Abstract
Summer heat stress decreases the pregnancy rate in cattle and has been thought to be associated with the early embryonic death caused by the elevation of maternal body temperature. In vitro cultures have been widely used for the evaluation of effects of heat stress on oocytes, fertilization, preimplantation, and embryonic development. Susceptibility to heat stress is present in developmental stages from oocytes to cleavage-stage (before embryonic gene activation, EGA) embryos, leading to a consequent decrease in developmental competence. On the other hand, advanced-stage embryos such as morula or blastocysts have acquired thermotolerance. The mechanism for the developmental stage-dependent change in thermotolerance is considered to be the accumulation of antioxidants in embryos in response to heat-inducible production of reactive oxygen species. The supplementation of antioxidants to the culture media has been known to neutralize the detrimental effects of heat stress. Besides, EGA could be involved in acquisition of thermotolerance in later stages of embryos. Morulae or blastocysts can repair heat-induced unfolded proteins or prevent DNA damage occurring in processes such as apoptosis. Therefore, embryo transfer (ET) that can bypass the heat-sensitive stage could be a good solution to improve the pregnancy rate under heat stress. However, frozen-thawed ET could not improve the pregnancy rate as expected. Frozen-thawed blastocysts were more sensitive to heat stress and showed less proliferation upon heat exposure, compared to fresh blastocysts. Therefore, further research is required to improve the reduction in pregnancy rates due to summer heat stress.
Keywords: Bovine, Heat stress, In vitro culture, Oxidative stress, Preimplantation embryo
Recently, global warming has had an impact worldwide. The elevation of ambient temperature, especially in summer, is a critical issue in the agricultural industry. Livestock productivity is also affected by summer heat stress, indicated by reduction in milk yield, dietary intake, daily gain, and reproductivity. One of the most important effects of heat stress in the livestock industry is the decrease in cattle reproductivity. It has been reported since the 1990s that the pregnancy rate by artificial insemination (AI) in dairy cattle dramatically decreased in the summers [1,2,3,4,5]. A major reason for this adverse effect is considered the elevation of maternal body temperature, which disrupts the ovarian and uterine functions [4, 6,7,8,9,10,11,12]. Besides, it is well known that heat stress induces embryonic mortality [13,14,15,16,17,18,19,20,21]. Elevation of maternal body temperature affects various aspects related to pregnancy such as secretion of reproductive hormones, oocyte quality, success of fertilization, and embryo development [5, 7,8,9, 12, 16, 21]. In vitro embryo production technique enables us to artificially culture oocytes and embryos at any temperature and evaluate the mechanism of heat stress-induced early embryonic death in detail. Gendelman et al. had reported that the in vitro culture model is reliable and would be relevant for evaluating the effect of heat stress on oocytes and embryos [22]. Therefore, many in vitro studies have been performed, through the period from oocyte maturation to preimplantation development, by mimicking the maternal body temperature in summer.
Moreover, it has been suggested that heat stress not only affects cell function directly, but also disrupts the DNA or organellar functions by inducing oxidative stress [12, 23, 24]. Oxidative stress agents like reactive oxygen species (ROS) induce DNA damage (leading to apoptosis), lipid peroxidation, and disrupt the mitochondrial function, causing abnormal gene expression and protein synthesis and finally resulting in cell death [23, 24]. Therefore, one factor that adversely affects oocytes and embryos has been considered to be the increase of oxidative stress originating from heat stress [25,26,27]. Besides, the change from maternal gene expression to embryonic gene expression could be associated with thermotolerance in oocytes and embryos. Therefore, this review discusses the stage-dependent change in thermotolerance in relation to oxidative stress and gene expression.
Effect of Heat Stress on Preimplantation Embryos
Effect of heat stress on oocyte maturation
It has been suggested that exposing cows to heat stress reduces the oocyte quality and affects subsequent embryo development [12, 22, 28,29,30]. To understand the mechanism underlying the effect of heat stress on germinal vesicle (GV)-stage cumulus-oocyte complexes (COCs), in vitro studies were widely performed. GV-stage COCs exposed to high temperatures from 40.0°C to 41.0°C show impaired oocyte nuclear and cytoplasmic maturation, increased abnormal spindle formation, and decreased developmental competence after IVF [12, 31,32,33,34,35,36,37,38]. Thus, heat-stressed oocytes fail to mature and are arrested at the metaphase I stage [12, 32]. Some reports suggest that oocytes are more susceptible to the elevation of temperature during the first 12 h of maturation because the exposure of oocytes to heat stress would hasten the progress of cytoplasmic and nuclear maturation [33,34,35, 39].
Heat stress in GV-stage COCs compromises oocyte functions and induces apoptosis [34, 35, 40, 41], disrupts cytoskeleton components [34], and alters maternal transcription [12, 22, 42], ATP content [43], and mitochondrial functions. Heat stress changes not only oocyte function, but also cumulus cell functions such as matrix metallopeptidase 9 (MMP9) and progesterone secretion [42]. These changes affect the oocyte’s protein synthesis and disrupt the oocyte maturation and further development [12, 42, 43].
Heat stress also induces oxidative stress leading to elevation in ROS levels in oocytes [12, 25, 40, 44]. ROS damage the DNA and induce apoptosis or dysfunction of cellular organelles such as the mitochondria [12]. Thus, ROS production in heat-stressed oocytes would disrupt oocyte quality and viability. However, researchers showed that supplementation of antioxidants during in vitro maturation at high temperature improved oocyte maturation and developmental competence of embryos [12, 25, 40]. Glutathione (GSH), a thiol-containing peptide, is a major antioxidant component in the oocyte stage that scavenges ROS [25, 45]. Some antioxidants increased the glutathione levels and decreased ROS levels of oocytes exposed to heat stress. This indicates that the administration of antioxidants in heat stress conditions could decrease thermal-oxidative stress in oocytes and improve fertility.
Effect of heat stress on in vitro fertilization
In vivo studies suggest that there is a correlation between the ambient temperature on the day of AI and the conception rate [4, 7, 18, 46]. In the case of higher body temperature or ambient temperature on the day of insemination, lower pregnancy rates were observed [4, 18, 46]. In vitro studies have also reported that high temperature during fertilization reduced embryonic competence [37, 44, 47]. However, it is difficult to reveal how heat stress affects the success of fertilization, which involves two factors (oocyte and sperm) that could both be damaged by the high temperature.
As mentioned, oocyte quality is reduced by heat stress. It has also been reported that sperm motility, integrity, and function are reduced by the elevation of temperature [48,49,50,51]. Pre-incubation of sperm at 40.0°C to 42.0°C for 4 h decreased sperm motility and integrity, and increased sperm damage [47, 48, 51]. However, there was no adverse effect on developmental competence when oocytes were fertilized at normal temperature (38.5°C) with sperm pre-incubated at 40.0°C to 42.0°C for 4 h [48, 51].
On the other hand, in vitro fertilization performed at 40.0°C or 41.0°C for 6 h decreased both cleavage and blastocysts rates significantly [47]. There was no difference observed in sperm penetration rate but polyspermy was induced by heat stress [47]. These results indicate that the anti-polyspermy mechanism of oocytes could have been disrupted by heat stress and increase of polyspermy could be part of the reason for the disturbed embryonic development. Zona pellucida (ZP) and cortical granules have important roles in inhibiting polyspermy in mammalian oocytes [52,53,54,55]. Zygotes exposed to high temperatures demonstrated changes in factors associated with prevention of polyspermy by the cortical granule migration by showing shorter ZP digestion time (weaker ZP-hardening) and lower UCHL1 (Ubiquitin C-terminal hydrolase-L1) gene expression [47]. Thus, it can be suggested that heat stress could disrupt the anti-polyspermy mechanism, increase polyspermy, and lead to decreased developmental competence.
Besides the effects on the anti-polyspermy mechanism, direct damage of zygotes can also suppress fertilization success and developmental competence. Mouse zygotes exposed to heat stress showed higher ROS levels and reduced developmental competence [44, 56]. We also evaluated ROS levels and expression levels of genes related to heat stress (heat shock protein 70 (HSP70); HSPA1) in zygotes exposed to 40.0°C for 6 h during in vitro fertilization. It has been reported that the GSH content at the zygote stage is much lower than that at the oocyte stage, which suggests that zygotes would be more sensitive to heat stress. Therefore, the intensity of H2O2 emission, which reflects ROS content in zygotes, was higher in heat stress-fertilized zygotes [47]. Besides, HSPA1 gene expression was induced by heat treatment.
During fertilization, it is likely that oocytes are more sensitive to heat-induced damage than sperm. Low fertilization rates might be due to sperm damage, whereas low embryonic competence could be due to the increase in polyspermy, zygote DNA damage, and changes in maternal transcript abundance associated with oxidative stress originating from heat stress.
Effect of heat stress on embryo development
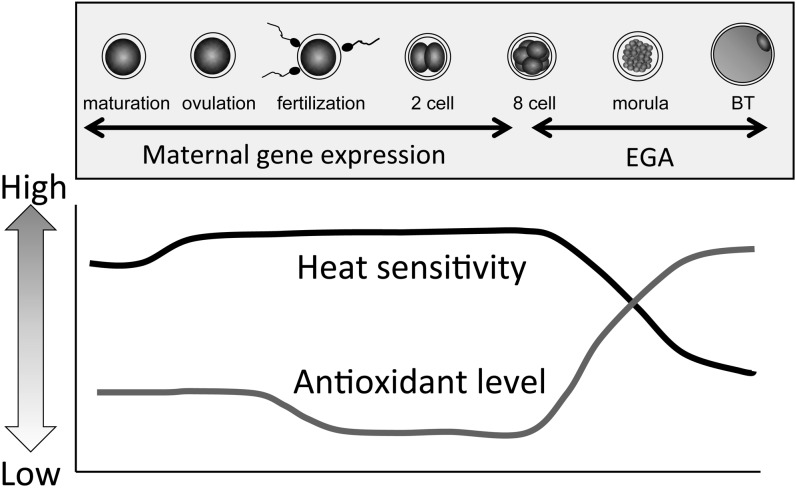
Experimental exposure to heat stress during preimplantation development in maternal body revealed the reduction of the proportion of embryos developing to blastocyst stage [16, 18]. Preimplantation stage embryos have been recognized as stress-sensitive, and the early embryonic loss happens during preimplantation stages. The heat sensitivity of embryos is considered stage dependent [21, 26, 57]. Thus, early stage embryos such as the 1-to 8-cell stage embryo are more susceptible to elevated temperatures than advanced stage embryos such as morulae or blastocysts [26, 27, 58,59,60]. To a varying degree, heat exposure of 40.0°C to 42.0°C significantly decreased developmental competence of 1-to 8-cell stage embryos, but showed little to no effect at morula and blastocyst stages (Fig. 1) [26, 58, 59, 61]. Besides, even if they survived the initial heat stress, early stage embryos exposed to elevated temperatures showed a lower total cell number, especially a reduced trophectoderm cell number in blastocysts [26, 59, 62, 63]. Heat exposure of the early stage embryos causes microfilament and microtubule disruption and mitochondrial swelling, which results in organellar damage [64, 65]. Moreover, the elevation of temperature increases the number of apoptotic cells in 2-cell-stage embryos [66, 67]. Thus, this evidence indicates that heat stress directly damages early stage embryos and leads to decreased developmental competence.
Fig. 1.
Schematic illustration of thermotolerance dynamics during bovine preimplantation development (EGA, embryonic gene activation; BT, blastocysts).
On the other hand, oxidative stress originating from the elevation of temperature is another factor. ROS plays an important role in mediating deleterious effects of elevated temperature on in vivo embryonic development in mouse [44, 56]. Therefore, we exposed embryos at various stages (Days 0, 2, 4 and 6, Day 0 = the day of fertilization) to a temperature of 41.0°C for 6 h in vitro and evaluated the embryonic development and oxidative stress level [26]. Embryos from heat-sensitive stage derived from Days 0 and 2 showed higher ROS levels and lower GSH levels by heat stress, but embryos in Days 4 and 6 had neither their ROS nor GSH levels affected by heat stress (Fig. 1). The amount of the antioxidant glutathione is low during early development and does not increase until the 9-to 16-cell stage [25, 45, 68]. Besides, 2-to 8-cell stage embryos exposed to heat stress in the presence of antioxidants did not show decreased developmental competence and their ROS level was similar to that of embryos not exposed to heat stress [62, 63, 69]. These results indicate that oxidative stress originating from the elevation of temperature could be the main factor inhibiting normal embryonic development and prevention of this increase in oxidative stress would result in the survival of embryos under heat stress.
Based on the aforementioned, the nature of the mechanisms involved in the stage-dependent thermotolerance and antioxidant function can be questioned. One possibility for the high susceptibility of early stage embryos to heat stress is that transcription is limited in this stage [70]. From the zygote to the 8-cell stage embryo, gene expression is of maternal (oocyte) origin; embryonic gene activation (EGA) starts after the 8-cell stage and increases dramatically after the morula stage (Fig. 1) [70]. HSP70, encoded by HSPA1, plays an important role as a molecular chaperone and in the prevention of apoptosis due to various stress conditions including heat stress [71]. Interestingly, HSP70 synthesis and HSPA1 transcription increase in early stage embryos even though EGA does not occur by then [39, 72, 73]. Moreover, HSPA1 transcription level is much higher in 2-cell embryos than in thermotolerant morula stage embryos [59, 67]. However, the fold change in the HSPA1 mRNA level, of heat-stressed morulae is much higher (9.72-fold over morula control) than that of heat-stressed 2-cell stage embryos (1.83-fold over 2-cell control) [59]. An important point here is that HSPs can be divided into two categories: constitutive and inducible [74]. Constitutive HSPs are essential for normal proliferation and are expressed stably under unstressed conditions. On the other hand, expression of inducible HSPs is activated by stress conditions such as heat stress. Thus, the higher HSPA1 expression level in early stage embryos could be that of constitutive HSPs and might contribute less to the heat stress response. Inducible HSPs could be activated by heat stress and mediate the detrimental effect of temperature elevation in advanced stages such as the morula stage [75]. However, this interpretation of HSP70 expression in preimplantation embryos could be controversial. The precise function of HSPs in the heat stress response in embryos is still unclear and needs further consideration.
Global gene expression analysis in thermotolerant morula stage embryos revealed that the expression of some genes related to HSPs was induced by heat stress [75]. Antioxidant genes were less affected by heat stress in the morula stage as this heat stress did not change the ROS production in Days 4 and 6 embryos [26] and antioxidant glutathione levels increased in advanced stage embryos [25, 45]. In addition, expression of genes involved in ubiquitin pathways, which are critical for removal of protein damaged by external stress, was induced by heat stress [75]. Thus, thermotolerance of advanced stage embryos is due in part to developmental mechanisms that prevent accumulation of denatured proteins and oxidative stress damage.
Effect of heat stress on blastocyst
According to previous reports, advanced preimplantation embryos and blastocyst stage embryos acquire thermotolerance and thus would be less damaged on exposure to heat stress [25,26,27, 58, 76]. Therefore, an embryo transfer (ET) that can bypass the heat-sensitive stage (the oocyte maturation, fertilization, and early embryonic stage) is the most promising technology to improve the low pregnancy rate in summer [1, 27, 57, 77,78,79,80]. It has been reported that fresh superovulation or IVF-derived ETs improved the summer pregnancy rate in dairy cattle [17, 19, 79, 81,82,83]. Moreover, ET following AI improved the pregnancy rate of dairy cattle [81, 84]. On the other hand, the quality and the number of embryos derived from superovulation decreased under heat stress conditions [15, 16, 18]. To produce embryos for fresh ET in summer would be inefficient and the utilization of fresh ET must be limited. Therefore, some researchers have evaluated cryopreserved ETs derived from superovulation or IVF. However, the efficacy of cryopreserved ETs in improving summer conception rate in dairy cattle could be controversial or may not be observed [20, 81, 82]. These aspects can be an obstacle to the dissemination of ET techniques to improve the low pregnancy rate in summer.
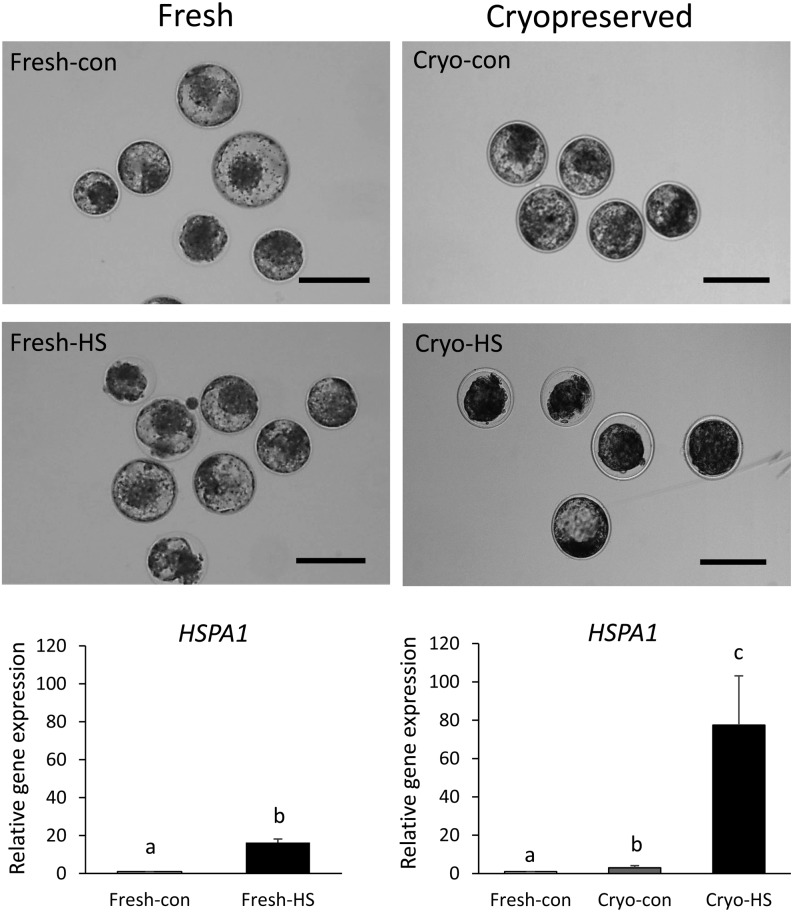
In consideration of these findings, we hypothesized that the cryopreserved blastocysts will be more susceptible to heat stress than fresh blastocysts, possibly owing to thermotolerance. Therefore, the effect of heat stress treatment on viability and gene expression of frozen-thawed cryopreserved blastocysts was evaluated. Firstly, the morphology and gene expression of Day 7 blastocysts exposed to 41.0°C for 6 h were evaluated. Even though HSPA1 expression was induced, expression of blastocyst-specific genes POU5F1 and IFNT and survival rates of embryos were not affected by heat exposure (Fig. 2) [85]. However, the second experiment showed that the viability and the diameter of embryos highly decreased during heat exposure to 41.0°C for 6 h after thawing cryopreserved blastocysts (Fig. 2). Besides, HSPA1 expression was dramatically induced (Fig. 2) and IFNT expression decreased in heat-exposed cryopreserved blastocysts [85]. Thus, these results indicate that the thermotolerance of cryopreserved blastocysts reduces in the process of freezing. Heat stress damage after ET could cause a low conception rate in blastocysts subjected to cryopreserved ET during the summer. Vasques et al. suggested that heat stress after cryopreserved ET retarded trophoblastic function, leading to embryo death [81]. This report agreed with our result that heat-exposed frozen-thawed blastocysts showed lower IFNT expression. Therefore, it is necessary to improve cattle pregnancy in summer by utilizing the ET technique to produce more thermotolerant cryopreserved embryos.
Fig. 2.
Representative images and HSPA1 gene expression of blastocysts exposed to heat stress with or without cryopreservation. Fresh-con: blastocysts incubated at 38.5°C for 6 h from 174 hpi (hours post insemination); Fresh-HS: blastocysts incubated at 41.0°C for 6 h from 174 hpi; Cryo-con: blastocysts incubated at 38.5°C for 6 h after thawing; Cryo-HS: blastocysts incubated at 41.0°C for 6 h after thawing. Scale bar, 200 μm. Relative gene expression (Fresh-con = 1) of HSPA1 gene (mean ± SE), Different letters above bars indicated P < 0.05. Modified from [85].
On the other hand, vitrification has been considered a less cryo-damaging technique than the slow-freezing cryopreservation techniques, because vitrification can eradicate the damage due to ice crystal formation [86]. The viability of embryos after thawing is higher when derived from vitrification than from slow-freezing [86]. Therefore, some researchers applied vitrified ET for improving low summer conception rate in dairy cattle. The vitrified ET improved summer conception rate compared to AI [87]. However, it is still not comparable to fresh ET technique, and the vitrification technique has not become widespread for domestic animals due to challenges in handling observed during ET. Therefore, further studies on such culture methods or freezing processes are needed to apply the cryopreserved (slow-freezing and vitrified) ET to improve the low summer conception rate.
Conclusion and Future Perspectives
Previous studies showed that increase of antioxidant capacity by the supplementation of various antioxidants or chemicals improved oocyte and embryo quality and improved developmental competence under heat stress conditions [12, 25, 40, 41, 45, 60, 62, 63, 69, 88]. Chemical treatments could induce thermotolerance in oocytes or embryos by increasing HSP70 synthesis or anti-apoptosis functions [12, 88]. Modification of embryo cryopreservation could improve the pregnancy rate by employing in vitro frozen ET. It is likely that culturing blastocysts with some cryoprotectants before freezing or using vitrification could increase the cryotolerance, improve embryo survival after thawing [89,90,91], and increase the ET pregnancy rate in summer [87]. Moreover, the genetic differences affecting thermotolerance in oocytes or embryos have been reported between breeds of cattle [38, 80, 92]. Recently, global gene analysis has been applied for domestic animals to reveal differences in single nucleotide polymorphisms (SNPs) and expression of specific genes associated with thermotolerance or fertility in breeds [74, 80]. Studies like these could provide more information on the physiological impacts of heat stress on embryonic developmental competence and could utilize cattle selection or new techniques to improve fertility under heat stress conditions.
In conclusion, heat stress shows its adverse effects through the period from oocyte maturation to preimplantation development. Most of the detrimental effects of heat stress could be due to the oxidative stress originating from the elevation of temperature (Fig. 1). Heat stress and oxidative stress could damage organelles directly or induce DNA damage (leading to apoptosis), alter gene expression, and reduce developmental competence in preimplantation embryos. Oocytes, zygotes, and early stage embryos have low potential for scavenging ROS emissions and cannot mediate heat stress. On the other hand, it is likely that the advanced stage embryos after EGA would have higher ROS scavenging ability and higher ability for the repair of DNA damage or unfolded proteins. Although a number of reports provide proof of the effects of heat stress on preimplantation embryos, further studies would be required to reveal the mechanism underlying thermotolerance and develop techniques to improve the low pregnancy rate due to heat stress.
Acknowledgments
I would like to express my gratitude to the Society for Reproduction and Development (SRD) for granting me with a Young Investigator Award and the opportunity for writing this review. I also thank Dr Masashi Takahashi, Dr Peter J Hansen, and Dr Kenichi Yamanaka for their kind advice and suggestions for my study. I sincerely appreciate the Kumamoto Prefectural Meat Inspection Office for providing the bovine ovaries. Finally, I thank my colleagues and staff in the Kyushu Okinawa Agricultural Research Center, NARO for their contribution and help with my research.
References
- 1.Hansen PJ, Aréchiga CF. Strategies for managing reproduction in the heat-stressed dairy cow. J Anim Sci 1999; 77(Suppl 2): 36–50. [DOI] [PubMed] [Google Scholar]
- 2.García-Ispierto I, López-Gatius F, Santolaria P, Yániz JL, Nogareda C, López-Béjar M, De Rensis F. Relationship between heat stress during the peri-implantation period and early fetal loss in dairy cattle. Theriogenology 2006; 65: 799–807. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Tsuruta S, Bertrand JK, Misztal I, Lawlor TJ, Clay JS. Trends for conception rate of Holsteins over time in the southeastern United States. J Dairy Sci 2009; 92: 4641–4647. [DOI] [PubMed] [Google Scholar]
- 4.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J Reprod Dev 2011; 57: 450–456. [DOI] [PubMed] [Google Scholar]
- 5.De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi RJ. Causes of declining fertility in dairy cows during the warm season. Theriogenology 2017; 91: 145–153. [DOI] [PubMed] [Google Scholar]
- 6.Berman A, Folman Y, Kaim M, Mamen M, Herz Z, Wolfenson D, Arieli A, Graber Y. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J Dairy Sci 1985; 68: 1488–1495. [DOI] [PubMed] [Google Scholar]
- 7.Sartori R, Sartor-Bergfelt R, Mertens SA, Guenther JN, Parrish JJ, Wiltbank MC. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci 2002; 85: 2803–2812. [DOI] [PubMed] [Google Scholar]
- 8.Sartori R, Rosa GJ, Wiltbank MC. Ovarian structures and circulating steroids in heifers and lactating cows in summer and lactating and dry cows in winter. J Dairy Sci 2002; 85: 2813–2822. [DOI] [PubMed] [Google Scholar]
- 9.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cowa review. Theriogenology 2003; 60: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 10.West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci 2003; 86: 2131–2144. [DOI] [PubMed] [Google Scholar]
- 11.Collier RJ, Dahl GE, VanBaale MJ. Major advances associated with environmental effects on dairy cattle. J Dairy Sci 2006; 89: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 12.Roth Z. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Cellular and molecular mechanisms of heat stress related to bovine ovarian function. J Anim Sci 2015; 93: 2034–2044. [DOI] [PubMed] [Google Scholar]
- 13.Dutt RH. Critical period for early embryo mortality in ewes exposed to high ambient temperature. J Anim Sci 1963; 22: 713–719. [Google Scholar]
- 14.Biggers BG, Geisert RD, Wetteman RP, Buchanan DS. Effect of heat stress on early embryonic development in the beef cow. J Anim Sci 1987; 64: 1512–1518. [DOI] [PubMed] [Google Scholar]
- 15.Putney DJ, Drost M, Thatcher WW. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between Days 1 to 7 post insemination. Theriogenology 1988; 30: 195–209. [DOI] [PubMed] [Google Scholar]
- 16.Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS. Embryonic development in super ovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 1989; 19: 37–51. [Google Scholar]
- 17.Putney DJ, Drost M, Thatcher WW. Influence of summer heat stress on pregnancy rates of lactating dairy cattle following embryo transfer or artificial insemination. Theriogenology 1989; 31: 765–778. [DOI] [PubMed] [Google Scholar]
- 18.Ealy AD, Drost M, Hansen PJ. Developmental changes in embryonic resistance to adverse effects of maternal heat stress in cows. J Dairy Sci 1993; 76: 2899–2905. [DOI] [PubMed] [Google Scholar]
- 19.Drost M, Ambrose JD, Thatcher MJ, Cantrell CK, Wolfsdorf KE, Hasler JF, Thatcher WW. Conception rates after artificial insemination or embryo transfer in lactating dairy cows during summer in Florida. Theriogenology 1999; 52: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 20.Dobson H, Tebble JE, Smith RF, Ward WR. Is stress really all that important? Theriogenology 2001; 55: 65–73. [DOI] [PubMed] [Google Scholar]
- 21.Hansen PJ, Drost M, Rivera RM, Paula-Lopes FF, al-Katanani YM, Krininger CE, 3rd, Chase CC., Jr. Adverse impact of heat stress on embryo production: causes and strategies for mitigation. Theriogenology 2001; 55: 91–103. [DOI] [PubMed] [Google Scholar]
- 22.Gendelman M, Roth Z. In vivo vs. in vitro models for studying the effects of elevated temperature on the GV-stage oocyte, subsequent developmental competence and gene expression. Anim Reprod Sci 2012; 134: 125–134. [DOI] [PubMed] [Google Scholar]
- 23.Loven DP. A role for reduced oxygen species in heat induced cell killing and the induction of thermotolerance. Med Hypotheses 1988; 26: 39–50. [DOI] [PubMed] [Google Scholar]
- 24.Lord-Fontaine S, Averill-Bates DA. Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: protection by glucose. Free Radic Biol Med 2002; 32: 752–765. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JL, King WA, Kawarsky SJ, Ealy AD. Responsiveness of early embryos to environmental insults: potential protective roles of HSP70 and glutathione. Theriogenology 2001; 55: 209–223. [DOI] [PubMed] [Google Scholar]
- 26.Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol Reprod Dev 2004; 67: 77–82. [DOI] [PubMed] [Google Scholar]
- 27.Hansen PJ. To be or not to bedeterminants of embryonic survival following heat shock. Theriogenology 2007; 68(Suppl 1): S40–S48. [DOI] [PubMed] [Google Scholar]
- 28.Rocha A, Randel RD, Broussard JR, Lim JM, Blair RM, Roussel JD, Godke RA, Hansel W. High environmental temperature and humidity decrease oocyte quality in Bos taurus but not in Bos indicus cows. Theriogenology 1998; 49: 657–665. [DOI] [PubMed] [Google Scholar]
- 29.Al-Katanani YM, Paula-Lopes FF, Hansen PJ. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J Dairy Sci 2002; 85: 390–396. [DOI] [PubMed] [Google Scholar]
- 30.Takuma T, Sakai S, Ezoe D, Ichimaru H, Jinnouchi T, Kaedei Y, Nagai T, Otoi T. Effects of season and reproductive phase on the quality, quantity and developmental competence of oocytes aspirated from Japanese black cows. J Reprod Dev 2010; 56: 55–59. [DOI] [PubMed] [Google Scholar]
- 31.Ju JC, Parks JE, Yang X. Thermotolerance of IVM-derived bovine oocytes and embryos after short-term heat shock. Mol Reprod Dev 1999; 53: 336–340. [DOI] [PubMed] [Google Scholar]
- 32.Payton RR, Romar R, Coy P, Saxton AM, Lawrence JL, Edwards JL. Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol Reprod 2004; 71: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 33.Edwards JL, Saxton AM, Lawrence JL, Payton RR, Dunlap JR. Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J Dairy Sci 2005; 88: 4326–4333. [DOI] [PubMed] [Google Scholar]
- 34.Roth Z, Hansen PJ. Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol Reprod 2004; 71: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 35.Roth Z, Hansen PJ. Disruption of nuclear maturation and rearrangement of cytoskeletal elements in bovine oocytes exposed to heat shock during maturation. Reproduction 2005; 129: 235–244. [DOI] [PubMed] [Google Scholar]
- 36.Roth Z. Heat stress, the follicle, and its enclosed oocyte: mechanisms and potential strategies to improve fertility in dairy cows. Reprod Domest Anim 2008; 43(Suppl 2): 238–244. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama S, McGowan M, Phillips N, Kafi M, Young M. Effects of increased ambient temperature during IVM and/or IVF on the in vitro development of bovine zygotes. Reprod Domest Anim 2007; 42: 271–274. [DOI] [PubMed] [Google Scholar]
- 38.Paula-Lopes FF, Lima RS, Satrapa RA, Barros CM. Physiology and Endocrinology Symposium: influence of cattle genotype (Bos indicus vs. Bos taurus) on oocyte and preimplantation embryo resistance to increased temperature. J Anim Sci 2013; 91: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 39.Lannett Edwards J, Hansen PJ. Elevated temperature increases heat shock protein 70 synthesis in bovine 2-cell embryos and compromises function of maturing oocytes. Biol Reprod 1996; 55: 340–346. [DOI] [PubMed] [Google Scholar]
- 40.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. The effects of cysteine addition during in vitro maturation on the developmental competence, ROS, GSH and apoptosis level of bovine oocytes exposed to heat stress. Zygote 2012; 20: 249–259. [DOI] [PubMed] [Google Scholar]
- 41.Balboula AZ, Yamanaka K, Sakatani M, Kawahara M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity has a crucial role in the developmental competence of bovine COCs exposed to heat-shock during in vitro maturation. Reproduction 2013; 146: 407–417. [DOI] [PubMed] [Google Scholar]
- 42.Rispoli LA, Payton RR, Gondro C, Saxton AM, Nagle KA, Jenkins BW, Schrick FN, Edwards JL. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013; 146: 193–207. [DOI] [PubMed] [Google Scholar]
- 43.Hooper LM, Payton RR, Rispoli LA, Saxton AM, Edwards JL. Impact of heat stress on germinal vesicle breakdown and lipolytic changes during in vitro maturation of bovine oocytes. J Reprod Dev 2015; 61: 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozawa M, Hirabayashi M, Kanai Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 2002; 124: 683–689. [DOI] [PubMed] [Google Scholar]
- 45.Hansen PJ. Possible roles for heat shock protein 70 and glutathione in protection of the mammalian preimplantation embryo from heat shock. Ann Rev Biomed Sci 1999; 1: 5–29. [Google Scholar]
- 46.Moghaddam A, Karimi I, Pooyanmehr M. Effects of short-term cooling on pregnancy rate of dairy heifers under summer heat stress. Vet Res Commun 2009; 33: 567–575. [DOI] [PubMed] [Google Scholar]
- 47.Sakatani M, Yamanaka K, Balboula AZ, Takenouchi N, Takahashi M. Heat stress during in vitro fertilization decreases fertilization success by disrupting anti-polyspermy systems of the oocytes. Mol Reprod Dev 2015; 82: 36–47. [DOI] [PubMed] [Google Scholar]
- 48.Chandolia RK, Reinertsen EM, Hansen PJ. Short communication: lack of breed differences in responses of bovine spermatozoa to heat shock. J Dairy Sci 1999; 82: 2617–2619. [DOI] [PubMed] [Google Scholar]
- 49.Argov N, Sklan D, Zeron Y, Roth Z. Association between seasonal changes in fatty-acid composition, expression of VLDL receptor and bovine sperm quality. Theriogenology 2007; 67: 878–885. [DOI] [PubMed] [Google Scholar]
- 50.Pérez-Crespo M, Pintado B, Gutiérrez-Adán A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol Reprod Dev 2008; 75: 40–47. [DOI] [PubMed] [Google Scholar]
- 51.Hendricks KEM, Martins L, Hansen PJ. Consequences for the bovine embryo of being derived from a spermatozoon subjected to post-ejaculatory aging and heat shock: development to the blastocyst stage and sex ratio. J Reprod Dev 2009; 55: 69–74. [DOI] [PubMed] [Google Scholar]
- 52.Vincent C, Pickering SJ, Johnson MH. The hardening effect of dimethylsulphoxide on the mouse zona pellucida requires the presence of an oocyte and is associated with a reduction in the number of cortical granules present. J Reprod Fertil 1990; 89: 253–259. [DOI] [PubMed] [Google Scholar]
- 53.Sekiguchi S, Kwon J, Yoshida E, Hamasaki H, Ichinose S, Hideshima M, Kuraoka M, Takahashi A, Ishii Y, Kyuwa S, Wada K, Yoshikawa Y. Localization of ubiquitin C-terminal hydrolase L1 in mouse ova and its function in the plasma membrane to block polyspermy. Am J Pathol 2006; 169: 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coy P, Grullon L, Canovas S, Romar R, Matas C, Aviles M. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 2008; 135: 19–27. [DOI] [PubMed] [Google Scholar]
- 55.Susor A, Liskova L, Toralova T, Pavlok A, Pivonkova K, Karabinova P, Lopatarova M, Sutovsky P, Kubelka M. Role of ubiquitin C-terminal hydrolase-L1 in antipolyspermy defense of mammalian oocytes. Biol Reprod 2010; 82: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 56.Matsuzuka T, Ozawa M, Nakamura A, Ushitani A, Hirabayashi M, Kanai Y. Effects of heat stress on the redox status in the oviduct and early embryonic development in mice. J Reprod Dev 2005; 51: 281–287. [DOI] [PubMed] [Google Scholar]
- 57.Hansen PJ. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 2007; 68(Suppl 1): S242–S249. [DOI] [PubMed] [Google Scholar]
- 58.Edwards JL, Hansen PJ. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev 1997; 46: 138–145. [DOI] [PubMed] [Google Scholar]
- 59.Sakatani M, Alvarez NV, Takahashi M, Hansen PJ. Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J Dairy Sci 2012; 95: 3080–3091. [DOI] [PubMed] [Google Scholar]
- 60.Bonilla AQS, Oliveira LJ, Ozawa M, Newsom EM, Lucy MC, Hansen PJ. Developmental changes in thermoprotective actions of insulin-like growth factor-1 on the preimplantation bovine embryo. Mol Cell Endocrinol 2011; 332: 170–179. [DOI] [PubMed] [Google Scholar]
- 61.Rivera RM, Hansen PJ. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001; 121: 107–115. [PubMed] [Google Scholar]
- 62.Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J Reprod Dev 2007; 53: 605–614. [DOI] [PubMed] [Google Scholar]
- 63.Sakatani M, Yamanaka K, Kobayashi S, Takahashi M. Heat shock-derived reactive oxygen species induce embryonic mortality in in vitro early stage bovine embryos. J Reprod Dev 2008; 54: 496–501. [DOI] [PubMed] [Google Scholar]
- 64.Rivera RM, Kelley KL, Erdos GW, Hansen PJ. Alterations in ultrastructural morphology of two-cell bovine embryos produced in vitro and in vivo following a physiologically relevant heat shock. Biol Reprod 2003; 69: 2068–2077. [DOI] [PubMed] [Google Scholar]
- 65.Rivera RM, Kelley KL, Erdos GW, Hansen PJ. Reorganization of microfilaments and microtubules by thermal stress in two-cell bovine embryos. Biol Reprod 2004; 70: 1852–1862. [DOI] [PubMed] [Google Scholar]
- 66.Paula-Lopes FF, Hansen PJ. Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol Reprod 2002; 66: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 67.Fear JM, Hansen PJ. Developmental changes in expression of genes involved in regulation of apoptosis in the bovine preimplantation embryo. Biol Reprod 2011; 84: 43–51. [DOI] [PubMed] [Google Scholar]
- 68.Lim JM, Liou SS, Hansel W. Intracytoplasmic glutathione concentration and the role of β-mercaptoethanol in preimplantation development of bovine embryos. Theriogenology 1996; 46: 429–439. [DOI] [PubMed] [Google Scholar]
- 69.Ealy AD, Drost M, Barros CM, Hansen PJ. Thermoprotection of preimplantation bovine embryos from heat shock by glutathione and taurine. Cell Biol Int Rep 1992; 16: 125–131. [DOI] [PubMed] [Google Scholar]
- 70.Memili E, First NL. Zygotic and embryonic gene expression in cow: a review of timing and mechanisms of early gene expression as compared with other species. Zygote 2000; 8: 87–96. [DOI] [PubMed] [Google Scholar]
- 71.Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci 2007; 32: 595–610. [DOI] [PubMed] [Google Scholar]
- 72.Chandolia RK, Peltier MR, Tian W, Hansen PJ. Transcriptional control of development, protein synthesis, and heat-induced heat shock protein 70 synthesis in 2-cell bovine embryos. Biol Reprod 1999; 61: 1644–1648. [DOI] [PubMed] [Google Scholar]
- 73.Al-Katanani YM, Hansen PJ. Induced thermotolerance in bovine two-cell embryos and the role of heat shock protein 70 in embryonic development. Mol Reprod Dev 2002; 62: 174–180. [DOI] [PubMed] [Google Scholar]
- 74.Driver AM, Khatib H. Physiology and Endocrinology Symposium: heat shock proteins: potentially powerful markers for preimplantation embryonic development and fertility in livestock species. J Anim Sci 2013; 91: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 75.Sakatani M, Bonilla L, Dobbs KB, Block J, Ozawa M, Shanker S, Yao J, Hansen PJ. Changes in the transcriptome of morula-stage bovine embryos caused by heat shock: relationship to developmental acquisition of thermotolerance. Reprod Biol Endocrinol 2013; 11: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krininger CE, 3rd, Stephens SH, Hansen PJ. Developmental changes in inhibitory effects of arsenic and heat shock on growth of pre-implantation bovine embryos. Mol Reprod Dev 2002; 63: 335–340. [DOI] [PubMed] [Google Scholar]
- 77.Rutledge JJ. Use of embryo transfer and IVF to bypass effects of heat stress. Theriogenology 2001; 55: 105–111. [DOI] [PubMed] [Google Scholar]
- 78.Hansen PJ. Embryo transfer as a tool for fertility enhancement of dairy cattle in hot climates. Acta Sci Vet 2006; 34(Suppl 1): 145–157. [Google Scholar]
- 79.Demetrio DG, Santos RM, Demetrio CG, Vasconcelos JL. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J Dairy Sci 2007; 90: 5073–5082. [DOI] [PubMed] [Google Scholar]
- 80.Hansen PJ. Cellular and molecular basis of therapies to ameliorate effects of heat stress on embryonic development in cattle. Anim Reprod 2013; 10: 322–333. [Google Scholar]
- 81.Vasques MI, Horta AEM, Marques CC, Sasser RG, Humblot P. Levels of bPSPB throughout single and twin pregnancies after AI or transfer of IVM/IVF cattle embryos. Anim Reprod Sci 1995; 38: 279–289. [Google Scholar]
- 82.Ambrose JD, Drost M, Monson RL, Rutledge JJ, Leibfried-Rutledge ML, Thatcher MJ, Kassa T, Binelli M, Hansen PJ, Chenoweth PJ, Thatcher WW. Efficacy of timed embryo transfer with fresh and frozen in vitro produced embryos to increase pregnancy rates in heat-stressed dairy cattle. J Dairy Sci 1999; 82: 2369–2376. [DOI] [PubMed] [Google Scholar]
- 83.Al-Katanani YM, Drost M, Monson RL, Rutledge JJ, Krininger CE, 3rd, Block J, Thatcher WW, Hanse PJ. Pregnancy rates following timed embryo transfer with fresh or vitrified in vitro produced embryos in lactating dairy cows under heat stress conditions. Theriogenology 2002; 58: 171–182. [DOI] [PubMed] [Google Scholar]
- 84.Tani M, Hayashida T, Tomokawa K, Mito Y, Funakoshi D, Tani C, Sakatani M, Takahashi M, Kitahara G, Kamimura S. Effect of embryo transfer following artificial insemination (ETFAI) on reproductive performance in dairy cows in South-Western Japan. J Vet Med Sci 2010; 72: 627–629. [DOI] [PubMed] [Google Scholar]
- 85.Mori M, Hayashi T, Isozaki Y, Takenouchi N, Sakatani M. Heat shock decreases the embryonic quality of frozen-thawed bovine blastocysts produced in vitro. J Reprod Dev 2015; 61: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandawala AA, Harvey SC, Roy TK, Fowler KE. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016; 86: 1637–1644. [DOI] [PubMed] [Google Scholar]
- 87.Stewart BM, Block J, Morelli P, Navarette AE, Amstalden M, Bonilla L, Hansen PJ, Bilby TR. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J Dairy Sci 2011; 94: 3437–3445. [DOI] [PubMed] [Google Scholar]
- 88.Khan I, Lee KL, Xu L, Mesalam A, Chowdhury MM, Joo MD, Ihsan-Ul-Haq, Mirza B, Kong IK. Improvement of in vitro-produced bovine embryo treated with coagulansin-A under heat-stressed condition. Reproduction 2017; 153: 421–431. [DOI] [PubMed] [Google Scholar]
- 89.Block J, Bonilla L, Hansen PJ. Efficacy of in vitro embryo transfer in lactating dairy cows using fresh or vitrified embryos produced in a novel embryo culture medium. J Dairy Sci 2010; 93: 5234–5242. [DOI] [PubMed] [Google Scholar]
- 90.Mori M, Kuwano T, Kamori T, Isozaki Y, Nishihara T, Yamauchi N, Hattori MA. Effect of ATP-binding cassette subfamily B member 1 on bovine blastocyst implantation. Theriogenology 2014; 81: 683–688. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi T, Inaba Y, Somfai T, Kaneda M, Geshi M, Nagai T, Manabe N. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod Fertil Dev 2013; 25: 589–599. [DOI] [PubMed] [Google Scholar]
- 92.Hansen PJ. Genetic variation in resistance of the preimplantation bovine embryo to heat shock. Reprod Fertil Dev 2014; 27: 22–30. [DOI] [PubMed] [Google Scholar]