Abstract
Background
This study aimed to evaluate the effects of electro-acupuncture (EA) on neuroplasticity associated with the expressions of neurotrophic factors (NTFs) and their receptors in rats subjected to spinal cord transection (SCT).
Material/Methods
A total of 144 rats were randomly divided into 3 groups (n=48 per group): sham-operated group, SCT group, and EA (electro-acupuncture) group. Rats in SCT and EA groups received spinal cord transection at T10–T11 vertebral levels. Then, EA group rats received EA treatment. Reverse transcription polymerase chain reaction was used to detect NTFs and receptors at the mRNA level. In situ hybridization (ISH) and immunohistochemistry (IHC) were used to detect the expression of NTFs and their receptors. Basso, Beattie, Bresnahan (BBB) scores and cortical somato-sensory evoked potentials (CSEP) were evaluated to assess the recovery of motor and sensory functions. We also measured BDA (Biotinylated dextran amine) axonal tracing, CGRP (Calcitonin gene-related peptide), GAP-43 (Growth-associated protein), and synaptophysin immunohistochemistry (IHC).
Results
EA treatment led to obvious improvement in hindlimb locomotor and sensory functions. CNTF, FGF-2, and TrkB mRNA were significantly upregulated, while NGF, PDGF, TGF-β1, IGF-1, TrkA, and TrkC mRNA were concomitantly downregulated in the caudal spinal segment (CSS) following EA. Immunohistochemistry demonstrated an increased number of CGRP fibers, GAP-43, and synaptophysin profiles in the CSS in the EA rats.
Conclusions
EA may promote the recovery of neuroplasticity in rats subjected to SCT. This could be attributed to the systematic regulation of NTFs and their receptors after EA.
MeSH Keywords: Electroacupuncture, Immunohistochemistry, Neurotrophic Factor, Spinal Cord Neuroplasticity
Background
Spinal cord injury (SCI) often results in functional deficits, inflammation, and tissue degradation [1]. It has many causes, such as traffic accidents and work-related juries. Researchers have made great efforts to develop several therapeutic strategies, including administration of growth factors [2], tissue bridges [3], various cell types [4,5], and artificial scaffolds [1,6]. However, at present, there remains a lack of effective treatments for SCI.
Acupuncture has been practiced in China for thousands of years. It has been reported to have therapeutic effects in patients suffering from SCI [7,8], apoplexy [9], and inflammatory diseases [10]. Electro-acupuncture (EA) is a widely-used method of acupuncture today. Increasing studies have used spinal cord-transected rat models to mimic SCI, and demonstrated that EA stimulation leads to neuroprotection and neuronal function recovery in spinal cord-transected rats [11–13]. However, the underlying mechanisms have not been fully elucidated.
A promising therapeutic strategy for SCI is to promote functional recovery of neurons. cAMP and Schwann cells are able to promote axonal growth and functional recovery after SCI [14], and neurotrophic factors (NTFs) and transplants are reported to increase regeneration and functional recovery of axons after SCI [15]. NTFs are important factors influencing neuronal plasticity following nerve injury [16]. A rich body of evidence demonstrates that EA therapy can change the expression of NTFs and strengthen spinal neuroplasticity following SCI [17,18]. Recently, Mo et al. reported that electro-acupuncture at Governor Vessel Acupoints (Dazhui and Mingmen) increased the expression of neurotrophin-3 in rats with SCI [11]. Liu et al. found that EA therapy downregulates platelet-derived growth factor (PDGF) [19]. However, there are few systematic analyses of NTFs by using EA after spinal cord transection (SCT).
Therefore, the present study aimed to investigate the effect of EA on multiple NTFs and their receptors in the caudal spinal segment (CSS) to the site of transection in spinal cord-transected rats. We also investigated the possible recovery of sensory and motor functions and axonal regeneration.
Material and Methods
Animals
Sprague-Dawley rats (180–220 g) were provided by the Laboratory Animal Center of Sichuan University. These experiments were approved by the Animal Care and Use Committee of Sichuan University (No. 20090920002). A total of 144 rats were randomly divided into 3 groups (n=48 per group): sham-operated controls group (group I), SCT group (group II), and EA group (group III). SCT group rats received spinal cord transection surgery between T10 and T11 vertebral levels. EA group rats received SCT surgery and then EA treatment.
Surgical procedures
Surgical SCT [11–13] was performed in SCT group and EA group rats. Briefly, after deep anaesthetizing with chloral hydrate, the rats were incised in the midline on the back and the muscles were retracted. T11 vertebral lamina was removed followed by incision of the dura mater. Subsequently, the spinal cord between T10 and T11 vertebral levels was transected. Finally, the wound was sutured. The sham-operated control group only received laminectomy without touching the spinal cord.
Electro-acupuncture
The rats of the SCT group were given 1, 3, 7, 14, and 28 days (n=8 for each point of time) of EA therapy at 2 pairs of acupoints, Zusanli-Xuanzhong and Futu-Sanyinjiao. These acupoints were identified according to previous studies [19,20]. These acupoints were stimulated in pairs (Zusanli and Xuanzhong, or Futu and Sanyingjiao), with each pair being stimulated on alternate days for 30 min (frequency=98 HZ). The stimulating electrodes were changed and their polarity reversed after 15 min.
Reverse transcription polymerase chain reaction (RT-PCR)
To determine the expression patterns of NTFs and their receptors in each group, 8 rats of the SCT group and the EA group were kept alive for 1, 3, 7, and 14 days post-operation (dpo), separately, and used for RT-PCR analysis. Rats were sacrificed after receiving an overdose of chloral hydrate, and then the spinal cords at T10 were harvested. Total RNA was extracted from each spinal cord sample (50 mg). cDNA was generated using the cDNA Synthesis kit (Fermentas, EU). Primers used for PCR amplification of nerve growth factor (NGF), PDGF, ciliary neurotrophic factor (CNTF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), tropomyosin receptor kinase (Trk)A, TrkB, TrkC, and tumor growth factor (TGF)-β1 cDNA samples are shown in Table 1. The mRNA level of each gene is expressed by the gray-scale intensity relative to β-actin level (internal control).
Table 1.
Primers for RT-PCR.
| Gene | primers | Production (bp) | Annealing temperature (°C) |
|---|---|---|---|
| β-actin | Sense: 5′GTAAAGACCTCTATGCCAACA 3′ Antisense: 5′GGACTCATCGTACTCCTGCT 3′ |
227 | 52.5 |
| NGF | Sense: 5′AAGCCCACTGGACTAAACT 3′ Antisense: 5′ ACCTCCTTGCCCTTGATG 3′ |
370 | 51 |
| BDNF | Sense: 5′ TCCCTGGCTGACACTTTT 3′ Antisense: 5′ATTGGGTAGTTCGGCATT 3′ |
466 | 50 |
| NT-3 | Sense: 5′CGTCCCTGGAAATAGTCATACGG 3′ Antisense: 5′GACAGATGCCAATTCATGTTCTT 3′ |
857 | 54 |
| PDGF | Sense: 5′CTGCTGCTACCTGCGTCTGG 3′ Antisense: 5′GCACTGCACATTGCGGTTATT 3′ |
391 | 55 |
| TGF-β1 | Sense: 5′GTGAGCACTGAAGCGAAAGC3′ Antisense: 5′TAATGGTGGACCGCAACAAC 3′ |
332 | 54 |
| CNTF | Sense: 5′CTTTCGCAGAGCAAACACCT 3′ Antisense: 5′CATCCCATCAGCCTCATTTT 3′ |
422 | 52 |
| IGF-1 | Sense: 5′GGCACTCTGCTTGCTCACCTT 3′ Antisense: 5′GCCTGTGGGCTTGTTGAAGTAAAA3′ |
130 | 57 |
| FGF-2 | Sense: 5′TCCCAAGCGGCTCTACT 3′ Antisense: 5′ACTCCAGGCGTTCAAAGA 3′ |
301 | 51 |
| TrkA | Sense: 5′GCTGGGAGCAGGAGGATTT 3′ Antisense: 5′GATGCTGTTCCACGGCTTT 3′ |
417 | 54 |
| TrkB | Sense: 5′GGTTCTACAACGGAGCCATAC 3′ Antisense: 5′GTCTTCATAGAGGACTTCAGGGT 3′ |
248 | 56 |
| TrkC | Sense: 5′AAGCCCACCCACTACAACAAT 3′ Antisense: 5′ AAAGAGGACCACCAGAAGGAC 3′ |
252 | 54 |
NGF – nerve growth factor; PDGF – platelet derived growth factor; CNTF – ciliary neurotrophic factor; TGF-β1 – tumor growth factor-β1; IGF – insulin-like growth factor; FGF – fibroblast growth factor; TrkA – tropomyosin receptor kinase A; TrkB – tropomyosin receptor kinase B; TrkC – tropomyosin receptor kinase C.
In situ hybridization
For detecting the expression and location of NTFs and receptors, 8 rats were randomly chosen from each group for in situ hybridization (ISH) and immunohistochemistry (IHC). The spinal cords of rats were harvested, and cut into sections at 20-μm thicknesses. The sections were fixed in 4% paraformaldehyde, and acetylated with 0.25% acetic anhydride. Prior to hybridizing in hybridization solution with the probes of NTFs for 12–16 h (37°C), the sections were prehybridized in a hybridization solution without probes. Subsequently, the sections were blocked at 37°C for 1 h, immersed in sheep anti-digoxygenin-alkaline phosphatase (AP) antibody (1: 1000) at 4°C overnight, and finally visualized with blue and purple sedimentation (Table 2).
Table 2.
Probes of NTFs and receptors for in situ hybridization.
| NTFs | Probes | Length (bp) |
|---|---|---|
| NGF | 5′ GCTGTGATCAGAGTGTAGAACAACATGGAC 3′ | 30 |
| BDNF | 5′CCCAATGAAGAAAACAATAAGGACGCA3′ | 27 |
| NT-3 | 5′ATTACCAGAGCACCCTGCCCAAAGC3′ | 25 |
| PDGF | 5′ CCACACCAGGAAGTTGGCATTG 3′ | 22 |
| TGF-β1 | 5′ TAGATTGCGTTGTTGCGGTCCACCATTAGC 3′ | 30 |
| CNTF | 5′ TCTCTTGGAGTCGCTCTGCCTCAGTCATCT 3′ | 30 |
| IGF-1 | 5′AACGATCAGAGTAGTGGTATTTCACC3′ | 26 |
| FGF-2 | 5′CGGGAAGGCGCCGCTGCCGCC 3′ | 30 |
| TrkA | 5′CCTCCCACACGGTAATAGAT3′ | 20 |
| TrkB | 5′CTTGGCTATTAGTGAGTCCCCATTGTTCA3′ | 29 |
| TrkC | 5′ CCTTGAGATGTCCGTGATGTTGATACTGGCGT3′ | 32 |
NGF – nerve growth factor; PDGF – platelet derived growth factor; CNTF – ciliary neurotrophic factor; TGF-β1 – tumor growth factor-β1; IGF – insulin-like growth factor; FGF – fibroblast growth factor; TrkA – tropomyosin receptor kinase A; TrkB – tropomyosin receptor kinase B; TrkC – tropomyosin receptor kinase C.
Immunohistochemistry
The tissue sections of spinal cords at 20 μm thicknesses were processed for immunohistochemistry as described previously [21]. Briefly, the sections were incubated with rabbit anti-rat polyclonal antibodies at 4°C overnight (Table 3). Negative controls were incubated in 2% goat serum instead of the primary antibody. Finally, the sections were detected by diaminobenzidine staining. Immuno-reactive staining was observed under a light microscope (Leica, Bensheim, Germany).
Table 3.
Polyclonal antibodies of NTFs for immunohistochemistry.
| Primary antibody | Dilution | Source | Company |
|---|---|---|---|
| NGF | 1: 1,00 | Rabbit | Chemicom |
| BDNF | 1: 500 | Rabbit | Santa |
| NT-3 | 1: 1,000 | Rabbit | Chemicom |
| PDGF | 1: 1,000 | Rabbit | Chemicom |
| TGF-β1 | 1: 20,000 | Rabbit | Chemicom |
| CNTF | 1: 2000 | Rabbit | Santa |
| IGF-1 | 1: 200 | Rabbit | Chemicom |
| FGF-2 | 1: 100 | Rabbit | Santa |
| TrkA | 1: 1000 | Rabbit | Santa |
| TrkB | 1: 500 | Rabbit | Santa |
| TrkC | 1: 800 | Rabbit | Chemicom |
| GAP-43 | 1: 10,000 | Rabbit | Santa |
| Synaptophysin | 1: 500 | Rabbit | Chemicom |
| 5-HT | 1: 1000 | Rabbit | RD |
| CGRP | 1: 500 | Rabbit | Chemicom |
NGF – nerve growth factor; PDGF – platelet derived growth factor; CNTF – ciliary neurotrophic factor; TGF-β1 – tumor growth factor-β1; IGF – insulin-like growth factor; FGF – fbroblast growth factor; TrkA – tropomyosin receptor kinase A; TrkB – tropomyosin receptor kinase B; TrkC – tropomyosin receptor kinase C; GAP-growth associated protein; 5-HT – 5-hydroxytryptamine; CGRP – calcitonin gene-related peptide.
Biotinylated dextran amine (BDA) tracing
For evaluating BDA axonal tracing, animals were anesthetized at 4 weeks post-operation, and biotinylated dextran amine was injected as previously described [13]. The rats were sacrificed after 3 weeks and prepared for histological analysis. The spinal cord was post-fixed in 4% paraformaldehyde for 3 days. Transverse sections (30 μm) were made on the injured site and on spinal segments rostral and caudal to the lesion. Some sections were used to detect whether the BDA-labeled regenerating axons were present. The remaining sections were used for immuno-histochemistry.
Hindlimb locomotor functions
Restoration of hindlimb locomotor function of the rats (n=8 for each group) was evaluated using the Basso, Beattie, Bresnahan (BBB) rat rating scale [22]. Briefly, motor performance of each rat was evaluated at 8 to 9: 00 am in an open-field arena at 1, 2, 3, and 4 weeks after injury. All the behavior evaluations were scored by 3 investigators who were blind to the operative procedure.
Measurement of cortical somatosensory evoked potentials (CSEP)
In order to evaluate the restoration of sensory functions, CSEP at 14, 21, 28 days post-operation was recorded by using a KEYPOINT Electromyography instrument (Medtronic, Minnesota, USA). The CSEP was measured as previously described [13]. The right common peroneal nerve was located based on surface landmarks and a stimulating electrode placed over the nerve. A recording electrode was placed over the dura overlying the somatosensory cortex through a small craniotomy (2 mm left of the midline and 2 mm anterior to the posterior fontanelle). The reference electrode was inserted on the nose, and the ground electrode was inserted on the tail. The stimulus intensity was set high enough to produce a marked muscle twitch in the hind limb (amplitude ~1.1 mA, duration of stimulation ~0.2 ms, and frequency ~3 Hz). The CSEP Tracings represented the average of 200 responses.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software. All data are expressed as mean ±SEM (standard error of the mean). Differences of the data among the 3 groups were analyzed using one-way analysis of variance (ANOVA) followed by least significant difference (LSD)-q test. P<0.05 was regarded as statistical significance.
Results
Gene expression changes in the CSS
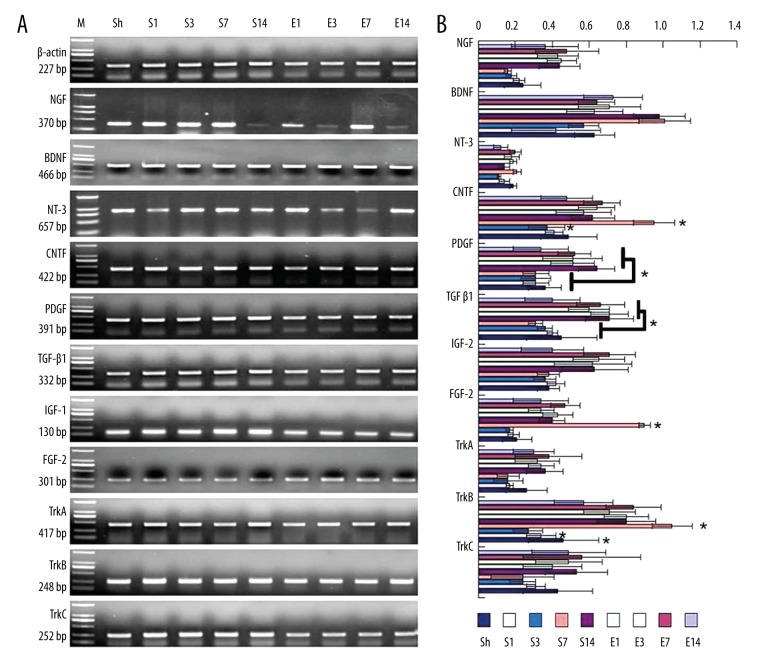
The mRNA expression of NTFs and their receptors in the sham-operated group, the SCT group, and the EA group were detected by using RT-PCR. As shown in Figure 1, mRNA expression of TrkA, TrkB, TrkC, NGF, PDGF, CNTF, IGF, FGF, and TGF-β1 were detected in the spinal cord of sham-operated rats. Compared to the sham-operated group, the SCT group showed an obvious increase in the mRNA level of PDGF and TGF-β1 at 1, 3, 7, and 14 dpo, FGF-2 at 1dpo, CNTF at 1 and 3 dpo, and TrkB at 1, 7, and 14 dpo in the CSS (P<0.05). However, no significant difference was observed in the expression of TrkA, TrkC, NGF, or IGF-I after SCT. Furthermore, in the EA group, NGF, PDGF, TGF-β1, IGF-1, TrkA and TrkC were significantly decreased (P<0.05), while CNTF, FGF-2 and TrkB were significantly increased in the CSS to the site of transection in comparison with those in the SCT group (P<0.05).
Figure 1.
Gene expression of NTFs and receptors in the CSS. (A) mRNA level of NTFs and receptors. (B) Quantitative analysis. M – marker; Sh – sham-operated rats; S – SCT rats. E – EA rats. S1, S3, S7 and S14 – 1 dpo, 3 dpo, 7 dpo, and 14 dpo of SCT rats. * P<0.05 among different groups.
In situ hybridization
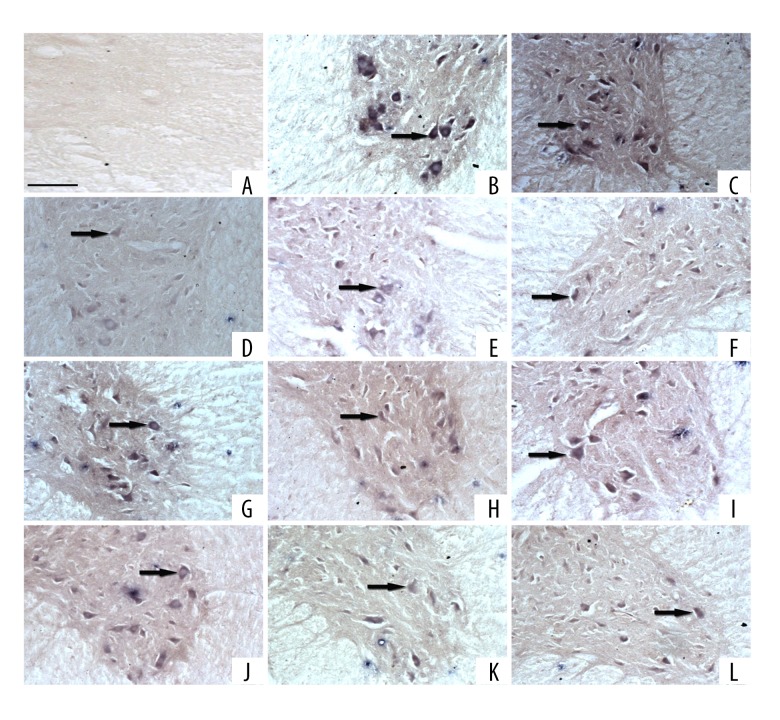
In situ hybridization revealed that these NTFs and receptors mRNA were detected in the cytoplasm of most neurons and in some glia cells in the spinal cord (Figure 2A–2L).
Figure 2.
Location of mRNA for NTFs and receptors in in situ hybridization. (A) Begative control (200×, scale bar=200 μm). (B) NGF. (C) BDNF. (D) NT-3. (E) PDGF. (F) TGF-β1. (G) CNTF. (H) IGF-1. (I) FGF-2. (J) TrkA. (K) TrkB. (L) TrkC.
Immunohistochemical findings
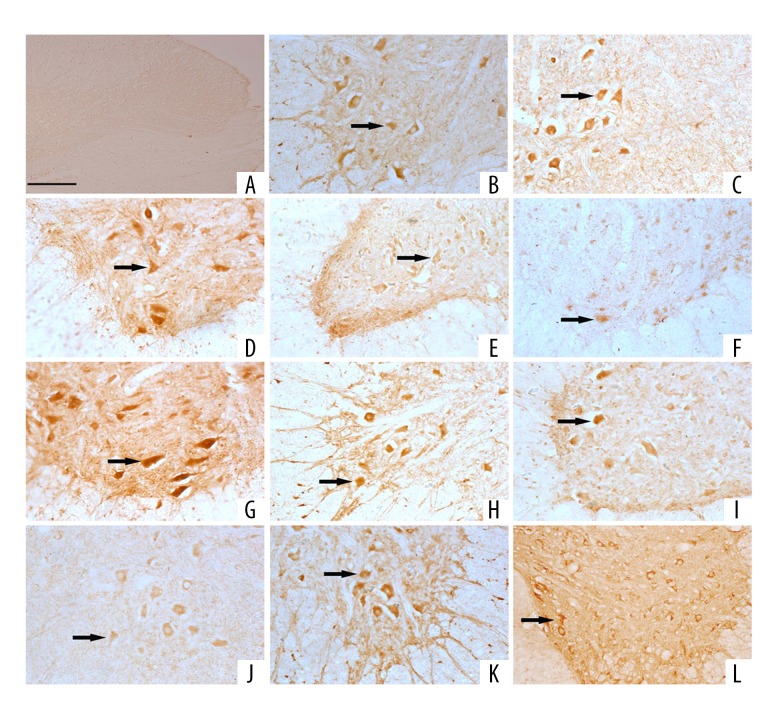
Positive staining for NGF, PDGF, TGF-β1, CNTF, TrkA, TrkB, TrkC, IGF-1, and FGF-2 were observed in spinal neurons, including motor neurons of ventral horn and sensory neurons located in the dorsal horn. Positive staining for PDGF, CNTF, and TGF-β1were also found in some astrocytes (Figure 3A–3L).
Figure 3.
Positive immunostaining for NTFs and receptors in spinal motor neurons. (A) Negative control (200×, scale bar=200 μm). (B) NGF. (C) BDNF. (D) NT-3. (E) PDGF. (F) TGF-β1. (G) CNTF. (H) IGF-1. (I) FGF-2. (J) TrkA. (K) TrkB. (L) TrkC.
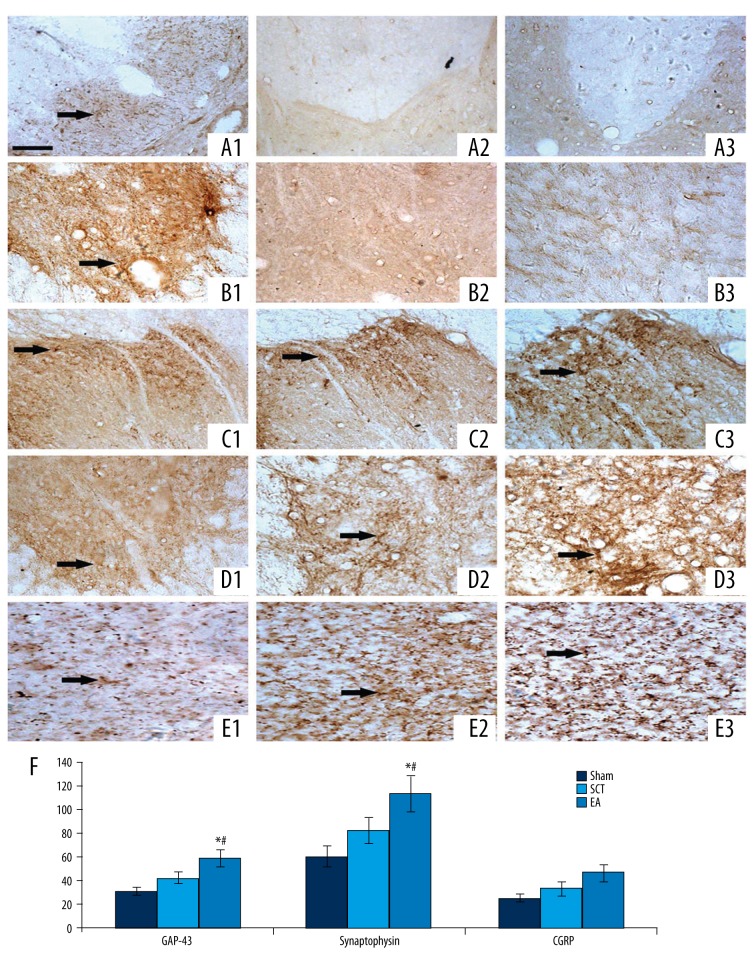
Fibers of spinal lamina I, II, IV, V and motor neurons of ventral horns in the CSS were positively stained for CGRP in the sham control group and the SCT group (Figure 4C1, C2), respectively. The CGRP immunopositive staining was significantly strengthened in the CSS in the EA group (Figure 4C3).
Figure 4.
Axonal regeneration and growth. (A1–A3) Staining of BDA-labeled axons in sham-operated rats, SCT rats, and EA rats, respectively. (B1–B3) Staining of 5-HT. (C1–C3) CGRP immuno-reactive staining. (D1–D3) GAP-43 immuno-reactive staining. (E1–E3) Synaptophysin immuno-reactive staining. (F) Quantitative analyses of GAP-43, synaptophysin and CGRP.* P<0.05 compared to sham group rats; # P<0.05 compared to SCT group rats. A, Scale bar=100 μm.
Axonal tract tracing
As shown in Figure 4A1, BDA-labeled axons were detected in the CSS of the sham-operated rats. Nevertheless, BDA-labeled axons were not observed in the same region in SCT group or EA group rats (Figure 4A2, A3).
5-HT immunopositive staining was found only in the sham-operated rats (Figure 4B1–B3). GAP-43 (Figure 4C1–C3) and synaptophysin (Figure 4D1–D3) immunopositive staining were markedly enhanced in the EA group relative to the sham-operated group and the SCT group (P>0.05).
BBB evaluation in hindlimbs
Figure 5 shows that the baseline BBB score of the sham rats was 21. The BBB score then gradually increased from 1 week to 4 weeks after injury. Moreover, the BBB score of the EA group was significantly elevated compared to that of the SCT group at 3 and 4 weeks after the operation (P<0.05). These data suggest that EA effectively promotes locomotor functional recovery in hindlimbs.
Figure 5.
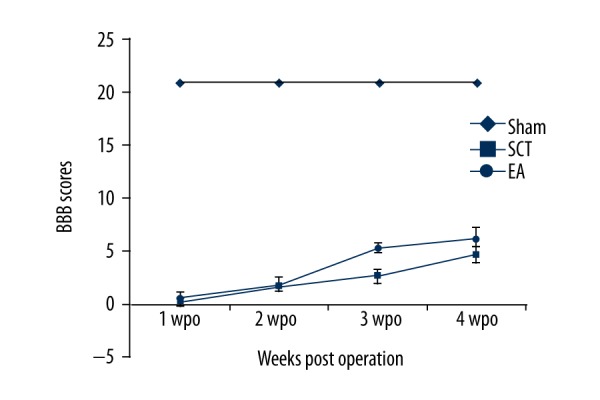
BBB score. The BBB score of the EA rats was significantly increased compared to that of the SCT rats at 3 and 4 weeks after the operation.
CSEP
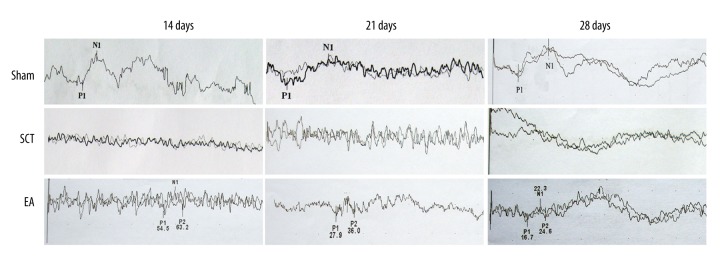
The mean latencies of P1 and N1 and the amplitude of P1-N1 of the acquired curves at different time points for all 3 groups are shown in Table 4. However, waves of P1 and N1 could not be detected in the SCT rats. The waves of P1 and N1 at 14, 21, and 28 dpo in EA rats (bottom line) were weaker compared to the sham-operated rats (Figure 6).
Table 4.
The records of cortical somatosensory evoked potential test (M±SEM).
| Time point | P1 latency (ms) | N1 latency (ms) | P1–N1 amplitude (μv) |
|---|---|---|---|
| Sham operation | 11.10±3.26 | 22.34±4.03 | 8.37±4.40 |
| SCT 14dpo | — | — | --- |
| 21dpo | — | — | --- |
| 28dpo | — | — | --- |
| EA 14dpo | 54.40±14.10# | 59.30a±9.89# | 5.37±0.58 |
| 21dpo | 25.5±10.06* | 28.00±7.06* | 5.37±0.58 |
| 28dpo | 22.17±5.37* | 26.33±4.41* | 6.06±0.32 |
Numbers refer to mean (M) ± standard error of mean (SEM).’—’ – latencies of P1 and N1 infinitely lengthened; ‘---’ – no P1 and N1 amplitude;
P<0.05 compare to sham operation group;
P<0.05 compared to EA 14dpo.
EA – acupuncture. dpo – days post operation.
Figure 6.
Detection of sensory function. The normal latencies of P1 and N1 were recorded in sham group rats, but were not observed in SCT group rats.
Discussion
The present study aimed to investigate the possible effects of EA stimulation on systematic regulation of multiple gene expressions in SCT rats. Changes in the expression of NTFs and tyrosine kinase receptors were observed in the CSS. Parallel to the gene changes, we found partial axonal regeneration and recovery of functions.
In the present study, CNTF, FGF-2, and TrkB were upregulated in the CSS, while others, like NGF, PDGF, TGF-β1, IGF-1 TrkA, and TrkC, were downregulated after EA treatment. These findings suggest that NTFs and their receptors play different roles in the CSS of SCT rats with EA treatment. EA may induce neural plasticity through systematic upregulation of NTFs or their receptors. BDNF, combined with its functional receptor TrkB, plays an important role in inducing neurite outgrowth of both sensory [23] and motor neurons [24]. In addition, FGF-2 has been shown to promote the outgrowth of neurites from ventral spinal cord neurons [25]. CNTF is a potent factor that induces growth of axons from sensory and motor neurons of the spinal cord [26]. These NTFs and receptors are important to the recovery of the injured spinal cord [23,27]. Our data provide direct evidence to map the regulated genes by EA stimulation in injured spinal cord, and provide a basis for finding new strategies using NTF synergistic administration for treatment of SCI in future clinical trials.
Significant decreases of NGF, PDGF, TGF-β1, IGF-1, TrkA, and TrkC were detected in CSS after EA treatment. We found that downregulated NTFs may function together with upregulated NTF like FGF-2, depending on the systematic regulatory mechanism. PDGF, also served as a cerebrovascular permeant, plays a regulatory role in the release of MCP-1 (monocyte chemoattractant protein-1) in astrocytes. The increase in MCP-1 contributes to the neuroinflammation and blood-brain barrier disruption [28]. It has been reported that PDGF induces the activation of astrocytes and the release of MCP-1, which further results in blood-brain barrier disruption [29]. In addition, TGF-β1 has been shown to suppress anti-inflammation and proinflammatory mediators. A previous study has suggested that increased PDGF and TGF-β1 result in the proliferation of astrocytes, further leading to scar formation [30]. Therefore, it is hypothesized that the downregulation of PDGF and TGF-β1 after EA may benefit the recovery of injured spinal cords. The effect of EA on neuroplasticity might result from the inhibition of neuroinflammation, which is associated with the systematic regulation of NTFs, according to our findings.
In this study, we also found that EA treatment increased the levels of GAP-43, synaptophysin, and immunopositive reactivity of CGRP in CSS. CGRP is a well-known marker for sensory axons transmitting pain sensation [31]. The expression of CGRP is increased in motoneurons of the spinal cord in rats after peripheral axotomy [32]. CGRP, which is closely associated with nociceptive neurons, is co-localized with GAP-43 in growing neurites [33]. The expressions of CGRP and GAP-43 play key roles in regenerative neurite growth after spinal lesions. Increases in the number of CGRP-positive axons, GAP-43, and synaptophysin immunopositive reactivity (representing the regrowth of cone and synaptic formation) in the CSS suggests that nerve regeneration, cone regrowth, and synaptic formation are promoted in the CSS by EA treatment, which might be used to reconstruct local circuitry for further functional recovery.
Recovery of motor function was also found in the EA rats. The BBB scores in the EA rats were higher than in the SCT rats at 14, 21, and 28 dpo. These observations suggest that EA improves hindlimb locomotor function after SCT injury. In addition, significantly reduced N1 and P1 latencies were found in the EA rats at 2, 3, and 4 weeks post-operation compared to those in SCT rats, indicating that partial recovery of sensory functions was found after EA. The amplitude of P1-N1 was detected again by CSEP recordings after EA treatment, even though they were still lower than normal levels. Of these, N1 and P1 recovered to near normal levels, whereas P1 was double that of the normal values at 28 dpo. Hence, the results indicate that significant improvement in sensory function occurred in the EA rats. As we did not find regenerating corticospinal axons in CSS, it is possible that there are some subcortical contributions to the functional recovery, by rearrangement of local spinal circuits and systematic modulation of NTFs in the EA rats.
Conclusions
EA stimulation promotes neuroplasticity and functional recovery of injured spinal cords by differentially regulating the expression of NTFs and receptors in the CSS. NTFs play essential roles in neuroplasticity and functional recovery of hindlimbs in spinal cord-transected rats induced by EA treatment. EA may be a potential therapeutic strategy for spinal cord injury. Clinical trials evaluating EA are warranted.
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest to state.
Source of support: This work was supported by the National Natural Science Foundation of China, grant no. 81501173
References
- 1.Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32(1):41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Awad BI, Carmody MA, Steinmetz MP. Potential role of growth factors in the management of spinal cord injury. World Neurosurg. 2015;83(1):120–31. doi: 10.1016/j.wneu.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi S, Iseda T, Nishio T. Effects of an embryonic repair graft on recovery from spinal cord injury. Prog Brain Res. 2004;143(143):155–62. doi: 10.1016/S0079-6123(03)43015-X. [DOI] [PubMed] [Google Scholar]
- 4.Kabu S, Yue G, Kwon BK, Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J Control Release. 2015;219:141–54. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumagai G, Tsoulfas P, Toh S, et al. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Exp Neurol. 2013;248(5):369–80. doi: 10.1016/j.expneurol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Gao M, Lu P, Bednark B, et al. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34(5):1529–36. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang SH, Tu WZ, Zou EM, et al. Neuroprotective effects of different modalities of acupuncture on traumatic spinal cord injury in rats. Evid Based Complement Alternat Med. 2014;2014:431580. doi: 10.1155/2014/431580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DC, Lee JY, Moon YJ, et al. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol Dis. 2010;39(3):272–82. doi: 10.1016/j.nbd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Moffet HH. Acupuncture for upper-extremity rehabilitation in chronic stroke. Arch Phys Med Rehabil. 2006;87(4):593–94. doi: 10.1016/j.apmr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Woźniak PR, Stachowiak GP, Oszukowski PJ. Anti-phlogistic and immunocompetent effects of acupuncture treatment in women suffering from chronic pelvic inflammatory diseases. Am J Chin Med. 2009;31(6):315–20. doi: 10.1142/S0192415X03000990. [DOI] [PubMed] [Google Scholar]
- 11.Mo YP, Yao HJ, Lv W, et al. Effects of electroacupuncture at governor vessel acupoints on neurotrophin-3 in rats with experimental spinal cord injury. Neural Plast. 2016;2016:2371875. doi: 10.1155/2016/2371875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y, Yan Q, Ruan JW, et al. Electro-acupuncture promotes survival, differentiation of the bone marrow mesenchymal stem cells as well as functional recovery in the spinal cord-transected rats. BMC Neuroscience. 2009;10(1):35. doi: 10.1186/1471-2202-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu SJ, Zheng SS, Dan QQ, et al. Effects of Governor Vessel electroacupuncture on the systematic expressions of NTFs in spinal cord transected rats. Neuropeptides. 2014;48(4):239–47. doi: 10.1016/j.npep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–16. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 15.Bregman BS, Coumans JV, Dai HN, et al. Chapter 18 Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog Brain Res. 2002;137(02):257–73. doi: 10.1016/s0079-6123(02)37020-1. [DOI] [PubMed] [Google Scholar]
- 16.Cowansage KK, Ledoux JE, Monfils MH. Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 17.Sun WW, Zhao W, Wang TH. Effects of electro-acupuncture on PDGF expression in spared dorsal root ganglion and associated dorsal horn subjected to partial dorsal root ganglionectomy in cats. Neurosci Lett. 2008;431(2):112–17. doi: 10.1007/s11064-007-9451-5. [DOI] [PubMed] [Google Scholar]
- 18.Xu DU. Effect of electroacupuncture on brain derivd neurotrophic factor and nerve function of spinal cord injury rats. Journal of Clinical Acupuncture & Moxibustion. 2009 [in Chinese] [Google Scholar]
- 19.Liu F, Zou Y, Liu S. Electro-acupuncture treatment improves neurological function associated with downregulation of PDGF and inhibition of astrogliosis in rats with spinal cord transection. J Mol Neurosci. 2013;51(2):629–35. doi: 10.1007/s12031-013-0035-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Qi JG, Zhang W, et al. Electro-acupuncture induced NGF, BDNF and NT-3 expression in spared L6 dorsal root ganglion in cats subjected to removal of adjacent ganglia. Neurosci Res. 2007;59(4):399–405. doi: 10.1016/j.neures.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Li X-F, Russell J, et al. Changes in tumor hypoxia induced by mild temperature hyperthermia as assessed by dual-tracer immunohistochemistry. Radiother Oncol. 2008;88(2):269–76. doi: 10.1016/j.radonc.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 23.Sharma HS. Neuroprotective effects of neurotrophins and melanocortins in spinal cord injury. Ann NY Acad Sci. 2005;1053(1053):407–21. doi: 10.1111/j.1749-6632.2005.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 24.Kishino A, Ishige Y, Tatsuno T, et al. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144(2):273–86. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y, Shiojima T, Ikeda K, et al. Acidic and basic fibroblast growth factors enhance neurite outgrowth in cultured rat spinal cord neurons. Neurol Res. 1995;17(1):70–72. doi: 10.1080/01616412.1995.11740289. [DOI] [PubMed] [Google Scholar]
- 26.Oyesiku NM, Wigston DJ. Ciliary neurotrophic factor stimulates neurite outgrowth from spinal cord neurons. J Comp Neurol. 1996;364(1):68–77. doi: 10.1002/(SICI)1096-9861(19960101)364:1<68::AID-CNE6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Teng YD, Mocchetti I, Taveira-Dasilva AM, et al. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19(16):7037–47. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentes ME, Durham SK, Swerdel MR, et al. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155(12):5769–76. [PubMed] [Google Scholar]
- 29.Bethel-Brown C, Yao H, Hu G, Buch S. Platelet-derived growth factor (PDGF)-BB-mediated induction of monocyte chemoattractant protein 1 in human astrocytes: implications for HIV-associated neuroinflammation. J Neuroinflammation. 2012;9(1):262. doi: 10.1186/1742-2094-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silberstein FC, Simone RD, Levi G, Aloisi F. Cytokine-regulated expression of platelet-derived growth factor gene and protein in cultured human astrocytes. J Neurochem. 1996;66(4):1409–17. doi: 10.1046/j.1471-4159.1996.66041409.x. [DOI] [PubMed] [Google Scholar]
- 31.Jang JH, Nam TS, Paik KS, Leem JW. Involvement of peripherally released substance P and calcitonin gene-related peptide in mediating mechanical hyperalgesia in a traumatic neuropathy model of the rat. Neurosci Lett. 2004;360(3):129–32. doi: 10.1016/j.neulet.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi K, Senba E, Morita Y, et al. Alpha-CGRP and beta-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Neuroscience Research Supplements. 1990;7(4):299–304. doi: 10.1016/0169-328x(90)90080-w. [DOI] [PubMed] [Google Scholar]
- 33.Ondarza AB, Ye ZC. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184(1):373–80. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]