Abstract
Offspring of parents with chronic pain are at increased risk for pain and adverse mental and physical health outcomes (Higgins et al, 2015). Although the association between chronic pain in parents and offspring has been established, few studies have addressed why or how this relation occurs. Identifying mechanisms for the transmission of risk that leads to the development of chronic pain in offspring is important for developing preventive interventions targeted to decrease risk for chronic pain and related outcomes (eg, disability and internalizing symptoms). This review presents a conceptual model for the intergenerational transmission of chronic pain from parents to offspring with the goal of setting an agenda for future research and the development of preventive interventions. Our proposed model highlights 5 potential mechanisms for the relation between parental chronic pain and pediatric chronic pain and related adverse outcomes: (1) genetics, (2) alterations in early neurobiological development, (3) pain-specific social learning, (4), general parenting and family health, and (5) exposure to stressful environment. In addition, the model presents 3 potential moderators for the relation between parent and child chronic pain: (1) the presence of chronic pain in a second parent, (2) timing, course, and location of parental chronic pain, and (3) offspring’s characteristics (ie, sex, developmental stage, race or ethnicity, and temperament). Such a framework highlights chronic pain as inherently familial and intergenerational, opening up avenues for new models of intervention and prevention that can be family centered and include at-risk children.
Keywords: Chronic pain, Parents, Offspring, Children
Chronic pain poses a significant burden to affected individuals, their families, and society.66 Estimates of chronic pain not associated with disease in the general adult population range from 10% to 40%,68 whereas persistent, severe, and disabling chronic pain occurs in 3% to 8% of the population and incurs additional burden and cost 9,150,154 Similarly, in adolescents, moderate-to-severe chronic pain costs the United States approximately $19.5 billion a year.52 Although the societal cost of chronic pain in families has not been calculated, there is some evidence that it would be high as chronic pain symptoms tend to aggregate in families.59
A recent systematic review and meta-analysis identified increased risk for pain and adverse mental and physical health outcomes for offspring of parents with chronic pain.59 Although there is increasing evidence for the relation between parent and child chronic pain, this systematic review identified gaps in the literature regarding how and why certain children with a parent with chronic pain may be at greater risk for poor outcomes than others. Identifying mechanisms for the transmission of risk for chronic pain in offspring is important for developing preventive interventions to decrease risk for chronic pain and common comorbidities (eg, functional disability, other somatic symptoms, and poor psychological functioning).
The current review aims to provide an integrative conceptual model for the transmission of risk for chronic pain from parents to offspring. The literature on the relation between parental chronic pain and outcomes in offspring contains substantial heterogeneity regarding study methodology, sample populations, and research questions.59 Thus, our model serves to provide a hypothesized overarching framework to spur, guide, and organize future research in the field. We do not advocate that these proposed relationships are rigid, but instead fluid and plausible based on underlying theoretical frameworks and the existing literature.
Our goals for outlining a conceptual framework specific to the transmission of risk for chronic pain are to (1) set an agenda for future interdisciplinary research on risk and resiliency for chronic pain and related outcomes in children of parents with chronic pain and (2) lay a foundation for the development of preventive interventions. First, we provide a brief overview of the theoretical underpinnings, key terms, and components of our model. Next, we devote sections to describing research relevant to hypothesized mechanisms and moderators in the model. Finally, we draw conclusions regarding the current state of the research and future directions.
1. Overview of the model
Our model is adapted from (1) a model of the intergenerational transmission of maternal depression49; (2) models of family coping with maternal illness4,88; (3) an integrative model of parent and family factors in pediatric chronic pain103; and (4) a model of pain vulnerability mechanisms.33 Our conceptual model proposes that having a parent with chronic pain heightens a child’s risk for chronic pain through genetic, neurobiological, pain-specific social learning, general parenting and family health, and family stress pathways. We provide an overview of the existing literature supporting plausible mechanisms (eg, genetics, modeled pain, and disability) that contribute to vulnerabilities within the child (eg, pain-related cognitions and pain sensitivity), ultimately leading to adverse outcomes (eg, chronic pain, pain-related disability, and poor psychological functioning). Although our model and review of the literature focus on chronic pain not related to disease, similar mechanisms may also operate in disease-related pain (eg, arthritis, sickle cell disease, inflammatory bowel disease, etc.) or in other contexts (eg, pain chronification after surgery or acute injury).
The model focuses on parental chronic pain as a specific risk factor that may increase the probability that offspring will develop chronic pain and related comorbidities (Fig. 1). There are likely many families where a parent may have chronic pain and offspring do not develop chronic pain or other adverse outcomes. Thus, our model focuses on outlining mechanisms, moderators, and vulnerabilities that may explain why some offspring of parents with chronic pain are at increased risk and develop adverse outcomes while others do not.
Figure 1.
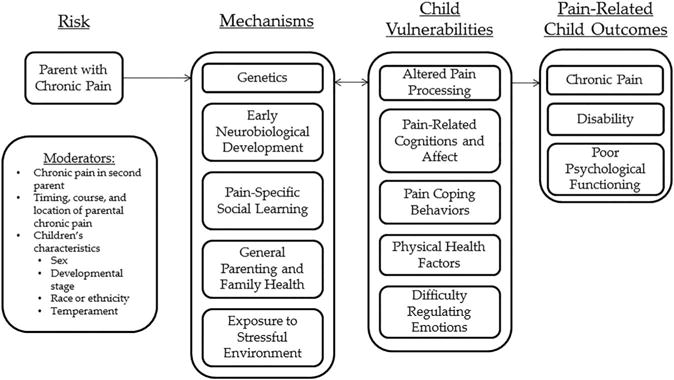
Conceptual model of Intergenerational transmission of chronic pain risk.
The outcomes addressed in our model correspond with the adverse outcomes identified in the meta-analysis on offspring of parents with chronic pain.59 We focus on the development of pediatric chronic pain and associated disability as the primary outcome and also highlight poor psychological functioning (eg, internalizing or externalizing behavior problems, poor social competence, or low self-esteem) as an outcome that may occur in combination with or independent from children’s pain outcomes. Many of the proposed mechanisms and vulnerabilities are associated with psychological outcomes in addition to pain outcomes.49
We posit that parental chronic pain influences the development of chronic pain in offspring through specific mechanisms or mediators (ie, how or why a specific effect occurs). Our proposed model highlights 5 potential mechanisms for the relation between parental chronic pain and children’s outcomes: (1) genetics, (2) alterations in early neurobiological development, (3) pain-specific social learning, (4), general parenting and family health, and (5) exposure to stressful environment. Next, we focus on moderators or variables that might explain when, and under what circumstances, offspring are at greatest risk for developing chronic pain in the context of parental chronic pain. We discuss 3 possible moderators of this relation: (1) the presence of chronic pain in a second parent, (2) timing, course, and location of parental chronic pain, and (3) offspring’s characteristics (ie, sex, developmental stage, race or ethnicity, and temperament).
In our model, vulnerabilities refer to factors that could be influenced by having a parent with chronic pain. These vulnerabilities could also be influenced by other biological, psychological, or contextual processes outside of parental chronic pain; thus, there is not a direct correspondence between mechanisms and vulnerabilities. Although we will not devote entire sections to these vulnerabilities, which are commonly proposed in models of pediatric chronic pain,5,152 we highlight within our discussion of mechanisms and moderators 5 potential vulnerabilities that could characterize offspring at greatest risk for the development of chronic pain: (1) altered pain processing, (2) offspring’s own pain-related cognitions and affect, (3) paincoping behaviors, (4) physical health factors, and (5) difficulty processing and regulating emotions.
The outlined mechanisms, moderators, and vulnerabilities likely interact over time to influence the development of chronic pain and related outcomes in offspring of parents with chronic pain. The current state of the literature allows for few conclusive statements regarding timing of effects or specific interactions. We propose that the development of chronic pain in offspring is the result of multiple complex interactions among mechanisms, child vulnerabilities, and moderators.
2. Potential mechanisms for the transmission of risk
2.1. Mechanism 1: genetics
Offspring of a parent with chronic pain may be at increased genetic risk for heightened sensory and psychological components of pain which could contribute to the development of chronic pain. Heritability estimates for genetic influences on the development of chronic pain in children and adolescents vary depending on the type of pain and age.58,97,132,136 These studies generally rely on twin designs and compute estimates for the amount of variability accounted for by genetic, shared environmental, and nonshared environmental factors. For example, a study of Danish twins computed heritability estimates for low back pain by age and found for young adolescents (age: 12–15 years), common environmental factors represented the strongest component.58 However, for older adolescents (age: 16–18 years), genetic and nonshared environmental factors comprised the model with the best overall fit. Thus, for children and young adolescents, shared environmental factors (eg, family context) may have greater influence on chronic pain development than genetic factors in some pain phenotypes. The studies mentioned above represent the few that have examined genetic influences on the presentation of pediatric chronic pain.
Genetic research from studies on the development of chronic pain in adults may inform future research on genetic mechanisms that influence the development of pediatric chronic pain. Genetic factors contribute to roughly 50% of the risk for chronic pain in adults and influence both sensory and psychological factors that commonly co-occur with chronic pain.35 Alterations in pain processing represents one vulnerability in our model which may have more genetic influence than others. Indeed, heightened pain sensitivity has been linked with genetic variants of the catecholamine-O-methyltransferase (COMT) gene.36 There is some evidence that variants of the COMT gene interact with psychological factors (eg, perceived stress) to increase risk of chronic pain development.127 For children, genetic components likely interact with the parent and child’s life experiences, environment, and characteristics to influence the development of chronic pain.
Psychological components that often predict or co-occur with chronic pain are also influenced by genetic factors.35 With regards to pain-specific psychological factors, a recent twin study139 estimated genetic factors to account for 37% of the variance in pain catastrophizing. In further analyses, the genetic factors influencing pain catastrophizing were found to be unrelated to genetic factors influencing cold-pressor task outcomes. Thus, the genetic risk for some psychological components of the pain experience may be distinct from the genetic risk for sensory components of the pain experience (ie, laboratory pain sensitivity).44 In a study of genetic risk for temporomandibular disorder (TMD) onset, prostaglandin-endoperoxide synthase 1 (PTGS1, rs3842803) was associated with global psychological symptoms, and amyloid-β (A4) precursor protein (APP, rs466448) was associated with stress and negative affectivity,130 indicating that more global psychological factors predictive of chronic pain development are influenced by genetic factors. In addition to pain, offspring of parents with chronic pain are more likely to experience externalizing and internalizing problems.70 Genetic contributions to these intermediate psychological phenotypes could help elucidate the transmission of risk for multiple outcomes in the context of parental chronic pain.
2.2. Mechanism 2: early neurobiological development
Having a parent with chronic pain has the potential to impact neurobiological features and function during critical periods early in development. These neurobiological changes, in turn, may increase vulnerability to chronic pain, particularly pain processing and ability to process and regulate emotions. Although we primarily address mothers in this section, parents of both sexes certainly contribute to their children’s ongoing neurobiological functioning across development. First, we address 2 important factors through which maternal chronic pain may influence the neurobiological development of their offspring during the prenatal and perinatal periods: (1) maternal stress and (2) maternal health behaviors. Then, we discuss how neurobiological features may influence vulnerabilities for the development of chronic pain and related outcomes in offspring of mothers with chronic pain.
2.2.1. Maternal stress during prenatal and perinatal periods
Disruptions of fetal neurobiological development may be due to maternal chronic pain or comorbidities such as somatic and depressive symptoms altering maternal neuroendocrine or hypothalamic—pituitary—adrenal (HPA) axis function,137,163 which in turn may alter fetal neuroendocrine systems and DNA methylation.13 Similar mechanisms have been demonstrated in the case of maternal depression.13,89 Maternal stress and cortisol levels during pregnancy impact the development of infant cardiac vagal tone,111 which is strongly associated with children’s later abilities to regulate emotions. Higher maternal cortisol levels during pregnancy were associated with infants’ slower behavioral distress recovery and larger cortisol responses in response to a painful procedure at age 2.30 There is also some evidence that early life pain responses (at age 5) are associated with somatic symptoms at age 12,115 suggesting increased pain response vulnerability can lead to poor outcomes across development.
2.2.2. Maternal health behaviors during prenatal and perinatal periods
Early neurobiological development could be disrupted due to maternal health and health behaviors that co-occur with chronic pain. There is a growing body of the literature demonstrating that maternal physical activity, obesity, and high-fat diets affect offspring health and development.93 These processes are important to consider as obesity is associated with chronic musculoskeletal pain and multisite pain in community and epidemiological samples of adolescents and adults.31,60,100
Classes of medications commonly prescribed for chronic pain management have the potential to negatively impact fetal development. Opioid pain medications disrupt endocrine system and HPA axis function.3,28,32 Prenatal exposure to opioids is associated with negative behavioral and cognitive outcomes for offspring, in both human and animal studies,34,45,106 and changes in analgesia in animals,138 suggesting opioid exposure might lead to changes in a child’s pain processing system; note that the majority of human research in this area has focused on prenatal opioid exposure among offspring of mothers addicted to opioids.45 Antiepileptic medications also carry increased risk of congenital abnormalities, preterm delivery, and low birth weight.6,46,147 A meta-analysis showed that antidepressant use during pregnancy is related to increased risk for low birth weight and low Apgar scores116 and is associated with increased anxiety in offspring at 3 years of age.14 Thus, exposure to medications commonly used to treat chronic pain or comorbid conditions might lead to increases in a number of vulnerabilities for chronic pain development, including difficulty regulating emotions.
2.2.3. Neurobiological features in offspring of parents with chronic pain during childhood and adolescence
Minimal work has been done to examine neurobiological features or functioning of the offspring of parents with chronic pain. A recent review outlines brain networks that are likely candidates for increasing vulnerability for pain chronicity, including the descending pain modulatory system and the reward-motivation network.33 Similar networks may be vulnerable in children with parents of chronic pain, and examining them early in life and across development will be key. Initial work in this area has shown that 11- to 15-year-old children of mothers with chronic pain show differences in brain activity during positive and negative emotion processing and differences during emotional inhibition tasks compared with controls.27 One other study found that healthy children with stronger inhibitory control and working memory reported less stress and unpleasantness during painful stimuli,148 suggesting that inhibition and attention networks may play a role in chronic pain development.
2.3. Mechanism 3: pain-specific social learning
Pain-specific social learning mechanisms that contribute to pain vulnerabilities in children are likely to be interdependent and mutually influence each other. Social learning theory7 is a theoretical framework that can guide hypotheses regarding how children may learn responses to pain from parents. According to this theory, children learn behaviors through observations of models and reinforcement. Parents with chronic pain may model maladaptive pain behaviors and reinforce children’s maladaptive pain behaviors through protective or solicitous responding to children’s pain complaints. Both parental modeling of pain behaviors and parental reinforcement of children’s pain behaviors may contribute to the development of children’s pain beliefs and responses to pain which could increase risk for the development of chronic pain and related disability. Similarly, transmission of resilience, including pain acceptance, could occur through modeling and reinforcement.
Evidence for the hypothesis that parental modeling of pain behavior influences children’s pain responses comes from one experimental study that manipulated parental responses to laboratory pain in the presence of their children.48 The effect of modeling has also been inferred based on the identification of pain models.101 Because evidence for the role of parental modeling of pain behaviors is limited, the goal of this section is to place parental pain behaviors in the broader context of parents’ own cognitive and affective responses to pain to set an agenda for further research. When a parent with chronic pain holds strong beliefs that pain represents a significant threat, this could lead to increased vulnerability for chronic pain and related disability in their children through parental modeling of maladaptive pain behaviors (eg, activity avoidance) and protective responses to children’s pain.
Pain catastrophizing is a cognitive—affective factor that could influence parental pain behaviors and responses to children’s pain behaviors and is associated with poor outcomes such as reduced quality of life, higher functional disability, and higher depressive symptoms in adults and adolescents.90,107 Pain catastrophizing refers to the negative cognitive tendency to magnify the threatening value of pain sensations and functions to facilitate avoidance of pain and communicate distress to others.25 Parents who catastrophize about their own pain or their children’s pain likely communicate high threat information about pain to children through verbal and nonverbal means.81,99,107,159 In turn, parental verbal and nonverbal pain behaviors are observed by children and may influence the development of children’s pain-related beliefs. Parent catastrophizing about children’s pain and children’s own catastrophizing about pain have consistently been associated with higher levels of pain-related disability in youth,50,90,160 and mothers and children have similar tendencies to catastrophize.90 Pain treatment can reduce catastrophizing in both parents and children.156
Parents high in catastrophizing about their own pain or their children’s pain behave differently toward their children in pain situations through restricting activities that might be painful.16,81 Parental responses to children’s pain and distress may be informed by their own pain experiences and beliefs. In a study comparing children of adult patients with chronic pain with children of adults without chronic pain, parents with chronic pain reported higher levels of catastrophizing about their children’s pain and protective responses to children’s pain.158 Parental responses that are solicitous or protective (eg, letting their child stay home from school and giving their child special treats or gifts) are associated with higher levels of pain, disability, and catastrophizing in pediatric patients with chronic pain.124 Children’s own levels of pain catastrophizing and emotional distress are associated with parents’ solicitous or protective responses to children’s pain and may drive the relation between parents’ responses to children’s pain and children’s symptoms and disability.19,54,151 Laboratory studies have further corroborated the relation between parental attention or solicitousness and children’s laboratory pain responses.18,153
In contrast, parents with chronic pain who are able to accept their own pain may respond to their children’s pain with acceptance which could facilitate higher levels of emotional and physical functioning for both parents and children.92,126 Parents’ acceptance of children’s pain has been associated with fewer attempts to engage in protective responses to children’s pain and lower levels of functional disability in the child.129 Acceptance of children’s pain likely parallels parents’ acceptance of their own pain, but to our knowledge, this has not been studied. Parents who are able to accept pain likely exhibit higher physical and emotional functioning than parents who struggle to accept both their own pain and their children’s pain. In addition to acceptance, a number of other pain-specific psychological factors in parents may contribute to resilient child outcomes, including active coping, problem solving skills, and optimism.23,63,64,105
2.4. Mechanism 4: general parenting and health habits
2.4.1. General parenting
Parents with chronic pain may influence the development of chronic pain and other poor outcomes in their children through engaging in a number of general parenting behaviors often linked with adverse child outcomes (eg, permissive parenting, difficulties providing consistency and warmth, and difficulty responding effectively to child distress43,158). Parental warmth is consistently associated with psychological outcomes for children, including lower levels of warmth being related to increased internalizing problems.74,82 Maternal responsiveness to distress is positively associated with a child’s ability to regulate negative affect and emotions effectively.29,75 Parents with chronic pain may be less able to respond to their child’s distress,158 which can impact children’s ability to develop negative affect regulation.76 Negative affect regulation has been tightly linked with pain in neuroanatomy40,121 and is also associated with pain experiences on a day-to-day basis.22 Poor negative affect regulation increases risk for the development of internalizing problems.38,39,125 Parent—child communication and interaction styles investigated in the context of pediatric chronic pain have found associations between high conflict and low adolescent autonomy with increased depressive symptoms86 and lower autonomy than expected for age in youth with pain.42
Attachment is also an important domain of parent—child relationships to consider,2,12 as adult attachment style has been linked with parenting behaviors20 and a number of pain outcomes, including pain self-efficacy.96 Children with a parent with chronic pain may form insecure attachment styles, such as anxious attachment, that may increase their tendency to respond to a threat, such as pain, with passive coping strategies which may increase risk for the development of chronic pain.79,95 Investigating the extent to which parental chronic pain affects overall parenting behaviors and responsiveness to their children is important for further understanding how parental chronic pain may contribute to the development of pediatric chronic pain and comorbid conditions. Many of these parenting and parent—child relationship mechanisms have the potential to be protective, and in that capacity might act as mechanisms for resilience, or as moderators that might attenuate the impact of other risk factors.
2.4.2. Physical activity and general health habits
One’s family strongly influences the development of health and illness-related attitudes, beliefs, and behaviors,73 and parents are salient models of health behaviors for their children.47,142 Because of impairments in physical functioning, parents with chronic pain may model less physical activity and engage in less physical activity with their children. Parenting that includes logistical support for physical activity and modeling or joint physical activity participation is associated with lower child body mass index (BMI) and increased child activity levels.128 Low levels of physical activity have a negative impact on physical fitness and function,65,164 important factors in the development and persistence of pain.15 In epidemiological samples, higher levels of sedentary activity are associated with increased pain in girls,60 whereas higher activity is associated with reduced risk for chronic pain.80 In addition, objectively measured activity levels have shown that lower physical activity is associated with greater pain and disability among youth with chronic pain and healthy youth.72,108,161 Thus, engagement in physical activity may be particularly important for youth at risk for chronic pain.155 Parents who have chronic pain may be less involved in their children’s activities and provide fewer opportunities for physical activity for their children.158
A number of other general health factors will be important to examine as potential mechanisms, including BMI, diet, and health care utilization. Higher BMI is a risk factor in and of itself for the persistence of chronic musculoskeletal8 or headache pain57,112 in children and adolescents and is associated with activity limitations in youth with pain.162 In addition, dietary interventions have been shown to reduce pain in adults with irritable bowel syndrome,55 and pain and psychological distress in adults with headache,110 suggesting that diet and nutrition habits within a family might influence pain outcomes. Health care utilization may also play a role, as parental health status has been associated with pain-related medical visits in children,141 and mother and child health care visit frequency are positively correlated in families in which the mother has irritable bowel syndrome.146 Families who catastrophize about pain may be more likely to seek frequent medical care which, in turn, may further increase pain catastrophizing. Examination of these health factors as potential mechanisms might yield additional targets for preventive interventions.
2.5. Mechanism 4: exposure to stressful environment
The environmental context surrounding a family where a parent has chronic pain, particularly stressors, could contribute to the development of chronic pain in their children. Chronic pain is both influenced by stress and poses a significant chronic stressor for individuals suffering with chronic pain syndromes and their families.67 Poor family functioning and family stress, such as low cohesion (ie, low positive emotional bonding) and high levels of marital conflict, chronic sources of stress, have consistently been shown to contribute to pain-related disability in youth.87 Children raised in families with high conflict and low warmth are at high risk for mental and physical health disorders because of vulnerabilities for alterations in stress response systems, difficulties with emotion processing, and poor health behaviors.113 Chronic stressors give the body little or no time to adapt or recover from the cognitive and physiological demands of the stressor and can produce a prolonged stress response associated with physiological dysregulation and stress-related diseases.69 The extent to which an event, such as parental chronic pain or financial challenges associated with parental pain-related disability, represents a stressor and is associated with adverse consequences can be modulated by one’s appraisal of the situation and attempts to cope with the situation.83
Children of parents with chronic pain likely experience unique stressors related to their parents’ health.143 Parents with chronic pain and pain-related disability may have difficulties completing household chores and everyday tasks that parents often complete to care for their families. Children of parents with chronic pain may feel pressure to help take care of their family and assume adult roles at an early age or try to assume responsibility for their parent’s pain.144 In addition, the financial burden of chronic pain due to health care utilization and lost productivity for adults could create additional stress for the family.66 Thus, the unique challenges of children with parents with chronic pain represent significant chronic stressors.
3. Potential moderators of risk
3.1. Moderator 1: pain status and the presence of second parent
The number of parents with chronic pain may influence the development of chronic pain in their children in a dose—response relation where having 1 parent with chronic pain increases risk for pediatric chronic pain, but having 2 parents with chronic pain poses greatest risk for pediatric chronic pain. The cumulative effect of having 2 parents with chronic pain has been reported in both clinical and community populations.61,123 Having a healthy co-parent may buffer children from the negative effects of parental pain, as he or she may be able to provide support for the other parent or provide children with positive parenting that may be difficult for the parent with pain to provide at times (eg, physical activity parenting). In some families where a parent has chronic pain, a healthy co-parent may not be present or available (eg, single-parent homes). There is some evidence that pediatric patients with chronic pain are more likely to come from single-parent homes1,71 and living in a single-parent home may be an important moderator when examining the association between parental chronic pain and the development of chronic pain in children.
3.2. Moderator 2: timing, course, and location of parental chronic pain
The timing, course, and location of parental chronic pain may moderate the relation between parental chronic pain and the development of pediatric chronic pain, though to our knowledge, no studies have examined this association. Longer duration of exposure to parental chronic pain provides additional repeated opportunities for children to observe and adopt pain-related behaviors, fears, and responses. In addition, early life exposure to maternal distress is consistently associated with poor psychological and physical health outcomes in children,78,131 and early life exposure to maternal pain may be similar. Timing of parental chronic pain with regards to children’s developmental stages may be most relevant for determining when in childhood to target preventive interventions for both the parent and child.
Regarding the bodily location of parental chronic pain, some studies have found a stronger relation between parent and child chronic pain for congruent pain locations than incongruent locations,53,119 whereas others have not.102 Some studies test multiple pain locations and find a relation between parent and child chronic pain for a specific congruent body location (eg, head pain), but not for other congruent body locations.11,133 Social Learning Theory would suggest that a child would be more likely to learn pain and pain behaviors from their parents when sharing the same pain location. Indeed, the literature examining illness behaviors has identified that childhood learning may be illness specific and may not generalize to other conditions.84,157 Thus, stronger relations between pediatric chronic pain and parental chronic pain may occur when pain locations are the same.
However, chronic pain may represent a unitary disease state regardless of location, especially when pain is reported across multiple pain sites.165 In some cases, the number of parental chronic pain sites may be related to children’s chronic pain severity more than a specific body location.119,120 The presence of multiple pain sites may be a marker of central sensitization which reflects alterations in the central nervous system with regards to pain processing.134 Therefore, parents with multiple chronic pain sites, reflecting possible central sensitization, may have children at greatest risk for developing widespread chronic pain because of underlying genetic bases and social learning that may be specific to multisite pain or comorbid pain conditions.
3.3. Moderator 3: children’s characteristics
A number of child characteristics may moderate the relation between parental chronic pain and the development of pediatric chronic pain.
3.3.1. Sex
Girls seem to be more vulnerable to the influence of maternal pain and illness,77 as well as to the influence of maternal responses to child pain.17,153 Girls also have a greater risk of musculoskeletal pain persisting from preadolescence to adolescence compared with boys and seem to be more likely to develop persistent pain in the context of other risk factors (eg, depressive symptoms and hypermobility) than boys.41 However, nonexperimental studies of the relation between parent and child chronic pain have not found differences between male and female offspring.61,123 Because chronic pain is most common in females, many of these larger studies have unequal numbers of males and females reporting chronic pain and may have insufficient power to detect sex-specific effects.
3.3.2. Race or ethnicity
African Americans, compared with non-Hispanic whites, report higher chronic pain ratings and greater evoked pain responsiveness in adulthood.37,109,114 In adolescence, African Americans have been shown to exhibit similar heightened evoked pain responsiveness compared with non-Hispanic whites.98 Thus, a child’s race or ethnicity may increase risk for heightened pain or the development of chronic pain in the context of parental pain. In addition, the extent to which certain mechanisms (eg, parental modeling or reinforcement) influence the intergenerational transmission of risk for chronic pain may differ by a child’s ethnicity or culture.
3.3.3. Developmental stage
Children’s developmental stage may influence both parental responses to children’s pain and children’s tendencies to express chronic pain complaints. The relation between parent and child pain may be strongest for children in early puberty stages vs those in later puberty stages.140 As children become adolescents, they strive to achieve more autonomy from their parents and thus may direct more attention to peers than parents.94 Thus, parental chronic pain may have greater influence on the development of children’s pain than adolescents’ pain. Similarly, the exposure to parental pain during critical early developmental periods may be more relevant for neurobiological mechanisms.
3.3.4. Temperament
Temperament is evident early in infancy and refers to individual differences in affective, attentional, and motor responses.117 These individual differences have been categorized into 3 broad factors commonly studied in early and middle childhood: (1) positive affectivity, (2) negative affectivity, and (3) effortful control.117,118 These factors could all moderate the extent to which offspring of parents with chronic pain are at risk for the development of pediatric chronic pain.
Children’s positive affectivity may serve as a resilience resource in the context of chronic pain and may be one factor that could buffer the relation between parental chronic pain and risk for the development of pediatric chronic pain.24 However, children characterized by high levels of negative affectivity, which is often linked with neuroticism, tend to experience higher levels of fear, anger, and sadness and have more difficulty engaging in coping efforts to modulate their own affect intensity in the face of challenges, such as pain.21 Pediatric patients with chronic pain experience higher rates of lifetime anxiety and depressive disorders than pain-free individuals122 suggesting higher levels of negative affectivity may confer greater risk for pediatric chronic pain, but whether these factors result from or contribute to the development of chronic pain in children is unknown.
Effortful control refers to the ability to modulate attention and inhibit automatic responses. Children with an attentional bias towards pain could be more susceptible parental modeling and pain responses because of their attentional bias towards threatening pain information and difficulty shifting attention in the context of a threat such as pain.51 This may be particularly true for children with low effortful control and a high tendency to catastrophize about pain.56
4. Summary and future directions
The comprehensive intergenerational framework described here provides a starting point for examining potential mechanisms and moderators through which parental chronic pain might increase the risk for pain and related poor outcomes in children. Such a framework highlights chronic pain as inherently familial and intergenerational, opening up avenues for new models of intervention and prevention that can be family centered and include at-risk children. Parents suffering from chronic pain are not simply isolated patients who need effective treatment for themselves, but as parents may also hold the key to improving outcomes for their own children and for future generations. We have proposed a number of possible mechanistic pathways that might contribute to the development of chronic pain and related outcomes in the offspring of parents with chronic pain. This model has strong theoretical underpinnings from Social Learning Theory, as well as from existing models of intergenerational transmission of depression and physical illness. Although some portions of the model are supported by the existing literature, it has been inferred that many of these mechanisms exist based on high rates of pain in offspring. By elucidating specific pathways that can be tested, knowledge can be gained which will provide avenues and targets for prevention efforts, which have not traditionally been pursued in chronic pain. Additional research will help support or refute aspects of this conceptual model.
Many of these mechanisms and moderators have been studied in the context of other mental and physical health conditions (eg, depression and obesity), but the chronic pain literature lags behind. Drawing from these other knowledge bases and our review of the literature, we propose future directions in 3 key areas: (1) basic science, (2) prevention, and (3) intervention (Fig. 2). Finally, we will address current methodological issues that need to be addressed to move this area of research forward.
Figure 2.
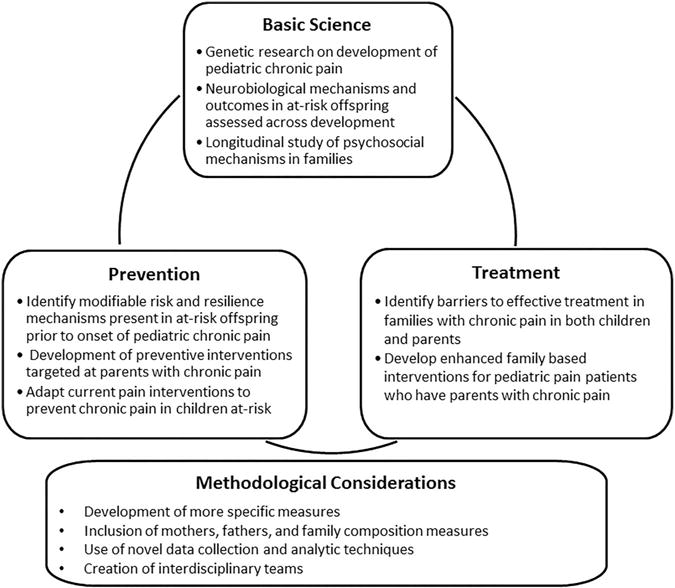
Future directions for research on the intergenerational transmission of chronic pain risk.
4.1. Basic science
Although there is a growing body of work focused on biological factors contributing to the development of chronic pain, these factors have rarely been examined in the development of chronic pain in children. In adults, results from a large candidate gene study examining the development of TMD suggest that genetic polymorphisms may be associated with intermediate clinical, psychological, and sensory phenotypes predictive of TMD onset in the absence of direct associations with the onset of the disorder.130 Similar methodologies could help discover genetic polymorphisms present in children of parents with chronic pain which confer greater risk for chronic pain development. In addition, research examining gene by environment interactions could elucidate when and under what circumstances each of these mechanisms increases risk for the development of chronic pain in children.
Work is also needed to better understand the potential impact of parental chronic pain on early neurobiological development and the potential long-term consequences of these early changes in childhood or adolescence. Examining brain networks proposed to contribute to pain chronicity (eg, descending pain modulatory system and reward-motivation network) early in development and over time among the offspring of parents with chronic pain may provide additional information about the ways in which parental chronic pain influences children’s neurobiology. In addition to neuroimaging, assessment of HPA axis functioning, physiological responses, and laboratory pain responses might inform this area of study.
There is a great need for intergenerational and longitudinal studies. Although a few studies have examined samples of parents with chronic pain, eg,85 the majority of this research has consisted of cross-sectional studies of group differences in the children of parents with a chronic pain condition compared with children of healthy control parents. Following high-risk children over time will allow for testing the proposed mechanisms and establishing targets for preventive interventions.
4.2. Prevention
Although our framework has primarily focused on mechanisms which increase risk, many children of parents with chronic pain do not develop pediatric chronic pain or related outcomes. Understanding protective factors in both parents with chronic pain and their offspring will help identify targets for promoting resilience in parents with chronic pain and at-risk children and adolescents with the goal of pediatric chronic pain prevention. Prevention of the onset of chronic pain is a new frontier in pediatric pain. Existing treatments that are effective for youth with chronic pain might be adapted to serve as preventive interventions. Consideration should also be given to circumstances (eg, surgery and presentation in primary care with pain) and points in development that might provide good opportunities to deliver these preventive interventions to high-risk youth. Developing and testing preventive interventions through randomized controlled trials could also advance our understanding of timing effects and causal mechanisms in the intergenerational transmission of risk for chronic pain.
4.3. Intervention
Close to 50% of pediatric patients with chronic pain presenting to tertiary medical clinics have at least 1 parent with chronic pain.91 We primarily discussed our model in the context of the development of chronic pain, but many of these mechanisms could also operate to maintain chronic pain and related comorbidities in children. For pediatric patients with chronic pain, it may be difficult to make lifestyle changes and maintain gains when parents hold strong beliefs regarding their own pain and engage in disability behaviors. Supplementing current interdisciplinary interventions for pediatric chronic pain with components that target parents’ own chronic pain and disability may improve treatment efficacy. Researching and understanding barriers to treatment in families with chronic pain could provide targets for improving current treatments to address the needs of these families.
4.4. Methodological considerations
To conduct longitudinal studies on mechanisms for the intergenerational transmission of chronic pain from parents to offspring, greater attention is needed on how we measure and assess chronic pain in families. Although a number of epidemiological studies have examined the impact of the presence of chronic pain in mothers and fathers on offspring,62 additional work is needed to assess the impact of other important dimensions of parental pain (eg, pain behaviors and pain-related disability) when considering chronic pain and other poor outcomes in offspring.104 Additional information about heritability might also be gleaned by assessing the presence of chronic pain in first- and second-degree biological relatives. Interview methods have been used successfully to assess family history of alcoholism and yield continuous scores of family history density which are associated with offspring risk for substance use disorders.26,135 Measuring the timing of parental chronic pain in relation to children’s developmental stage, duration of parental chronic pain, and chronic pain location and severity will help further our knowledge of these moderators. In addition, it will be important to include both mothers and fathers in this research with careful attention to family compositions as a potential moderating variable. The development of additional measures is needed to test specific mechanisms (eg, social learning) in the model.
Currently, measures of parent responses to children’s pain are limited and have typically been investigated with a single-parent self-report measure.145 Observational tasks and measures that assess moment-to-moment interactions between parent—child dyads are needed to establish the behaviors that parents exhibit in response to children’s pain, and behavioral or emotional responses that children may exhibit in response to parents’ pain. A number of studies have examined parent—child dyads in the context of acute pain or laboratory tasks,10,149 but this research has yet to be conducted in the context of parental chronic pain. In addition, using data collection and analytic techniques (eg, electronic momentary assessment and actigraphy) to capture daily variations in parental pain characteristics and stressors and child responses to parental pain might yield unique perspective on the synchrony of parent—child experiences and may provide insight into the implementation of prevention programs that could target symptoms and behaviors in daily life.
Finally, execution of many of these future research directions requires the creation of interdisciplinary teams to conduct complex studies examining interactions among mechanisms. These interdisciplinary research teams should be comprised a variety of disciplines, such as geneticists, neurobiologists, medical providers, psychologists, and physical therapists. Many of the pathways outlined interact, and thus researchers must interact to examine these specific interactive components between biology, psychology, and clinical presentation. These interdisciplinary teams will also be needed to execute effective family-based preventive interventions and interventions for children of parents with chronic pain. It is also important to note that this topic bridges both pediatric and adult chronic pain research. Teams comprised both pediatric and adult pain researchers could help bridge this gap to design studies that effectively capture mechanisms operating to maintain pain in both parents and their children. Forming diverse teams of researchers committed to carrying out this vision and addressing these current methodological issues and gaps is one of the first steps. It will take years of research to establish or refute each of these mechanisms, but the potential benefit of developing effective preventive interventions and interventions for children of parents with chronic pain is a worthwhile pursuit.
Supplementary Material
Acknowledgments
The first author thanks her dissertation committee chair, Lynn Walker, as well as committee members Stephen Bruehl, Judy Garber, and Craig Smith for their helpful input. The authors additionally thank Tonya Palermo and Christine Chambers for their feedback on early versions of the theoretical model.
Conflict of interest statement
A. L. Stone is an international trainee with Pain in Child Health (PICH), a Strategic Training Initiative in Health Research of the Canadian Institutes for Health Research (CIHR). A. C. Wilson’s work on this project was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD082200).
Footnotes
Supplemental media
A supplemental video associated with this article can be found online at http://links.lww.com/PAIN/A298.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
References
- 1.Aasland A, Flato B, Vandvik IH. Psychosocial factors in children with idiopathic musculoskeletal pain: a prospective, longitudinal study. Acta Paediatr. 1997;86:740–6. doi: 10.1111/j.1651-2227.1997.tb08578.x. [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth MS. Infant—mother attachment. Am Psychol. 1979;34:932–7. doi: 10.1037//0003-066x.34.10.932. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM, Buonocore M, Merlo L, Galandra C, Sotgiu A, Bacchella L, Ungaretti M, Demartini L, Bonezzi C. Chronic pain therapy and hypothalamic-pituitary-adrenal axis impairment. Psychoneuroendocrinology. 2011;36:1032–9. doi: 10.1016/j.psyneuen.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Armistead L, Klein K, Forehand R. Parental physical illness and child functioning. Clin Psychol Rev. 1995;15:409–22. [Google Scholar]
- 5.Asmundson GJ, Noel M, Petter M, Parkerson HA. Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res Manag. 2012;17:397–405. doi: 10.1155/2012/908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ban L, Fleming KM, Doyle P, Smeeth L, Hubbard RB, Fiaschi L, Tata LJ. Congenital anomalies in children of mothers taking antiepileptic drugs with and without periconceptional high dose folic acid use: a population-based cohort study. PLoS One. 2015;10:e0131130. doi: 10.1371/journal.pone.0131130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandura A. Social learning theory. Englewood Cliffs: Prentice Hall; 1977. [Google Scholar]
- 8.Bell LM, Byrne S, Thompson A, Ratnam N, Blair E, Bulsara M, Jones TW, Davis EA. Increasing body mass index z-score is continuously associated with complications of overweight in children, even in the healthy weight range. J Clin Endocrinol Metab. 2007;92:517–22. doi: 10.1210/jc.2006-1714. [DOI] [PubMed] [Google Scholar]
- 9.Blyth FM, March LM, Brnabic AJM, Cousins MJ. Chronic pain and frequent use of health care. PAIN. 2004;111:51–8. doi: 10.1016/j.pain.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Boerner KE, Noel M, Birnie KA, Caes L, Petter M, Chambers CT. Impact of threat level, task instruction, and individual characteristics on cold pressor pain and fear among children and their parents. Pain Pract. 2015 doi: 10.1111/papr.12306. [DOI] [PubMed] [Google Scholar]
- 11.Borge AI, Nordhagen R. Recurrent pain symptoms in children and parents. Acta Paediatr. 2000;89:1479–83. doi: 10.1080/080352500456688. [DOI] [PubMed] [Google Scholar]
- 12.Bowlby JA. Secure base: parent-child attachment and healthy human development. New York: Basic Books; 1988. [Google Scholar]
- 13.Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10:408–17. doi: 10.1080/15592294.2015.1039221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandlistuen RE, Ystrom E, Eberhard-Gran M, Nulman I, Koren G, Nordeng H. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol. 2015;44:1397–1407. doi: 10.1093/ije/dyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns JW, Johnson BJ, Mahoney N, Devine J, Pawl R. Cognitive and physical capacity process variables predict long-term outcome after treatment of chronic pain. J Consult Clin Psychol. 1998;66:434–9. doi: 10.1037//0022-006x.66.2.434. [DOI] [PubMed] [Google Scholar]
- 16.Caes L, Vervoort T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child’s pain and its relationship with activity restriction: the mediating role of parental distress. PAIN. 2011;152:212–22. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Chambers CT, Craig KD, Bennett SM. The impact of maternal behavior on children’s pain experiences: an experimental analysis. J Pediatr Psychol. 2002;27:293–301. doi: 10.1093/jpepsy/27.3.293. [DOI] [PubMed] [Google Scholar]
- 18.Chambers CT, Craig KD, Bennett SM. The impact of maternal behavior on children’s pain experiences: an experimental analysis. J Pediatr Psychol. 2002;27:293–301. doi: 10.1093/jpepsy/27.3.293. [DOI] [PubMed] [Google Scholar]
- 19.Claar RL, Simons LE, Logan DE. Parental response to children’s pain: the moderating impact of children’s emotional distress on symptoms and disability. PAIN. 2008;138:172–9. doi: 10.1016/j.pain.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Cohn DA, Cowan PA, Cowan CP, Pearson J. Mothers’ and fathers’ working models of childhood attachment relationships, parenting styles, and child behavior. Dev Psychopathol. 1992;4:417–31. [Google Scholar]
- 21.Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: implications for depression in childhood and adolescence. J Clin Child Adolesc Psychol. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- 22.Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. PAIN. 2007;131:162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousins LA, Cohen LL, Venable C. Risk and resilience in pediatric chronic pain: exploring the protective role of optimism. J Pediatr Psychol. 2015;40:934–42. doi: 10.1093/jpepsy/jsu094. [DOI] [PubMed] [Google Scholar]
- 24.Cousins LA, Kalapurakkel S, Cohen LL, Simons LE. Topical review: resilience resources and mechanisms in pediatric chronic pain. J Pediatr Psychol. 2015;40:840–45. doi: 10.1093/jpepsy/jsv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. PAIN. 2003;104:639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 26.Cservenka A, Casimo K, Fair D, Nagel B. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–19. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cservenka A, Stein H, Wilson AC, Nagel BJ. Neurobiological phenotypes of familial chronic pain in adolescence: a pilot fMRI study. J Pain. 2015;16:913–25. doi: 10.1016/j.jpain.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9:28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Davidov M, Grusec JE. Untangling the links of parental responsiveness to distress and warmth to child outcomes. Child Dev. 2006;77:44–58. doi: 10.1111/j.1467-8624.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 30.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deere KC, Clinch J, Holliday K, McBeth J, Crawley EM, Sayers A, Palmer S, Doerner R, Clark EM, Tobias JH. Obesity is a risk factor for musculoskeletal pain in adolescents: findings from a population-based cohort. PAIN. 2012;153:1932–8. doi: 10.1016/j.pain.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Demarest SP, Gill RS, Adler RA. Opioid endocrinopathy. Endocr Pract. 2015;21:190–8. doi: 10.4158/EP14339.RA. [DOI] [PubMed] [Google Scholar]
- 33.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 34.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K, Kerzner LS, Bateman BT. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;9:340–50. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–23. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg N, Cumberland A, Spinrad TL, Fabes RA, Shepard SA, Reiser M, Murphy BC, Losoya SH, Guthrie IK. The relations of regulation and emotionality to children’s externalizing and internalizing problem behavior. Child Dev. 2001;72:1112–34. doi: 10.1111/1467-8624.00337. [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg N, Spinrad TL, Eggum ND. Emotion-related self-regulation and its relation to children’s maladjustment. Annu Rev Clin Psychol. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 41.El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M. Prognosis of non-specific musculoskeletal pain in preadolescents: a prospective 4-year follow-up study till adolescence. PAIN. 2004;110:550–9. doi: 10.1016/j.pain.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Evans S, Meldrum M, Tsao JCI, Fraynt R, Zeltzer LK. Associations between parent and child pain and functioning in a pediatric chronic pain sample: a mixed methods approach. Int J Disabil Hum Dev. 2010;9:11–21. doi: 10.1515/ijdhd.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans S, Shipton EA, Keenan T. The relationship between maternal chronic pain and child adjustment: the role of parenting as a mediator. J Pain. 2006;7:236–43. doi: 10.1016/j.jpain.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Fillingim RB. Heritability of catastrophizing: the biopsychosocial model in action. PAIN. 2015;156:357–8. doi: 10.1097/01.j.pain.0000460338.16353.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fodor A, Tímár J, Zelena D. Behavioral effects of perinatal opioid exposure. Life Sci. 2014;104:1–8. doi: 10.1016/j.lfs.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Fujii H, Goel A, Bernard N, Pistelli A, Yates LM, Stephens S, Han JY, Matsui D, Etwell F, Einarson TR, Koren G, Einarson A. Pregnancy outcomes following gabapentin use: results of a prospective comparative cohort study. Neurology. 2013;80:1565–70. doi: 10.1212/WNL.0b013e31828f18c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallant AR, Tremblay A, Perusse L, Despres JP, Bouchard C, Drapeau V. Parental eating behavior traits are related to offspring BMI in the Quebec Family Study. Int J Obes (Lond) 2013;37:1422–6. doi: 10.1038/ijo.2013.14. [DOI] [PubMed] [Google Scholar]
- 48.Goodman JE, McGrath PJ. Mothers’ modeling influences children’s pain during a cold pressor task. PAIN. 2003;104:559–65. doi: 10.1016/S0304-3959(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 49.Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. 1999;106:458–90. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 50.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. PAIN. 2006;123:254–63. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Goubert L, Vlaeyen JW, Crombez G, Craig KD. Learning about pain from others: an observational learning account. J Pain. 2011;12:167–74. doi: 10.1016/j.jpain.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment seeking adolescents in the United States. J Pain. 2014;15:925–33. doi: 10.1016/j.jpain.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groholt EK, Stigum H, Nordhagen R, Kohler L. Recurrent pain in children, socio-economic factors and accumulation in families. Eur J Epidemiol. 2003;18:965–75. doi: 10.1023/a:1025889912964. [DOI] [PubMed] [Google Scholar]
- 54.Guite JWP, McCue RLBA, Sherker JLP, Sherry DDMD, Rose JBMD. Relationships among pain, protective parental responses, and disability for adolescents with chronic musculoskeletal pain: the mediating role of pain catastrophizing. Clin J Pain. 2011;27:775–81. doi: 10.1097/AJP.0b013e31821d8fb4. [DOI] [PubMed] [Google Scholar]
- 55.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e65. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 56.Heathcote LC, Vervoort T, Eccleston C, Fox E, Jacobs K, Van Ryckeghem DM, Lau JY. The relationship between adolescents’ pain catastrophizing and attention bias to pain faces is moderated by attention control. PAIN. 2015;156:1334–41. doi: 10.1097/j.pain.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 57.Hershey AD, Powers SW, Nelson TD, Kabbouche MA, Winner P, Yonker M, Linder SL, Bicknese A, Sowel MK, McClintock W. Obesity in the pediatric headache population: a multicenter study. Headache. 2009;49:170–7. doi: 10.1111/j.1526-4610.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 58.Hestbaek L, Iachine IA, Leboeuf-Yde C, Kyvik KO, Manniche C. Heredity of low back pain in a young population: a classical twin study. Twin Res. 2004;7:16–26. doi: 10.1375/13690520460741408. [DOI] [PubMed] [Google Scholar]
- 59.Higgins KS, Birnie KA, Chambers CT, Wilson AC, Caes L, Clark AJ, Lynch M, Stinson J, Campbell-Yeo M. Offspring of parents with chronic pain: a systematic review and meta-analysis of pain, health, psychological, and family outcomes. PAIN. 2015;156:2256–66. doi: 10.1097/j.pain.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoftun GB, Romundstad PR, Rygg M. Factors associated with adolescent chronic non-specific pain, chronic multisite pain, and chronic pain with high disability: the Young-HUNT Study 2008. J Pain. 2012;13:874–83. doi: 10.1016/j.jpain.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult family linkage data from the HUNT study. Jama Pediatr. 2013;167:61–9. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- 62.Hoftun GB, Romundstad PR, Rygg M. Association of parental chronic pain with chronic pain in the adolescent and young adult: family linkage data from the HUNT Study. JAMA Pediatr. 2013;167:61–9. doi: 10.1001/jamapediatrics.2013.422. [DOI] [PubMed] [Google Scholar]
- 63.Hunt MA, Keefe FJ, Bryant C, Metcalf BR, Ahamed Y, Nicholas MK, Bennell KL. A physiotherapist-delivered, combined exercise and pain coping skills training intervention for individuals with knee osteoarthritis: a pilot study. Knee. 2013;20:106–12. doi: 10.1016/j.knee.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, Jones RH, Dieppe PA, Reeves BC. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Rheum. 2007;57:1211–19. doi: 10.1002/art.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hussey J, Gormley J, Bell C, Roche EF, Hoey H. Exercise tolerance and physical activity levels in children referred to a weight reduction clinic. Ir Med J. 2006;99:46–7. [PubMed] [Google Scholar]
- 66.Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington: Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 67.Jensen MP, Turner JA, Romano JM, Karoly P. Coping with chronic pain: a critical review of the literature. PAIN. 1991;47:249–83. doi: 10.1016/0304-3959(91)90216-K. [DOI] [PubMed] [Google Scholar]
- 68.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–9. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Kaasboll J, Lydersen S, Indredavik MS. Psychological symptoms in children of parents with chronic pain-the HUNT study. PAIN. 2012;153:1054–62. doi: 10.1016/j.pain.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Karabulut GS, Beser OF, Erginoz E, Kutlu T, Cokugras FC, Erkan T. The incidence of Irritable Bowel Syndrome in children using the Rome III criteria and the effect of trimebutine treatment. J Neurogastroenterol Motil. 2013;19:90–3. doi: 10.5056/jnm.2013.19.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashikar-Zuck S, Flowers SR, Verkamp E, Ting TV, Lynch-Jordan AM, Graham TB, Passo M, Schikler KN, Hashkes PJ, Spalding S, Banez G, Richards MM, Powers SW, Arnold LM, Lovell D. Actigraphy-based physical activity monitoring in adolescents with juvenile primary fibromyalgia syndrome. J Pain. 2010;11:885–93. doi: 10.1016/j.jpain.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerns RD, Rosenberg R. Pain-relevant responses from significant others: development of a significant-other version of the WHYMPI scales. PAIN. 1995;61:245–9. doi: 10.1016/0304-3959(94)00173-C. [DOI] [PubMed] [Google Scholar]
- 74.Khaleque A. Perceived parental warmth, and children’s psychological adjustment, and personality dispositions: a meta-analysis. J Child Fam Stud. 2012;22:297–306. [Google Scholar]
- 75.Kiff CJ, Lengua LJ, Zalewski M. Nature and nurturing: parenting in the context of child temperament. Clin Child Fam Psychol Rev. 2011;14:251–301. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S, Kochanska G. Child temperament moderates effects of parent—child mutuality on self-regulation: a relationship-based path for emotionally negative infants. Child Dev. 2012;83:1275–89. doi: 10.1111/j.1467-8624.2012.01778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korneluk YG, Lee CM. Children’s adjustment to parental physical illness. Clin Child Fam Psychol Rev. 1998;1:179–93. doi: 10.1023/a:1022654831666. [DOI] [PubMed] [Google Scholar]
- 78.Kozyrskyj AL, Mai X-M, McGrath P, HayGlass KT, Becker AB, MacNeil B. Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma. Am J Respir Crit Care Med. 2008;177:142–7. doi: 10.1164/rccm.200703-381OC. [DOI] [PubMed] [Google Scholar]
- 79.Laird KT, Preacher KJ, Walker LS. Attachment and adjustment in adolescents and young adults with a history of pediatric functional abdominal pain. Clin J Pain. 2015;31:152–8. doi: 10.1097/AJP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landmark T, Romundstad P, Borchgrevink PC, Kaasa S, Dale O. Associations between recreational exercise and chronic pain in the general population: evidence from the HUNT 3 study. PAIN. 2011;152:2241–7. doi: 10.1016/j.pain.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 81.Langer SL, Romano JM, Levy RL, Walker LS, Whitehead WE. Catastrophizing and parental response to child symptom complaints. Child Health Care. 2009;38:169–84. doi: 10.1080/02739610903038750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lansford JE, Sharma C, Malone PS, Woodlief D, Dodge KA, Oburu P, Pastorelli C, Skinner AT, Sorbring E, Tapanya S, Tirado LMU, Zelli A, Al-Hassan SM, Alampay LP, Bacchini D, Bombi AS, Bornstein MH, Chang L, Deater-Deckard K, Di Giunta L. Corporal punishment, maternal warmth, and child adjustment: a longitudinal study in eight countries. J Clin Child Adolesc Psychol. 2014;43:670–85. doi: 10.1080/15374416.2014.893518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazarus RS, Folkman S. Stress, appraisal, coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 84.Levy RL, Whitehead WE, Von Korff MR, Feld AD. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–6. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 85.Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, Christie D. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99:2442–51. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 86.Lewandowski AS, Palermo TM. Parent-teen interactions as predictors of depressive symptoms in adolescents with headache. J Clin Psychol Med Settings. 2009;16:331–38. doi: 10.1007/s10880-009-9173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewandowski AS, Palermo TM, Stinson J, Handley S, Chambers CT. Systematic review of family functioning in families of children and adolescents with chronic pain. J Pain. 2010;11:1027–38. doi: 10.1016/j.jpain.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis FM, Hammond MA, Woods NF. The family’s functioning with newly diagnosed breast cancer in the mother: the development of an explanatory model. J Behav Med. 1993;16:351–70. doi: 10.1007/BF00844777. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, Overcash F, Kurtzberg J, Jirtle R, Iversen ES, Forman MR, Hoyo C. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7:735–46. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. Clin J Pain. 2013;29:681–8. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. J Pain. 2006;7:244–51. doi: 10.1016/j.jpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 92.McCracken LM, Carson JW, Eccleston C, Keefe FJ. Acceptance and change in the context of chronic pain. PAIN. 2004;109:4–7. doi: 10.1016/j.pain.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 93.McCullough LE, Mendez MA, Miller EE, Murtha AP, Murphy SK, Hoyo C. Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics. 2015;10:597–606. doi: 10.1080/15592294.2015.1045181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McElhaney KB, Allen JP, Stephenson JC, Hare AL. Attachment and autonomy during adolescence. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. 3rd. Vol. 1. Hoboken, NJ: John Wiley & Sons, Inc; 2009. pp. 358–403. Chapter 11. [Google Scholar]
- 95.Meredith P, Ownsworth T, Strong J. A review of the evidence linking adult attachment theory and chronic pain: presenting a conceptual model. Clin Psychol Rev. 2008;28:407–29. doi: 10.1016/j.cpr.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Meredith P, Strong J, Feeney JA. Adult attachment, anxiety, and pain self-efficacy as predictors of pain intensity and disability. PAIN. 2006;123:146–54. doi: 10.1016/j.pain.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 97.Mikkelsson M, Kaprio J, Salminen JJ, Pulkkinen L, Rose RJ. Widespread pain among 11-year-old Finnish twin pairs. Arthritis Rheum. 2001;44:481–5. doi: 10.1002/1529-0131(200102)44:2<481::AID-ANR68>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 98.Morris MC, Walker L, Bruehl S, Hellman N, Sherman AL, Rao U. Race effects on temporal summation to heat pain in youth. PAIN. 2015;156:917–22. doi: 10.1097/j.pain.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muris P, Field AP. The role of verbal threat information in the development of childhood fear. “Beware the Jabberwock!”. Clin Child Fam Psychol Rev. 2010;13:129–50. doi: 10.1007/s10567-010-0064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osborne RB, Hatcher JW, Richtsmeier AJ. The role of social modeling in unexplained pediatric pain. J Pediatr Psychol. 1989;14:43–61. doi: 10.1093/jpepsy/14.1.43. [DOI] [PubMed] [Google Scholar]
- 102.Oster J. Recurrent abdominal pain, headache and limb pains in children and adolescents. Pediatrics. 1972;50:429–36. [PubMed] [Google Scholar]
- 103.Palermo TM, Chambers CT. Parent and family factors in pediatric chronic pain and disability: an integrative approach. PAIN. 2005;119:1–4. doi: 10.1016/j.pain.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 104.Palermo TM, Holley AL. The importance of family environment in pediatric chronic pain. JAMA Pediatrics. 2013;167:93–4. doi: 10.1001/jamapediatrics.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palermo TM, Law EF, Bromberg M, Fales J, Eccleston C, Wilson AC. Problem-solving skills training for parents of children with chronic pain: a pilot randomized controlled trial. PAIN. 2016;157:1213–23. doi: 10.1097/j.pain.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, Ely EW, Grijalva CG, Cooper WO. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135:842–50. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745–58. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rabbitts JA, Holley AL, Karlson CW, Palermo TM. Bidirectional associations between pain and physical activity in adolescents. Clin J Pain. 2014;30:251–8. doi: 10.1097/AJP.0b013e31829550c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rahim-Williams B, Riley JL, III, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13:522–40. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramsden CE, Zamora D, Makriyannis A, Wood JT, Mann JD, Faurot KR, MacIntosh BA, Majchrzak-Hong SF, Gross JR, Courville AB, Davis JM, Hibbeln JR. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain. 2015;16:707–16. doi: 10.1016/j.jpain.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rash JA, Campbell TS, Letourneau N, Giesbrecht GF. Maternal cortisol during pregnancy is related to infant cardiac vagal control. Psychoneuroendocrinology. 2015;54:78–89. doi: 10.1016/j.psyneuen.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 112.Ravid S, Shahar E, Schiff A, Gordon S. Obesity in children with headaches: association with headache type, frequency, and disability. Headache. 2013;53:954–61. doi: 10.1111/head.12088. [DOI] [PubMed] [Google Scholar]
- 113.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330. [PubMed] [Google Scholar]
- 114.Riley JL, III, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. PAIN. 2002;100:291–8. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 115.Rocha EM, Prkachin KM. Temperament and pain reactivity predict health behavior seven years later. J Pediatr Psychol. 2007;32:393–9. doi: 10.1093/jpepsy/jsl036. [DOI] [PubMed] [Google Scholar]
- 116.Ross LE, Grigoriadis S, Mamisashvili L, Vonderporten EH, Roerecke M, Rehm J, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70:436–43. doi: 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- 117.Rothbart MK. Temperament: A developmental framework. In: Strelau J, Angleitner A, editors. Explorations in temperament: International perspectives on theory and measurement. New York: Plenum Press; 1991. pp. 61–74. [Google Scholar]
- 118.Rothbart MK, Bates JE. Temperament. In: Eisenberg N, editor. Handbook of child psychologyâ Volâ 3â Social, emotional, and personality development. New York: Wiley; 1998. pp. 105–76. [Google Scholar]
- 119.Saunders K, Von Korff M, Leresche L, Mancl L. Relationship of common pain conditions in mothers and children. Clin J Pain. 2007;23:204–13. doi: 10.1097/AJP.0b013e31802d7807. [DOI] [PubMed] [Google Scholar]
- 120.Schanberg LE, Keefe FJ, Lefebvre JC, Kredich DW, Gil KM. Social context of pain in children with juvenile primary fibromyalgia syndrome: parental pain history and family environment. The Clin J Pain. 1998;14:107–15. doi: 10.1097/00002508-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 121.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, Horst SN, Smith CA, Garber J, Walker LS. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132:475–82. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sherman AL, Bruehl S, Smith CA, Walker LS. Individual and additive effects of mothers’ and fathers’ chronic pain on health outcomes in young adults with a childhood history of functional abdominal pain. J Pediatr Psychol. 2013;38:365–75. doi: 10.1093/jpepsy/jss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sieberg CB, Williams S, Simons LE. Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain? J Pediatr Psychol. 2011;36:1043–51. doi: 10.1093/jpepsy/jsr043. [DOI] [PubMed] [Google Scholar]
- 125.Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74:1869–80. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 126.Simons LE, Sieberg CB, Kaczynski KJ. Measuring parent beliefs about child acceptance of pain: a preliminary validation of the Chronic Pain Acceptance Questionnaire, parent report. PAIN. 2011;152:2294–300. doi: 10.1016/j.pain.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Slade GD, Sanders AE, Ohrbach R, Bair E, Maixner W, Greenspan JD, Fillingim RB, Smith S, Diatchenko L. COMT diplotype amplifies effect of stress on risk of temporomandibular pain. J Dent Res. 2015;94:1187–95. doi: 10.1177/0022034515595043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sleddens EF, Kremers SP, Hughes SO, Cross MB, Thijs C, De Vries NK, O’Connor TM. Physical activity parenting: a systematic review of questionnaires and their associations with child activity levels. Obes Rev. 2012;13:1015–33. doi: 10.1111/j.1467-789X.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 129.Smith AM, Sieberg CB, Odell S, Randall E, Simons LE. Living life with my child’s pain: The Parent Pain Acceptance Questionnaire (PPAQ) Clin J Pain. 2015;31:633–41. doi: 10.1097/AJP.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Weir B, Maixner W, Diatchenko L. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic Architecture of TMD in the OPPERA prospective cohort study. J Pain. 2013;14(suppl 12):T91–101.e103. doi: 10.1016/j.jpain.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spence SH, Najman JM, Bor W, O’Callaghan MJ, Williams GM. Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. J Child Psychol Psychiatry. 2002;43:457–69. doi: 10.1111/1469-7610.00037. [DOI] [PubMed] [Google Scholar]
- 132.Stahl MK, El-Metwally AA, Mikkelsson MK, Salminen JJ, Pulkkinen LR, Rose RJ, Kaprio JA. Genetic and environmental influences on non-specific neck pain in early adolescence: a classical twin study. Eur J Pain. 2013;17:791–8. doi: 10.1002/j.1532-2149.2012.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. PAIN. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 134.Staud R, Rodriguez ME. Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheum. 2006;2:90–8. doi: 10.1038/ncprheum0091. [DOI] [PubMed] [Google Scholar]
- 135.Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–20. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- 136.Svensson DA, Larsson B, Bille B, Lichtenstein P. Genetic and environmental influences on recurrent headaches in eight to nine-year-old twins. Cephalalgia. 1999;19:866–72. doi: 10.1046/j.1468-2982.1999.1910866.x. [DOI] [PubMed] [Google Scholar]
- 137.Tak LM, Cleare AJ, Ormel J, Manoharan A, Kok IC, Wessely S, Rosmalen JGM. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183–94. doi: 10.1016/j.biopsycho.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 138.Timár J, Sobor M, Király KP, Gyarmati S, Riba P, Al-Khrasani M, Fürst S. Peri, pre and postnatal morphine exposure: exposure-induced effects and sex differences in the behavioural consequences in rat offspring. Behav Pharmacol. 2010;21:58–68. doi: 10.1097/FBP.0b013e3283359f39. [DOI] [PubMed] [Google Scholar]
- 139.Trost ZA, Strachan EB, Sullivan MC, Vervoort TD, Avery ARE, Afari NF. Heritability of pain catastrophizing and associations with experimental pain outcomes: a twin study. PAIN. 2015;156:514–20. doi: 10.1097/01.j.pain.0000460326.02891.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsao JC, Li N, Parker D, Seidman LC, Zeltzer LK. Pubertal status moderates the association between mother and child laboratory pain tolerance. Pain Res Manag. 2014;19:23–9. doi: 10.1155/2014/390368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsao JCI, Evans S, Seidman LC, Zeltzer LK. Health care utilization for pain in children and adolescents: a prospective study of laboratory and non-laboratory predictors of care-seeking. Int J Adolesc Med Health. 2011;23:287–92. doi: 10.1515/ijamh.2011.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Turbin MS, Jessor R, Costa FM, Dong Q, Zhang H, Wang C. Protective and risk factors in health-enhancing behavior among adolescents in china and the United States: does social context matter? Health Psychol. 2006;25:445–54. doi: 10.1037/0278-6133.25.4.445. [DOI] [PubMed] [Google Scholar]
- 143.Umberger WA, Risko J, Covington E. The forgotten ones: challenges and needs of children living with disabling parental chronic pain. J Pediatr Nurs. 2015;30:498–507. doi: 10.1016/j.pedn.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Umberger WA, Risko J, Covington E. The forgotten ones: challenges and needs of children living with disabling parental chronic pain. J Pediatr Nurs. 2015;30:498–507. doi: 10.1016/j.pedn.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 145.Van Slyke DA, Walker LS. Mothers’ responses to children’s pain. Clin J Pain. 2006;22:387–91. doi: 10.1097/01.ajp.0000205257.80044.01. [DOI] [PubMed] [Google Scholar]
- 146.van Tilburg MAL, Levy RL, Walker LS, Von Korff M, Feld LD, Garner M, Feld AD, Whitehead WE. Psychosocial mechanisms for the transmission of somatic symptoms from parents to children. World J Gastroenterol. 2015;21:5532–41. doi: 10.3748/wjg.v21.i18.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014;261:579–88. doi: 10.1007/s00415-013-7239-x. [DOI] [PubMed] [Google Scholar]
- 148.Verhoeven K, Dick B, Eccleston C, Goubert L, Crombez G. The role of executive functioning in children’s attentional pain control: an experimental analysis. PAIN. 2014;155:413–21. doi: 10.1016/j.pain.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 149.Vervoort T, Caes L, Trost Z, Notebaert L, Goubert L. Parental attention to their child’s pain is modulated by threat-value of pain. Health Psychol. 2012;31:623–31. doi: 10.1037/a0029292. [DOI] [PubMed] [Google Scholar]
- 150.Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. PAIN. 1990;40:279–91. doi: 10.1016/0304-3959(90)91125-3. [DOI] [PubMed] [Google Scholar]
- 151.Vowles KE, Cohen LL, McCracken LM, Eccleston C. Disentangling the complex relations among caregiver and adolescent responses to adolescent chronic pain. PAIN. 2010;151:680–6. doi: 10.1016/j.pain.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 152.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walker LS, Williams SE, Smith CA, Garber J, Van Slyke DA, Lipani TA. Parent attention versus distraction: impact on symptom complaints by children with and without chronic functional abdominal pain. PAIN. 2006;122:43–52. doi: 10.1016/j.pain.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D. Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general population. Spine (Phila Pa 1976) 2003;28:1195–202. doi: 10.1097/01.BRS.0000067430.49169.01. [DOI] [PubMed] [Google Scholar]
- 155.Wedderkopp N, Kjaer P, Hestbaek L, Korsholm L, Leboeuf-Yde C. High-level physical activity in childhood seems to protect against low back pain in early adolescence. Spine J. 2009;9:134–41. doi: 10.1016/j.spinee.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 156.Welkom JS, Hwang WT, Guite JW. Adolescent pain catastrophizing mediates the relationship between protective parental responses to pain and disability overtime. J Pediatr Psychol. 2013;38:541–50. doi: 10.1093/jpepsy/jst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Whitehead WE, Crowell MD, Heller BR, Robinson JC, Schuster MM, Horn S. Modeling and reinforcement of the sick role during childhood predicts adult illness behavior. Psychosom Med. 1994;56:541–50. doi: 10.1097/00006842-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 158.Wilson AC, Fales JL. Parenting in the context of chronic pain: a controlled study of parents with chronic pain. Clin J Pain. 2015;31:689–98. doi: 10.1097/AJP.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wilson AC, Lewandowski AS, Palermo TM. Fear-avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Res Manag. 2011;16:178–82. doi: 10.1155/2011/296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilson AC, Moss A, Palermo TM, Fales JL. Parent pain and catastrophizing are associated with pain, somatic symptoms, and pain-related disability among early adolescents. J Pediatr Psychol. 2014;39:418–26. doi: 10.1093/jpepsy/jst094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain. 2012;13:121–30. doi: 10.1016/j.jpain.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wilson AC, Samuelson B, Palermo TM. Obesity in children and adolescents with chronic pain: associations with pain and activity limitations. Clin J Pain. 2010;26:705–11. doi: 10.1097/AJP.0b013e3181e601fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wingenfeld K, Nutzinger D, Kauth J, Hellhammer DH, Lautenbacher S. Salivary cortisol release and hypothalamic pituitary adrenal axis feedback sensitivity in fibromyalgia is associated with depression but not with pain. J Pain. 2010;11:1195–202. doi: 10.1016/j.jpain.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 164.Wittmeier KD, Mollard RC, Kriellaars DJ. Physical activity intensity and risk of overweight and adiposity in children. Obesity (Silver Spring) 2008;16:415–20. doi: 10.1038/oby.2007.73. [DOI] [PubMed] [Google Scholar]
- 165.Yunus MB. Central Sensitivity Syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–52. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


