, which may also explain why we observed attenuated coagulation at the 2HR time point compared to admission. Second, we did not observe significant changes in TEG and platelet function parameters between non-VTE and VTE patients at specific time points during the recovery period. Ideally, identifying abnormal values would be a more definitive clinical tool in predicting VTE. The lack of difference between groups may be contributed to by the lower number of VTE patients compared to non-VTE patients, which may have reduced the power of the analysis. Furthermore, the limited number of VTE patients in the total patient cohort also prevents the analysis of these responses at each clinical site.
CONCLUSIONS
Our study showed that despite relative hypercoagulable TEG parameters and enhanced platelet function on admission, the onset of recovery from coagulopathy and platelet dysfunction following severe trauma is delayed in patients with VTE. This suggests that a hypercoagulable state at admission may be a critical time point in determining if a patient is vulnerable for VTE development over time. Compensatory mechanisms, such as an enhanced role for functional fibrinogen, may also be the underlying cause of VTE formation. Clot lysis also remained attenuated for a longer period of time, suggesting that VTE development may also be linked to suppressed fibrinolysis rather than the early onset of clot formation. Future studies examining plasma markers of clot stability and lysis are warranted to identify the mechanisms that contribute to VTE development in patients with severe traumatic injury.
Acknowledgments
The authors would like to thank the PROPPR Study Group and Clinical Sites for their participation in the study:
PROPPR Study Group:
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee Cochair); Jeanette Podbielski, RN; and Angela M. Beeler, BS.
Data Coordinating Center: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; and Michael O. Gonzalez, MS.
Core Laboratory: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; and David Clark, BS.
Resuscitation Outcomes Consortium: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; and Kellie Sheehan, BSN.
Systems Biology Committee: Alan Hubbard, PhD (Cochair); and Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD (Cochair); and Jeanne Callum, MD (Cochair).
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; and Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; and Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees Cochair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; and Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Christopher N. Miller, MD; Helen Hancock, BS, MT(ASCP); and Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; and Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research–Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr., DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; and Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; and Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; and Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; and Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Jean-Francois Pittet, MD (Anesthesia Chair); Patrick L. Bosarge, MD; Albert T. Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS; and Marisa B. Marques, MD.
Oregon Health and Science University: Martin A. Schreiber, MD; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD; Richard M. Scanlan, MD; and Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; and Connie Colavecchia, BSc, MLT.
GRANT FUNDING
The PROPPR trial was sponsored by the US National Heart, Lung, and Blood Institute (U01HL077863), the US Department of Defense, as well as Defense Research and Development Canada in partnership with the Canadian Institutes of Health Research (CIHR), Institute of Circulatory and Respiratory Health (CRR-120612).
References
- 1.Van Haren RM, Valle EJ, Thorson CM, et al. Hypercoagulability and other risk factors in trauma intensive care unit patients with venous thromboembolism. J Trauma Acute Care Surg. 2014;76:443–449. doi: 10.1097/TA.0b013e3182a9d11d. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 3.Karcutskie CA, Meizoso JP, Ray JJ, et al. Association of mechanism of injury with risk for venous thromboembolism after trauma. JAMA Surg. 2017;152:35–40. doi: 10.1001/jamasurg.2016.3116. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin DF, Wade CE, Champion HR, et al. Thromboembolic complications following trauma. Transfusion. 2009;49(Suppl 5):256S–263S. doi: 10.1111/j.1537-2995.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopez JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res. 2009;123(Suppl 4):S30–34. doi: 10.1016/S0049-3848(09)70140-9. [DOI] [PubMed] [Google Scholar]
- 6.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72:1470–1475. doi: 10.1097/TA.0b013e31824d56ad. discussion 1475–1477. [DOI] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-teg) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–772. doi: 10.1016/j.surg.2009.06.054. discussion 772–764. [DOI] [PubMed] [Google Scholar]
- 8.Allen CJ, Murray CR, Meizoso JP, et al. Coagulation profile changes due to thromboprophylaxis and platelets in trauma patients at high-risk for venous thromboembolism. Am Surg. 2015;81:663–668. [PubMed] [Google Scholar]
- 9.Harr JN, Moore EE, Chin TL, et al. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74:756–762. doi: 10.1097/TA.0b013e3182826d7e. discussion 762–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harr JN, Moore EE, Chin TL, et al. Postinjury hyperfibrinogenemia compromises efficacy of heparin-based venous thromboembolism prophylaxis. Shock. 2014;41:33–39. doi: 10.1097/SHK.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frith D, Brohi K. The pathophysiology of trauma-induced coagulopathy. Curr Opin Crit Care. 2012;18:631–636. doi: 10.1097/MCC.0b013e3283599ab9. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Darlington DN, Cap AP. Procoagulant and fibrinolytic activity after polytrauma in rat. Am J Physiol Regul Integr Comp Physiol. 2016;310:R323–329. doi: 10.1152/ajpregu.00401.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–1229. doi: 10.1097/TA.0b013e31828b7fa1. discussion 1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsey MT, Fabian TC, Shahan CP, et al. A prospective study of platelet function in trauma patients. J Trauma Acute Care Surg. 2016;80:726–732. doi: 10.1097/TA.0000000000001017. discussion 732–723. [DOI] [PubMed] [Google Scholar]
- 15.Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: An unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudson MM, Gomez D, Haas B, et al. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: A new look at an old disease. Ann Surg. 2011;254:625–632. doi: 10.1097/SLA.0b013e3182300209. [DOI] [PubMed] [Google Scholar]
- 18.Van Gent JM, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis? J Trauma Acute Care Surg. 2014;76:1270–1274. doi: 10.1097/TA.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 19.Yeh DD, Hwabejire JO, DeMoya MA, et al. Sternal fracture--an analysis of the national trauma data bank. J Surg Res. 2014;186:39–43. doi: 10.1016/j.jss.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Kornblith LZ, Kutcher ME, Redick BJ, et al. Fibrinogen and platelet contributions to clot formation: Implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76:255–256. doi: 10.1097/TA.0000000000000108. discussion 262–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13:1878–1887. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White NJ, Newton JC, Martin EJ, et al. Clot formation is associated with fibrinogen and platelet forces in a cohort of severely injured emergency department trauma patients. Shock. 2015;44(Suppl 1):39–44. doi: 10.1097/SHK.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangkuhl K, Shuldiner AR, Klein TE, et al. Platelet aggregation pathway. Pharmacogenet Genomics. 2011;21:516–521. doi: 10.1097/FPC.0b013e3283406323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore EE, Moore HB, Gonzalez E, et al. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016;56(Suppl 2):S110–114. doi: 10.1111/trf.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karasu A, Baglin TP, Luddington R, et al. Prolonged clot lysis time increases the risk of a first but not recurrent venous thrombosis. Br J Haematol. 2016;172:947–953. doi: 10.1111/bjh.13911. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer ME, Lisman T, Doggen CJ, et al. Synergistic effects of hypofibrinolysis and genetic and acquired risk factors on the risk of a first venous thrombosis. PLoS Med. 2008;5:e97. doi: 10.1371/journal.pmed.0050097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traby L, Kollars M, Eischer L, et al. Prediction of recurrent venous thromboembolism by clot lysis time: A prospective cohort study. PLoS One. 2012;7:e51447. doi: 10.1371/journal.pone.0051447. [DOI] [PMC free article] [PubMed] [Google Scholar]