Abstract
Approximately 25% of melanoma patients with locoregional metastases are nonresponsive to new molecular target therapy and immunotherapy. When metastases are located in the pelvis, melphalan hypoxic perfusion can be an optional treatment. Because methylation of MGMT promoter increases the efficacy of alkylating agents, studies on melanoma outcome of patients treated with melphalan regional chemotherapy should consider this epigenetic change. This study aims to evaluate whether the survival of stage III melanoma patients treated with melphalan regional chemotherapy may be correlated with MGMT methylation status. The metastatic tissues of 27 stage III melanoma patients with locoregional metastases located in the pelvis subjected to melphalan hypoxic pelvic perfusion were examined. The methylation status of the MGMT promoter was investigated by MS-MLPA probes analysis and the presence of the BRAF V600E mutation was analyzed by CAST-PCR. The median survival times were estimated using the Kaplan–Meier curves and were stratified according to the clinicopathological characteristics of patients and lesions. The overall median survival time was 17 months. The 1-year, 3-year, and 5-year survival rates were 66.7, 18.5, and 7.4%, respectively. Disease stage, burden, and percentage of MGMT methylation significantly affected survival. We estimated an MGMT promoter methylation cut-off of at least 14%, which was significantly associated with a longer survival after melphalan regional chemotherapy. Our data suggest that MGMT promoter methylation could be an important factor in determining which melanoma patients should receive melphalan regional chemotherapy, but its prognostic significance in the routine clinical setting needs to be clarified in a larger study.
Keywords: melanoma, melphalan, methylation, MGMT, pelvis, perfusion
Introduction
Approximately 25% of melanoma patients with locoregional metastases are nonresponsive to new molecular target therapy and immunotherapy 1. For these patients, regional chemotherapy with alkylating agents could be an option on the basis of the results of several published reports for the lower extremity 2,3 and pelvis 4–9. Being highly reactive molecules, the alkylating agents induce cell death by binding to DNA 10. There are two types of alkylating drugs: monofunctional, including temozolomide and dacarbazine, which produce a cross-link between adjacent strands of DNA, and bifunctional, such as melphalan, which produces interstrand and intrastrand cross-links; melphalan also alkylates RNA and protein structures 11. DNA lesions produced by melphalan require several and complex repair mechanisms 12. Many DNA adducts can be repaired by DNA repair enzymes such as the O6-methylguanine-DNA methyltransferase (MGMT) that repairs O6-alkylguanine adducts and reverses the cytotoxicity induced by the two types of alkylating agents 13,14. Overexpression of MGMT prevents the death of cancer cells induced by alkylating agents; in contrast, a lack of MGMT could increase the efficacy of alkylating chemotherapy. Considering that the MGMT gene is not commonly mutated or deleted, the inactivation of this gene may be caused by epigenetic changes such as hypermethylation at the promoter site or at exon 1 with loss of MGMT expression 10.
The hypothesis of this study is that the survival of melanoma patients treated with melphalan regional chemotherapy may be correlated with the MGMT methylation status. The carcinogenesis process is very complex in relation to the variety of melphalan action mechanisms and DNA repair systems; thus, an accurate homogeneity of both patient sample and regional chemotherapy treatment procedure is absolutely necessary to evaluate the hypothesis.
We investigated whether epigenetic silencing of MGMT could influence the survival of stage III melanoma patients affected by metastases in the pelvic and/or inguinal regions subjected to melphalan monotherapy hypoxic pelvic perfusion.
Patients and methods
Patients
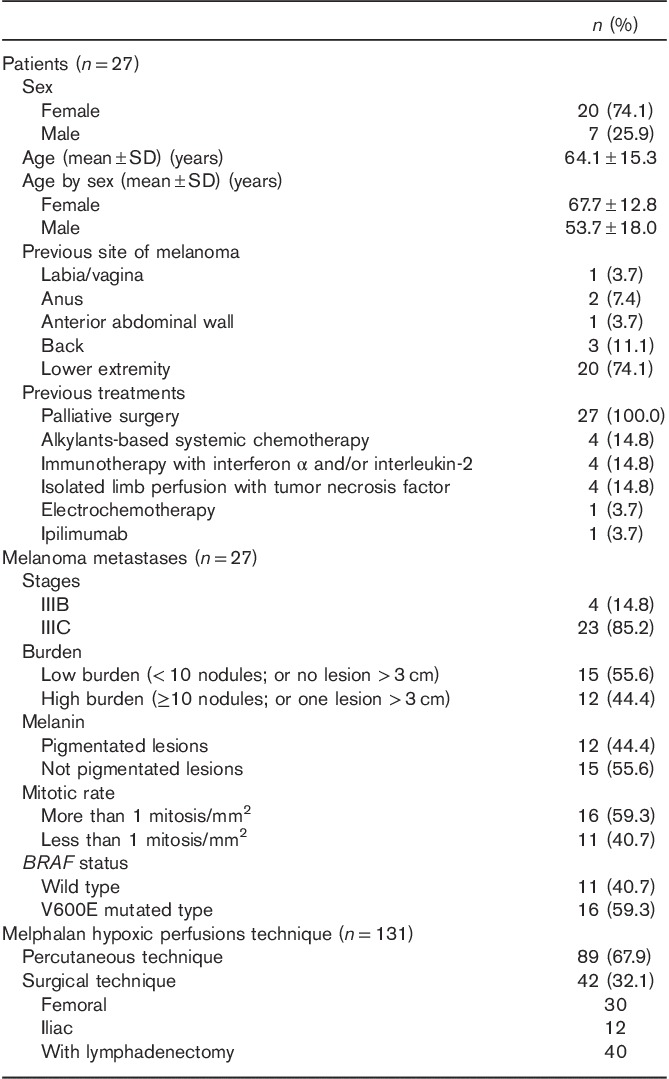
This is a retrospective review of a subset of a prospective database reflecting a larger trial of melanoma patients undergoing melphalan hypoxic perfusion (ClinicalTrials.gov Identifier NCT01920516). The subset included 27 patients (Table 1) with pelvic and/or inguinal melanoma locoregional metastases at stages IIIB and IIIC 15. These patients, requiring melphalan hypoxic pelvic perfusion, were referred only to our institution to achieve homogeneity of performance. From June 2003 to July 2012, 27 patients received 131 treatments at the University of L’Aquila, L’Aquila, Italy. Before the beginning of the treatments, all patients were in progression after previous therapies. All patients had Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were classified as ‘High Burden’ when they had at least 10 lesions or one lesion more than 3 cm in size or as ‘Low Burden’ when they had less than 10 lesions and no lesion more than 3 cm in size 16.
Table 1.
Characteristics of 27 patients and 131 procedures
Patients with associated lesions lower than the middle part of the thigh requiring a larger perfusion compartment and patients with macroscopic extra-pelvic disease were excluded from the study. Moreover, patients receiving any kind of chemotherapy, immune-therapy, or target therapy after the last melphalan treatment were considered ineligible.
The study was approved by the Local Ethics Committee and was carried out according to the Declaration of Helsinki Principles. Written informed consent was obtained from all participants.
Study design
Eligibility criteria for hypoxic pelvic perfusion have been published previously 8. The clinical protocol provided repetition of both perfusion and palliative cytoreductive surgery at a time interval of ~6–7 weeks. Criteria for excision were as follows: (a) the debulking of deep masses and superficial lesions and (b) the study of histological/molecular parameters in metastatic tissue. Excision was always attempted and avoided only when bleeding or a difficult wound closure was anticipated. Melphalan hypoxic pelvic perfusions were performed using surgical or percutaneous approaches. The percutaneous perfusion technique was not performed when the diameter of the common femoral artery was 7 mm or less in relation to the risk of vessel dissection. The rationale and timing of repetition were based on two previous pilot studies 8,17, showing that the relapse was always observed in the presence of residual tumor and that the progression started within 8 weeks when aggressive disease was present. In case of a complete response, the purpose was to bolster the clinical results obtained until 1 year of progression-free survival was achieved; the treatments were not repeated if the patient did not consent or the general condition worsened. In patients achieving a partial response or stable disease, the purpose was to repeat the combined treatment in each case with residual tumor. In general, after a combined treatment, some metastases disappear or are excised, but new metastases can grow. Treatments were not repeated if the patient did not consent, locoregional metastases progressed more than 20% in dimensions and numbers, simultaneous distant relapses occurred, or the general condition of the patient worsened.
Hypoxic pelvic perfusion techniques
The perfusion was performed under general anesthesia as described previously 8. Hypoxic perfusion with hemofiltration included three phases: isolation, perfusion, and hemofiltration.
First phase
The first phase is the isolation phase in which the blood flow to the aorta and the inferior cava vein is blocked by using endovascular balloon catheters and at the level of the thighs by using pneumatic cuffs. The isolation phase can be performed by a surgical 8 or a percutaneous approach 9,18,19 (Fig. 1). When perfusion had to be repeated at the same site, the endovascular balloon catheters were positioned using a percutaneous approach. This procedure was not performed when the diameter of the common femoral artery was 7 mm or less, which made vessel dissection risky. When an associated lymphadenectomy was necessary, the catheters were positioned by exposing the femoral artery and vein through a short longitudinal incision in the groin. When this procedure was performed more than two or three times in the same patient and the femoral vessels were surrounded by fibrous tissue, the iliac vessels were exposed. In the surgical technique, after systemic heparinization (150 U/kg heparin), a 3-lumen, 12-French balloon catheter (pfm medical ag, Cologne, Germany) was introduced into the inferior vena cava through the saphenous vein (or iliac vein) and into the aorta through the femoral artery (or iliac artery); the catheters were positioned below the renal vessels and above the aortic and venous bifurcation using a guide wire under fluoroscopic guidance. One of the three lumens of the catheter was used for blood circulation and the other lumens were used for inflating the balloons and positioning the guide wire. The percutaneous procedure requires puncturing the femoral vessels, which was performed using two 11-French introducers, each with a hemostatic valve and a dilatator (Radifocus; Terumo, Tokyo, Japan). The arterial introducer was 25 cm long and the venous introducer was 10 cm long. We also used two double-lumen 8-French balloon catheters (pfm medical ag). In each catheter, one lumen was used to inflate the balloons and the other lumen was used to position the guide wire. Blood circulation and drug perfusion took place in a long, hollow cylindrical space between the introducer wall and the catheter, with blood flowing through a ring surface at the top of the space. To suspend the blood flow, the balloons were inflated with the radiopaque dye diatrizoate diluted in an isotonic sodium chloride solution. To complete the isolation of the pelvis, two large-cuff orthopedic tourniquets were placed around each of the thighs and inflated just before starting the perfusion.
Fig. 1.
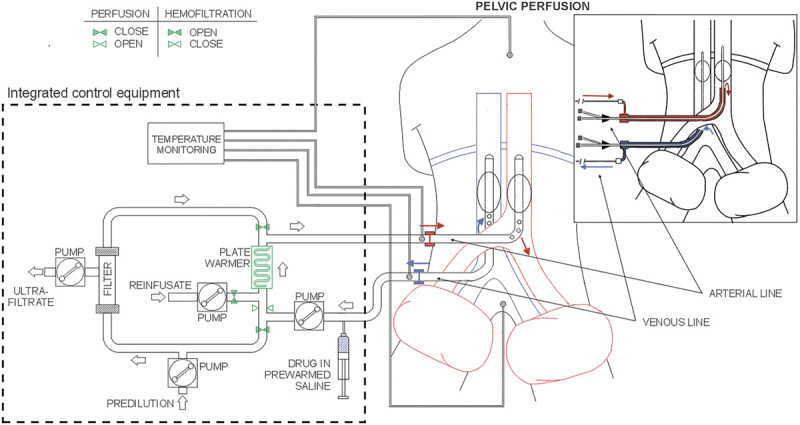
Schema of surgical and percutaneous hypoxic pelvic perfusion with hemofiltration.
Second phase
The second phase is the perfusion phase in which perfusion occurs through extracorporeal blood circulation. The infusion channels of the arterial and venous surgical catheters or the hemostatic valve channels of the 11-French introducers were connected to a hypoxic perfusion tubing set mounted on a regional cancer therapy-dedicated circulation device (Performer LRT; RanD, Medolla, Italy). The circuit was primed with an isotonic sodium chloride solution containing heparin (10 000 U/l). Once the requisite blood flow (~100 ml/min) in the extracorporeal circuit (aspiration from the inferior cava vein and infusion into the aorta) was established, drug perfusion was started. According to previous pilot studies 8,17, 30 mg/m2 of melphalan, diluted in 250 ml of an isotonic sodium chloride solution containing 16 mg of dexamethasone sodium phosphate, was administered through the circuit over a 3-min period. The extracorporeal circuit (Fig. 1) connected to the circulation device contains a heating element and a hemofiltration module, both of which are controlled by the device during the perfusion phase and the subsequent hemofiltration phase. Perfusion was administered for 20 min. The temperature loss was ~1°C/m of the tubing set. The length of the tubing set was 5 m; hence, the temperature required to ensure normothermia was 42°C at the outlet level of the heating element. The circulation device has sensors that monitor and regulate the blood flow, withdrawal pressure, infusion pressure, circuit and patient temperatures, hemofiltration parameters, and save the data.
Third phase
The third phase is the hemofiltration phase. After perfusion, the catheter balloons and pneumatic cuffs were deflated and normal circulation was restored. Hemofiltration was then administered for 60 min through the circuit. The blood flow was increased to ~200 ml/min and, to guarantee this value, the aorta was the withdrawal site. The temperature at the outlet level of the heating element was decreased to 39°C. A polyamide hemofilter with a surface area of 2.1 m2 was used for filtration. After filtration, the catheters were withdrawn and the vessels were repaired. Approximately 30 min of compression hemostasis was administered after the percutaneous technique. Protamine was injected at 200 IU/kg to reverse the anticoagulant effects of heparin.
Tumor response criteria and adverse events
Response was determined 30 days after each perfusion. Tumor response was due to perfusion that involved all lesions; additionally, the surgical excision concurred to response. For deep masses and lymph nodes, computed tomography, nuclear magnetic resonance, and PET were used to evaluate responses that were classified using the Response Evaluation Criteria in Solid Tumors, version 1.1 20; responses of patients treated before 2009 were retrospectively reclassified. Responses of superficial lesions were assessed on the basis of inspection and photocomparing. Adverse events were evaluated using CTCAE v4.03 (National Cancer Institute, Bethesda, Massachusetts, USA).
Pathology and molecular diagnostics
Metastatic lesions were surgically excised at the first melphalan treatment. For each patient, one specimen was subjected to histopathological review and molecular analysis. The growth pattern, the mitotic rate (<1 or ≥1 mitosis/m2), the presence of melanin-producing cells (pigmented or not pigmented cells), and tumor-infiltrating lymphocyte were assessed in the histopathological report.
For all excised lesions, DNA was isolated from five formalin-fixed paraffin-embedded tissue sections, each 10 µm in thickness, using the DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentrations and the quality of DNA were determined using the Qubit fluorometer (Thermo-Fisher, Foster City, California, USA).
The methylation status of the MGMT promoter was tested using the MS-MLPA probe mix prepared by MRC-Holland (Amsterdam, the Netherlands) (Salsa MS-MLPA Kit ME011 MMR), which includes six probes specific for the MGMT promoter region containing an Hha1 recognition site. The procedure was performed according to the manufacturer’s protocol. Hha1 (R6441; Promega, Madison, Wisconsin, USA), a methylation-sensitive restriction enzyme that cuts unmethylated GCGC sites, was applied to each set of samples. The resultant PCR fragments were separated by capillary gel electrophoresis on a 3500 Genetic-Analyzer (Thermo-Fisher). The CpGenome universal methylated DNA and unmethylated DNA (Chemicon; Millipore, Billerica, Massachussetts, USA) were included as controls in each set of MS-MLPA. The methylation status was quantified using GeneMarker software (version 1.5; Soft Genetics, State College, Pennsylvania, USA). To compensate for the differences in the efficiency of the PCR for the individual samples, the peak value of each probe was normalized by dividing it by the peak of the control probes. To evaluate the methylation status, the methylation ratio was calculated by the average obtained by dividing each normalized peak value of the digested sample by that of the corresponding undigested sample. This value corresponds to the percentage of methylated sequences.
Mutational BRAF V600E status was investigated using competitive allele-specific hydrolysis probes (TaqMan), PCR technology (CAST) 21 (Thermo-Fischer).
Statistical analysis
The analysis provided descriptive statistics estimated with a 95% confidence interval. Median survival times (MST) was estimated using the Kaplan–Meier product limit estimator. We recorded deaths because of pathologically confirmed locoregional metastases.
Overall survival times were stratified according to the clinical variables that potentially affect survival. Log-rank tests were used to assess the significance of the differences between the groups. Hazard ratios were estimated using a proportional hazard Cox regression model. The statistical analysis was carried out using STATA software, version 14 (StataCorp, College Station, Texas, USA). Data were presented as mean±SD.
Results
Patients’ and tumors’ characteristics
A total of 27 melanoma patients (20 women and seven men) with pelvic and/or inguinal locoregional metastases were enrolled in this study. The demographic and clinical characteristics of patients and related tumors are listed in Table 1. According to the histopathological review of tumors, all surgical specimens had an epithelioid cell pattern, whereas no one showed tumor-infiltrating lymphocytes.
Treatment characteristics and clinical outcomes
Overall, 131 melphalan perfusion procedures including 42 surgical and 89 percutaneous approaches were performed. The median number of treatments received by each patient was 4 (mean: 4.8±3.2). No deaths occurred during the 131 procedures performed and in the postoperative period. One patient, after a first partial response, refused further treatments.
Figure 2 shows the responses to each treatment for each patient. The overall response rate after the first treatment was 100.0%, a complete response being observed in two of 27 (7.4%) patients and a partial response in 25 of 27 (92.6%) patients. No patients experienced disease progression within 30 days after the first treatment. Figure 3 shows the percentages of responses in the following treatments.
Fig. 2.
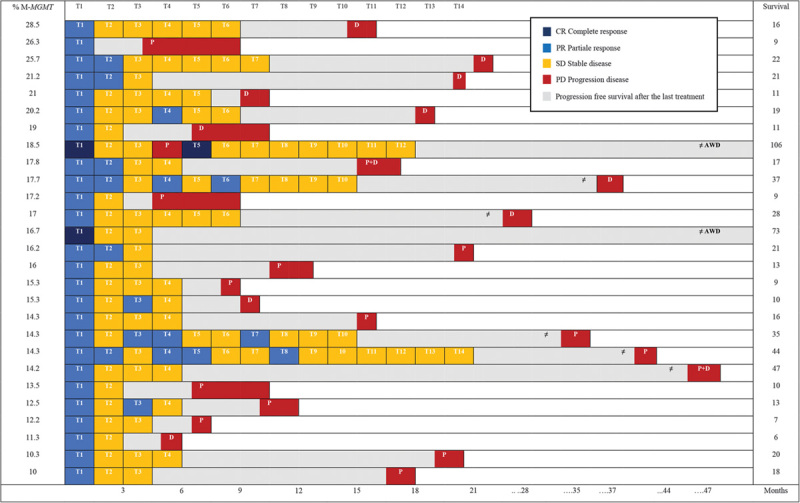
Responses and survival after 131 procedures in 27 patients. Each T column shows a separate treatment; each row corresponds to the outcomes of each patient following the treatments. AWD, alive without disease; D, distant site of progression; % M-MGMT, percentage of MGMT promoter methylation; P, pelvic site of progression; T, treatment.
Fig. 3.
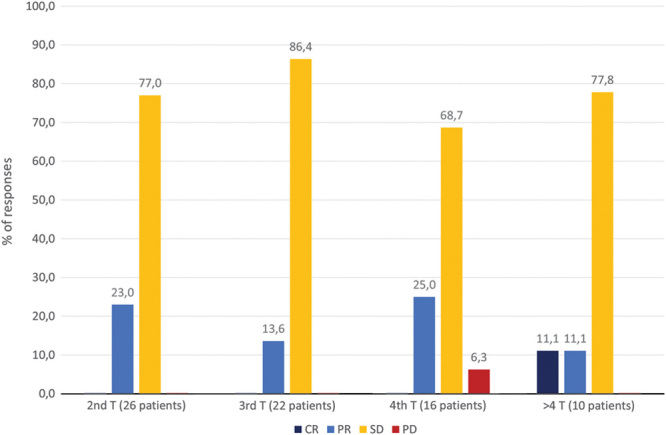
Percentages of responses in the treatments (T) following the first. CR, complete response; PD, progression disease; PR, partial response; SD, stable disease.
Adverse events
No technical (i.e. balloon rupture), hemodynamic, or vascular complications occurred during the procedures. Three cases of grade 2 seroma [3/42 (7.1%)], three cases of grade 2 persistent leakage of lymphatic fluid from the incision [3/42 (7.1%)], and one case grade 1 wound infection [1/27 (2.4%)] were registered in the group of 42 surgical procedures (associated with lymphadenectomy in 40 cases). Five cases of grade 1 inguinal hematoma [5/89 (5.6%)] were observed in the group of 89 percutaneous procedures. Eighteen cases of grade 1 bone marrow hypocellularity [18/131 (13.7%)], 12 cases of grade 2 [12/131 (9.2%)], and five cases of grade 3 [5/131 (3.8%)] were registered in all 131 procedures. Treatments were interrupted for hematological toxicity in two of 27 (7.4%) patients, in one patient after four procedures and in the second one after 14 procedures. Nausea and vomiting (grade 1) were reported after 16 procedures. Two cases of grade 1 alopecia were observed. Local toxicity, mainly because of scrotum edema (grade 1), and pelvic pain (grade 1), was reported in two patients subjected to lymphadenectomy.
Survival
The overall MST was 17 months (range: 10–28 months); the mean survival time was 24±22.3 months. The 1-year, 3-year, and 5-year survival rates were 66.7, 18.5, and 7.4%, respectively. Table 2 shows that disease stage, number and size of nodules, and percentage of MGMT methylation significantly affected survival, whereas sex, age, mitosis, melanin pigmentation, and BRAF V600E status did not.
Table 2.
Survival according to age, sex, stage, BRAF V600E status, burden, mitosis, cellular melanin pigmentation, number of treatments, and MGMT methylation
The overall MST of six (22.2%) patients who interrupted treatments because of worsening of general conditions was 27.5 months (mean: 30.2±13.6 months). This was significantly longer (P=0.01) than that of 14 (51.8%) patients who interrupted treatments because of progression (median: 12.0; mean: 15.0±8.7 months). No significant difference was found between patients who interrupted treatments because of worsening of general condition and the five patients (18.5%) who interrupted treatments for retired consent (median: 18.0; mean: 15.6 +/− 6.1 months).
Follow-up
No patients were lost at follow-up and all deaths were tumor related in our patients. The median follow-up duration was 17 months (range: 10–28 months), with a mean of 24.0±22.3; 25 of 27 (92.6%) patients died because of melanoma and two of 27 (7.4%) patients are alive without evidence of disease after 73 and 106 months. Among the 25 patients who died, progression in the pelvis was registered in 16 stage IIIC patients (two of them also had a distant relapse) and in one stage IIIB patient, whereas seven stage IIIC and one stage IIIB patients experienced distant tumor progression (Fig. 2). The patient who was alive without disease after 106 months showed a pelvic progression after the fourth treatment, followed by a complete response after a new treatment.
MGMT promoter methylation and BRAF status
MGMT promoter methylation was present in all tumor samples [27/27 (100.0%)], with a median percentage methylation of 17.0% (Fig. 2). To confirm that MGMT promoter methylation was not induced by previous alkylating agents or chemotherapy, a statistical correlation by the Mann–Whitney test was performed (P=0.41). Figure 4a shows the MGMT promoter methylation percentage estimated for each sample according to the survival times. A statistical association between methylation of the MGMT promoter and increase in survival times of melanoma patients treated with melphalan regional chemotherapy (Log-Rank: 5.46, P=0.01, hazard ratio=0.32) was detected. A cut-off value of 14% was found that can significantly differentiate different survival groups of patients (Fig. 4b).
Fig. 4.
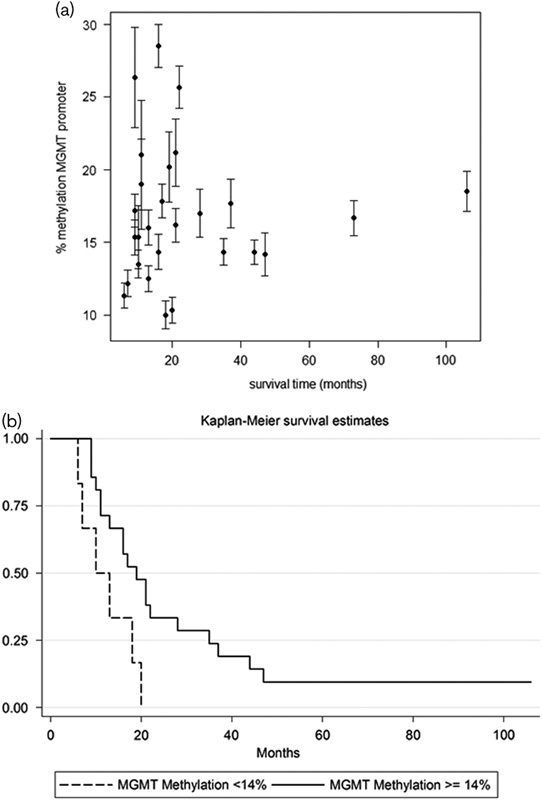
(a) MGMT promoter methylation percentage estimated for each sample in relation to the survival times. Each estimation (±SE) originated from the six probes’ peak values. (b) Cut-off value of 14% significantly differentiating different survival groups of patients.
The mutational analysis of the BRAF gene identified the V600E mutation in 16 of 27 (59.3%) metastases. No association was detected between BRAF V600E status and MGMT promoter methylation (P=0.37).
Discussion
This study showed that melphalan hypoxic perfusion is a safe and effective therapy for stage III melanoma patients with pelvic locoregional metastases who fail current systemic therapies. Survival was significantly influenced by stage, burden, and MGMT methylation. In particular, it has been found that high levels of MGMT promoter methylation in melanoma metastases were associated with longer overall survival. Moreover, in our cases, we identified a significant MGMT methylation cut-off of at least 14% useful to differentiate the patients who will experience prolonged survival.
Methylation of DNA is the main type of epigenetic modifications in humans able to modulate or silencing gene expression 22. In particular, methylation of normally unmethylated sites, known as cytidine phosphate guanosine islands at the promoter region of genes, is correlated with reduced levels of gene transcription. Tumors with a methylated MGMT promoter and exon 1 have transcriptional silencing of MGMT 10. Conflicting reports on MGMT protein expression and in-vitro melphalan sensitivity have been published 23,24,25.
In melanoma patients, no correlation between MGMT methylation and outcomes after temozolamide systemic chemotherapy has been found in a large study by Hassel et al. 26. Similarly, the study by Beasley et al. 27 showed that the response to temozolamide-isolated limb infusion for metastatic patients was not related to MGMT promoter methylation status. In contrast, Tuominen et al. 28 observed a significant association of MGMT promoter methylation with the response rate and progression-free survival after temozolomide systemic chemotherapy.
To date, our work is the first study showing a positive association between the MGMT promoter methylation and a prolonged survival following melphalan locoregional treatment in stage III melanoma patients. This finding was probably a result of the exclusion of stage IV patients and the high homogeneity of perfusion procedures. Interestingly, in our samples, we estimated an MGMT promoter methylation cut-off of at least 14%, which was significantly associated with a longer survival after melphalan regional chemotherapy. This cut-off value could be useful to dichotomize metastatic melanoma patients into two prognostic groups. Similar results were reported for glioblastoma 29. However, we observed that in seven patients with MGMT promoter methylation higher than 18.5%, the median survival was 16 months, which is lower than the overall MST of 17 months. This could be related to the following: (i) all patients were high burden; (ii) these patients could have other additional very effective repair mechanisms in response to melphalan-induced DNA damage, such as nucleotide excision repair and homologous recombination 12. Further studies on higher number of samples should be carried out to validate this result.
The main limitation of our study was the small sample size, which could not have provided an adequate statistical power in some subgroup analyses; however, this small sample size was obtained on the basis of rigorous selection criteria that provided high homogeneity both of patients and of melphalan locoregional procedures. Moreover, the assessment of MGMT promoter methylation was performed using only the MS-MLPA method. Despite the advantages of this method, such as the absence of a bisulfite conversion reaction 29, other molecular methods could be useful to confirm MGMT methylation findings. Finally, no statistical correlation was found between MGMT status and response. In relation to the regional impact of the treatment, one expects a more relevant influence of MGMT status on response than on survival. Given the small sample size, the high number of treatments per patient, and the heterogeneity of burden, the statistical power was rather low.
The determination of MGMT methylation status may be an important factor in determining which melanoma patients should receive melphalan regional chemotherapy, but its prognostic significance in the routine clinical setting should be established in a larger multicenter prospective controlled study.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Merlino G, Herlyn M, Fisher DE, Bastian BC, Flaherty KT, Davies MA, et al. The state of melanoma: challenges and opportunities. Pigment Cell Melanoma Res 2016; 29:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroon BBR, Kroon HM, Noorda EM, Vrouenraets BC, Klaase JM, van Slooten GW, et al. Aigner KR, Stephens FO. Isolated limb perfusion for melanoma. Induction chemotherapy, 2th ed Berlin: Springer; 2016. 355–373. [Google Scholar]
- 3.Huismans AM, Kroon HM, Kam PCA, Thompson JF. Aigner KR, Stephens FO. Isolated limb infusion. Induction chemotherapy, 2th ed Berlin: Springer; 2016. 375–390. [Google Scholar]
- 4.Stehlin JS, Clark RL, White EC, Smith JL, Jr, Griffin AC, Jesse RH, Jr, et al. Regional chemotherapy for cancer: experiences with 116 perfusions. Ann Surg 1960; 151:605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan RF, Schramel RJ, Creech O., Jr Value of perfusion in pelvic surgery. Dis Colon Rectum 1963; 6:297–300. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JF, Waugh RC, Saw RPM, Kam PCA. Isolated limb infusion with melphalan for recurrent melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat 1994; 7:188–192. [Google Scholar]
- 7.Wanebo HJ, Chung MA, Levy AI, Turk PS, Vezeridis MP, Belliveau JF. Preoperative therapy for advanced pelvic malignancy by isolated pelvic perfusion with the balloon-occlusion technique. Ann Surg Oncol 1996; 3:295–303. [DOI] [PubMed] [Google Scholar]
- 8.Guadagni S, Russo F, Rossi CR, Pilati PL, Miotto D, Fiorentini G, et al. Deliberate hypoxic pelvic and limb chemoperfusion in the treatment of recurrent melanoma. Am J Surg 2002; 183:28–36. [DOI] [PubMed] [Google Scholar]
- 9.Bonvalot S, de Baere T, Mendiboure J, Paci A, Farace F, Drouard-Troalen L, et al. Hyperthermic pelvic perfusion with tumor necrosis factor-α for locally advanced cancers: encouraging results of a phase II study. Ann Surg 2012; 255:281–286. [DOI] [PubMed] [Google Scholar]
- 10.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. New Engl J Med 2000; 343:1350–1354. [DOI] [PubMed] [Google Scholar]
- 11.Arai H, Yamauchi T, Uzui K, Ueda T. Leukemia cells are sensitized to temozolomide, carmustine and melphalan by the inhibition of O6-methylguanine DNA methyltransferase. Oncol Lett 2015; 10:845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo CY, Chou WC, Wu CC, Wong TS, Kakadiya R, Lee TC, et al. Repairing of N-mustard derivative BO-1055 induced DNA damage requires NER, HR, and MGMT-dependent DNA repair mechanisms. Oncotarget 2015; 6:25770–25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan CH, Liu WL, Cao H, Wen C, Chen L, Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis 2013; 4:e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahrer J, Kaina B. O6-methylguanine-DNA methyltransferase in the defense against N-nitroso compounds and colorectal cancer. Carcinogenesis 2013; 34:2435–2442. [DOI] [PubMed] [Google Scholar]
- 15.Balch CM, Gershenwald JE, Song SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009, 27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman J, Ariyan C, Rafferty B, Brady MS. Factors associated with response, survival, and limb salvage in patients undergoing isolated limb infusion. J Surg Oncol 2014; 109:405–409. [DOI] [PubMed] [Google Scholar]
- 17.Guadagni S, Santinami M, Patuzzo R, Pilati PL, Miotto D, Deraco M, et al. Hypoxic pelvic and limb perfusion with melphalan and mitomycin C for recurrent limb melanoma: a pilot study. Melanoma Res 2003; 13:51–58. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JF, Liu M, Waugh RC, Watson LD, Sheldon DM, Stephen MS, et al. A percutaneous aortic ‘stop-flow’ infusion technique for regional cytotoxic therapy of the abdomen and pelvis. Reg Cancer Treat 1994; 7:202–207. [Google Scholar]
- 19.Ricci S, Rossi G, Roversi R, Cavallo G, Romanelli M, Roversi M, et al. Antiblastic locoregional perfusion with control of the aorto-caval flow: technique of percutaneous access. Radiol Med 1997; 93:246–252. [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response valuation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 21.Didelot A, Le Corre D, Luscan A, Cazes A, Pallier K, Emile JF, et al. Competitive allele specific TaqMan PCR for KRAS, BRAF and EGFR mutation detection in clinical formalin fixed paraffin embedded samples. Exp Mol Pathol 2012; 92:275–280. [DOI] [PubMed] [Google Scholar]
- 22.Episkopou H, Kyrtopoulos SA, Sfikakis PP, Dimopoulos MA, Souliotis VL. The repair of melphalan-induced DNA adducts in the transcribed strand of active genes is subject to a strong polarity effect. Mutat Res 2011; 714:78–87. [DOI] [PubMed] [Google Scholar]
- 23.Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinogenic activity of different antineoplastic drugs. Int J Cancer 1996; 65:506–512. [DOI] [PubMed] [Google Scholar]
- 24.Passagne I, Evrard A, Depeille P, Cuq P, Cupissol D, Vian L. O(6)-methylguanine DNA-methyltransferase (MGMT) overexpression in melanoma cells induces resistance to nitrosoureas and temozolomide but sensitizes to mitomycin C. Toxicol Appl Pharmacol 2006; 211:97–105. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto Y, Augustine CK, Yoo JS, Zipfel PA, Selim MA, Pruitt SK, et al. Defining regional infusion treatment strategies for extremity melanoma: comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol Cancer Ther 2007; 6:1492–1500. [DOI] [PubMed] [Google Scholar]
- 26.Hassel JC, Sucker A, Edler L, Kurzen H, Moll I, Stresemann C, et al. MGMT gene promoter methylation correlates with tolerance of temozolomide treatment in melanoma but not with clinical outcome. Br J Cancer 2010; 103:820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beasley GM, Speicher P, Augustine CK, Dolber PC, Peterson BL, Sharma K, et al. A multicenter phase I dose escalation trial to evaluate safety and tolerability of intra-arterial temozolomide for patients with advanced extremity melanoma using normothermic isolated limb infusion. Ann Surg Oncol 2015; 22:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuominen R, Jewell R, van den Oord JJ, Wolter P, Stierner V, Lindholm C, et al. MGMT promoter methylation is associated with temozolomide response and prolonged progression-free survival in disseminated cutaneous melanoma. Int J Cancer 2015; 136:2844–2853. [DOI] [PubMed] [Google Scholar]
- 29.Park CK, Kim JW, Yim SY, Lee AR, Han JH, Kim CY, et al. Usefulness of MS-MLPA for detection of MGMT promoter methylation in the evaluation of pseudoprogression in glioblastoma patient. Neuro Oncol 2011; 13:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]


