Abstract
Age-associated decline in oocyte quality is common in mammals. Oocytes take a long time to reach their full-grown size in large animals, and maternal physical conditions profoundly affect follicle development. Aging affects the oocyte itself as well as the surrounding environment, such as granulosa cells and follicular fluid. This review discusses age-associated changes that occur in granulosa cells and follicular fluid in cows and suggests that age-associated decline in granulosa cells and follicular fluid hampers proper oocyte development.
Keywords: Aging, Granulosa cells, Follicular fluids
In industrialized countries, changes in working patterns and late marriages decrease the opportunities that women have to become pregnant. In humans, fertility declines once women exceed 35 years of age [1, 2], and accumulating evidence has shown that the quality of oocytes declines as the donor age increases [3]. To manage the rapidly aging population and falling birth rate, it is of great importance to determine the factors involved with this age-associated decline in oocyte quality. Assisted reproductive technologies (ART), such as artificial insemination, embryo transfer, and in vitro production of embryos, have advanced in domestic industries, especially in cattle production, and almost all cows are now derived from artificial insemination and embryo transfer in Japan. Cows are known to have follicular wave, follicle selection, and ovulation patterns similar to those in humans and have a long reproductive life compared to rodents [4,5,6,7,8]. In the 1970s, a field-based study demonstrated age-associated decline in bovine reproductive performance [9]. In addition to developments in reproductive technologies, age-associated decline in the reproductive performance of cows is supported by evidences; for example, the number of embryos collected from aged cows (13–16 years old) following super-ovulation was lower than that collected from their younger daughters [10]. We have previously reported specific features of bovine oocytes that are associated with aging: premature progression of nuclear maturation [11], high abnormal fertilization rate following in vitro fertilization [12], low developmental ability [13], shorter telomere length [14], low lipid content in oocytes (unpublished data), and a low level of histone acetylation in germinal vesicle-stage oocytes [15]. In line with this, we conducted a comprehensive gene expression analysis of oocytes using next-generation sequencing technology (NGS) and compared between young (25–40 months) and aged (> 120 months) cows. The results showed that differentially expressed genes between in vitro-matured oocytes from aged and young cows were associated with mitochondrial dysfunction [16]. That study also showed that oocytes from aged cows had higher levels of reactive oxygen species (ROS) compared with those from their younger counter parts. In studies in mammals, age-related decline in oocyte quality is associated with mitochondrial DNA damage, decreased mitochondrial membrane potential, and downregulation of both mitochondria- and ATP-related genes [17,18,19,20,21]. Consistent with this, we observed that in vitro-matured oocytes collected from aged cows had a lower mitochondrial DNA copy number compared to that in their younger counterparts, and there was a negative correlation between mitochondrial DNA copy number in oocytes and donor age [12]. Similarly, eight-cell-stage embryos derived from cows of advanced age (> 180 months) had a low mitochondrial DNA copy number [13]. However, despite accumulating findings on age-associated abnormalities in oocytes, the primary question remains unsolved: whether these abnormalities in oocytes are caused by lifelong accumulation of molecular and cellular damages in the oocytes, or if they are induced by other factors such as the surrounding environment that support oocytes growth. This review focuses on age-associated changes in granulosa cells and follicular fluid, as well as the relationship between the environment and oocyte qualities.
Granulosa Cells Collected from Aged Cows
In ovaries, most oocytes are quiescent in the form of primordial follicles, which contain several granulosa cells. Once follicles are activated, the morphology of granulosa cells changes from flattened to cuboidal, and they proliferate to become multi-layered, surrounding the oocytes. At the preantral follicle stage, oocytes and granulosa cell complexes form an antral like cavity, which is filled with follicular fluid. The interactions among oocytes and granulosa cells, as well as follicular fluid, are crucial for oocytes growth [22]. Oocyte growth and granulosa cell number are affected by physical conditions. When the diameters of oocytes collected from antral follicles (AF; 3–5 mm in diameter) were compared between young and aged cows, those from aged cows were smaller than those from younger cows [23], which is consistent with previous reports in humans [24]. Interestingly, when comparing the diameter of bovine oocytes collected from early antral follicles (EAFs; 0.5–0.7 mm in diameter), oocytes from aged cows had larger diameters than those from their younger counterparts. In line with this, the number of granulosa cells surrounding the oocyte in AFs was lower in aged cows than in their younger counterparts, whereas those in EAFs were similar between the two age groups [23]. Furthermore, the developmental ability of oocyte-granulosa cell complexes collected from the EAFs of aged cows was lower than that in the younger cows. These results prompted us to address the relationship between granulosa cells and oocyte growth.
Molecular Background of Granulosa Cell Proliferation
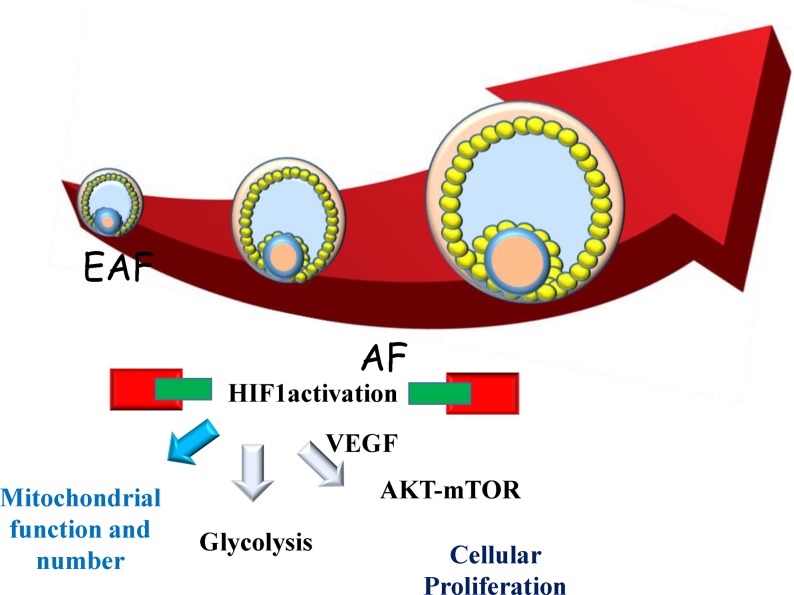
To better understand the molecular background of follicular development, we conducted RNA-seq of porcine granulosa cells of EAFs (0.5–0.7 mm in diameter) and AFs (1–3 mm in diameter). The analysis predicted that inhibition of TP53 and SP1 is a key top upstream regulator, indicating that downregulating apoptosis is necessary during rapid granulosa proliferation along with follicular development [25]. In addition, those authors showed that HIF1 activation is predicted to be an upstream regulator. HIF1 is a basic helix-loop-helix transcriptional factor comprised of a heterodimeric complex of alpha (HIF1A) and beta (ARNT) subunits, which regulates versatile cellular metabolisms, including glycolysis and angiogenesis. In addition, most genes associated with glycolysis were upregulated in granulosa cells during the transition of EAFs to AFs [25]. In the follicle, oxygen concentration is maintained at low levels [26], which is the normal condition for granulosa cell proliferation in vivo. To examine the effect of hypoxic conditions on the metabolism and proliferation of granulosa cells, granulosa cells were cultured under high or low oxygen concentrations, and gene expression was compared between the two oxygen levels using NGS [27]. In that study, hypoxia induced cellular proliferation and increased the expression levels of HIF1 and HIF1-regulated genes concomitant with the activation of glycolytic genes and the suppression of oxidative phosphorylation (OXPHOS)-related genes. In line with this, hypoxia increased the protein levels of HIF1 and the activation of PFK. It was also reported that low oxygen stimuli in mice enhanced the expression of glycolytic genes in GCs, as well as the survival rate and development of follicles [28]. Furthermore, oocytes themselves activate glycolytic genes through the secretion of cytokines in mice and pigs [29,30,31]. Contrary to the activation of glycolysis, hypoxic conditions decreased mitochondrial functions and quantity, as determined by mitochondrial mass and DNA copy number [27]. These data indicate that high glycolysis and low OXPHOS represent a primary metabolic state of granulosa cells for follicle growth (Fig. 1). During follicular development, the number of granulosa cells expands such that EAFs contains 8000 granulosa cells, which increases to 1390000 in AFs in pigs [32]. Gene expression analysis shows the apparent upregulation of VEGF during the EAF–AF transition [25]. Furthermore, Shiratsuki et al. [27] showed that low oxygen-induced granulosa cell proliferation is dependent on the HIF1-VEGF-AKT-mTOR pathways (Fig. 1). The importance of VEGF on the level of AKT phosphorylation required for granulosa cells proliferation was examined in rats [33]. Furthermore, Rico et al. [34] used Vegfaδ/δ mice to show that VEGFA deficiency decreases HIF-1α-dependent signaling and consequently decreases antrum formation and luteal formation. From these findings, we can speculate that hypoxia-induced activation of HIF1, activation of the VEGF-AKT-mTOR pathway, and the metabolic shift from OXPHOS to glycolysis are important events for granulosa cell proliferation in developing follicles. However, how aging affects this pathway in granulosa cells remains unclear.
Fig. 1.
Comprehensive gene expression analysis and protein analysis of granulosa cells show that granulosa cells proliferate during follicle development from an early antral follicle (EAFS; 0.5–0.7 mm in diameter) to an antral follicle (AFs; 1–3 mm in diameter), depending on the activation of the HIF1-VEGF-AKT-mTOR pathway and glycolysis as well as the suppression of mitochondrial functions. [25, 27].
Gene Expression in Granulosa Cells
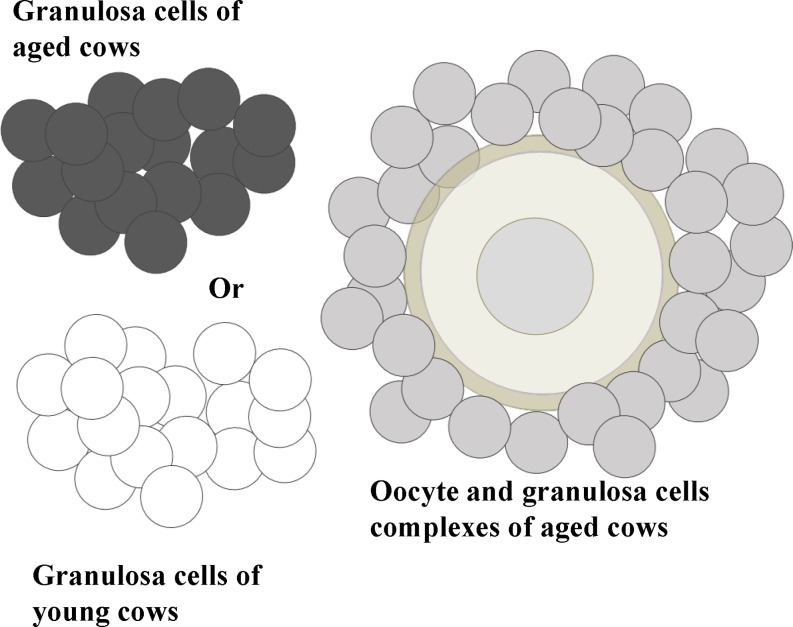
Several studies have investigated granulosa cell marker genes associated with the high developmental competence of oocytes by comparing large healthy follicles and subordinate follicles [35,36,37,38,39]. We conducted RNA-seq using granulosa cells collected from the EAFs of young and aged cows and found that all marker genes related to subordinate follicles were expressed at higher levels in the granulosa cells of aged cows than in those from young cows. Furthermore, many genes related to healthy large follicles were expressed at lower levels in the granulosa cells of aged cows [26]. These results suggest that granulosa cells from the EAFs of aged cows have similar gene expression profiles to those from subordinate follicles. Furthermore, Ingenuity Pathway Analysis (IPA; Qiagen) showed that the genes that were significantly differentially expressed between the granulosa cells of young and aged cows were associated with the oxidative stress response. In line with this prediction, the expression levels of genes related to anti-oxidative ability were lower in granulosa cells from aged cows, and the GSH content in the granulosa cells of aged cows was also significantly lower than that in their younger counterparts [26]. Decreased levels of SOD1, SOD2, and catalase mRNA and proteins in granulosa cells derived from the AFs of older women (≥ 38 years) have also been reported [40]. These findings raise the possibility that age-associated events in oocytes result from the deterioration of granulosa cells surrounding the oocytes. To examine this hypothesis, when oocytes and granulosa cell complexes (OGCs) derived from the EAFs of aged cows were co-incubated with granulosa cells collected from the EAFs of aged or young cows (Fig. 2), only co-incubation with GCs derived from young cows improved the development of OGCs in vitro, the ATP content, and the development of in vitro-grown oocytes (Table 1). These results show that the ability of granulosa cells to support oocyte development deteriorates in aged cows. In the following section, we address how low granulosa cell number and quality affect oocytes, and what is responsible for the low quality of granulosa cells.
Fig. 2.
Oocyte and granulosa cell complexes (OGCs) were collected from the early antral follicles of aged cows and cultured for 16 days. OGCs were cultured with or without a granulosa cell mass, which was collected from randomly selected OGCs of either young or aged cows. The granulosa cell mass was incorporated into OGCs and assisted the development of OGCs.
Table 1. Effect of origns of granulosa cells orign on oocyte growth.
| GC addition | Day 16 Antrum formation | Oocyte ATP (pM) | 24 h after IVF > 8 cell (Mean ± SE%) |
| – | 56.2 ± 4.2 a | 1.94 ± 0.12 a | 12.0 ± 3.0 ab |
| Aged | 69.4 ± 4.1 ab | 2.21 ± 0.14 ab | 5.0 ± 3.1 a |
| Young | 73.3 ± 5.3 b | 2.41 ± 0.10 b | 25.0 ± 5.7 b |
Oocyte and granulosa cell complexes (OGCs) collected from aged cows were cultured with or without additional granulosa cells (GC) for 16 days (Fig. 2), at which antrum formation was determined. GC were collected from OGCs of young or aged cows. In vitro grown oocytes were examined for their ATP content and developmental ability following in vitro maturation and fertilization.
Number of Granulosa Cells and Oocytes
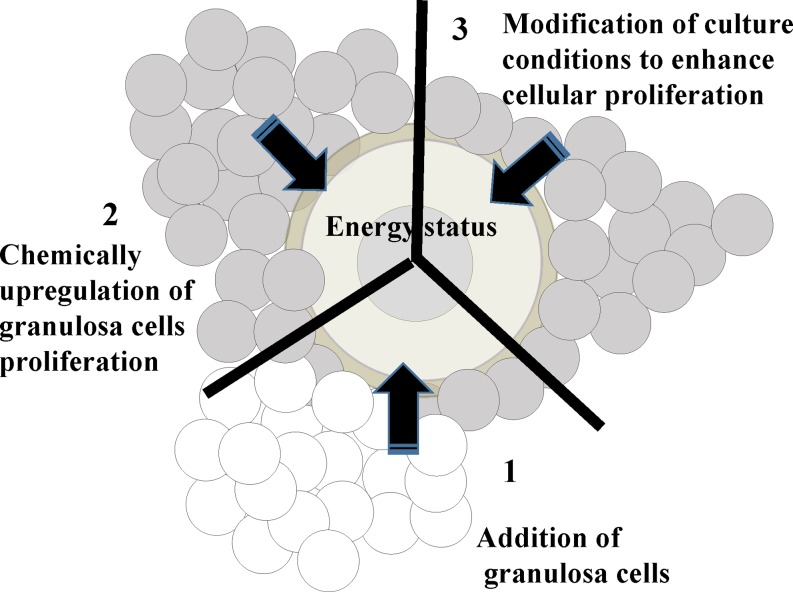
Granulosa cells proliferate along with follicle development and support oocyte development via gap junctional communication and cytokines secreted from each cell in an autocrine- and paracrine-dependent manner [41]. Although myriad studies have addressed the mutual communication that occurs between oocytes and granulosa cells [42, 43], the significance of granulosa cell number for oocyte quality has not been determined. It is well known that metabolites derived from surrounding cells are used to generate ATP in the mitochondria of oocytes [44]. During the growth phase, oocytes accumulate lipids as small droplets in their cytoplasm [45], and oocytes use these lipids as an important energy source for nuclear maturation [46] and early development [47]. We addressed the relationship between the lipid content of oocytes and the number of surrounding cells in porcine ovaries and found a close significant relationship among the average number of granulosa cells contained within a follicle, the average number of cumulus cells surrounding an oocyte, and the average lipid content in an oocyte [48]. Furthermore, this relationship was confirmed in in vitro-developed OGCs. In that study, we cultured OGCs derived from the EAFs of gilts for 16 days; after which, the granulosa cell number, the glucose consumption of the OGCs, and the lipid and ATP content in the enclosed oocytes were compared. The ATP and lipid content in oocytes grown in vitro were significantly correlated with the number of granulosa cells surrounding the oocytes and glucose consumption of the OGCs. Interestingly, granulosa cell number in OGCs was related to the level of histone H4K12 acetylation in enclosed oocytes grown in vitro [48]. During oocyte growth, histone acetylation increases with chromatin condensation [49, 50], and a high level of H4K12 acetylation was observed in human, bovine, and porcine germinal vesicle-stage oocytes [51,52,53]. Energy sufficiency results in high ATP generation, as well as protein acetylation, in cells [54, 55]. Furthermore, acetyl-CoA is derived from citrate, which originates from the mitochondria, and is used for protein acetylation [56]. Next, we hypothesized that the number of granulosa cells that surround the oocyte is a determinant factor for the energy status of the oocytes and that the number of granulosa cells affects the lipid and ATP content in oocytes, as well as their acetylation levels. To examine this notion, we examined the relationship between the energy status of oocytes and the granulosa cell number by in vitro culture of OGCs from the following three possibilities (Fig. 3): 1) Does artificially increasing the number of granulosa cells result in the high-energy status of oocytes grown in vitro? 2) Does chemically upregulating granulosa cell proliferation increase the energy status of oocytes grown in vitro? 3) Does modifying the culture conditions to enhance cellular proliferation result in the high-energy status of oocytes grown in vitro? In all trials, we found that granulosa cell number positively correlated with increased lipid, ATP, and histone acetylation levels in oocytes grown in vitro [15, 57, 58]. Thus, we conclude that the number of granulosa cells surrounding oocytes profoundly regulates the energy status of oocytes, which reflects the ATP, lipid, and acetylation levels found in oocytes (Fig. 4). Together with these results, we speculated that the low acetylation levels and low lipid content of oocytes derived from aged cows were attributable to the low number of granulosa cells in the follicle and that increasing the number of granulosa cells surrounding the oocyte may improve the quality of oocytes in older females.
Fig. 3.
1: Effect of adding granulosa cells to the oocytes and granulosa cell complexes (OGCs) on the energy status of oocytes grown in vitro. OGCs derived from the early antral follicles (EAFs) of young cows were cultured with an additional granulosa cell mass collected from other young cows for 16 days; after which, ATP and lipid content and histone acetylation levels were examined [15]. 2: Effect of chemical stimulation of granulosa cell proliferation on the energy status of oocytes grown in vitro. OGCs of porcine EAFs were cultured in medium supplemented with or without high glucose and insulin, after which the ATP and lipid contents of oocytes grown in vitro were examined [58]. 3: Effects of modifying culture conditions for the proliferation of granulosa cells on the energy status of oocytes grown in vitro. OGCs of EAFs were cultured on acrylamide gels, which enhanced granulosa cell proliferation; after which, ATP and lipid contents as well as the acetylation levels of proteins and histones were compared to those cultured without acrylamide gels [57].
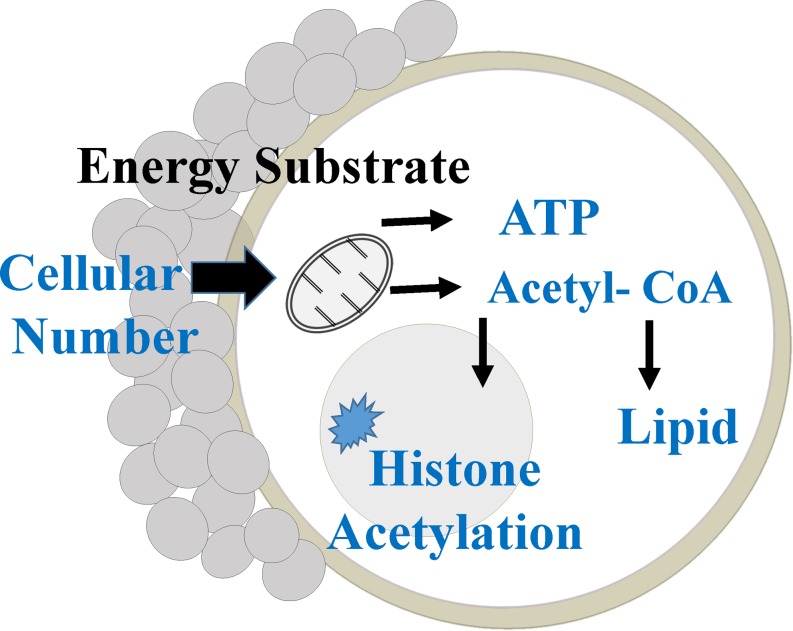
Fig. 4.
Schematic design of factors affecting ATP levels, lipid content, and acetylation of oocytes. Granulosa cells provide energy substrates to oocytes; mitochondria use these substrates to generate ATP, and acetyl CoA derived from mitochondrial citrate is used to generate lipids and acetylation of proteins in oocytes.
Age-associated Changes in the Characteristics of Granulosa Cells
The characteristics of granulosa cells change as the donor ages. In the AFs of aged cows, the number of granulosa cells in follicles was lower than that contained by their younger counterparts [15]. Consistent with this, the levels of proliferation, as measured by the BrdU assay, was low in the granulosa cells of AFs from aged cows [59]. The telomere length of cells is closely linked to their proliferation activity, and an age-associated reduction in telomere length was observed in the granulosa cells of both the EAFs and AFs of cows [14]. The telomere length of granulosa cells or cumulus cells may represent a potential marker for oocyte ability in older women [60]. A comparison of telomere length between oocytes and granulosa cells revealed significant positive correlations in the AFs of cows [14]. Telomeres shorten owing to the end replicable problem, and telomeres are vulnerable to DNA damage due to their GC-rich sequence [61]. The extended exposure of oocytes and granulosa cells to oxidative stress during their lifespan may damage telomeres in both cell types. In line with this, the frequency of double-stranded breaks in DNA from granulosa cells was higher in aged monkeys than in their younger counterparts [62]. Moreover, the amount of reactive oxygen and oxidative damage was higher in the ovaries of aged mice than in those of younger counterparts [63], and low ovary anti-oxidative activities were observed in the granulosa cells of aged humans [40]. Furthermore, high levels of p38 MAPK expression induced by high levels of ROS were observed in human granulosa cells [64]. Consistent with this report, we observed higher p38 MAPK expression in granulosa cells from aged cows compared to that in young cows [65]. Together with these results, it is speculated that age-associated high ROS generation in ovaries plays a role in the high level of DNA damage and telomere shortening that occurs in granulosa cells. Thus, the accumulation of molecular and cellular damage, including shorter telomere lengths, prevents granulosa cells from actively proliferating; however, the molecular targets of high ROS and the causal factor for high ROS generation remain elusive.
Follicular Fluid Affects Granulosa Cell Proliferation and Oocyte Maturation
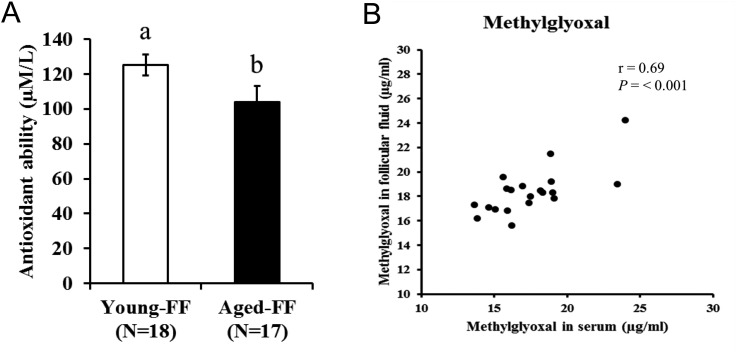
Oocytes grow in follicles, which are filled with follicular fluid derived from serum and granulosa cell secretions. The characteristics of follicular fluid reflect those of the serum and are affected by physical conditions and aging in cows [65, 66]. Follicular fluid contents include hormones, proteins, peptides, amino acids, and molecules with antioxidant and anti-apoptotic abilities [67]. An imbalance in the pro-oxidant and antioxidative ability of follicular fluid is suggested as a causal factor for low oocyte quality [22], because low glutathione peroxidase and superoxide dismutase were reported in the follicular fluid and cumulus cells of aged women [68, 69]. Furthermore, when culturing oocytes in medium containing follicular fluid derived from aged cows, the ROS content in oocytes was greater compared with that in oocytes cultured in follicular fluid derived from younger cows [65]. Moreover, low anti-oxidative abilities in the follicular fluid of aged cows were observed (Fig. 5-A). Takeo et al. [65] reported that supplementation of in vitro-maturation medium with follicular fluid from aged cows induced fast closure of gap-junctions, high abnormal fertilization, and low developmental competence in oocytes, similar to age-induced abnormalities in bovine oocytes. In the context of a possible causal candidate for the age-associated deleterious effects of follicular fluid, it was reported that supplementation of maturation medium with advanced glycation end products (AGEs) induced oocyte abnormalities in similar ways to induction by the follicular fluid of oocytes from aged cows [65]. Consistent with this, a positive correlation between pentosidine (Pent), a type of AGE, and aging in women was reported [70]. In addition, Pent accumulation in oocytes from primordial and primary follicles was observed in aged women [71]. Considering that AGEs in the follicular fluid of cows were closely associated to that in the serum (Fig. 5-B), age-associated physical changes indirectly hamper proper oocyte development.
Fig. 5.
A: Antioxidant ability of follicular fluid. Follicular fluid was collected from the antral follicles of ovaries, which were collected from young (27–50 months of age) and aged (> 120 months age) Japanese Black cows from a slaughterhouse. Antioxidative abilities were measured using a test kit for potential antioxidant (PAO; Japan Institute for the Control of Aging, NIKKEN SEIL, Shizuoka, Japan). B: Correlation of methylglyoxal between follicular fluid and serum. Follicular fluid was collected from Japanese Black cows following collection of serum and slaughter. Methylglyoxal was measured using the Methylglyoxal (MG) competitive ELISA kit (Cell Biolabs, San Diego, CA, USA).
Perspective
In large domestic animals, oocytes take a long time to reach their full growth size, and the surrounding environment is crucial for their proper development. Studies have revealed that aging affects granulosa cell quality and quantity as well as the characteristics of follicular fluid, which in turn deteriorates oocyte development. This raises the possibility that, under proper conditions, some of the age-associated decline in oocytes associated with older females could be improved.
Acknowledgments
This study was supported by the Promotion and Mutual Aid Corporation for Private Schools of Japan and the Ministry of Education, Culture, Sports, Science, and Technology (Grants-in-Aid for Scientific Research [S0801025]) and the Grant-in-Aid for Scientific Research C (KAKENHI, grant number: 16K07996) from the Japan Society for the Promotion of Science. I express my sincere gratitude to Professor Koji Kimura and Dr Shuichi Matsuyama for kindly providing follicular fluid and serum from young and aged cows.
References
- 1.Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril 1996; 65: 783–790. [DOI] [PubMed] [Google Scholar]
- 2.Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril 2004; 81: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 3.Assisted reproductive technology success rates 2005, National summary and fertility clinic reports. [DOI] [PubMed] [Google Scholar]
- 4.Adams GP. Comparative patterns of follicle development and selection in ruminants. J Reprod Fertil Suppl 1999; 54: 17–32. [PubMed] [Google Scholar]
- 5.Ireland JJ, Mihm M, Austin E, Diskin MG, Roche JF. Historical perspective of turnover of dominant follicles during the bovine estrous cycle: key concepts, studies, advancements, and terms. J Dairy Sci 2000; 83: 1648–1658. [DOI] [PubMed] [Google Scholar]
- 6.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003; 80: 116–122. [DOI] [PubMed] [Google Scholar]
- 7.Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod 2003; 69: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 8.Adams GP, Jaiswal R, Singh J, Malhi P. Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 2008; 69: 72–80. [DOI] [PubMed] [Google Scholar]
- 9.Erickson BH, Reynolds RA, Murphree RL. Ovarian characteristics and reproductive performance of the aged cow. Biol Reprod 1976; 15: 555–560. [DOI] [PubMed] [Google Scholar]
- 10.Malhi PS, Adams GP, Mapletoft RJ, Singh J. Oocyte developmental competence in a bovine model of reproductive aging. Reproduction 2007; 134: 233–239. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Iwata H, Goto H, Shiratuki S, Tanaka H, Monji Y, Kuwayama T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol Reprod Dev 2010; 77: 595–604. [DOI] [PubMed] [Google Scholar]
- 12.Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev 2011; 23: 424–432. [DOI] [PubMed] [Google Scholar]
- 13.Takeo S, Goto H, Kuwayama T, Monji Y, Iwata H. Effect of maternal age on the ratio of cleavage and mitochondrial DNA copy number in early developmental stage bovine embryos. J Reprod Dev 2013; 59: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kin A, Kansaku K, Sumiya M, Itami N, Sirasuna K, Kuwayama T, Iwata H. Effect of aging on telomeres of oocytes and granulosa cells in cows. J Mamm Ova Res 2017; 34: 37–43. [Google Scholar]
- 15.Sugiyama M, Sumiya M, Shirasuna K, Kuwayama T, Iwata H. Addition of granulosa cell mass to the culture medium of oocytes derived from early antral follicles increases oocyte growth, ATP content, and acetylation of H4K12. Zygote 2016; 24: 848–856. [DOI] [PubMed] [Google Scholar]
- 16.Takeo S, Kawahara-Miki R, Goto H, Cao F, Kimura K, Monji Y, Kuwayama T, Iwata H. Age-associated changes in gene expression and developmental competence of bovine oocytes, and a possible countermeasure against age-associated events. Mol Reprod Dev 2013; 80: 508–521. [DOI] [PubMed] [Google Scholar]
- 17.Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod 2001; 16: 909–917. [DOI] [PubMed] [Google Scholar]
- 18.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 2004; 13: 2263–2278. [DOI] [PubMed] [Google Scholar]
- 19.Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion 2004; 4: 577–600. [DOI] [PubMed] [Google Scholar]
- 20.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005; 309: 481–484. [DOI] [PubMed] [Google Scholar]
- 21.Wang LY, Wang DH, Zou XY, Xu CM. Mitochondrial functions on oocytes and preimplantation embryos. J Zhejiang Univ Sci B 2009; 10: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 2015; 103: 303–316. [DOI] [PubMed] [Google Scholar]
- 23.Itami N, Kawahara-Miki R, Kawana H, Endo M, Kuwayama T, Iwata H. Age-associated changes in bovine oocytes and granulosa cell complexes collected from early antral follicles. J Assist Reprod Genet 2014; 31: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valeri C, Pappalardo S, De Felici M, Manna C. Correlation of oocyte morphometry parameters with womans age. J Assist Reprod Genet 2011; 28: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munakata Y, Kawahara-Miki R, Shiratsuki S, Tasaki H, Itami N, Shirasuna K, Kuwayama T, Iwata H. Gene expression patterns in granulosa cells and oocytes at various stages of follicle development as well as in in vitro grown oocyte-and-granulosa cell complexes. J Reprod Dev 2016; 62: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 1997; 12: 1047–1055. [DOI] [PubMed] [Google Scholar]
- 27.Shiratsuki S, Hara T, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol 2016; 437: 75–85. [DOI] [PubMed] [Google Scholar]
- 28.Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am J Physiol Endocrinol Metab 2014; 306: E893–E903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 2009; 27: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, OBrien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007; 134: 2593–2603. [DOI] [PubMed] [Google Scholar]
- 31.Matsuno Y, Onuma A, Fujioka YA, Emori C, Fujii W, Naito K, Sugiura K. Effects of porcine oocytes on the expression levels of transcripts encoding glycolytic enzymes in granulosa cells. Anim Sci J 2016; 87: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 32.Oi A, Tasaki H, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Effects of reaggregated granulosa cells and oocytes derived from early antral follicles on the properties of oocytes grown in vitro. J Reprod Dev 2015; 61: 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irusta G, Abramovich D, Parborell F, Tesone M. Direct survival role of vascular endothelial growth factor (VEGF) on rat ovarian follicular cells. Mol Cell Endocrinol 2010; 325: 93–100. [DOI] [PubMed] [Google Scholar]
- 34.Rico C, Dodelet-Devillers A, Paquet M, Tsoi M, Lapointe E, Carmeliet P, Boerboom D. HIF1 activity in granulosa cells is required for FSH-regulated Vegfa expression and follicle survival in mice. Biol Reprod 2014; 90: 135. [DOI] [PubMed] [Google Scholar]
- 35.Greenaway J, Gentry PA, Feige JJ, LaMarre J, Petrik JJ. Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod 2005; 72: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 36.Chen AQ, Wang ZG, Xu ZR, Yu SD, Yang ZG. Analysis of gene expression in granulosa cells of ovine antral growing follicles using suppressive subtractive hybridization. Anim Reprod Sci 2009; 115: 39–48. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi KG, Ushizawa K, Hosoe M, Takahashi T. Differential genome-wide gene expression profiling of bovine largest and second-largest follicles: identification of genes associated with growth of dominant follicles. Reprod Biol Endocrinol 2010; 8: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction 2010; 140: 835–852. [DOI] [PubMed] [Google Scholar]
- 39.Mora JM, Fenwick MA, Castle L, Baithun M, Ryder TA, Mobberley M, Carzaniga R, Franks S, Hardy K. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol Reprod 2012; 86: 153–: 1–14.. [DOI] [PubMed] [Google Scholar]
- 40.Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod 2006; 12: 655–660. [DOI] [PubMed] [Google Scholar]
- 41.Pepling M. Oocyte development before and during folliculogenesis. In: Oocyte Physiology and Development in Domestic Animals. Wiley-Blackwell; 2013: 1−19.
- 42.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res 2009; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binelli M, Murphy BD. Coordinated regulation of follicle development by germ and somatic cells. Reprod Fertil Dev 2010; 22: 1–12. [DOI] [PubMed] [Google Scholar]
- 44.Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci 2015; 72: 251–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva RC, Báo SN, Jivago JL, Lucci CM. Ultrastructural characterization of porcine oocytes and adjacent follicular cells during follicle development: lipid component evolution. Theriogenology 2011; 76: 1647–1657. [DOI] [PubMed] [Google Scholar]
- 46.Elis S, Desmarchais A, Maillard V, Uzbekova S, Monget P, Dupont J. Cell proliferation and progesterone synthesis depend on lipid metabolism in bovine granulosa cells. Theriogenology 2015; 83: 840–853. [DOI] [PubMed] [Google Scholar]
- 47.Jeong WJ, Cho SJ, Lee HS, Deb GK, Lee YS, Kwon TH, Kong IK. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology 2009; 72: 584–589. [DOI] [PubMed] [Google Scholar]
- 48.Munakata Y, Ichinose T, Ogawa K, Itami N, Tasaki H, Shirasuna K, Kuwayama T, Iwata H. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology 2016; 86: 1789–1798.e1. [DOI] [PubMed] [Google Scholar]
- 49.Franciosi F, Lodde V, Goudet G, Duchamp G, Deleuze S, Douet C, Tessaro I, Luciano AM. Changes in histone H4 acetylation during in vivo versus in vitro maturation of equine oocytes. Mol Hum Reprod 2012; 18: 243–252. [DOI] [PubMed] [Google Scholar]
- 50.Bui HT, Van Thuan N, Kishigami S, Wakayama S, Hikichi T, Ohta H, Mizutani E, Yamaoka E, Wakayama T, Miyano T. Regulation of chromatin and chromosome morphology by histone H3 modifications in pig oocytes. Reproduction 2007; 133: 371–382. [DOI] [PubMed] [Google Scholar]
- 51.Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 2007; 133: 85–94. [DOI] [PubMed] [Google Scholar]
- 52.Racedo SE, Wrenzycki C, Lepikhov K, Salamone D, Walter J, Niemann H. Epigenetic modifications and related mRNA expression during bovine oocyte in vitro maturation. Reprod Fertil Dev 2009; 21: 738–748. [DOI] [PubMed] [Google Scholar]
- 53.van den Berg IM, Eleveld C, van der Hoeven M, Birnie E, Steegers EA, Galjaard RJ, Laven JS, van Doorninck JH. Defective deacetylation of histone 4 K12 in human oocytes is associated with advanced maternal age and chromosome misalignment. Hum Reprod 2011; 26: 1181–1190. [DOI] [PubMed] [Google Scholar]
- 54.Morrish F, Noonan J, Perez-Olsen C, Gafken PR, Fitzgibbon M, Kelleher J, VanGilst M, Hockenbery D. Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. J Biol Chem 2010; 285: 36267–36274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghanta S, Grossmann RE, Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol 2013; 48: 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morciano P, Carrisi C, Capobianco L, Mannini L, Burgio G, Cestra G, De Benedetto GE, Corona DF, Musio A, Cenci G. A conserved role for the mitochondrial citrate transporter Sea/SLC25A1 in the maintenance of chromosome integrity. Hum Mol Genet 2009; 18: 4180–4188. [DOI] [PubMed] [Google Scholar]
- 57.Munakata Y, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Polyacrylamide gel as a culture substrate improves in vitro oocyte growth from porcine early antral follicles. Mol Reprod Dev 2017; 84: 44–54. [DOI] [PubMed] [Google Scholar]
- 58.Itami N, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Promotion of glucose utilization by insulin enhances granulosa cell proliferation and developmental competence of porcine oocyte grown in vitro. Zygote 2017; 25: 65–74. [DOI] [PubMed] [Google Scholar]
- 59.Goto H, Iwata H, Takeo S, Nisinosono K, Murakami S, Monji Y, Kuwayama T. Effect of bovine age on the proliferative activity, global DNA methylation, relative telomere length and telomerase activity of granulosa cells. Zygote 2013; 21: 256–264. [DOI] [PubMed] [Google Scholar]
- 60.Cheng EH, Chen SU, Lee TH, Pai YP, Huang LS, Huang CC, Lee MS. Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum Reprod 2013; 28: 929–936. [DOI] [PubMed] [Google Scholar]
- 61.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol Life Sci 2007; 64: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, Wu X, Keefe DL, Liu L. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet 2015; 32: 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod 2011; 84: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito M, Miyado K, Nakagawa K, Muraki M, Imai M, Yamakawa N, Qin J, Hosoi Y, Saito H, Takahashi Y. Age-associated changes in the subcellular localization of phosphorylated p38 MAPK in human granulosa cells. Mol Hum Reprod 2010; 16: 928–937. [DOI] [PubMed] [Google Scholar]
- 65.Takeo S, Kimura K, Shirasuna K, Kuwayama T, Iwata H. Age-associated deterioration in follicular fluid induces a decline in bovine oocyte quality. Reprod Fertil Dev 2016. (In press). [DOI] [PubMed] [Google Scholar]
- 66.Tanaka H, Shibano K, Monji Y, Kuwayama T, Iwata H. Liver condition affects bovine oocyte qualities by changing the characteristics of follicular fluid and plasma. Reprod Domest Anim 2013; 48: 619–626. [DOI] [PubMed] [Google Scholar]
- 67.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 2009; 7: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod 2003; 9: 639–643. [DOI] [PubMed] [Google Scholar]
- 69.Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod 2009; 15: 411–419. [DOI] [PubMed] [Google Scholar]
- 70.Jinno M, Takeuchi M, Watanabe A, Teruya K, Hirohama J, Eguchi N, Miyazaki A. Advanced glycation end-products accumulation compromises embryonic development and achievement of pregnancy by assisted reproductive technology. Hum Reprod 2011; 26: 604–610. [DOI] [PubMed] [Google Scholar]
- 71.Matsumine M, Shibata N, Ishitani K, Kobayashi M, Ohta H. Pentosidine accumulation in human oocytes and their correlation to age-related apoptosis. Acta Histochem Cytochem 2008; 41: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]