Abstract
Maternal obesity is a major risk factor for pregnancy complications, causing inflammatory cytokine release in the placenta, including interleukin-1β (IL-1β), IL-6, and IL-8. Pregnant women with obesity develop accelerated systemic and placental inflammation with elevated circulating advanced glycation end products (AGEs). IL-1β is a pivotal inflammatory cytokine associated with obesity and pregnancy complications, and its production is regulated by NLR family pyrin domain-containing 3 (NLRP3) inflammasomes. Here, we investigated whether AGEs are involved in the activation of NLRP3 inflammasomes using human placental tissues and placental cell line. In human placental tissue cultures, AGEs significantly increased IL-1β secretion, as well as IL-1β and NLRP3 mRNA expression. In human placental cell culture, although AGE treatment did not stimulate IL-1β secretion, AGEs significantly increased IL-1β mRNA expression and intracellular IL-1β production. After pre-incubation with AGEs, nano-silica treatment (well known as an inflammasome activator) increased IL-1β secretion in placental cells. However, after pre-incubation with lipopolysaccharide to produce pro-IL-1β, AGE treatment did not affect IL-1β secretion in placental cells. These findings suggest that AGEs stimulate pro-IL-1β production within placental cells, but do not activate inflammasomes to stimulate IL-1β secretion. Furthermore, using pharmacological inhibitors, we demonstrated that AGE-induced inflammatory cytokines are dependent on MAPK/NF-κB/AP-1 signaling and reactive oxygen species production in placental cells. In conclusion, AGEs regulate pro-IL-1β production and inflammatory responses, resulting in the activation of NLRP3 inflammasomes in human placenta. These results suggest that AGEs, as an endogenous and sterile danger signal, may contribute to chronic placental cytokine production.
Keywords: Advanced glycation end products, Inflammation, NLRP3 inflammasome, Placenta
Maternal obesity has recently been increasing in developed countries [1]. Obesity is a major risk factor in pregnancy complications, such as gestational diabetes, spontaneous miscarriage, intrauterine growth restriction, and preeclampsia [1, 2]. Obesity represents a low-grade chronic systemic inflammation [3], and maternal obesity increases the risk of the offspring developing obesity and insulin resistance in the later stages of life [4,5,6,7,8].
The placenta is a vital organ for pregnancy and produces cytokines to regulate placental function [9]. Levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor-α (TNFA), are increased in the serum and placental tissue of pregnant women with obesity when compared with those in pregnant women of normal weight [4, 8, 10, 11]. In addition, pregnant women with obesity also develop accelerated systemic and placental inflammation with elevated circulating advanced glycation end products (AGEs) [10, 12]. AGEs consist of heterogeneous, reactive, and irreversibly crosslinked molecules formed from the non-enzymatic glycation of proteins, lipids, and nucleic acids [13, 14]. In general, AGEs interact with the receptor for advanced glycation end product (RAGE) and/or toll-like receptor 4 (TLR4) to induce inflammatory responses (the production of cytokines) [15, 16]. However, the mechanism underlying these inflammatory responses via AGEs during pregnancy remains unclear.
IL-1β is one of the pivotal inflammatory cytokines associated with obesity and pregnancy complications, and its production is regulated by nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 (NLRP3) inflammasomes [17,18,19,20]. NLRP3 inflammasomes comprise three different proteins—NLRP3, an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 (CASP1, known as IL-1β-converting enzyme). In most cells, optimal IL-1β production is dependent on two separate signals [17]. Signal 1 is a stimulus involving lipopolysaccharide (LPS) and is needed to produce the IL-1β precursor (pro-IL-1β) in cells before inflammasome activation. Signal 2 consists of IL-1β secretion and is regulated by CASP1 activation, which is induced by NLRP3 inflammasome activators, such as ATP, nano-silica, nigericin, or cholesterol crystal [17,18,19,20]. NLRP3 inflammasomes are involved in the pathogenesis of inflammatory diseases, including metabolic syndrome, type 2 diabetes, cardiovascular diseases, gout, and silicosis [17,18,19,20]. It has been reported that uric acid and nano-silica, a known NLRP3 activator, stimulates IL-1β secretion via the NLRP3 inflammasome in human trophoblast cells [21]. In addition, we and other groups have recently reported that pregnancy complications are improved in NLRP3-deficient mice because placental inflammation is suppressed [22,23,24]. However, the role of NLRP3 inflammasomes in placental function is poorly understood.
In the present study, we hypothesized that obesity-related AGEs stimulate IL-1β production and secretion via the NLRP3 inflammasome. To test this hypothesis, we investigated the effect of AGEs on mRNA expression of NLRP3 inflammasome constituted molecules and IL-1β secretion using human placental tissues and human trophoblast cell line in vitro.
Materials and Methods
Tissue collection
Human placentae were obtained from a total of three women, who delivered healthy, singleton infants at term (patients who gave informed consent were obtained using protocols approved by the Jichi Medical University Ethics Committee). Tissues were obtained within 10 min of delivery and dissected fragments were placed in ice-cold phosphate buffered saline (PBS). Placental tissue was blunt dissected to remove visible connective tissue and cut into small pieces (40–60 mg wet weight). These placental tissue pieces were placed on a cell culture insert membrane (0.4 µm membrane, 24-well plate, Thermo Fisher Scientific, Waltham, MA, USA) with 1 ml Dulbecco’s modified Eagle’s medium/F-12 (DMEM/F-12; Life Technologies, Carlsbad, CA, USA) supplemented with antibiotics including amphotericin B and gentamicin (Sigma-Aldrich, St. Louis, MO, USA), and 5% fetal calf serum (FCS, ICN Pharmaceuticals, Costa Mesa, CA, USA).
Cell culture of human trophoblast cell line
Human first-trimester trophoblast cells (Sw.71, trophoblast cell line) were kindly provided by Professor Gil Mor [25]. The Sw.71 cells produced various types of cytokines, including IL-1β, IL-6, IL-8, and TNFA, were used as in vitro model of uterine implantation [26]. It has also been reported that Sw.71 cells express key inflammasome components, including NLRP3 and ASC [27]. Cells were cultured in DMEM/F-12 supplemented with antibiotics, sodium pyruvate (Wako Pure Chemical Industries, Osaka, Japan), non-essential amino acids (Wako Pure Chemical Industries), and 5% FCS. After reaching confluence, the cells were harvested and plated at a concentration of 1 × 105 cells/well in a 48-well culture plate (Thermo Fisher Scientific).
Experimental conditions
Placental tissue culture experiment; human placental tissues were incubated with glycoaldehyde-AGEs-BSA (200 μg/ml; BioVision, Milpitas, CA, USA), BSA as a control for AGEs (BioVision), or LPS (100 ng/ml; Sigma-Aldrich) for 6 h at 37°C. Supernatant and tissues were collected for enzyme-linked immunosorbent assay (ELISA), western blotting, and real-time RT-PCR, and stored at –20°C or –80°C before use.
Placental cell culture experiment; Sw.71 placental cells were washed twice in PBS and treated with AGEs-BSA (200 μg/ml), BSA, or LPS (100 ng/ml) for 24 h at 37°C. After serum starvations and priming with AGEs or LPS for 24 h, cells were treated with nano-silica (100 μg/ml, Micromod Partikeltechnologie GmbH, Rostock, Germany) for 24 h at 37°C, to investigate the role of AGEs on inflammasome activation. To further explore the relationships between AGEs and inflammatory cytokine production, various pharmacological inhibitors, including SB203580 [a mitogen-activated protein kinase (MAPK) p38 inhibitor, 20 μM, Merck Millipore, Belize, MA, USA], SP600125 [a Jun N-terminal kinase (JNK) inhibitor, 20 μM, Merck Millipore], Bay 11-7082 (a NF-κB activation inhibitor, 2 μM, Merck Millipore), SR11302 (an AP-1 inhibitor, 10 μM, Merck Millipore), N-acetyl-L-cysteine (NAC, an antioxidant, 5 mM, Wako Pure Chemical Industries), or diphenyleneiodonium (DPI, a NADPH inhibitor, 2 μM, Sigma-Aldrich) were pre-incubated in the medium for 1 h. Supernatant and cell lysate were collected for ELISA, real-time RT-PCR, or western blot analysis, and stored at –20°C or –80°C before use.
Determination of cytokines
Levels of IL-1β, IL-6, or IL-8 were determined using a human ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Real-time RT-PCR
Total RNA was prepared using ISOGEN (Nippon Gene, Toyama, Japan) according to the manufacturer’s instructions. RNA extraction and cDNA production were performed using a commercial kit (ReverTra Ace; Toyobo, Osaka, Japan). Real-time RT-PCR was performed using the CFX ConnectTM Real Time PCR (Bio-Rad, Hercules, CA, USA) and a commercial kit (Thunderbird SYBR qPCR Mix; Toyobo) to detect mRNA expressions of IL-1β, NLRP3, ASC, CASP1, IL-6, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following antisense and sense primers were used: IL-1β (5′- TGATGGCTTATTACAGTGGCAATG-3′ and 5′- GTAGTGGTGGTGGGAGATTCG-3′, NM000576), NLRP3 (5′-GAGAGACCTTTATGAGAAAGCA-3′ and 5′-GCATATCACAGTGGGATTCGAA-3′, BC143363), ASC (5′-AACCCAAGCAAGATGCGGAAG-3′ and 5′-TTAGGGCCTGGAGGAGCAAG-3′, BC013569), CASP1 (5′- GAAGCTCAAAGGATATGGAAACAAA -3′ and 5′-AAGACGTGTGCGGCTTGACT-3′, X65019), IL-6 (5′- AAATTCGGTACATCCTCGACGG -3′ and 5′- GGAAGGTTCAGGTTGTTTTCTGC-3′, M54894) and GAPDH (5′- AAATGAGCCCCAGCCTTCT-3′ and 5′- AGGATGTCAGCGGGAGCCGG-3′, M33197). RT-qPCR was performed in duplicate with a final reaction volume of 20 µl containing 10 µl SYBR Green, 7.8 µl distilled water, 0.1 µl 100 µM forward and reverse primers, and 2 µl of cDNA template. The amplification program consisted of a 5 min denaturation at 95°C followed by 40 cycles of amplification (95°C for 15 sec, 60°C for 30 sec, and 72°C for 20 sec). Expression levels of each target gene were normalized to corresponding GAPDH threshold cycle (CT) values using the ΔΔ CT comparative method [28].
Western blot analysis
Lysates from the cell culture were prepared using RIPA buffer (Wako Pure Chemical Industries). Cells were washed with cold PBS and incubated with RIPA buffer for 15 min on ice. Cell lysates were subsequently transferred into 1.5 ml tubes and centrifuged at 12,000 × g for 20 min at 4°C. Supernatants were transferred to a fresh tube and stored at –80°C before analysis. A total of 10 µg protein was loaded per lane and separated by 10% SDS-PAGE. The expression of pro-IL-1β and β-actin (ACTB) was analyzed by western blot. After transfer onto polyvinylidene fluoride membranes, nonspecific antibody binding was blocked for 1 h at room temperature using Immunoblock (DS Pharma Biomedical, Osaka, Japan). Then, membranes were incubated for 24 h at 4°C with anti-IL-1β antibody (1:1000, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-ACTB antibody (1:10000, Sigma-Aldrich), followed by an incubation for 1 h with secondary antibody conjugated horseradish peroxidase (HRP; 1:1000, GE Healthcare, UK, Buckinghamshire, UK). Immunoreactive bands were visualized by Western BLoT Quant HRP Substrate (GE Healthcare) using ImageQuant LAS 4000 (GE Healthcare). The results represent at least 3 independent experiments. Quantitative analysis of bands was performed using Image J (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Differences between treatment groups were identified using unpaired t-tests. Multiple comparisons were made using one-way analysis of variance (ANOVA), followed by Bonferroni’s multiple comparison test. A P-value of < 0.05 was considered statistically significant.
Results
Effects of AGEs on inflammatory cytokine production in human placental tissues
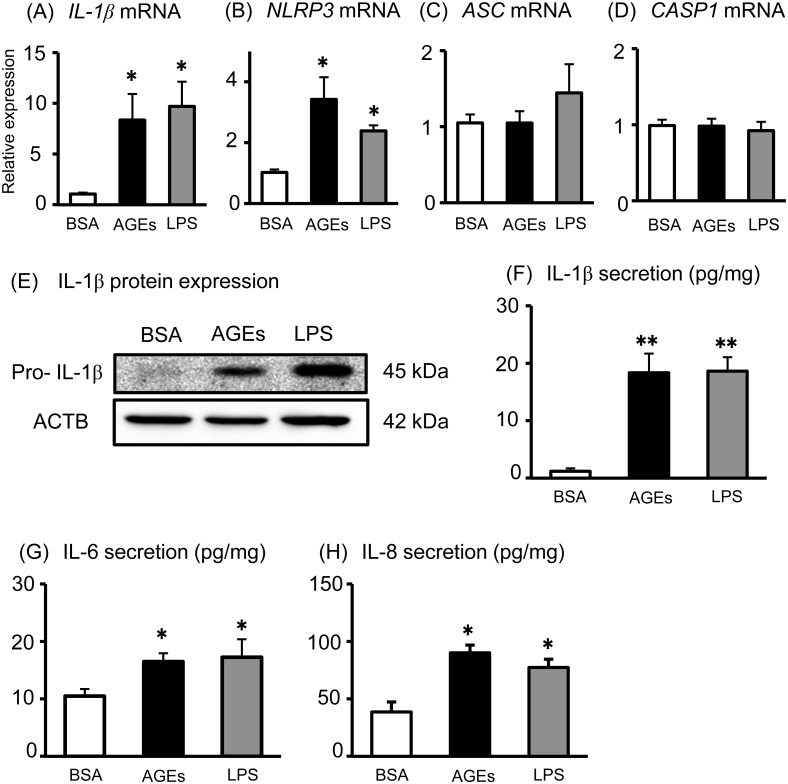
First, we tested whether AGEs could induce inflammatory responses using healthy human placental tissue cultures. LPS was used as a positive control for induction of inflammatory responses. Both AGE and LPS treatments significantly increased IL-1β and NLRP3 mRNA expression (Fig. 1A and B), but did not affect ASC and CASP1 mRNA expression in human placental tissues (Fig. 1C and D). On the other hand, both AGEs and LPS significantly increased pro-IL-1β production (Fig. 1E) and IL-1β secretion (Fig. 1F) in human placental tissues. In the present study, proinflammatory cytokines, including IL-6 and IL-8, were used as positive markers for inflammatory responses, because we previously reported that AGEs and LPS stimulated IL-6 and IL-8 secretion from human placental cells [16]. As expected, both AGEs and LPS significantly increased IL-6, and IL-8 secretion from human placental tissues (Fig. 1G and H). These findings suggest that AGEs up-regulate inflammatory cytokine production and secretion in human placenta.
Fig. 1.
Effects of AGEs on inflammatory cytokine production in human placental tissues. Human placental tissues were incubated for 6 h with BSA, AGEs (200 μg/ml), or LPS (100 ng/ml). (A–D) After incubation, the IL-1β, NLRP3, ASC and CASP1 mRNA levels were measured using qRT-PCR. Pro-IL-1β and ACTB protein levels were subsequently quantified in the tissue lysates by western blot (E). IL-1β (F), IL-6 (G), and IL-8 (H) levels in the supernatant were determined using ELISA. Data are expressed as means ± SEM (n = 4). Significant differences were identified using ANOVA followed by Bonferroni’s multiple comparison test; * P < 0.05 and ** P < 0.01.
Effects of AGEs on IL-1β production in the Sw.71 human placental cell line
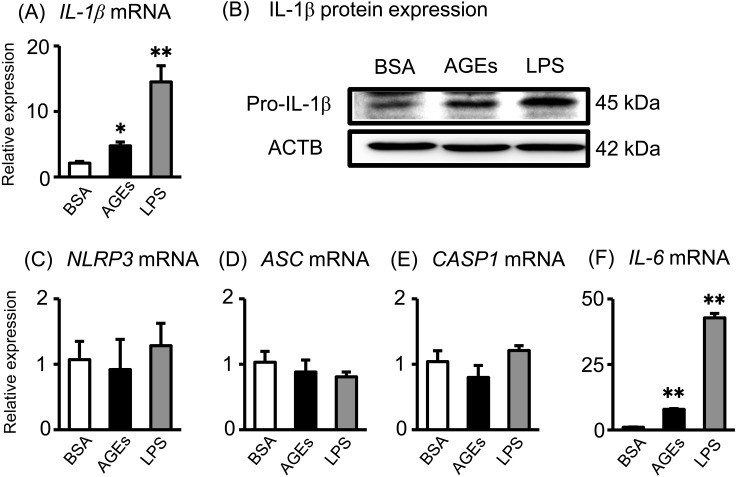
Next, we investigated the effects of AGEs and LPS on IL-1β production systems in Sw.71 cells (a human placental cell line). Both AGEs and LPS significantly increased IL-1β mRNA expression (Fig. 2A) and pro-IL-1β protein production (Fig. 2B) in Sw.71 cells. Contrary to the human placental tissue data, AGE and LPS treatment did not affect NLRP3, ASC and CASP1 mRNA expression (Fig. 2C–E). Previously, we reported that AGEs and LPS stimulate inflammatory cytokine secretion, including IL-6 and IL-8, from human Sw.71 placental cells [16]. To support the previous results, both AGEs and LPS significantly increased IL-6 mRNA expression in Sw.71 cells (Fig. 2F).
Fig. 2.
Effects of AGEs on IL-1β production in the Sw.71 human placental cell line. (A and B) Sw.71 cells were incubated for 24 h with BSA or AGEs (200 μg/ml), and LPS (100 ng/ml); The IL-1β mRNA levels were measured using qRT-PCR. Pro-IL-1β and ACTB protein levels were subsequently quantified in the cell lysates by western blot. (C–F) After incubation, the NLRP3, ASC and CASP1 mRNA levels were measured using qRT-PCR. Significant differences were identified using ANOVA, followed by Bonferroni’s multiple comparison test; * P < 0.05 and ** P < 0.01.
Effects of AGEs on IL-1β secretion in the Sw.71 human placental cell line
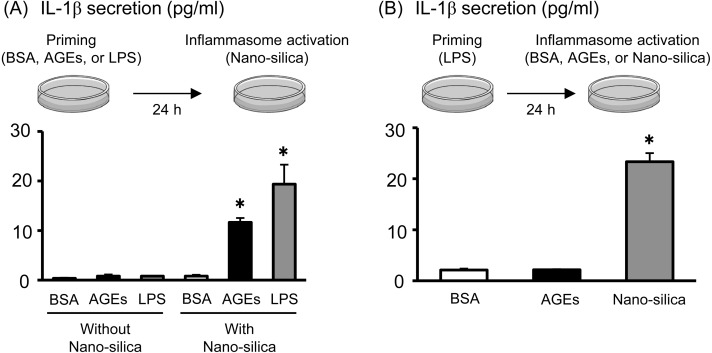
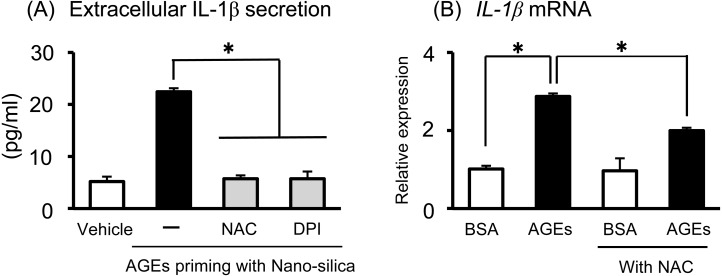
Two signals are required for mature IL-1β secretion from the NLRP3 inflammasome [17]. The first signal is the transcription and translation of pro-IL-1β by priming stimuli, including LPS. The second signal activates autocatalytic cleavage of pro-CASP1 to CASP1, to secrete IL-1β from pro-IL-1β. We used nano-silica for the second signal to activate the NLRP3 inflammasome. As shown in Fig. 3A (left side), both AGEs and LPS alone (without nano-silica) did not affect IL-1β secretion in Sw.71 placental cells. However, after AGE or LPS priming for 24 h to induce pro-IL-1β production within the cells, nano-silica treatment clearly stimulated IL-1β secretion in Sw.71 placental cells (Fig. 3A, right side), indicating the role of AGEs and LPS as “Signal 1” for pro-IL-1β production. We checked the potential role of AGEs as “Signal 2” to activate the inflammasome system in Sw.71 placental cells. After LPS priming, AGE treatment did not affect IL-1β secretion, whereas nano-silica treatment significantly stimulated IL-1β secretion in Sw.71 cells (Fig. 3B). These findings suggest that AGEs stimulate pro-IL-1β production within the cells, but do not activate the inflammasome to stimulate IL-1β secretion.
Fig. 3.
Effects of AGEs on inflammasome activation in the Sw.71 human placental cell line. (A) Sw.71 cells were pre-incubated for 24 h with BSA, AGEs (200 μg/ml), or LPS (100 ng/ml). After priming, cells were treated with nano-silica (100 μg/ml) for 24 h to activate NLRP3 inflammasomes. (B) Sw.71 cells were pre-incubated for 24 h with LPS (100 ng/ml). After priming, cells were treated with BSA, AGE (200 μg/ml) or nano-silica (100 μg/ml) for 24 h. IL-1β levels in the supernatant were determined using ELISA. Data are expressed as mean ± SEM (n = 4). Significant differences were identified using ANOVA, followed by Bonferroni’s multiple comparison test; * P < 0.05 and ** P < 0.01.
Intracellular AGE signaling pathways on inflammatory cytokine production in the Sw.71 human placental cell line
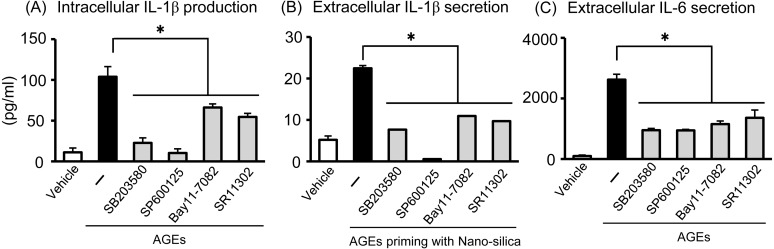
It is well known that MAPK and transcription factors, including NF-κB and AP-1 are important in AGE and LPS signal transduction [29]. To elucidate the signaling mechanisms, we aimed to clarify which of these pathways is involved in AGE- and LPS-amplified inflammatory cytokine production using specific pharmacological inhibitors to block each individual signaling pathway. Blockage of each individual pathway (SB203580 as a p38 MAPK inhibitor, SP600125 as a JNK inhibitor, Bay11-7082 as a NF-κB inhibitor, and SR11302 as an AP-1 inhibitor) significantly attenuated AGEs-induced intracellular IL-1β production (Fig. 4A), extracellular IL-1β secretion stimulated by nano-silica (Fig. 4B), and extracellular IL-6 secretion (Fig. 4C) in human Sw.71 placental cells. These findings indicate that the activation of all of these four pathways is necessary for effective induction of cytokines by AGEs in human Sw.71 cells.
Fig. 4.
Intracellular AGE signaling pathways on inflammatory cytokine production in the Sw.71 human placental cell line. Sw.71 cells were pre-incubated for 1 h with specific pharmacological inhibitors (SB203580 as a p38 MAPK inhibitor, SP600125 as a JNK inhibitor, Bay11-7082 as a NF-κB inhibitor, and SR11302 as an AP-1 inhibitor). (A) After inhibitor treatments, cells were incubated for 24 h with AGEs (200 μg/ml). (B) After inhibitor treatments, cells were incubated for 24 h with AGEs (200 μg/ml), followed by nano-silica (100 μg/ml) treatment for 24 h. (C) After inhibitor treatments, cells were incubated for 24 h with AGEs (200 μg/ml). Intracellular IL-1β, and IL-1β and IL-6 levels in the supernatant were measured using ELISA. Data are expressed as mean ± SEM (n = 4). Significant differences were identified using ANOVA, followed by Bonferroni’s multiple comparison test; * P < 0.05.
Role of ROS for AGE-induced cytokine production in the Sw.71 human placental cell line
The increase in inflammatory cytokine secretion after AGE treatment led us to hypothesize that reactive oxygen species (ROS) production involves AGE-induced inflammation in Sw.71 cells. Indeed, we already reported that AGEs stimulated ROS production and that AGE-induced ROS production is important for stimulating cytokine production, including IL-6 and IL-8 [16]. To investigate the importance of ROS for AGE-induced IL-1β production, we tested the effect of the antioxidants NAC and DPI on Sw.71 cells. Treatment with NAC or DPI significantly decreased the extracellular IL-1β secretion induced by AGE priming with nano-silica (Fig. 5A). In addition, we showed that pre-treatment of NAC attenuated AGE-induced IL-1β mRNA expression (Fig. 5B). Thus, our findings indicated that ROS production is an upstream factor in AGE-induced inflammatory cytokine production, including IL-1β and IL-6, in human placental cells.
Fig. 5.
ROS pathways of AGEs and their effect on inflammatory cytokine production in the Sw.71 human placental cell line. Sw.71 cells were pre-incubated for 1 h with specific pharmacological inhibitors (NAC as an antioxidant, DPI as a NADPH inhibitor). (A) After inhibitor treatments, cells were incubated for 24 h with AGEs (200 μg/ml), and then treated with nano-silica (100 μg/ml) for 24 h. IL-1β levels in the supernatant were determined using ELISA. (B) After NAC treatments, cells were incubated for 24 h with AGEs (200 μg/ml). The IL-1β mRNA levels were measured using qRT-PCR. Data are expressed as mean ± SEM (n = 4). Significant differences were identified using ANOVA, followed by Bonferroni’s multiple comparison test; * P < 0.05.
Discussion
Increasing evidence indicates that AGEs and IL-1β are associated with preeclampsia and obesity in pregnant women [1, 2, 30,31,32]. In the present study, we investigated whether AGEs are involved in IL-1β production and secretion via NLRP3 inflammasomes in human placenta. Our novel results demonstrated that (1) in human placental tissues, AGEs directly increase both the transcription and secretion of IL-1β; (2) In human placental cell line, AGEs stimulate pro-IL-1β production, resulting in the acceleration of mature IL-1β secretion by NLRP3 inflammasome activation; (3) AGE-induced inflammatory cytokines are dependent on MAPK/NF-κB/AP-1 signaling and ROS production.
In human placental tissues, AGE treatment directly stimulated mature IL-1β secretion, together with an increase in IL-1β and NLRP3 mRNA transcription. Similar to our present results, it has been reported that AGEs increased the in vitro release of IL-1β from human placenta in a tissue culture system [12]. However, we showed that AGEs markedly induced pro-IL-1β production, regulating transcription of IL-1β mRNA, whereas AGEs did not stimulate IL-1β secretion in human placental cells. We believed that these differences regarding IL-1β secretion by AGEs were due to the conditional differences between placental cells (cell lines) and placental tissues (composed of many types of cells, including placental cells, endothelial cells, and immune cells). Indeed, numerous immune and inflammatory cells, including macrophages, NK cells, lymphocytes and neutrophils, reside in the placenta and decidua [9]. In addition, activated macrophages are the major secretors of IL-1β via NLRP3 inflammasomes. In fact, AGEs, as well as LPS, independently increased IL-1β secretion by human peritoneal macrophages in a dose-dependent manner [33]. In addition, inflammatory immune cells accumulate in the adipose tissues of obese patients, and AGEs directly increased IL-1β secretion without LPS priming in the adipose tissue of women with gestational diabetes [12]. These findings led us to hypothesize that (1) immune cells within human placental tissues respond to AGE treatment to induce IL-1β secretion, and (2) cell-to-cell interactions (communication between placenta cells and immune cells) within the placenta are important for AGEs to directly induce mature IL-1β secretion. Further investigation is needed to clarify the mechanisms of AGE-induced IL-1β secretion, its related signaling pathways, and the key cell types involved in IL-1β production and secretion in human placental tissues.
Next, we investigated the possible role of AGEs on IL-1β production and secretion using the human Sw.71 placental cell line. We showed that AGEs did not regulate mature IL-1β secretion, but regulated pro-IL-1β production, and nano-silica treatment stimulated IL-1β secretion after AGE-priming in placental cells. Therefore, these results indicate that AGEs act as “Signal 1” to produce pro-IL-1β, not as “Signal 2” to activate NLRP3 inflammasomes within human placental cells, suggesting that AGEs have a key role in pro-IL-1β production following NLRP3 inflammasome activation. Contrary to our present study, Song et al. [34] recently demonstrated that using human primary nucleus pulposus cells, AGEs alone can activate not only pro-IL-1β production as “Signal 1” within the cells, but also the NLRP3 inflammasome assembly on mitochondria as “Signal 2”, resulting in elicitation of mature IL-1β secretion. We considered cell type or cell condition (primary cells isolated from tissues or established cell lines) as possible reasons for differences in experimental outcome.
We further investigated the possible signaling pathways of AGE-induced inflammatory cytokine production in human Sw.71 placental cells. Recently, we reported that exposure to AGEs resulted in the activation of inflammatory responses, inducing IL-6 and IL-8 secretion, and AGE-induced IL-6 secretion was dependent on NF-κB activation through RAGE and/or TLR4 in human Sw.71 placental cells [16]. Accumulating evidence has indicated that activation of both MAPK (p38, JNK, extracellular signal-regulated kinase 1/2) and transcription factors, including NF-κB and AP-1, are required for AGE-amplified inflammatory cytokine production [29, 35, 36]. In the present study, we confirmed that both MAPK (p38 and JNK) and NF-κB/AP-1 are essential for AGE-induced IL-1β production and secretion, as well as IL-6 secretion, in human placental cells.
ROS are harmful mediators of inflammation, and ROS inhibition suppresses NLRP3 inflammasome activation and inflammatory responses [22]. We previously reported that AGEs significantly stimulated ROS production, ROS inhibitor experiments confirmed that ROS are essential for IL-6 secretion by AGEs in human Sw.71 cells [16]. In the present study, we used the ROS inhibitors NAC (an antioxidant) and DPI (an NADPH oxidase inhibitor) to examine the importance of ROS, and found that these significantly blocked AGE-induced IL-1β production, due to the inhibition of IL-1β mRNA transcription, resulting in the suppression of IL-1β secretion via NLRP3 inflammasome activation. Indeed, ROS generation is essential as an upstream factor for NLRP3 inflammasome-dependent IL-1β secretion [37]. Therefore, it appears that ROS production is important for both inflammatory cytokine production and inflammasome activation in placental cells.
In conclusion, we demonstrated that exposure to AGEs resulted in the activation of NLRP3 inflammasome via pro-IL-1β production and inflammatory responses, inducing IL-6 and IL-8 secretion in human placenta. MAPK/NF-κB/AP-1 signaling and ROS production play pivotal roles in AGE-induced inflammatory responses. These results suggest that AGEs, as an endogenous and sterile danger signal, may contribute to chronic placental cytokine production, resulting in pregnancy related complications such as preeclampsia. Further insight into the relationships between placental inflammation, obesity, and pregnancy complications will be useful for developing therapeutic strategies to treat these complications.
Conflict of interest: The authors declare no conflict of interest.
Acknowledgments
This study received grants from the Japan Society for the Promotion of Science (JSPS) through the Scientific Research (C) (KS and HS), and Strategic Research Project from Tokyo University of Agriculture (KS).
References
- 1.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16: 621–638. [DOI] [PubMed] [Google Scholar]
- 2.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006; 113: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 3.Prieto D, Contreras C, Sánchez A. Endothelial dysfunction, obesity and insulin resistance. Curr Vasc Pharmacol 2014; 12: 412–426. [DOI] [PubMed] [Google Scholar]
- 4.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 2008; 29: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 2010; 151: 4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jungheim ES, Louden ED, Chi MM, Frolova AI, Riley JK, Moley KH. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biol Reprod 2011; 85: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 2011; 152: 4158–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aye IL, Jansson T, Powell TL. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol 2013; 381: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offsprings health. Nat Med 2013; 19: 548–556. [DOI] [PubMed] [Google Scholar]
- 10.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Hauguel-de Mouzon S. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011; 19: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011; 32: 247–254. [DOI] [PubMed] [Google Scholar]
- 12.Lappas M. Activation of inflammasomes in adipose tissue of women with gestational diabetes. Mol Cell Endocrinol 2014; 382: 74–83. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988; 318: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 14.John WG, Lamb EJ. The Maillard or browning reaction in diabetes. Eye (Lond) 1993; 7: 230–237. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 2013; 56: 739–744. [DOI] [PubMed] [Google Scholar]
- 16.Shirasuna K, Seno K, Ohtsu A, Shiratsuki S, Ohkuchi A, Suzuki H, Matsubara S, Nagayama S, Iwata H, Kuwayama T. AGEs and HMGB1 increase inflammatory cytokine production from human placental cells, resulting in an enhancement of monocyte migration. Am J Reprod Immunol 2016; 75: 557–568. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 2010; 327: 296–300. [DOI] [PubMed] [Google Scholar]
- 18.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M. Role of the inflammasome in myocardial infarction. Trends Cardiovasc Med 2011; 21: 37–41. [DOI] [PubMed] [Google Scholar]
- 21.Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1β production by human first trimester trophoblast. PLoS ONE 2013; 8: e65237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirasuna K, Usui F, Karasawa T, Kimura H, Kawashima A, Mizukami H, Ohkuchi A, Nishimura S, Sagara J, Noda T, Ozawa K, Taniguchi S, Takahashi M. Nanosilica-induced placental inflammation and pregnancy complications: Different roles of the inflammasome components NLRP3 and ASC. Nanotoxicology 2015; 9: 554–567. [DOI] [PubMed] [Google Scholar]
- 23.Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, Takahashi M. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology 2015; 156: 4281–4292. [DOI] [PubMed] [Google Scholar]
- 24.Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, Bock F, Nazir S, Huebner H, Mertens PR, Fischer KD, Zenclussen AC, Offermanns S, Aharon A, Brenner B, Shahzad K, Ruebner M, Isermann B. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 2016; 128: 2153–2164. [DOI] [PubMed] [Google Scholar]
- 25.Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 2009; 30: 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmberg JC, Haddad S, Wünsche V, Yang Y, Aldo PB, Gnainsky Y, Granot I, Dekel N, Mor G. An in vitro model for the study of human implantation. Am J Reprod Immunol 2012; 67: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Uric acid induces trophoblast IL-1β production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol 2011; 65: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Zhao S, Tang J, Li Z, Zhong T, Liu Y, Chen D, Zhao M, Li Y, Gong X, Deng P, Wang JH, Jiang Y. Advanced glycation end products and lipopolysaccharide synergistically stimulate proinflammatory cytokine/chemokine production in endothelial cells via activation of both mitogen-activated protein kinases and nuclear factor-kappaB. FEBS J 2009; 276: 4598–4606. [DOI] [PubMed] [Google Scholar]
- 30.Chekir C, Nakatsuka M, Noguchi S, Konishi H, Kamada Y, Sasaki A, Hao L, Hiramatsu Y. Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta 2006; 27: 225–233. [DOI] [PubMed] [Google Scholar]
- 31.Alexander KL, Mejia CA, Jordan C, Nelson MB, Howell BM, Jones CM, Reynolds PR, Arroyo JA. Differential receptor for advanced glycation end products expression in preeclamptic, intrauterine growth restricted, and gestational diabetic placentas. Am J Reprod Immunol 2016; 75: 172–180. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Zhang Y, Yue C, Ye Y, Chen P, Peng W, Wang Y. Accumulation of advanced glycation end products involved in inflammation and contributing to severe preeclampsia, in maternal blood, umbilical blood and placental tissues. Gynecol Obstet Invest 2016. [DOI] [PubMed] [Google Scholar]
- 33.Rashid G, Korzets Z, Bernheim J. Advanced glycation end products stimulate tumor necrosis factor-alpha and interleukin-1 beta secretion by peritoneal macrophages in patients on continuous ambulatory peritoneal dialysis. Isr Med Assoc J 2006; 8: 36–39. [PubMed] [Google Scholar]
- 34.Song Y, Wang Y, Zhang Y, Geng W, Liu W, Gao Y, Li S, Wang K, Wu X, Kang L, Yang C. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J Cell Mol Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lappas M, Permezel M, Rice GE. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Endocrinol 2007; 193: 269–277. [DOI] [PubMed] [Google Scholar]
- 36.Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, Diamanti-Kandarakis E, Papavassiliou AG. Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-κB and JNK-AP-1 signaling pathways. Cell Mol Life Sci 2016; 73: 1685–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA 2008; 105: 9035–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]