Abstract
Kisspeptin, which is encoded by the Kiss1 gene, and its receptor, the G protein-coupled receptor 54 (Kiss1r), play important roles in the regulation of reproductive functions in mammals. Several studies have shown that the Kiss1 and Kiss1r genes are expressed in the rat, primate, and human ovaries, and that the ovarian kisspeptin system plays a pivotal role in ovulation at the proestrous stage in adulthood. The purpose of this study was to evaluate development-related changes in the expression of ovarian Kiss1 and Kiss1r genes and in kisspeptin levels, and to identify the regulatory factors for these genes during the prepubertal period. The serum kisspeptin level was also measured to examine whether ovarian kisspeptin affects serum kisspeptin levels. Variations in the ovarian Kiss1 and Kiss1r mRNA levels were observed during the prepubertal period in female rats, with levels peaking around postnatal days 20 and 15, respectively. Nevertheless, the ovarian kisspeptin content per total protein level was stably maintained. Serum kisspeptin levels at postnatal days 30 and 35 were higher than those at earlier postnatal days. The pattern of the ovarian Kiss1 mRNA levels was similar to that of the serum luteinizing hormone (LH) levels, and the ovarian Kiss1 mRNA level increased after injection with human chorionic gonadotropin (HCG) on postnatal day 20, but not on postnatal days 10 and 30. These data indicate that ovarian Kiss1 and Kiss1r mRNA levels are increased on postnatal days 20 and 15, respectively, and that changes in the serum LH level and the ovarian sensitivity to LH may be involved in the alteration of ovarian Kiss1 mRNA levels.
Keywords: Development, kisspeptin, Kiss1, Kiss1r, Ovary
Kisspeptin, which is encoded by the Kiss1 gene, and its receptor, the G protein-coupled receptor 54 (Kiss1r), play pivotal roles in the regulation of reproductive functions in mammals [1,2,3]. Kisspeptin acts as a stimulator of the gonadotropin-releasing hormone (GnRH) in the hypothalamus, and regulates the female ovulatory cycle in experimental animals and humans [4, 5]. Kisspeptin and Kiss1r are also involved in sexual maturation through the activation of the hypothalamic-pituitary-gonadal axis, e.g., of the GnRH-gonadotropin-gonadal hormone, during the pubertal period in both males and females [5]. This relationship between kisspeptin, Kiss1r, and reproductive functions was first reported in 2003 [4, 5]. Subsequently, the precise mechanism by which hypothalamic kisspeptin regulates GnRH secretion, and its involvement in physiological and pathological reproductive conditions have been elucidated by rigorous research [6,7,8,9].
As noted above, although kisspeptin acts primarily at the hypothalamic level to regulate GnRH secretion, it is possible that kisspeptin at other sites also plays a role in reproductive functions. Several studies have shown that the Kiss1 and Kiss1r genes are expressed in rat, primate, and human ovaries [10, 11], and that ovarian Kiss1 levels and kisspeptin immune reactivities vary drastically based on the estrous cycle in female rats [12, 13]. In addition, it has been reported that the downregulation of the ovarian Kiss1 gene induces ovulatory dysfunction in adult female rats [11, 13]. These data indicate that ovarian kisspeptin, as well as hypothalamic kisspeptin, may act as positive regulators of ovulation in adulthood. However, the expression of ovarian Kiss1 and Kiss1r during the prepubertal period have not yet been fully examined. Thus, the purpose of this study was to evaluate the developmental changes in the ovarian Kiss1/Kiss1r gene expression and the amounts of kisspeptin, and to identify the regulatory factors of these genes during the prepubertal period. A recent study suggested that the kisspeptin produced in the gonadal tissue (testis) may be secreted into the serum in male rats [14]. Therefore, we also measured the serum kisspeptin levels during the developmental period, and compared their pattern to that of the expression of the ovarian Kiss1 gene.
Materials and Methods
Animals
Pregnant Sprague-Dawley rats were purchased from Charles River Japan (Tokyo, Japan) and housed under controlled lighting (12-h light, 12-h dark; lights on at 0800 h, and off at 2000 h) and temperature (24°C) conditions. The day on which the pups were born was defined as postnatal day 1. Twelve female pups were randomly assigned to a dam on postnatal day 2. The rats were weaned on postnatal day 21, and housed in groups of three or four per cage. The male pups were not used in this study. All animal experiments were conducted in accordance with the ethical standards of the animal care and use committee of the University of Tokushima.
Experiment 1: Development-related changes in the levels of hypothalamic and ovarian Kiss1 and Kiss1r mRNAs, ovarian kisspeptin, serum luteinizing hormone (LH), follicle stimulating hormone (FSH), and kisspeptin levels
Female rats were randomly selected from each dam or cage on postnatal days 10, 15, 20, 25, 30, and 35 (n = 7 to 15 each). They were killed by decapitation, and their blood, brain, and ovaries were collected between 0900 h and 1100 h. The rat brains and ovaries were stored at −80°C, and the sera were stored at −20°C. Thereafter, the levels of hypothalamic and ovarian Kiss1 and Kiss1r mRNAs, and the (unilateral) ovarian kisspeptin, serum LH, FSH, and kisspeptin levels were measured.
Experiment 2: Effects of injection with human chorionic gonadotropin (HCG) on ovarian Kiss1 mRNA levels during the prepubertal period
Female rats were randomly selected, as described above, on postnatal days 10, 20, and 30. Rats at each time point were divided into three groups (n = 7 per group). One group was intraperitoneally injected with saline, and brain and blood samples were collected 3 h after injection. The other groups were intraperitoneally injected with HCG (10 IU), and the brains and ovaries were collected 3 h and 6 h after injection. The samples were stored as described above. Thereafter, the hypothalamic and ovarian Kiss1 levels were measured as described below.
Serum assay
The serum LH level was measured using a radioimmunoassay (rLH [I-125] RIA kit, Institute of Isotopes, Tokyo, Japan). The sensitivity of the assay was 0.8 ng/ml, and the inter- and intra-assay coefficients of variation (CVs) were 7.7% and 6.5%, respectively. The serum FSH level was also measured using a radioimmunoassay (rFSH [I-125] RIA kit, Institute of Isotopes). The sensitivity of the assay was 1.6 ng/ml, and the inter- and intra-assay CVs were 7.9% and 4.2%, respectively. The serum kisspeptin levels were measured using an enzyme linked immunosorbent assay (Rat Kisspeptin 1 (KISS1) ELISA Kit, Wuxi Donglin Sci&Tech Development, Jiangsu, China). The sensitivity of the assay was 31.2 pg/ml, and the inter- and intra-assay CVs were < 12%, according to the manufacturer’s instructions.
Quantification of ovarian kisspeptin
Unilateral whole ovaries were homogenized in 500 μl of tissue protein extraction buffer (T-PER, Thermo Scientific, Rockford, IL, USA). The sample was then centrifuged and the kisspeptin in the supernatant was measured using an enzyme linked immunosorbent assay (Rat Kisspeptin 1 (KISS1) ELISA Kit, Cusabio Biotech, Wuhan, China) and the total amount of ovarian kisspeptin was calculated. The sensitivity of the assay was 156 pg/ml, and the inter- and intra-assay CVs were < 10% and < 8.0%, respectively. Total protein levels were measured in an equal volume of supernatant, using a Pierce BCA Protein Assay Kit (Thermo Scientific). The relative level of kisspeptin was calculated by dividing the total amount of ovarian kisspeptin by the total protein level.
Quantitative real-time polymerase chain reaction (RT-PCR)
Unilateral whole ovary and hypothalamic tissues were used for the quantitative real-time polymerase chain reaction. Hypothalamic tissues were dissected from frozen brains, as described previously [15]. Total RNA was isolated from the ovary and the hypothalamus using a TRIzol® reagent kit (Invitrogen, Carlsbad, CA), and an RNeasy® mini kit (Qiagen Gmbh, Hilden, Germany). cDNA was synthesized with oligo (deoxythymidine) primers at 50°C using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen). PCR analysis was performed using the StepOnePlusTM RT-PCR system (PE Applied Biosystems, Foster City, CA) and FAST SYBR® green. A standard curve was generated by diluting a representative sample, and the relative mRNA expression levels were quantified. The mRNA expression levels of Kiss1 and Kiss1r in the hypothalamus and ovary were normalized to those of GAPDH (hypothalamus) and β-actin (ovary), respectively. The forward and reverse primers, and annealing temperatures, are summarized in Table 1. The PCR conditions were as follows: initial denaturation and enzyme activation at 95°C for 20 sec, followed by 45 cycles of denaturation at 95°C for 3 sec, annealing for 30 sec, and a final extension step at 72°C for 1 min.
Table 1. Primer sequences.
| Primer | Sequence | Product size | Annealing T (ºC) |
| Kiss1 forward | ATG ATC TCG CTG GCT TCT TGG | 91 | 65 |
| Kiss1 reverse | GGT TCA CCA CAG GTG CCA TTT T | ||
| Kiss1r forward | TGT GCA AAT TCG TCA ACT ACA TCC | 193 | 65 |
| Kiss1r reverse | AGC ACC GGG GCG GAA ACA GCT GC | ||
| GAPDH forward | ATG GCA CAG TCA AGG CTG AGA | 64 | 64 |
| GAPDH reverse | CGC TCC TGG AAG ATG GTG AT | ||
| β-actin forward | CCG TAA AGA CCT CTA TGC CAA CA | 103 | 65 |
| β-actin reverse | GCT AGG AGC CAG GGC AGT AAT |
Statistical analyses
All data are presented as the mean ± standard deviation (SD). The statistical analyses were performed using a one-way analysis of variance (ANOVA), together with the Tukey-Kramer post-hoc test for comparison among the groups in Experiment 1. In contrast, a one-way ANOVA, together with Dunnett’s test, was used in Experiment 2, because HCG-induced changes from the baseline (saline injected group) were observed in this experiment. The statistical significance was defined as P < 0.05.
Results
Experiment 1: Development-related changes in the levels of hypothalamic and ovarian Kiss1 and Kiss1r mRNAs, serum LH, and serum kisspeptin
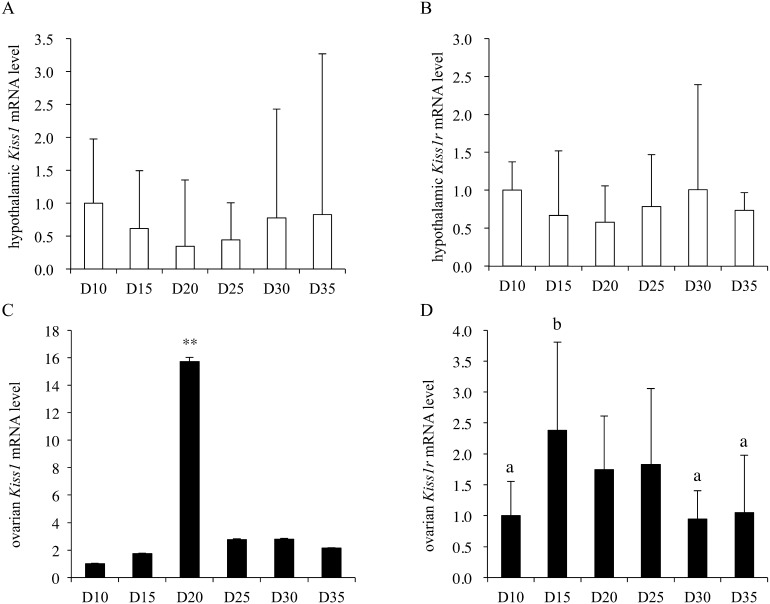
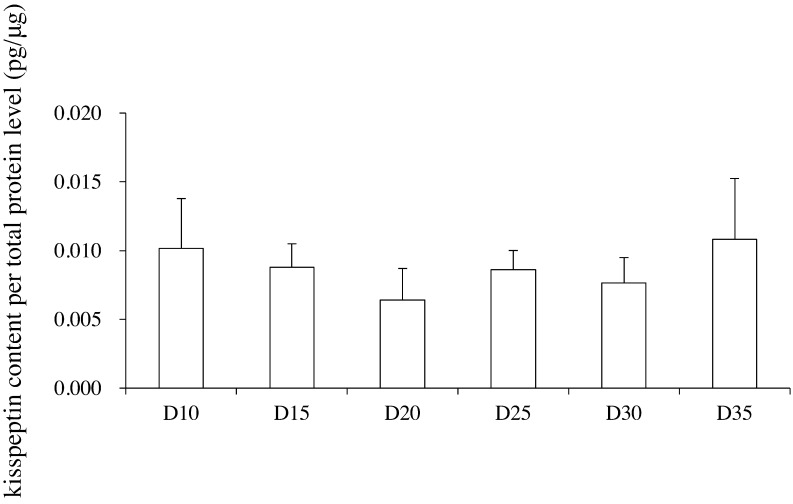
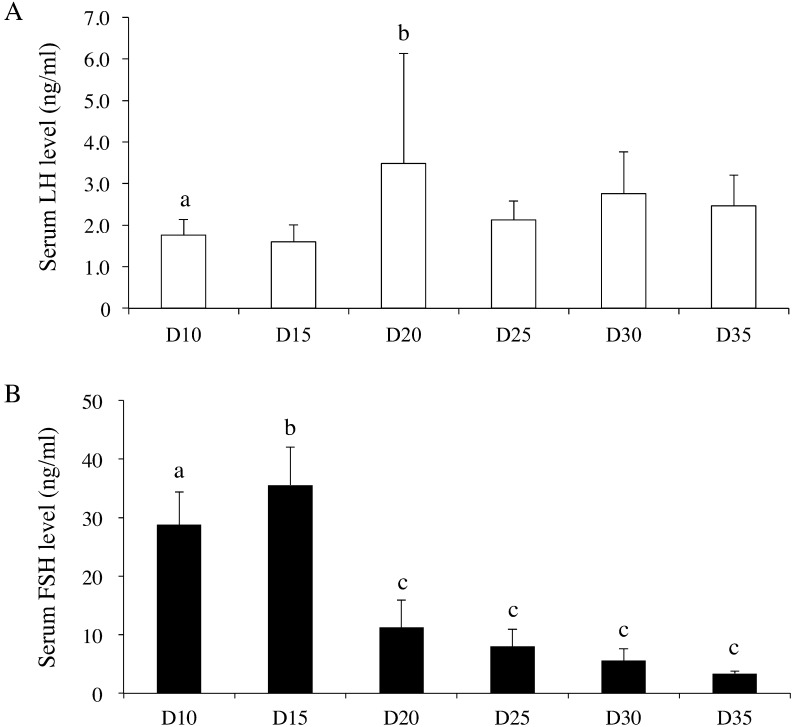
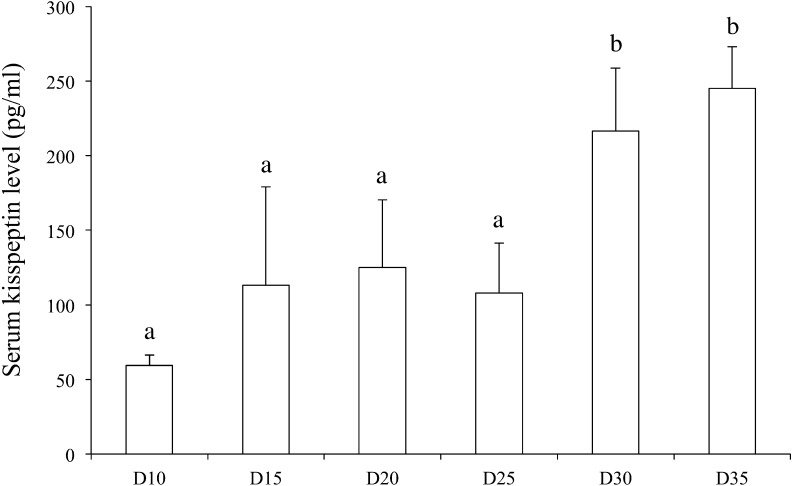
Hypothalamic Kiss1 mRNA levels varied during the developmental period (one-way ANOVA, F(5,40) = 2.59; P < 0.05); however, post-hoc analyses did not reveal any significant differences among any of the examined time points (Fig. 1A). The hypothalamic Kiss1r mRNA level did not change during the developmental period (Fig. 1B). The ovarian Kiss1 mRNA levels varied during the developmental period (one-way ANOVA, F(5,41) = 28.3; P < 0.01), and the Kiss1 mRNA level on postnatal day 20 was significantly higher than the levels at all other ages (Fig. 1C). Similarly, the ovarian Kiss1r mRNA levels also varied during the developmental period (one-way ANOVA, F(5,40) = 6.04; P < 0.01), and post-hoc analyses revealed that the Kiss1r mRNA level was significantly higher than the Kiss1 mRNA level on postnatal day 15, and was also significantly higher than the Kiss1r mRNA levels on postnatal days 10, 30, and 35 (Fig. 1D). The ovarian kisspeptin content per total protein level varied during the developmental period (one-way ANOVA, F(5,44) = 2.58; P < 0.05), but post-hoc analyses did not detect any significant differences among the values on any postnatal day (Fig. 2). Although serum LH levels were not statistically changed (one-way ANOVA, F(5,38) = 2.08; P = 0.09) during the developmental period, the serum LH level on postnatal day 20 was significantly higher than that on postnatal day 10 (Fig. 3A). On the other hand, serum FSH levels varied during the developmental period (one-way ANOVA, F(5,45) = 74.4; P < 0.01). The serum FSH levels on postnatal days 10 and 15 were significantly higher than those on postnatal days 20–35 (Fig. 3B). The serum kisspeptin levels varied during the developmental period (one-way ANOVA, F(8,63) = 15.5; P < 0.01), and post-hoc analyses revealed that the kisspeptin levels on postnatal days 30 and 35 were significantly higher than those on postnatal days 10 to 25 (Fig. 4).
Fig. 1.
Hypothalamic (A, B) and ovarian (C, D) Kiss1 and Kiss1r mRNA levels from day (D) 10 until D35 (n = 7 per group). The mRNA levels in the hypothalamus are expressed as values relative to GAPDH, and those in ovary are expressed as values relative to β-actin. The mRNA levels at D10 were set as 1.0. Data are presented as the mean ± SD. a, b: different letters indicate different significant differences (P < 0.05). **: P < 0.01 vs. all other time points.
Fig. 2.
Ovarian kisspeptin content per total protein level (pg/μg) in unilateral ovaries from day (D) 10 until D35 (n = 7–8 per group). The ovarian kisspeptin content per total protein level is expressed relative to the total protein level. Data are presented as the mean ± SD.
Fig. 3.
Serum LH (A) and FSH (B) levels from day (D) 10 until D35 (n = 7–8 per group). Data are presented as the mean ± SD. a–c: different letters indicate different significant differences (P < 0.05).
Fig. 4.
Serum kisspeptin levels from day (D) 10 until D35 (n = 7 per group). Data are presented as the mean ± SD. a, b: different letters indicate different significant differences (P < 0.05).
Experiment 2: Effects of injection with HCG on ovarian Kiss1 mRNA levels during the prepubertal period
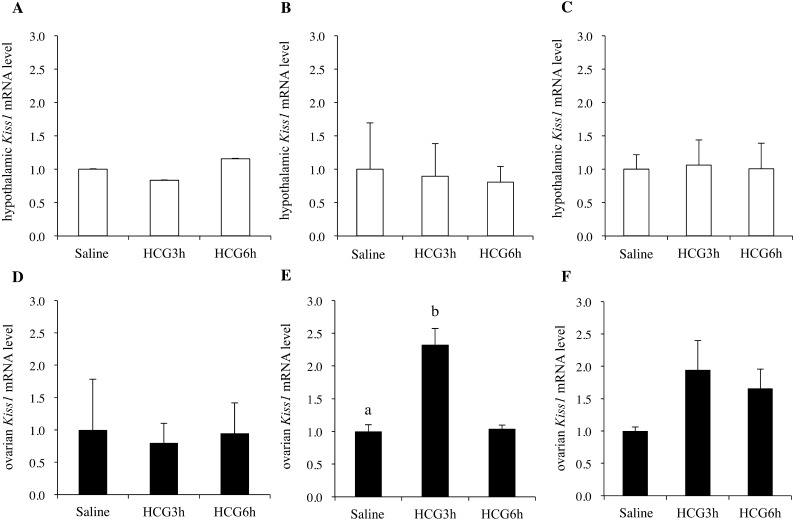
Hypothalamic Kiss1 mRNA levels did not change after injection with HCG on postnatal days 10, 20, and 30 (Figs. 5A–C). In contrast, the ovarian Kiss1 mRNA level on postnatal day 20 increased after injection with HCG, but this increase was not observed on postnatal days 10 or 30 (Figs. 5D–F).
Fig. 5.
Hypothalamic (A, B, C) and ovarian (D, E, F) Kiss1 mRNA levels 3 h after saline injection, or 3 h or 6 h after HCG administration in rats on days (D) 10 (A, D), D20 (B, E), and D30 (C, F) (n = 7 per group). The mRNA levels in the hypothalamus are expressed as values relative to GAPDH, and those in the ovary are expressed as values relative to β-actin. The mRNA levels of the saline-injected groups were set as 1.0. Data are presented as the mean ± SD. a, b: different letters indicate different significant differences (P < 0.05).
Discussion
In the present study, we showed that the levels of ovarian Kiss1 and Kiss1r mRNAs varied during development, in the prepubertal period in female rats. The expression levels were higher at around postnatal days 15 to 20, when compared to the levels on other days. This pattern of ovarian Kiss1 mRNA variation was somewhat similar to the results of a previous study, showing that ovarian Kiss1 mRNA levels on postnatal days 20 to 26 were higher than those on days 29 and 32 [12]. To the best of our knowledge, the present study is the first to show that the Kiss1r mRNA level increases on the same postnatal days as Kiss1, although the Kiss1r peak occurred slightly earlier. On the other hand, ovarian kisspeptin content per total protein level did not change on any experimental day. The present study does not identify why the changing patterns of Kiss1 mRNA levels do not correspond to changes in the ovarian kisspeptin content per total protein level. We speculate that some kisspeptin might be secreted into the plasma, and the concentration of kisspeptin might be stably maintained in the ovary. Another possibility is that kisspeptin might infiltrate the ovary from the blood or other tissues, such as the liver and adipose tissues, and that this mechanism maintains stable levels of ovarian kisspeptin. The patterns of ovarian Kiss1 and Kiss1r mRNA levels differed from those of hypothalamic Kiss1 and Kiss1r mRNA levels during the prepubertal period. Therefore, we speculated that ovarian Kiss1 and Kiss1r play roles in the development of ovarian functions, such as the maturation of ovarian follicles or the increased sensitivity to other stimulatory factors, and that ovarian and hypothalamic Kiss1 and Kiss1r systems may be separately regulated during the prepubertal period. However, we cannot exclude the possibility that the present study did not detect a pubertal increase in hypothalamic Kiss1 mRNA levels, due to an insufficient analysis of the estrous cycle after the onset of puberty.
It has been reported that kisspeptin and Kiss1r are mainly distributed in the theca layer in adult rats, primates, and humans in adulthood [11,12,13]. It has also been shown that ovarian kisspeptin and Kiss1r play pivotal roles in ovulation and in the regulation of the estrous cycle in rats. Ovarian Kiss1 and/or Kiss1r mRNA levels increase during the proestrous stage, i.e., the ovulatory period, and their levels decrease in a high-fat diet-induced anovulatory model [12, 13]. Because the ovulatory cycle has not yet been established during the prepubertal period, the roles of kisspeptin and Kiss1r during this period may differ from those in adulthood. Thus, further examination is needed to clarify the specific roles of ovarian kisspeptin system during sexual maturation.
It has been reported that an increase in ovarian Kiss1 mRNA levels in the proestrous stage was prevented by a GnRH antagonist, and that its expression level was restored by injection with HCG [12]. These data indicated that ovarian Kiss1 mRNA is mainly regulated by LH after the establishment of the estrous cycle. Therefore, we hypothesized that LH stimulates an increase in the ovarian Kiss1 mRNA level even during the prepubertal period, and that alterations in the LH level itself, or in the sensitivity of the ovarian kisspeptin system to LH might be involved in the upregulation of the ovarian Kiss1 mRNA levels at around postnatal day 20. As a result, the serum LH level increased on postnatal day 20, and the ovarian Kiss1 mRNA level increased after injection with HCG at the same time point, in the present study. Interestingly, such stimulatory effects of HCG on ovarian Kiss1 mRNA levels were not detected at other time points. These data indicate that changes in the serum LH level and the ovarian sensitivity to LH may be involved in the alteration of ovarian Kiss1 mRNA levels during the prepubertal period. The changing pattern of FSH during the developmental period was dissimilar to that of the ovarian Kiss1 mRNA level, and thus, we speculate that FSH may not be involved in the regulation of ovarian Kiss1 mRNA. In contrast, for Kiss1, the changing pattern of ovarian Kiss1r mRNA levels did not correspond to that of serum LH or FSH levels, suggesting that Kiss1r mRNA levels might not be regulated by gonadotropins. To our knowledge, the factor regulating ovarian Kiss1r gene expression has not been identified, and further comprehensive research is required to investigate these factors. Interestingly, in addition to the peak observed around the weaning period in this study, the ovarian Kiss1r mRNA level peaks before that of Kiss1 mRNA, around the time of the proestrous luteinizing hormone surge in adult female rats [16]. Although the physiological roles of such time-course changes in Kiss1 and Kiss1r gene expression levels remain unclear, they might act in a coordinated manner in the ovary, at each reproductive stage.
Recently, it has been reported that Kiss1 mRNA is also detectable in mouse testicular Leydig cells, and that testicular kisspeptin might be secreted into the peripheral plasma [14]. It has been reported that kisspeptin is produced in other tissues and organs, such as the adipose tissue and the liver [17, 18], and that some of the produced kisspeptin may be secreted into the plasma [18]. In this study, the changing pattern of serum kisspeptin levels did not correspond to that of the ovarian kisspeptin content per total protein level. Taken together, it is possible that the serum kisspeptin level may not reflect the kisspeptin level in a specific tissue or organ, but that it rather reflects comprehensive changes in the total production of kisspeptin.
In summary, we showed that the levels of ovarian Kiss1 and Kiss1r mRNAs varied during the prepubertal developmental period in female rats. Their expression levels peaked at around postnatal days 15 to 20. The pattern of the ovarian Kiss1 mRNA levels was similar to that of the serum LH levels, and the ovarian Kiss1 mRNA level increased after injection with HCG on postnatal day 20, but not at the other time points. These data indicated that changes in the serum LH level and in the ovarian sensitivity to LH may be involved in the alteration of ovarian Kiss1 mRNA levels during the prepubertal period. On the other hand, the ovarian kisspeptin content per total protein level varied little during the developmental period, whereas serum kisspeptin levels increased after puberty. Further examination is required to clarify the roles of the ovarian kisspeptin system in the ovary and other tissues during the developmental period. The present results indicate that the ovarian kisspeptin system may play a role in the ovary and other peripheral and central reproductive tissues.
References
- 1.Putteeraj M, Soga T, Ubuka T, Parhar ISA. A timed Kiss is essential for reproduction: lessons from mammalian studies. Front Endocrinol (Lausanne) 2016; 7: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: inside and outside the hypothalamus. J Reprod Dev 2016; 62: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uenoyama Y, Tomikawa J, Inoue N, Goto T, Minabe S, Ieda N, Nakamura S, Watanabe Y, Ikegami K, Matsuda F, Ohkura S, Maeda K, Tsukamura H. Molecular and epigenetic mechanism regulating hypothalamic Kiss1 gene expression in mammals. Neuroendocrinology 2016; 103: 640–649. [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, ORahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 6.Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Niki H, Kato T, Kuwahara A, Uemura H, Yasui T, Irahara M. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav 2014; 66: 309–316. [DOI] [PubMed] [Google Scholar]
- 7.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 2014; 20: 485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev 2015; 61: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassaneen A, Naniwa Y, Suetomi Y, Matsuyama S, Kimura K, Ieda N, Inoue N, Uenoyama Y, Tsukamura H, Maeda KI, Matsuda F, Ohkura S. Immunohistochemical characterization of the arcuate kisspeptin/neurokinin B/dynorphin (KNDy) and preoptic kisspeptin neuronal populations in the hypothalamus during the estrous cycle in heifers. J Reprod Dev 2016; 62: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, Shintani Y. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta 2004; 1678: 102–110. [DOI] [PubMed] [Google Scholar]
- 11.Gaytán F, Gaytán M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sánchez-Criado JE, Millar RP, Pellicer A, Fraser HM, Tena-Sempere M. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab 2009; 296: E520–E531. [DOI] [PubMed] [Google Scholar]
- 12.Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 2006; 147: 4852–4862. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Chen H, Yang S, Li Y, Wang B, Chen Y, Wu X. High-fat diet decreases the expression of Kiss1 mRNA and kisspeptin in the ovary, and increases ovulatory dysfunction in postpubertal female rats. Reprod Biol Endocrinol 2014; 12: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salehi S, Adeshina I, Chen H, Zirkin BR, Hussain MA, Wondisford F, Wolfe A, Radovick S. Developmental and endocrine regulation of kisspeptin expression in mouse Leydig cells. Endocrinology 2015; 156: 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwasa T, Matsuzaki T, Munkhzaya M, Tungalagsuvd A, Kuwahara A, Yasui T, Irahara M. Developmental changes in the hypothalamic mRNA levels of prepro-orexin and orexin receptors and their sensitivity to fasting in male and female rats. Int J Dev Neurosci 2015; 46: 51–54. [DOI] [PubMed] [Google Scholar]
- 16.Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod 2015; 93: 15. [DOI] [PubMed] [Google Scholar]
- 17.Brown RE, Imran SA, Ur E, Wilkinson M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol 2008; 281: 64–72. [DOI] [PubMed] [Google Scholar]
- 18.Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BWT, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab 2014; 19: 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]