Abstract
PURPOSE:
The purpose of this study was to examine the effectiveness and value of prophylactic 5-layer foam sacral dressings to prevent hospital-acquired pressure injury rates in acute care settings.
DESIGN:
Retrospective observational cohort.
SAMPLE AND SETTING:
We reviewed records of adult patients 18 years or older who were hospitalized at least 5 days across 38 acute care hospitals of the University Health System Consortium (UHC) and had a pressure injury as identified by Patient Safety Indicator #3 (PSI-03). All facilities are located in the United States.
METHODS:
We collected longitudinal data pertaining to prophylactic 5-layer foam sacral dressings purchased by hospital-quarter for 38 academic medical centers between 2010 and 2015. Longitudinal data on acute care, hospital-level patient outcomes (eg, admissions and PSI-03 and pressure injury rate) were queried through the UHC clinical database/resource manager from the Johns Hopkins Medicine portal. Data on volumes of dressings purchased per UHC hospital were merged with UHC data. Mixed-effects negative binomial regression was used to test the longitudinal association of prophylactic foam sacral dressings on pressure injury rates, adjusted for hospital case-mix and Medicare payments rules.
RESULTS:
Significant pressure injury rate reductions in US acute care hospitals between 2010 and 2015 were associated with the adoption of prophylactic 5-layer foam sacral dressings within a prevention protocol (−1.0 cases/quarter; P = .002) and changes to Medicare payment rules in 2014 (−1.13 cases/quarter; P = .035).
CONCLUSIONS:
Prophylactic 5-layer foam sacral dressings are an effective component of a pressure injury prevention protocol. Hospitals adopting these technologies should expect good value for use of these products.
Keywords: Longitudinal data analysis, Pressure injury, Pressure injury prevention, pressure ulcer, Prophylactic dressing
INTRODUCTION
Hospital-acquired pressure injuries (HAPIs) are common, costly, and deadly to acute and critically ill patients.1 They occur in 2.5 million patients per year, costing anywhere from $500 to $150,000 per case and totaling $11 billion annually in the United States.2,3 Moreover, full-thickness pressure injuries cause an astounding 60,000 deaths per year in the United States.4 Hospitals face a financial burden as a result of uncompensated care for full-thickness pressure injuries due to reimbursement policies set by the Centers for Medicare & Medicaid Services (CMS).5 In 2008, the CMS reduced payments related to hospital-acquired full-thickness pressure injuries.6 In addition, in October of 2014, the CMS began penalizing hospitals 1% of their total reimbursements if they fell into the lowest 25th percentile with respect to composite rates of pressure injuries and other hospital-acquired conditions.7
These CMS policies led to implementation of prevention protocols for pressure injuries in many hospitals. Pressure injury prevention standards were introduced by the Agency for Healthcare Research and Quality (AHRQ) in 1992 and have been routinely updated by expert organizations such as the National Pressure Ulcer Advisory Panel (NPUAP) and Wound, Ostomy, Continence Nurses Society, beginning with a skin assessment and risk assessment.8–10 Pressure injury prevention recommendations include frequent turning and repositioning; managing moisture and incontinence; selection of an appropriate support surface; managing nutrition; pressure redistribution, reducing friction, and shear; and pressure injury prevention education.8,10 Careful compliance with all parts of the protocol has shown to reduce pressure injuries, and is actually cost-saving at $55/patient per day relative to the cost of treating full-thickness wounds (ie, >$300/patient per day on average).3,11 However, the method for effectively mitigating loading forces has never been well defined, leaving opportunity for improvement.12
Recently, foam dressings have been used to cover the incisions of postsurgical patients or to cover complicated injuries to the skin (eg, punctures or burns) to prevent pressure injuries. These dressings are used to mitigate the loading forces applied to the tissues between the support surface and bony prominence, or between the skin and underlying connective tissues and a medical device. There is limited evidence supporting the efficacy of polyurethane foam dressings to date according to the NPUAP, which noted that the strength of evidence ranges from “B” to “C.”8 Brindle13 first reported a quality improvement study demonstrating the potential benefit of foam sacral dressings for pressure injury prevention in surgical intensive care patients, followed by a study of their use in patients undergoing cardiac surgery. Santamaria and colleagues14–17 explored the efficacy of prophylactic foam dressings applied to the sacrum and heels in prevention of pressure injuries, demonstrating both clinical effectiveness and cost-benefit of prophylactic foam dressings. A randomized trial by Kalowes and colleagues18 validated these reports of clinical efficacy, along with a systematic review of nonexperimental prospective studies, demonstrating the benefit of prophylactic foam dressings for sacral and heel pressure injury prevention.19
Although these studies have explored the efficacy of prophylactic foam dressings in clinical trial settings, no studies to date have looked at the effectiveness in a wide range of patient settings using an observational approach. The purpose of this study was to examine the effectiveness and value of the prophylactic 5-layer sacral dressing to prevent HAPI rates in the acute care setting.
METHODS
We conducted a retrospective observational cohort study of US acute care academic medical centers to examine the effectiveness and value of prophylactic 5-layer sacral dressings to prevent HAPI rates in the acute care setting. Among all types of dressings, 5-layer sacral dressings were examined based on the availability of data in combination with the fact that pressure injuries on the sacrum represent the highest proportion of coded cases.20 Rates on all pressure injuries between 2010 and 2015 were obtained from a representative sample of academic medical centers in the University Health System Consortium (UHC) institutions (https://www.uhc.edu, Chicago, Illinois) based on the selection criteria previously used by Padula and colleagues.11,21 The UHC clinical database/resource manager provides quarterly, hospital-level administrative data on patient hospitalizations throughout its system of more than 250 academic medical centers. These all-cause pressure injury rates from UHC were merged with quarterly, hospital-level data provided by a manufacturer of foam sacral dressings (Mölnlycke Health Care; https://www.monlycke.com, Norcross, Georgia) on the amount of prophylactic 5-layer foam sacral dressings purchased in terms of total volume and cost of each quarterly purchase under the stock-keeping unit.
Study Population
We applied longitudinal data analysis to this cohort so that each hospital could act as its own control, since hospitals varied in their start time with the use of prophylactic 5-layer foam sacral dressings over the 6-year period of observation. Data were managed longitudinally by hospital-quarter. Counts of pressure injuries obtained from UHC met the inclusion and exclusion criteria of AHRQ Patient Safety Indicator #3 (PSI-03, v. 5.0) for acute and critically ill patients.22 PSI-03 defines HAPIs as stage 3, 4, or unstageable pressure injuries (ICD-9 707.23-707.25) not present on admission after 5 days of length of stay in patients 18 years and older.
Following approval from the Johns Hopkins Institutional Review Board, hospital-level data were gathered from UHC, which provided aggregate hospital data on patient outcomes by quarter, including case-mix index (hospital-level case-mix per quarter), as well as hospitalized patient discharges and HAPI cases (counts of each). Patient-level data were not available from these data sources (UHC or Mölnlycke Health Care).
Statistical Analysis
The hospital-level cohort was divided into periods when prophylactic 5-layer foam sacral dressings were or were not purchased for use at the hospital. The average rates of PSI-03 pre- and postdressing purchase were compared using a student t test at the 95% confidence level.
We used 2-level mixed-effects negative binomial regression models to perform longitudinal data analysis of PSI-03 counts (PSI-03, pressure injury rate) over each quarter from 2010 to 2015 associated with adoption of prophylactic 5-layer foam sacral dressings.11 Negative binomial regression is specifically designed to analyze data where the main outcome measure is a count (eg, PSI-03 [HAPI stages 3, 4, and unstageable]) offset by a certain number of exposures (ie, inpatient hospitalizations, which are the denominator of the PSI-03 count), and this approach accounts for unbalanced skew between the mean and variance.23 We organized these data as a series of quarterly, hospital-level counts of PSI-03 (HAPI stages 3, 4, and unstageable) and inpatient hospitalizations and other hospital-level patient outcomes. Since UHC did not provide patient-level data, individual means were not included in the model. Changes in counts of PSI-03 (HAPI stages 3, 4, and unstageable) were studied over time, nested within hospitals, using total counts of quarterly admissions to weight each hospital's contribution to the overall regression model in order to control for variability in the number of patient hospitalizations between hospitals according to inclusion criteria in PSI-03. According to an initial calculation of power, 38 hospitals would be used in the analysis to detect a clinically meaningful and statistically significant reduction in pressure injuries by at least 1.0 case per 1000 hospitalizations, which Padula and colleagues11,24 previously noted as the demarcation for clinically meaningful reduction in HAPI prevention.
The mixed-effects regression models were developed and tested in multiple iterations using Stata (StataCorp, College Station, Texas, 2014). The first model iteration began by studying the quarterly associations between PSI-03 (ie, the dependent variable) and multiple predictors: prophylactic 5-layer foam sacral dressing use and cost; case-mix index (CMI); time; and changes in CMS reimbursement policy in 2014. Time-interaction with prophylactic dressing purchases was also tested in a separate regression model by multiplying time by the number of dressings purchased. We then applied a random-intercept to the regression model to allow hospitals to vary naturally by their baseline rates of PSI-03 prior to dressing adoption since hospitals began using prophylactic dressings at different points in the process toward improving pressure injury prevention.25
In order to perform a budget impact analysis and return-on-investment calculation of the value of prophylactic 5-layer foam sacral dressings to reduce long-term additional spending on pressure injury care for this cohort of 38 hospitals, we used the retail cost per prophylactic foam sacral dressing, the estimated cost per HAPI ($70,000 per PSI-03), and the estimated cost of a HAPI prevention protocol ($55/patient/day).3,26
RESULTS
UHC data on hospital-level patient outcomes from a representative sample of 38 of more than 240 academic medical centers were merged with data on purchase of prophylactic 5-layer foam sacral dressings. These observations amounted to 912 hospital-quarters, including 631 hospital-quarters when prophylactic foam 5-layer sacral dressings were available for pressure injury prevention. There were 1.03 million patients hospitalized at these 38 hospitals during the period of observation and 618 PSI-03 cases (Table 1).
TABLE 1. Aggregate Patient Characteristics by Hospital Among a Sample of 38 UHC Academic Medical Centers and Volumes of Prophylactic Sacral Dressings Purchased, 2010-2015; and Annual Rates of Admissions and Pressure Injuries.
| Aggregate Patient Characteristics by Hospital | |||||||
|---|---|---|---|---|---|---|---|
| N | Mean | Standard Error | Minimum | Maximum | |||
| UHC hospitals | 38 | ||||||
| Hospital-quarters | 912 | ||||||
| Case-mix index | 2.44 | 0.53 | 1.21 | 3.94 | |||
| Inpatients per hospital-quarter | 1,754 | 1,106 | 180 | 7,606 | |||
| PSI-03a per hospital-quarter | 1.27 | 1.64 | 0 | 10.00 | |||
| Prophylactic dressings Purchased | |||||||
| Hospital-quarters | 631 | ||||||
| Units of dressings acquiredb | 2,586 | 3,312 | 0 | 45,970 | |||
| Cost of dressings acquired, $b | 19,506.22 | 24,477.67 | 0.00 | 283,658.00 | |||
| Annual Rates of Admissions and Pressure Injuries | |||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
| Hospital-quartersc | 40 | 44 | 57 | 147 | 148 | 152 | 588 |
| Total admissions | 66,945 | 82,512 | 103,624 | 251,980 | 255,428 | 271,075 | 1,031,564 |
| PSI-03a per 1000 | 1.72 | 1.09 | 1.15 | 0.61 | 0.53 | 0.62 | |
| Dressings per 1000 | 355 | 1,085 | 1,701 | 1,043 | 1,401 | 2,662 | |
| Case-mix index | 2.50 | 2.39 | 2.43 | 2.43 | 2.45 | 2.45 | |
Abbreviations: UHC, University Health System Consortium; PSI-03, Patient Safety Indicator #3.
aAHRQ Patient Safety Indicator #3 for stage 3, 4, or unstageable pressure injury not present on admission.
bPer hospital-quarter.
cQuarters where a hospital is observed in UHC and purchases prophylactic dressings.
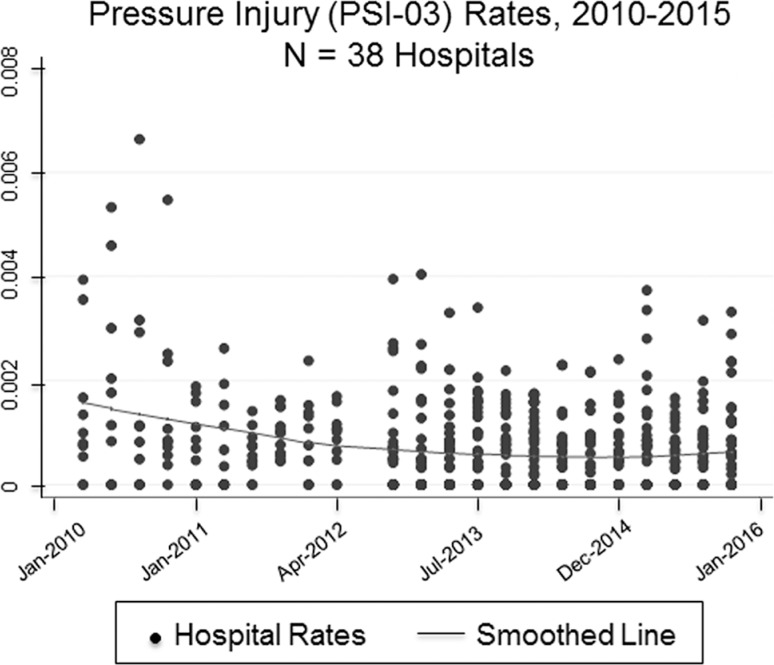
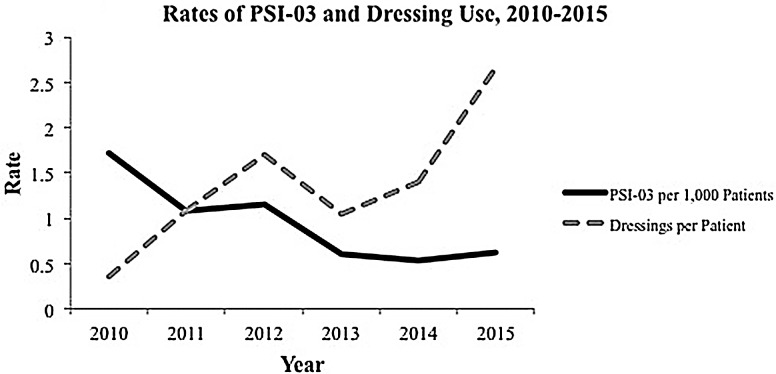
The average medical center experienced 1754 hospitalizations per quarter and 1.27 PSI-03 cases between quarters (Figure 1). Hospitals typically purchased 2586 units of prophylactic sacral dressing per quarter (ie, 90 days), which represented about 1.5 units per patient over an average length of stay of 7 days. These data align with the indication that a prophylactic dressing can be used continuously for 3 to 4 days. These units cost in the range of $7 to $8 per patient. The average hospital paid $19,506 per quarter to provide prophylactic foam sacral dressings to its high-risk patients. However, additional patients could have benefited from the prevention protocols beyond the PSI-03 patients included in the analysis. These hospitals generally observed a decrease in pressure injury rates associated with the increased use of prophylactic 5-layer foam sacral dressings per patient over time (Figure 2).
Figure 1.
Smoothed locally weighted regression of aggregate quarterly weights and scatterplot of hospital-level pressure injury rates relative to all hospitalized patients according to Agency for Healthcare Research and Quality Patient Safety Indicator #3 inclusion criteria.
Figure 2.
Decreasing rates of hospital-acquired pressure injury (Patient Safety Indicator #3) coinciding with increased use of prophylactic dressings per patient, 2010-2015.
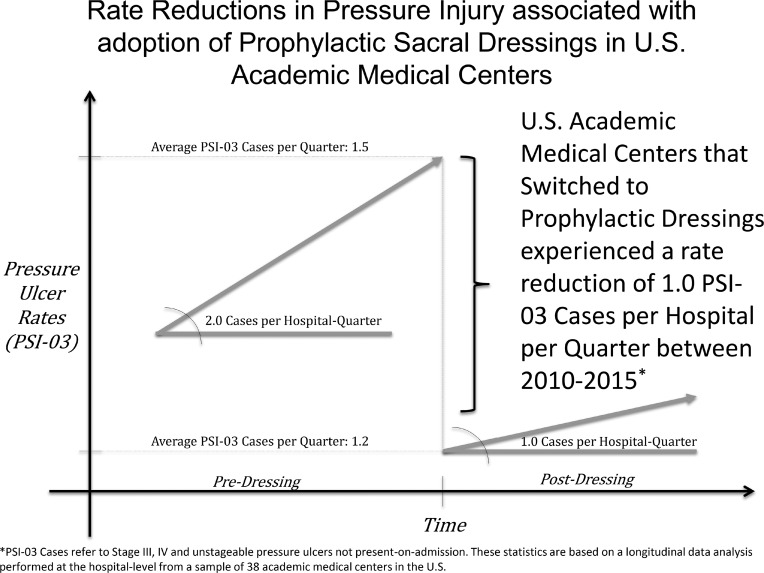
The average hospital-level PSI-03 count (HAPI stages 3, 4, and unstageable) during a quarter when prophylactic foam sacral dressings were available was 1.2 (SD = 0.045), compared to 1.5 (SD = 0.125) PSI-03 (HAPI stages 3, 4, and unstageable) during quarters when there were no dressings in a hospital. This 0.3 reduction in PSI-03 (HAPI stages 3, 4, and unstageable) per hospital-quarter represents a statistically significant improvement in pressure injury rates (P = .0063) according to a t test, and borders on being clinically meaningful depending on the size of the hospital relative to a 1 PSI-03 (HAPI stages 3, 4, and unstageable) case per 1000 reduction.
Longitudinal data analysis using a mixed-effects negative binomial regression with random intercept determined that the purchase of prophylactic 5-layer foam sacral dressing units was associated with significant reductions in PSI-03 (HAPI stages 3, 4, and unstageable), while controlling for CMI (Table 2). In general, the average hospital experienced a 1.0 case reduction in PSI-03 (HAPI stages 3, 4, and unstageable) per quarter following the introduction of prophylactic 5-layer foam sacral dressings (model 1).
TABLE 2. Results of Longitudinal Data Analysis of Pressure Injury Rates (AHRQ Patient Safety Indicator #3) Associated With Adoption of Prophylactic Foam Sacral Dressings Between Hospital-Quartersa.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Units of dressings acquired | −3.79E-05b | 1.80E-05 | −2.77E-05 | 9.90E-06 | ||
| Cost of dressings acquired, $ | −5.92E-06 | 2.24E-06 | 1.77E-04 | 7.61E-05 | ||
| Case-mix index | 0.331 | 0.224 | 0.340 | 0.225 | 0.315 | 0.232 |
| Intercept | −8.349 | 0.567 | −8.358 | 0.570 | −8.349 | 0.590 |
| Variance (intercept) | 0.457 | 0.153 | 0.467 | 0.155 | 0.535 | 0.175 |
| Log-likelihood | −739.021 | −737.674 | −735.126 | |||
Abbreviation: SE, standard error.
aCoefficients for time and CMS policy were insignificant, thus removed from the final model.
bItalicized values are significant (P < .05).
The total cost of these products purchased per quarter was also associated with significant reductions in PSI-03 (HAPI stages 3, 4, and unstageable), suggesting that a greater investment in these dressings resulted in greater reductions of PSI-03 (HAPI stages 3, 4, and unstageable) (model 2). However, the best-fit regression model controlled for units of prophylactic foam sacral dressings purchase and cost invested in measuring the association between dressings and pressure injury counts (model 3). Other covariates (eg, CMI, CMS reimbursement policy, time, and time-interactions) tested in the models did not show significant effects, or as great of a marginal effect on PSI-03 (HAPI stages 3, 4, and unstageable) as units of prophylactic 5-layer foam sacral dressings purchased. Despite these findings, CMI remained in the model to adjust the hospital-level cohort for overall risk of pressure injury development.
There were 1.72 PSI-03 HAPI cases per 1000 in 2010 compared to 0.62 cases in 2015 at an estimated cost of $70,000 per case. However, the average hospital in 2010 also purchased 355 prophylactic foam sacral dressings per 1000 compared to 2662 per 1000 in 2015 at a cost of $7.50 per dressing. Given our understanding of the patients admitted to these hospitals over 5 years, spending on pressure injuries decreased from $120/patient down to $43/patient, while the investment in prophylactic foam sacral dressings increased from $2.60/patient to $20/patient.
DISCUSSION
Hospitals that began using prophylactic 5-layer foam sacral dressings to prevent pressure injuries between 2010 and 2015 experienced statistically significant and clinically meaningful decreases in PSI-03 (HAPI stages 3, 4, and unstageable) rates (Figure 3). The facilities that invested in prophylactic 5-layer foam sacral dressings at a rate of 1 dressing per patient made a 100% return on investment in less than 1 year, not including litigation costs, which average settlement in a pressure injury malpractice lawsuit of $250,000.27
Figure 3.
Graphical depiction of the adjusted and unadjusted pressure injury rate reductions following adoption of prophylactic sacral dressings.
The association between prophylactic 5-layer foam sacral dressings and rate reductions outweighed the other most observable interruption during the same period, the addition of a CMS policy to penalize hospitals 1% of reimbursements, which fell into the lowest quartile of hospital-acquired condition rates.7 Hospital providers who are seeking to improve pressure injury prevention should consider bundling prophylactic 5-layer foam sacral dressings with other elements of prevention guidelines.
Pressure injury rates are on a downward trend from 2006 to 2015 as reported in the study by VanGilder and colleagues.28 The findings in our study on the effectiveness of prophylactic 5-layer foam sacral dressings in acute care hospitals may offer one explanation of the successful reduction in HAPIs many hospitals have achieved. According to an observational study by Padula and colleagues29 conducted between 2007 and 2012, most US acute care hospitals reported adherence to elements of the prevention guidelines endorsed by the AHRQ and the NPUAP. However, those hospitals, which bundled “skin care products (eg, dressings and creams)” with their prevention protocol, experienced the greatest reductions in pressure injury rates beyond simply adhering to the prevention protocol.24 Exactly which skin care products were used in hospitals remained unknown from that previous study. This study provides new evidence demonstrating an association between pressure injury rate reductions and prophylactic 5-layer foam sacral dressing use.
A caveat should be made from the findings of this study that despite a significant association between the purchase of prophylactic 5-layer foam sacral dressings and improved pressure injury rates, excessive spending does not prevent pressure injuries. Specifically, the purchase and actual use of the prophylactic 5-layer foam sacral dressing in combination with adherence to an evidence-based prevention protocol may improve outcomes. In fact, our findings indicate that high-performing hospitals using prophylactic 5-layer foam sacral dressings did so efficiently, averaging just 1 to 2 dressings over 5+ days per hospitalization. Furthermore, a hospital's investment in products for pressure injury prevention could be associated with a cultural paradigm shift among administration and staff; investment in a tangible product as part of a pressure injury prevention bundle results in staff who are more aware of this concerning issue and feel supported in their efforts to prevent pressure injuries.30
At this point in time, the AHRQ and the NPUAP have not updated prevention guidelines to include recommendations for use of any technologies that would improve prevention other than support surfaces (eg, pressure-relieving air-fluidized beds); however, prophylactic use of foam dressings is “recommended” as a technology to be considered.8 Study findings provide further support for the AHRQ and the NPUAP to include a recommendation for use of prophylactic foam sacral dressings as part of an evidence-based guideline for pressure injury prevention rather than just a consideration. Furthermore, current evidence shows that hospitals making an effort to follow AHRQ and NPUAP prevention guidelines carefully with regular updates from 2007 to 2012 witnessed the greatest reductions in pressure injury rates.11
Prophylactic 5-layer foam sacral dressings may be economical in addition to being effective at reducing pressure injury rates. According to Padula and colleagues,3 the threshold at which the prevention guidelines were no longer cost-effective was over $300 per patient per day. Since this study found the average cost to be just over $7 per dressing, this element fits well within societal willingness to pay for improving the quality of pressure injury prevention.31
Strengths and Limitations
This study has several limitations. First, hospital-level data limited our discernment of causality between prophylactic foam sacral dressing use and pressure injury prevention. We assume that these prophylactic dressings were used as indicated. Second, we assumed that hospitals could act as their own controls in the longitudinal design of the study since PSI-03 (HAPI stage 3, 4 and unstageable) counts were regressed between hospitals at times when we knew hospitals had purchased different amounts of 5-layer foam sacral dressings to predict a trajectory of HAPI rates. We did not specify any UHC hospitals that purchased zero prophylactic dressings during the observation period since the commercial data provided could not ensure that hospitals used a different branded prophylactic foams sacral dressing during unobserved periods. Third, due to an abundance of missing data from the UHC clinical database/resource manager in the third quarter of 2012, this quarter was omitted from the analysis. Fortunately, the mixed-effects regression design is generally robust to missing data when multiple other time points exist to regress across missing data points. Fourth, the rates of HAPIs are dependent upon accurate coding and reporting of PSI-03 (HAPI stages 3, 4, and unstageable) in UHC, which Meddings32 noted is not as accurate as measurement of surveillance data. Fortunately, the period of observation has not been associated with concerning shifts in PSI-03 coding, such as in 2009 when it was modified to include the first present-on-admission status indicator. Fifth, using an observational cohort of all-inclusive hospitalizations according to PSI-03 (HAPI stages 3, 4, and unstageable) increases the number needed to treat to detect a pressure injury since, in reality, these events are more common in noncontrolled settings as compared to clinical trial data referenced in previous research of dressing use. A study by Santamaria and colleagues14 detected small changes in pressure injury rates for a high-risk, critically ill patient population with a number needed to treat of 3, whereas this study needed greater volumes of patients to detect clinically meaningful reductions in PSI-03 (HAPI stages 3, 4, and unstageable) using real-world data. Sixth, this study only analyzed the effects of 5-layer foam sacral dressings on HAPI outcomes, and no other dressings. The comparative effectiveness of other types of dressings used prophylactically cannot be drawn from this study. Seventh, the study analyzed counts of all PSI-03 (HAPI stages 3, 4, and unstageable) cases regardless of body location. While sacral injuries constitute the expected majority of HAPIs, some of the variability in HAPI reduction through PSI-03 rates (HAPI stages 3, 4, and unstageable) is based on other, unobserved factors besides the use of sacral dressings.
CONCLUSIONS
A robust sample of acute care hospitals experienced significant reductions in counts of hospital-acquired stages 3, 4 and unstageable pressure injuries following the adoption of prophylactic 5-layer foam sacral dressings. On average, a hospital using a standard quantity of 1 to 2 dressings per hospitalized patient admission over 5+ days witnessed a 1.0 case reduction in PSI-03 (HAPI stages 3, 4, and unstageable) per quarter. Given that the average estimated cost of a PSI-03 ranges from $50,000 to 150,000, this implies that prophylactic 5-layer foam sacral dressings could save hospitals $200,000 to $600,000 per year in expenses associated with pressure injuries in addition to avoidance of CMS penalties for high hospital-acquired condition rates.3 Hospital purchasing directors and bedside clinicians should consider using these prophylactic foam sacral dressings in their pressure injury prevention protocol to improve HAPI rates and reduce costs.
ACKNOWLEDGMENTS
We thank C. Tod Brindle, MSN, RN, ET, CWOCN, for providing a review of current peer-reviewed literature on prophylactic dressings.
Footnotes
Dressing data for the study described in this article were provided freely by Mölnlycke Health Care. The author has relationships as a consultant and speakers bureau member to Mölnlycke Health Care. This arrangement has been reviewed and approved by The Johns Hopkins University in accordance with its conflict of interest policies.
REFERENCES
- 1.Padula WV, Duffy MP, Yilmaz T, Mishra MK. Integrating systems engineering practice with health-care delivery. Health Syst. 2014;3(3):159–164. [Google Scholar]
- 2.Lyder CH, Wang Y, Metersky M, et al. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc. 2012;60(9):1603–1608. [DOI] [PubMed] [Google Scholar]
- 3.Padula WV, Mishra MK, Makic MB, Sullivan PW. Improving the quality of pressure ulcer care with prevention: a cost-effectiveness analysis. Med Care. 2011;49(4):385–392. [DOI] [PubMed] [Google Scholar]
- 4.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: Final data for 2005. National Vital Statistics Reports, 56(10). Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 5.Kurtzman ET, Buerhaus PI. New Medicare payment rules: danger or opportunity for nursing? Am J Nurs. 2008;108(6):30–35. [DOI] [PubMed] [Google Scholar]
- 6.Wald HL, Kramer AM. Nonpayment for harms resulting from medical care: catheter-associated urinary tract infections. JAMA. 2007;298(23):2782–2784. [DOI] [PubMed] [Google Scholar]
- 7.CMS Media Relations. CMS to Improve Quality of Care During Hospital Inpatient Stays. In: Department of Health & Human Services, ed. Washington, DC: Centers for Medicare & Medicaid Services; 2014. [Google Scholar]
- 8.National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance, Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Perth, Australia: Cambridge Media; 2014. [Google Scholar]
- 9.Agency for Healthcare Research and Quality. AHRQ Toolkit Helps to Prevent Hospital-Acquired Pressure Ulcers: Research Activities. Vol Publication No. 371. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 10.Ratliff CR, Tomaselli N. WOCN update on evidence-based guideline for pressure ulcers. J Wound Ostomy Continence Nurs. 2010;37(5):459–460. [DOI] [PubMed] [Google Scholar]
- 11.Padula WV, Gibbons RD, Valuck RJ, et al. Are evidence-based practices associated with effective prevention of hospital-acquired pressure ulcers in US academic medical centers? Med Care. 2016;54(5):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qaseem A, Mir TP, Starkey M, Denberg TD. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162(5):359–369. [DOI] [PubMed] [Google Scholar]
- 13.Brindle CT. Outliers to the Braden Scale: identifying high-risk ICU patients and the results of prophylactic dressing use. World Counc Enterostomal Ther J. 2010;30(1):11. [Google Scholar]
- 14.Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J. 2015;12(3):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santamaria N, Santamaria H. An estimate of the potential budget impact of using prophylactic dressings to prevent hospital-acquired PUs in Australia. J Wound Care. 2014;23(11):583–584, 586, 588-589. [DOI] [PubMed] [Google Scholar]
- 16.Santamaria N, Liu W, Gerdtz M, et al. The cost-benefit of using soft silicone multilayered foam dressings to prevent sacral and heel pressure ulcers in trauma and critically ill patients: a within-trial analysis of the Border Trial. Int Wound J. 2015;12(3):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santamaria N, Gerdtz M, Liu W, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II Trial. J Wound Care. 2015;24(8):340–345. [DOI] [PubMed] [Google Scholar]
- 18.Kalowes P, Li M, Carlson C, et al. Use of a soft silicone, self-adherent, bordered foam dressing to reduce pressure ulcer formation in high-risk patients: a randomized clinical trial. Paper presented at: Journal of Wound Ostomy and Continence Nursing; 2013. [Google Scholar]
- 19.Davies P. Role of multi-layer foam dressings with Safetac in the prevention of pressure ulcers: a review of the clinical and scientific data. J Wound Care. 2016;25(1 suppl):S1, S4–S23. [PubMed] [Google Scholar]
- 20.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974–984. [DOI] [PubMed] [Google Scholar]
- 21.Padula WV, Makic MB, Wald HL, et al. Hospital-acquired pressure ulcers at academic medical centers in the United States, 2008-2012: tracking changes since the CMS Nonpayment Policy. Jt Comm J Qual Patient Saf. 2015;41(6):257–253. [DOI] [PubMed] [Google Scholar]
- 22.Farguhar M. AHRQ quality indicators. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 23.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: Jon Wiley & Sons, Inc; 2006. [Google Scholar]
- 24.Padula WV, Makic MB, Mishra MK, et al. Comparative effectiveness of quality improvement interventions for pressure ulcer prevention in academic medical centers in the United States. Jt Comm J Qual Patient Saf. 2015;41(6):246–256. [DOI] [PubMed] [Google Scholar]
- 25.Padula WV, Mishra MK, Weaver CD, Yilmaz T, Splaine ME. Building information for systematic improvement of the prevention of hospital-acquired pressure ulcers with statistical process control charts and regression. BMJ Qual Saf. 2012;21(6):473–480. [DOI] [PubMed] [Google Scholar]
- 26.Russo CA, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers Among Adults 18 Years and Older, 2006. Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research & Quality; 2008. [PubMed] [Google Scholar]
- 27.Bennett RG, O'Sullivan J, DeVito EM, Remsburg R. The increasing medical malpractice risk related to pressure ulcers in the United States. J Am Geriatr Soc. 2000;48(1):73–81. [DOI] [PubMed] [Google Scholar]
- 28.VanGilder C, Lachenbruch C, Algrim-Boyle C, Meyer S. The International Pressure Ulcer Prevalence™ Survey: 2006-2015: a 10-year pressure injury prevalence and demographic trend analysis by care setting. J Wound Ostomy Continence Nurs. 2007;44(1):20–28. [DOI] [PubMed] [Google Scholar]
- 29.Padula WV, Mishra MK, Makic MB, et al. Increased adoption of quality improvement interventions to implement evidence-based practices for pressure ulcer prevention in U.S. academic medical centers. Worldviews Evid Based Nurs. 2015;12(6):328–336. [DOI] [PubMed] [Google Scholar]
- 30.Padula WV, Valuck RJ, Makic MB, Wald HL. Factors influencing adoption of hospital-acquired pressure ulcer prevention programs in US academic medical centers. J Wound Ostomy Continence Nurs. 2015;42(4):327–330. [DOI] [PubMed] [Google Scholar]
- 31.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. [DOI] [PubMed] [Google Scholar]
- 32.Meddings JA. Using administrative discharge diagnoses to track hospital-acquired pressure ulcer incidence—limitations, links, and leaps. Jt Comm J Qual Patient Saf. 2015;41(6):243–245. [DOI] [PubMed] [Google Scholar]