Abstract
Inflammation affects all stages of tumorigenesis. A key signaling pathway leading to acute and chronic inflammation is through activation of the caspase-1 inflammasome. Inflammasome complexes are assembled on activation of certain nucleotide-binding domain, leucine-rich repeat containing proteins (NLRs), AIM2-like receptors (ALRs), or pyrin. Of these, NLRP1, NLRP3, NLRC4, NLRP6 and AIM2 influence the pathogenesis of cancer by modulating innate and adaptive immune responses, cell death, proliferation, and/or the gut microbiota. Activation of the inflammasome and IL-18 signaling pathways is largely protective in colitis-associated colorectal cancer, whereas excessive inflammation driven by the inflammasome or the IL-1 signaling pathways promotes breast cancer, fibrosarcoma, gastric carcinoma, and lung metastasis in a context-dependent manner. The clinical relevance of inflammasomes in multiple forms of cancer highlights their therapeutic promise as molecular targets. In this Review, we explore the crossroads between inflammasomes and the development of various tumors, and discuss possible therapeutic values in targeting the inflammasome for the prevention and treatment of cancer.
Keywords: AIM2, carcinogens, caspase-1, caspase-11, inflammasomes, IL-1β, IL-1R, IL-18, IL-18R, NLRP1, NLRP3, NLRC4, NLRP6, non-canonical inflammasomes, pyroptosis, therapy, tumors
Introduction
Inflammation triggered by microbial or danger signals drive many forms of cancer in humans (1). Inflammation associated with tumor development is triggered by a variety of immune cells, including macrophages, neutrophils, dendritic cells, natural killer (NK) cells, and T and B lymphocytes (2). A central mechanism driving inflammation in immune cells is orchestrated by the inflammasome, a cytoplasmic multimeric protein complex that provides a molecular platform for activation of the cysteine protease caspase-1 (3). Activated caspase-1 mediates proteolytic cleavage and release of the pro-inflammatory cytokines IL-1β and IL-18 and initiates an inflammatory form of programed cell death known as pyroptosis (3).
Certain members of the nucleotide-binding domain, leucine-rich repeat containing proteins (NLRs) and AIM2-like receptors (ALRs) form inflammasome complexes in response to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) (3). Mutations in genes encoding inflammasome components often lead to susceptibilities to cancer, infection, and autoinflammatory diseases in humans. In the context of cancer, polymorphisms in the gene encoding NLRP1 are linked to mesothelioma (4), melanoma (5) and epidermal hyperplasia (6); those of NLRP3 are associated with melanoma (5) and colorectal cancer (7); and those of AIM2 with colorectal cancer (8). Furthermore, our contemporary appreciation of the functional importance of inflammasomes in cancer is illuminated by mouse models. Here, we highlight recent development in our understanding of inflammasomes in cancer and outline the therapeutic potential of modulating inflammasome responses for use in anti-cancer therapies.
Protective roles of inflammasomes in cancer
The global inflammasome-initiating sensor of PAMPs and DAMPs, NLRP3, assembles a fully functional inflammasome complex by recruiting apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and caspase-1. Its ability to respond a variety of signals contributes to its biological importance in a number of diseases, including colorectal cancer, melanoma and transplantable tumors. Multiple studies have shown that mice lacking NLRP3 are hypersusceptible to colitis and colitis-associated colorectal cancer induced by the DNA damaging agent azoxymethane (AOM) and chemical colitogen dextran sulfate sodium (DSS) (9–12). However, another study has suggested that mice lacking NLRP3 were more resistant to DSS-induced colitis compared with wild-type mice (13), whereas a further study has found a similar tumor burden between wild-type mice and mice lacking NLRP3 treated with AOM and DSS (14). It is possible that differences in the gut microbiota between different animal facilities could have contributed to the differences observed in these studies. It is important to note that mice lacking ASC and caspase-1 are also susceptible to DSS-induced colitis and colitis-associated colorectal cancer (9–11,15), providing substantial evidence to favor a protective role of inflammasomes in an inflammatory model of colorectal cancer.
Bone marrow chimera studies have identified that signaling through the NLRP3 inflammasome in the hematopoietic, but not the stromal, compartment are essential for mediating protection against tumorigenesis (9,10). The ability of inflammasome sensors such as NLRP3 to mediate secretion of IL-18, a cytokine which contributes to epithelial barrier repair against damage, is a potential mechanism explaining the protective role of IL-18 against colitis-associated colorectal cancer (9–11,15–17) (Fig. 1A). In contrast with previous studies showing that mice lacking IL-18 are susceptible to DSS-induced intestinal inflammation and tumorigenesis (9,16,17), a study has found that mice with a conditional deletion of IL-18 in either epithelial cells or hematopoietic cells were more resistant to DSS-induced colitis compared with cohoused wild-type mice, indicating an IL-18–dependent function in both enterocytes and hematopoietic cells (18). Under cohousing conditions whereby mice harbor a similar microbiota profile, IL-18 might inhibit goblet cell maturation prior to the onset of colitis to drive pathology (18). However, injection of recombinant IL-18 into mice lacking inflammasome components reduces the prevalence of tumors in response to AOM and DSS (9), suggesting that this inflammasome-associated cytokine could be considered a potential candidate in immunotherapy against certain cases of colorectal cancer.
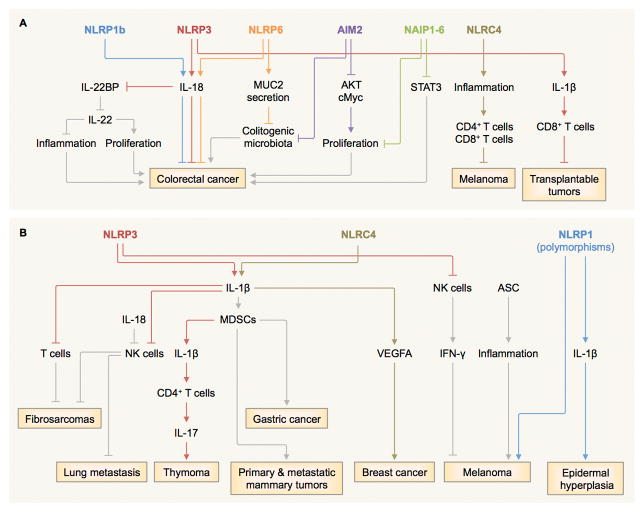
Figure 1. Diverse roles of inflammasome sensors in tumorigenesis.
A, NLRP1b, NLRP3 and NLRP6 mediate the production of IL-18, contributing to the protection against colitis-associated colorectal cancer (9–11,15,19,25–29). The IL-18 axis can also induce tumoricidal activity of NK cells against metastasized colonic tumor cells (19), downregulation IL-22 binding protein (IL-22BP) (20), and inhibiting the colonization of colitogenic microbiota (27) possibly through its role in MUC2 secretion by goblet cells (29,31). The NLRP3 inflammasome and the IL-1β–IL-1 receptor (IL-1R) signaling axis drives a T-cell response towards transplantable tumor cells (24). Mouse NAIP1–6 proteins control phosphorylation of STAT3 and the expression of genes encoding anti-apoptotic and proliferation-related molecules (32). NLRC4 controls the suppression of melanoma growth by amplifying inflammation in macrophages and potentiates production of IFN-γ in T cells (34). AIM2 inhibits phosphorylation of AKT and cMyc activities and stem cell proliferation, while preventing colonization of colitogenic microbiota (35,36). B, The NLRP3–IL-1β–IL-1R signaling axis suppresses the tumoricidal activity of NK cells and T cells and promotes methylcholanthrene (MCA)-induced fibrosarcomas (23). It also induces secretion of IL-17 by CD4+ T cells and dampens the anti-tumor efficacy of chemotherapeutic agents in thymoma (42). Overexpression of IL-1β mobilizes myeloid-derived suppressor cells (MDSCs) to the stomach and induces gastric cancer (40). IL-1 signaling drives accumulation of MDSCs and promotes primary and metastatic mammary tumors (41). Inflammasome-independent activity of NLRP3 suppresses NK cells and increases lung metastasis in certain models of melanoma (23,37). The NLRC4 inflammasome mediates expression of adipocyte-mediated vascular endothelial growth factor A (VEGFA) and accelerates the progression of breast cancer (43). In some cases, ASC increases the viability and growth of melanoma cells (38) and promotes inflammation in infiltrating myeloid cells and the development of skin cancer (39). Mutations in the gene encoding NLRP1 is linked to melanoma and epidermal hyperplasia in humans (5,6).
NLRP3 inflammasome-mediated secretion of IL-18 can also induce tumoricidal activity of NK cells against metastasized colonic tumor cells in the mouse liver (19). In addition, IL-18 promotes downregulation of the soluble IL-22 receptor IL-22 binding protein (20) (Fig. 1A). Controlled production of the IL-22 binding protein fine-tunes the biological activity of IL-22, a cytokine which exerts protective effects against intestinal damage at the peak of inflammation and promotes tumor development at later stages (20). IL-22 also maintains IL-18 expression in epithelial cells of the ileum, whereas IL-18 itself is required for IL-22 expression in CD4+ T cells and innate lymphoid cells (21).
The diametric roles of IL-18 have also been observed in lung metastasis. Recombinant IL-18 injected into mice twice within a week enhances the development of B16F10 metastases, whereas daily administration for 5 days reduces tumorigenesis (22). In addition, mice lacking IL-18 are more susceptible to B16–F10 tumor metastasis (23). It is possible to speculate that temporary exposure to IL-18 might drive inflammation and accelerate metastasis, whereas a sustained circuit of IL-18 might be fully required to enhance and shape anti-tumor immunosurveillance. Indeed, IL-18 has the capacity to fine-tune the activation status of NK cells (22,23). In cases whereby IL-18 is detrimental, the use of IL-18 binding protein to neutralize IL-18 might be beneficial in the treatment of certain types of cancer (22) (Fig. 2).
Figure 2. Therapeutic targets of the inflammasome pathway.
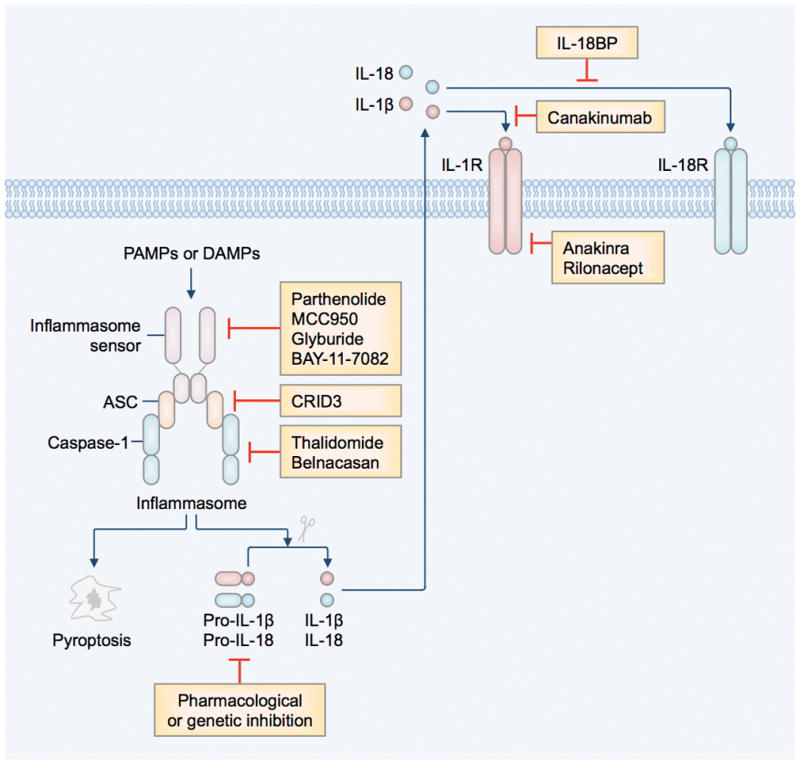
Recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) by inflammasome-initiating sensors leads to the activation of the inflammasome and initiation of pyroptosis and release of the bioactive form of IL-1β and IL-18 (3). IL-1β and IL-18 engage in autocrine and paracrine signaling pathways via the IL-1 receptor (IL-1R) and IL-18 receptor (IL-18R), respectively. The inflammasome signaling pathway can be inhibited by pharmacological inhibition of activation of the inflammasome (Parthenolide, MCC950, Glyburide, and BAY-11–7082) (46–48), ASC oligomerization (CRID3) (49), caspase-1 (Thalidomide and Belnacasan or VX-765) (45,50), and IL-1R (Anakinra or Kineret, and Rilonacept or Arcalyst) (44,51), or neutralizing IL-1β (Canakinumab or Ilaris) (52) or IL-18 (IL-18 binding proteins or IL-18BP) (53).
The NLRP3 inflammasome is also required for anti-cancer adaptive immune responses. The release of ATP by dying tumor cells treated with chemotherapeutic agents activates the NLRP3 inflammasome and the IL-1β–IL-1 receptor (IL-1R) signaling axis in dendritic cells (24) (Fig. 1A). This pathway drives an effective CD8+ T-cell response towards transplantable tumor cells (24). As a result, mice lacking components of the NLRP3 inflammasome do not respond to treatment with the chemotherapeutic drug oxaliplatin against implanted tumors owing to a loss of oxaliplatin–induced priming of CD8+ T cells (24).
In addition to NLRP3, other NLR sensors, including NLRP1b and NLRP6, mediate protection against tumorigenesis (25–30) (Fig. 1A). In mice, the NLRP1b inflammasome provides protection against colon tumorigenesis, mediating secretion of both IL-1β and IL-18 in stromal cells of the colon (25). NLRP6 has several inter-related mechanistic functions by which it confers protection against colon tumorigenesis in mice. NLRP6 has been proposed to activate caspase-1 and drive IL-18 production in the intestine in response to AOM and DSS treatment (26–28). The NLRP6–IL-18 signaling axis prevents the colonization of pro-colitogenic bacterial species TM7 and those of the Prevotellaceae family (27). Furthermore, NLRP6 is essential for MUC2 secretion by goblet cells to clear potentially colitogenic bacteria (29,31). NLRP6–dependent secretion of MUC2 in the intestinal epithelium have been shown to be dependent and independent of the inflammasome (29,31), an observation which could be attributed to differences in the gut microbiota and the mouse facilities housing the animals.
The ability of inflammasome sensors to provide protection against cancer does not always rely on the effector functions of caspase-1 and the cytokines processed by inflammasomes (Fig. 1A). Mouse NAIP1–6 proteins are components of the NLRC4 inflammasome and have been linked to the protection against AOM-DSS-induced colorectal cancer (32). The mechanism driving this response is independent of the NLRC4 inflammasome, but relates to the ability of NAIP proteins to inhibit hyperactivation of the transcription factor STAT3 and the expression of genes encoding anti-apoptotic and proliferation-related molecules (32). Further, there is evidence to suggest that adjuvant-based cancer immunotherapies targeting cytosolic NAIP proteins and surface-associated TLR5 could be beneficial. The NAIP proteins and TLR5 both recognize flagellin of certain bacteria (3). Enforced expression of flagellin in tumor cell lines, ensuring dual recognition by NAIP proteins and TLR5, induces tumor cell clearance by innate immune cells and activation of tumor-specific CD4+ and CD8+ T-cell responses in mice (33). These findings suggest that recognition of tumor cells by the inflammasome and other innate immune sensors could lead to desirable outcomes.
The role of NLRC4 itself in a mouse model of AOM-DSS-induced tumorigenesis is unclear; a study suggests that NLRC4 prevents colorectal tumorigenesis by inhibiting cellular proliferation and driving cell death (14), whereas another found no role for NLRC4 (10). NLRC4 can also amplify inflammatory signaling pathways in macrophages independently of inflammasome assembly and potentiates production of IFN-γ in CD4+ and CD8+ T cells to dampen melanoma tumor growth in mice (34).
In addition to NLRs, the DNA-sensing inflammasome sensor AIM2 can inhibit AOM-DSS-induced and spontaneous colorectal tumorigenesis via an inflammasome-independent mechanism (35,36) (Fig. 1A). AIM2 inhibits overt proliferation of intestinal stem cells and promotes cell death (35). Furthermore, AIM2 interacts with and limits the activation of DNA-dependent protein kinase DNA-PK to reduce phosphorylation of AKT that governs cell proliferation (36). In addition, AIM2 expression prevents colonization of colitogenic microbiota and reduces susceptibility of mice to colorectal tumorigenesis (35). Overall, there is substantial evidence to suggest that inflammasome sensors have tumor-suppressive roles in certain forms of cancer. These oncogenic inhibitory activities are dependent on the ability of inflammasome sensors to modulate cytokine production, engaging T cell activities, cellular proliferation and maturation, and the microbiota profile of the host (Fig. 1A).
Detrimental roles of inflammasomes in cancer
Activation of the inflammasome leads to inflammatory responses, and in some cases, anti-tumor immunity (Fig. 1B). NLRP3 activity is associated with increased lung metastasis when mice were injected intravenously, but not subcutaneously, with B16–F10 melanoma cells or RM-1 prostate carcinoma cells (23,37). In this case, mice lacking NLRP3 have a substantially reduced number of lung metastases compared with wild-type mice, whereas mice lacking caspase-1 and caspase-11 or IL-1R have a similar number of lung metastases compared with wild-type mice (23). The negative effect of NLRP3 is also observed when mice are vaccinated with wild-type dendritic cells pulsed with B16–F10 melanoma cell lysates prior to injection with B16–F10 melanoma, such that a greater proportion of vaccinated mice lacking NLRP3 survived compared with that of vaccinated wild-type mice (37). The deleterious effect of NLRP3 in the melanoma model is owing to its ability to suppress activation of NK cells that secrete IFN-γ and kill tumor cells (23) (Fig. 1B).
The inflammasome adaptor protein ASC also appears to have multiple biological activities that affect the outcome of tumorigenesis (38). A knockdown of the gene encoding ASC increases the viability and growth of primary melanoma cells whereas it reduces the viability and growth of metastatic melanoma cells, when these cells were injected into nude mice (38). Using cell-type-specific knockout mouse strains lacking ASC in a chemically-induced skin carcinogenesis model, ASC was found to limit keratinocyte proliferation and tumor formation, whereas it promotes inflammation in infiltrating myeloid cells and the development of tumors (39) (Fig. 1B). These findings further highlight the cell-type- and tissue-specific roles for inflammasome components in cancer.
In addition to IL-18, activation of the inflammasome leads to secretion of the inflammasome substrate IL-1β. IL-1β is involved in the pathogenesis of spontaneous gastric cancer or Helicobacter felis-induced gastric cancer (40). A transgenic mouse strain engineered to overexpress human IL-1β in the stomach is prone to developing gastric cancer due to increased mobilization of myeloid-derived suppressor cells (MDSCs) to the stomach (40). A deleterious role of IL-1 signaling is also supported by the finding that mice lacking IL-1R have a delayed accumulation of MDSCs and reduced primary and metastatic mammary tumors (41), suggesting that inflammation driven by the IL-1R signaling pathway is detrimental (Fig. 1B).
The relationship between IL-1R signaling and MDSCs in cancer is further demonstrated in a study showing that activation of the NLRP3 inflammasome by chemotherapeutic agents gemcitabine and 5-fluorouracil leads to IL-1β production in MDSCs (42). Production of IL-1β by MDSCs induces secretion of IL-17 by CD4+ T cells and dampens the anti-tumor efficacy of gemcitabine and 5-fluorouracil (42). The IL-1β–IL-1R signaling axis activated by the NLRP3 inflammasome has an adverse role in methylcholanthrene (MCA)-induced fibrosarcomas (23). In this case, IL-1β suppresses the tumoricidal activity of NK cells and T cells (23). Moreover, IL-1β produced as a result of activation of the NLRC4 inflammasome mediates expression of adipocyte-mediated vascular endothelial growth factor A and angiogenesis, which accelerates the progression of breast cancer (43). Furthermore, gain-of-function mutations in the gene encoding NLRP1 induce spontaneous inflammasome activation and IL-1 production and drives epidermal hyperplasia in humans (6) (Fig. 1B).
Owing to the detrimental effects of the IL-1R signaling pathway, treatment of mice with IL-1R antagonist IL-1Ra enhances the anti-tumor effect of gemcitabine and 5-fluorouracil (42). Furthermore, neutralizing IL-1β or IL-1R at early stages of tumorigenesis reduces the incidence of MCA-induced fibrosarcomas in mice (23). Inhibitors of IL-1 cytokines, such as Anakinra, have been suggested for use in prophylaxis or treatment of active myeloma (44) (Fig. 2). Similarly, thalidomide, an immunomodulator approved by the US Food and Drug Administration, can inhibit caspase-1 activation and is used for the treatment of malignant myeloma (45) (Fig. 2). Excessive inflammation induced by inflammasome activation and inflammasome substrates is a consistent theme which seems to explain the detrimental effects of inflammasomes in multiple forms of cancer. The complex and diametric roles of inflammasome components in different forms of cancer suggest that anti-cancer therapies must be tailor to the specific cancer type and stage of disease.
Conclusions
In this review, we provided a brief overview of the biological importance of inflammasomes in different forms of cancer. Activation of inflammasome sensors is largely beneficial in colitis-associated colorectal cancer largely owing to the epithelial healing effects of the IL-18 signaling pathway, regulation of cellular proliferation, maturation and cell death, and maintenance of a healthy gut microbiota. Identification of novel tumor-suppressive mechanisms of inflammasome sensors pushes the boundaries of the traditional roles of inflammasomes.
In other cases, inflammation triggered by inflammasomes and IL-1 signaling leads to suppression of anti-tumor immunity conferred by NK cells and T cells that is detrimental to the development of fibrosarcoma, melanoma, gastric carcinoma, and lung metastasis. As a result, boosting or reducing the activity of inflammasomes or their effector molecules could be efficacious by tailoring therapy to specific types of cancer. Several small molecules, antagonists and monoclonal antibodies are being developed against components of the inflammasome for use in therapies to control cancer (Fig. 2). However, inappropriate use of inflammasome modulatory therapies might lead to suppression of anti-tumor immunity and/or increased susceptibility to infection and the development of metabolic and autoinflammatory diseases.
Since inflammasome sensors regulate multiple signaling pathways beyond that of caspase-1, it is essential to understand which molecular mechanism is governed by inflammasome components in specific tumors. Furthermore, the pro-tumorigenic and anti-tumorigenic properties of inflammasomes are largely determined by the types of cells, tissues and organs involved. The use of tissue- and cell-type-specific conditional deletion approaches in mice would fully reveal the complex functions of inflammasomes in the progression of cancer. The biological relationship between inflammasomes and cancer provides promising avenue with which to explore new anti-cancer therapies.
Acknowledgments
Grant Support
Work from our laboratory is supported by the US National Institutes of Health (AI101935, AI124346, AR056296 and CA163507 to T.D.K.), the American Lebanese Syrian Associated Charities (to T.D.K.), and the R.G. Menzies Early Career Fellowship from the National Health and Medical Research Council of Australia (to S.M.M.).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 3.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunological reviews. 2015;265(1):6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girardelli M, Maestri I, Rinaldi RR, Tognon M, Boldorini R, Bovenzi M, et al. NLRP1 polymorphisms in patients with asbestos-associated mesothelioma. Infect Agent Cancer. 2012;7(1):25. doi: 10.1186/1750-9378-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma D, Bivik C, Farahani E, Synnerstad I, Fredrikson M, Enerback C, et al. Inflammasome polymorphisms confer susceptibility to sporadic malignant melanoma. Pigment Cell Melanoma Res. 2012;25(4):506–13. doi: 10.1111/j.1755-148X.2012.01008.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell. 2016;167(1):187–202. e17. doi: 10.1016/j.cell.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Ungerback J, Belenki D, Jawad ul-Hassan A, Fredrikson M, Fransen K, Elander N, et al. Genetic variation and alterations of genes involved in NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis. 2012;33(11):2126–34. doi: 10.1093/carcin/bgs256. [DOI] [PubMed] [Google Scholar]
- 8.Kim TM, Laird PW, Park PJ. The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell. 2013;155(4):858–68. doi: 10.1016/j.cell.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. Journal of immunology. 2010;185(8):4912–20. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. The Journal of experimental medicine. 2010;207(5):1045–56. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–91. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflammatory bowel diseases. 2011;17(6):1359–72. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59(9):1192–9. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 14.Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21635–40. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32(3):367–78. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. The Journal of experimental medicine. 2010;207(8):1625–36. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38(8):837–44. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 18.Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163(6):1444–56. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, et al. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015;43(4):751–63. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491(7423):259–63. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz M, Eidenschenk C, Ota N, Wong K, Lohmann U, Kuhl AA, et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 2015;42(2):321–31. doi: 10.1016/j.immuni.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–9. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 23.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, et al. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72(22):5721–32. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 24.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 25.Williams TM, Leeth RA, Rothschild DE, Coutermarsh-Ott SL, McDaniel DK, Simmons AE, et al. The NLRP1 inflammasome attenuates colitis and colitis-associated tumorigenesis. J Immunol. 2015;194(7):3369–80. doi: 10.4049/jimmunol.1402098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. Journal of immunology. 2011;186(12):7187–94. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–57. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9601–6. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birchenough GM, Nystrom EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352(6293):1535–42. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karki R, Man SM, Malireddi RK, Kesavardhana S, Zhu Q, Burton AR, et al. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature. 2016 doi: 10.1038/nature20597. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–59. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allam R, Maillard MH, Tardivel A, Chennupati V, Bega H, Yu CW, et al. Epithelial NAIPs protect against colonic tumorigenesis. The Journal of experimental medicine. 2015;212(3):369–83. doi: 10.1084/jem.20140474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4(120):120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 34.Janowski AM, Colegio OR, Hornick EE, McNiff JM, Martin MD, Badovinac VP, et al. NLRC4 suppresses melanoma tumor progression independently of inflammasome activation. J Clin Invest. 2016 doi: 10.1172/JCI86953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, et al. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162(1):45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JE, Petrucelli AS, Chen L, Koblansky AA, Truax AD, Oyama Y, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nature medicine. 2015;21(8):906–13. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 2010;70(24):10161–9. doi: 10.1158/0008-5472.CAN-10-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Luo Y, Dunn JH, Norris DA, Dinarello CA, Fujita M. Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Invest Dermatol. 2013;133(2):518–27. doi: 10.1038/jid.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H, et al. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci U S A. 2012;109(45):18384–9. doi: 10.1073/pnas.1209171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14(5):408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 43.Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM, et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun. 2016;7:13007. doi: 10.1038/ncomms13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–22. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller M, Sollberger G, Beer HD. Thalidomide inhibits activation of caspase-1. J Immunol. 2009;183(9):5593–9. doi: 10.4049/jimmunol.0900476. [DOI] [PubMed] [Google Scholar]
- 46.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285(13):9792–802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6(12):e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175(4):2630–4. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 51.Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, et al. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum. 2008;58(8):2432–42. doi: 10.1002/art.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhimolea E. Canakinumab. MAbs. 2010;2(1):3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10(1):127–36. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]