Abstract
This is the first systematic review, to our knowledge, of published studies investigating the gastrointestinal effects of A1-type bovine β-casein (A1) compared with A2-type bovine β-casein (A2). The review is relevant to nutrition practice given the increasing availability and promotion in a range of countries of dairy products free of A1 for both infant and adult nutrition. In vitro and in vivo studies (all species) were included. In vivo studies were limited to oral consumption. Inclusion criteria encompassed all English-language primary research studies, but not reviews, involving milk, fresh-milk products, β-casein, and β-casomorphins published through 12 April 2017. Studies involving cheese and fermented milk products were excluded. Only studies with a specific gastrointestinal focus were included. However, inclusion was not delimited by specific gastrointestinal outcome nor by a specific mechanism. Inclusion criteria were satisfied by 39 studies. In vivo consumption of A1 relative to A2 delays intestinal transit in rodents via an opioid-mediated mechanism. Rodent models also link consumption of A1 to the initiation of inflammatory response markers plus enhanced Toll-like receptor expression relative to both A2 and nonmilk controls. Although most rodent responses are confirmed as opioid-mediated, there is evidence that dipeptidyl peptidase 4 stimulation in the jejunum of rodents is via a nonopioid mechanism. In humans, there is evidence from a limited number of studies that A1 consumption is also associated with delayed intestinal transit (1 clinical study) and looser stool consistency (2 clinical studies). In addition, digestive discomfort is correlated with inflammatory markers in humans for A1 but not A2. Further research is required in humans to investigate the digestive function effects of A1 relative to A2 in different populations and dietary settings.
Keywords: β-casein, β-casomorphin, gastrointestinal tract, humans, in vitro, in vivo, inflammation, milk
Introduction
In this review, we address the scientific evidence for A1-type bovine β-casein (A1) compared with A2-type bovine β-casein (A2) being associated with gastrointestinal issues. We do this within a systematic framework in accordance with section 6 of the Australia New Zealand Food Standards Code (1) by using defined criteria and including in vivo and in vitro studies (all species) published through 12 April 2017. Articles that do not have an explicit relevance to gastrointestinal issues were excluded.
As background, β-casein makes up ∼30% of the total protein contained in bovine milk and may present as 1 of 2 major genetic types: A1 and A2 (2). The distinguishing structure between these 2 forms of β-casein is the presence of either histidine (His67) in A1 or proline (Pro67) in A2 at position 67 of this 209–amino acid protein, with A1 being consequential to a point mutation from Pro67 to His67 occurring in ancestors to modern European-type cattle (2). Consequently, the milk produced commercially in many countries contains a mixture of A1 and A2 (2). The His67 mutation is absent in purebred Asian and African cattle. Similarly, the presence of a histidine mutation at the equivalent position in other mammalian species, including humans, is either absent or extremely rare (3, 4).
Within modern European-type cattle, there are additional derivative β-casein proteins through mutations at other points of the protein chain, which can be grouped within the A1 and A2 types. The most important of these is type B β-casein, which, like A1, contains His67. Other A1 and A2 caseins can be considered minor. Most studies are not explicit as to the presence or absence of these minor variants and refer only to either “A1” or “A2.” Accordingly, throughout this review we use the same terminology but recognize that other unrecorded minor subvariants of these types may also have been present.
Although His67 within A1 is susceptible to proteolytic cleavage, Pro67 within A2 is not. Thus, A1s have the potential to release short β-casomorphin (BCM) opioid peptides, including BCM-7, during gastrointestinal digestion (Table 1). The avoidance of A1 is feasible within dairy-based diets through the consumption of goat, sheep, and buffalo milk or through the consumption of bovine milk from the native Asian and African bovine breeds, or through the consumption of milk from genetically selected herds of European-type cattle that are certified free of the His67 mutation. Such herds are being developed in many countries.
TABLE 1.
Structure of BCMs released from bovine milk1
| Peptide | Corresponding β-casein location | Structure |
| BCM-4 | 60–63 | Tyr-Pro-Phe-Pro |
| BCM-5 | 60–64 | Tyr-Pro-Phe-Pro-Gly |
| BCM-6 | 60–65 | Tyr-Pro-Phe-Pro-Gly-Pro |
| BCM-7 | 60–66 | Tyr-Pro-Phe-Pro-Gly-Pro-Ile |
| Pro8–BCM-8 | 60–67 (A2/A3) | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-Pro |
| His8–BCM-8 | 60–67 (A1/B) | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-His |
| Pro8–BCM-11 | 60–70 (A2/A3) | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-Pro-Asn-Ser-Leu |
| His8–BCM-11 | 60–70 (A1/B) | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-His-Asn-Ser-Leu |
A1, A1-type bovine β-casein; A2, A2-type bovine β-casein; A3, A3-type bovine β-casein; BCM, β-casomorphin.
Bovine milk that is free of A1 is now available commercially in a range of countries, including Australia, the United Kingdom, the United States, New Zealand, and The Netherlands, and is widely promoted as beneficial for people who suffer from milk intolerances. Infant formula containing casein but free of A1 is now marketed widely in China and Australia and is promoted commercially as being more gentle on the infant digestive system.
To our knowledge, this is the first scientific review to focus specifically on intolerances and gastrointestinal effects. An earlier review by Truswell (5) was not systematic, did not focus specifically on gastrointestinal effects, and has been criticized for ignoring relevant studies (6, 7). A review by the European Food Safety Authority (EFSA) undertaken in 2008 (2) noted that BCM-7 was an opioid peptide released by A1 but not A2, but did not find convincing evidence for physiologic effects in humans. The EFSA study did not investigate intolerances and gastrointestinal effects. In our study, we focus on the available science both before and subsequent to these studies. Although the underlying science of casomorphins has been known for >30 y, the scientific evidence specific to gastrointestinal effects has largely emerged since 2009.
Methods
Criteria for inclusion
Both in vitro and in vivo animal studies and human clinical trials that reported outcomes relevant to a comparison between the digestion and potential health impacts of A1 and A2 in the gastrointestinal system were included in the review. Studies involving milk, milk products, β-casein, and BCM peptides of various lengths were considered relevant. For in vivo animal and human clinical studies, inclusion was limited to those studies in which the test material was administered orally. Relevant outcome measures were as follows: release of BCM in actual or simulated gastrointestinal digestion of milk, fresh-milk products, or casein; opioid agonist activity after digestion of milk, milk products, or casein; and variations in biomarkers of bloating and abdominal pain or discomfort after the consumption of milk, fresh-milk products, or casein. However, the selection of studies was not delimited by any specific mechanism or outcome as long as it had a gastrointestinal relevance. Studies involving fermented and aged dairy products, such as cheese, were not included. The authors considered that the presence of cultures in fermented and aged products raises 2 separate questions—1) how much BCM is released during the cheese-making process and 2) how much BCM is released during in vitro and in vivo digestion—which are sufficiently complex to warrant a separate review.
Search methods
English-language literature searches were undertaken by using PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Scopus (https://www.elsevier.com/solutions/scopus), and ScienceDirect (http://www.sciencedirect.com/) (filtered for Agricultural and Biological Sciences; Biochemistry, Genetics and Molecular Biology; and Health Sciences), initially in March 2016, but updated through 12 April 2017 with the use of the following search terms: A2 AND Milk; beta-casein AND A1 OR A2; casomorphin; beta-casomorphin; beta-casomorphin-7; beta-casomorphine; beta-casomorphine-7; A1_betacasein OR A2_betacasein; and b-cm OR 7 bcm7 OR bcm-7. In addition, 1 relevant study (8) was identified from the EFSA DATEX Working Group report (3), 1 (9) was identified from a citation in a selected study (10), and 1 (11) was provided by one of the authors.
Study selection
Data were extracted manually and independently for each search database. Studies that measured outcomes relevant to potential direct effects of A1 compared with A2 in the gastrointestinal tract were selected. Studies that did not contain relevant outcome measures or that were only available as abstracts of conference presentations were excluded. Studies were assessed manually for bias on the basis of information provided in each publication (see Supplemental Tables 1–6).
Results
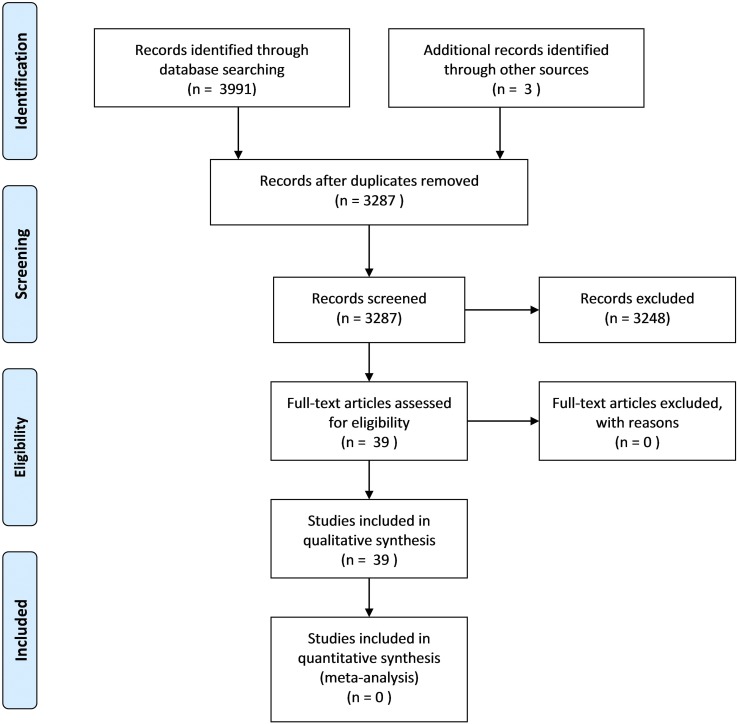
A total of 3287 unique studies were identified (Figure 1). The overwhelming majority of studies were excluded on the basis that the reported variables did not include oral exposure to the test materials for in vivo studies; did not include markers relevant to gastrointestinal bloating, abdominal pain or discomfort, or both; were not original results; or were reviews. Additional exclusion criteria included that the original references could not be sourced, were not available in English, or the references were conference papers or abstracts that contained data published elsewhere in full. Thirty-five studies were identified for review directly from the search results, and 4 were identified from other sources as described. Of these, 11 described the digestion of bovine milk or fresh-milk products within in vitro studies and 5 within in vivo studies, 11 reported bovine BCM activity in the gastrointestinal tract within in vitro studies, and 15 reported results from milk or bovine BCM within in vivo studies. Some studies were allocated to more than one category during review depending on the relevant content. Although mention of BCM activity was not a required condition for study selection, it was found that β-casein studies of gastrointestinal effects consistently referred to either BCM-related analyses or BCM-related explanatory hypotheses.
FIGURE 1.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Release of BCMs from β-casein
Eleven in vitro studies that reported enzymatic digestion of milk and milk products or casein, and 5 in vivo digestion studies (including those in humans), met the inclusion criteria for review (Supplemental Tables 1 and 2). One study reported both in vitro and in vivo data.
In vitro studies
The release of bioactive peptides from milk and milk products with opioid properties was first discovered in an in vitro system by Brantl and Teschemacher (12) in 1979 while searching for pronase-resistant opioids in bovine milk and milk products that were similar to those reported previously in plasma. Chloroform-methanol extracts of enzyme-digested commercial cow milk, casein digests, and milk-based baby food were found to inhibit contraction of isolated guinea pig ileum. Furthermore, this activity was blocked by the opioid antagonist (−)naloxone, suggesting that milk peptides in the extracts had μ-opioid receptor agonist activity. The fresh-milk samples were collected monthly over a 7-mo period from a local farm. Opioid activity was found in casein extracts prepared from milk collected in May, June, and October but not in July, August, September, or November. An explanation for this differential finding may reflect random sampling, herd composition, and health status. In a follow-up study, similar chloroform-methanol extracts of digested casein were shown to have μ-opioid receptor activity in isolated guinea pig ileum (13), and the active peptide was isolated and identified by using amino acid sequencing as BCM-7 (14, 15). The sequence identified was cross-mapped to bovine β-casein corresponding to the amino acid sequence 60–66 (16).
Subsequent studies have shown that longer BCM peptides, but not BCM-7 itself, can be released by simulated gastric digestion with pepsin, but that a multistage digestion system as is present in the human stomach and small intestine is necessary for the release of BCM-7 and other smaller BCMs. Svedberg et al. (17) reported that large amounts of BCM-7 immunoreactive material was released after simulated gastrointestinal digestion of β-casein (unspecified variant or variants) in a multienzyme system comprising the following: 1) pepsin; 2) lipase, amylases, proteases, and bile acids; and finally, 3) intestinal mucosal peptidase. Only small amounts of BCM-7 immunoreactive material were found after pepsin digestion alone. This finding was confirmed in a small follow-up study in 4 healthy adults fitted with intestinal tubes who consumed the same β-casein (17). The BCM-7 immunoreactive material identified in these studies was not further identified as BCM-7 itself and potentially indicates the release of a longer-chain peptide that includes the BCM-7 amino acid sequence. In another more recent study, pepsin digestion of commercial bovine β-casein released both His9 and Pro9 BCM-11 but not BCM-7 (18). These data indicate that pepsin alone is insufficient and that the release of BCM-7 occurs beyond the stomach.
It has been shown that pancreatin, a mixture of several digestive enzymes produced by the pancreas and only found in the small intestine (1), releases BCM-7 from His67 β-caseins (i.e., A1 and B β-casein types) but not Pro67 (i.e., A2 type) variants (16). Elastase was identified as the essential component enzyme in the pancreatin responsible for cleaving the Ile66–His67 bond to release BCM-7 from longer BCM peptides (19). More recently, De Noni (20) and Ul Haq et al. (21) both confirmed the release of BCM-7 after simulated gastrointestinal digestion of milk from A1/A1 and A1/A2 but not from A2/A2 cows. Ul Haq et al. (21) also verified that elutes containing BCM-7 showed opioid activity in an isolated rat ileum assay.
One research group has consistently reported small amounts of BCM-7 in fresh milk and in hydrolyzed milk from both A1/A1 and A2/A2 cows after an intensive 24-h acidic (pH 2.0) pepsin digestion (22, 23). The presence of BCM-7 in fresh milk is not confirmed elsewhere in the literature, and it has alternatively been proposed that this finding may be explained by proteolysis of caseins by enzymes associated with somatic cells in the milk, which are normally associated with clinical or subclinical mastitis (2, 24). However, it could be more likely that small amounts of BCM-7 detected by this group after pepsin digestion were the result of extended acidic hydrolysis rather than enzymatic action and are not reflective of human multienzyme gastrointestinal digestion in which gastric digestion may occur for <1 h and is unlikely to exceed 6 h (25). From the same research group, and by using an alternative pepsin-trypsin-elastase sequential digestion system, Cieślińska et al. (23) reported that the concentrations of BCM-7 were highest in milk hydrolysates from A1/A1 cows and 25- to 27-fold higher than that reported for pepsin digestion alone. In hydrolysates of milk from A1/A2 cows, the concentrations of BCM-7 measured were ∼50–60% of those from the A1/A1 milk. BCM-7 was also detected at low concentrations in milk hydrolysates from A2/A2 cows in this study, although the concentrations were consistently <10% of those measured from A1/A1 milk. Although this study was undertaken by using simulated gastric and intestinal digestion, the detection of BCM-7 from A2/A2 milk stands in contrast to other studies that have shown no release of BCM-7 from the milk of A2/A2 cows (19–21).
In vivo studies
BCMs were first identified in the gut contents after the consumption of 100 g of a commercial, acid-precipitated bovine casein in a small study with 2 mini-pigs fitted with a duodenal cannula (26). The duodenal extract contained 23 tyrosine-containing peptides from which electrophoresis produced a main band that was subsequently resolved by HPLC into 14 peptides, predominantly BCM-5, but also minor bands of BCM-7 and a BCM-4 amide.
A similar result was reported from a small study in 4 healthy men fitted with a gastric tube positioned in the proximal small intestine after the consumption of 1 L raw milk (β-casein variants not reported) (17). Analysis of the BCM-7 immunoreactive peptides recovered indicated that, although they contained the BCM-7 sequence, much of the material was suspected to be a longer-peptide sequence, possibly indicative of A2s. In a separate study, BCM-7 immunoreactive material, with chain lengths of 12–13 amino acids, was also found in the plasma of young dogs (aged 2 and 4 wk), but not in adult dogs, after oral administration of cow milk (β-casein variants not reported) and canine milk (27). All of these studies indicate the release of BCM peptides but do not directly show the release of BCM-7 itself from β-casein. In addition, because the β-casein variants were not identified, it is unclear from these studies alone whether the longer BCM peptides detected, including the [Pro8]BCM-11 identified by Meisel (26), came from A1 or A2. The formation of a number of β-casein peptides was also reported in samples collected from the stomach and duodenum from a small study in 6 adult humans after the consumption of milk and yogurt; however, no peptides that contained sequences corresponding to β-casein positions 60–70 were reported (7).
In a current single-blind parallel study, 16 healthy adults were randomly assigned to 2 groups of 8 participants (28). Each group consumed daily, for 9 d, a protein controlled meal (1.4 g · kg−1 · d−1), including a protein shake containing 30 g of either milk casein (equivalent to ∼1 L milk) or whey protein. The β-casein status of the source milk was not reported. On day 9, samples were withdrawn by nasojejunal tube, positioned to collect contents from the proximal jejunum, at multiple times postconsumption. Analysis of peptides was undertaken by using tandem MS. β-Casein was reported to be the predominant precursor of peptides recovered, accounting for 61.2% of total recovered casein peptides. There were 20 peptides identified that had amino acid sequences that included all or part of the β-casein 60–66 sequence (i.e., BCM-7) (Table 2). Six peptides cleaved before β-casein position 67, including BCM-7, were identified with collection frequencies between 0.73 and 0.14. The cumulative quantity of 4 of these peptides [β-casein57–66, β-casein58–66, β-casein59–66, and β-casein60–66 (the last being BCM-7)] was highest at 30 min postconsumption, at 3.60 ± 0.35 mg. An average total of 4 mg BCM-7 was recovered from the jejunal effluent ≤2 h postconsumption, corresponding to a concentration of 17 μmol/L in 304 mL (mean volume) of the jejunal effluent collected. Only 2 long peptides containing intact His67 were identified, β-casein59–72 and β-casein57–72, both with very low frequencies (0.06 and 0.02, respectively). A total of 14 peptides containing an intact Pro67 bond (i.e., peptides of the A2 type) were identified. The most frequently found was β-casein59–68 (i.e., Pro67–10 amino acid peptide) at a frequency of 0.65. Three other Pro67 peptides—β-casein57–68 (i.e., Pro67–12 amino acid peptide), β-casein58–68 (i.e., Pro67–11 amino acid peptide), and β-casein59–67(i.e., Pro67–9 amino acid peptide)—were identified with frequencies between 0.31 and 0.35. β-Casein60–68 (i.e., Pro67–BCM-9) was identified at a frequency of 0.15. These data support the in vitro data that His67 (i.e., A1 and B) β-casein is readily cleaved at position 67.
TABLE 2.
Frequency of detection of jejunal BCM peptides after consumption of bovine casein by healthy adults1
| β-Casein sequence | Peptide length | Amino acids | Frequency detected |
| Peptides cleaved at position 66 or 67 | |||
| 61–66 | 6 | PFPGPI | 0.42 |
| 60–65 | 6 | YPFPGP | 0.14 |
| 60–66 | 7 | YPFPGPI | 0.77 |
| 59–66 | 8 | VYPFPGPI | 0.67 |
| 58–66 | 9 | LVYPFPGPI | 0.73 |
| 57–66 | 10 | SLVYPFPGPI | 0.65 |
| Peptides containing intact His67 | |||
| 59–72 | 14 | VYPFPGPIHNSLPQ | 0.06 |
| 57–72 | 16 | SLVYPFPGPIHNSLPQ | 0.02 |
| Peptides containing intact Pro67 | |||
| 62–67 | 6 | FPGPIP | 0.01 |
| 63–68 | 6 | PGPIPN | 0.14 |
| 61–67 | 7 | PFPGPIP | 0.1 |
| 62–68 | 7 | FPGPIPN | 0.01 |
| 60–67 | 8 | YPFPGPIP | 0.2 |
| 61–68 | 8 | PFPGPIPN | 0.02 |
| 59–67 | 9 | VYPFPGPIP | 0.31 |
| 60–68 | 9 | YPFPGPIPN | 0.15 |
| 58–67 | 10 | LVYPFPGPIP | 0.11 |
| 59–68 | 10 | VYPFPGPIPN | 0.65 |
| 57–67 | 11 | SLVYPFPGPIP | 0.01 |
| 58–68 | 11 | LVYPFPGPIPN | 0.33 |
| 59–69 | 11 | VYPFPGPIPNS | 0.01 |
| 57–68 | 12 | SLVYPFPGPIPN | 0.35 |
Data from reference 28. BCM, β-casomorphin.
Mechanism of activity of β-casein
A total of 11 in vitro enzyme studies investigating the activity in digests of milk, milk products, or casein, and including one human colon cell culture system, were included for review (Supplemental Tables 3 and 4). Two studies reviewed under the preceding section that also included data on activity of casein digests were included in this section. All of these studies identified opioid-related mechanisms of activity. Studies that reported effects in vivo were allocated for review in the subsequent section.
BCM-7 has been identified as a source of milk-derived opioid activity and mapped to the β-casein peptide chain locations 60–66 (12–15). BCM-4, -5, -6, and -7 have all been shown subsequently to have μ-opioid agonist activity in a range of in vitro assays and analgesic activity in vivo (29, 30). After intracerebroventricular injection in rats, the most potent of the bovine BCMs was reportedly BCM-5, with a potency (half maximum inhibition of naloxone, IC50) one-tenth that of morphine (29).
A predigested enzyme hydrolysate of casein has also been shown to increase jejunal mucin secretion in vitro (31). BCM-7 also induced jejunal mucin secretion when administered intra-arterially in an isolated vascularly perfused rat jejunum system (32). Both effects were inhibited by naloxone, indicating μ-opioid receptor activity. BCM-7 also produced a dose- and time-dependent increase in mucin-like glycoprotein secretion by cultured rat and human colon cells that was inhibited by the μ-opioid receptor antagonist cyprodime (33).
Endogenous opioids, including both bovine and human BCM-7, have also been shown to reduce cellular redox status and methylation capacity, through opioid receptor–mediated inhibition of cysteine uptake by human intestinal Caco-2 cells, in an isolated cell system (34). The relative potency in this assay was morphine > bovine BCM-7 > human BCM-7. We note here for clarification that human BCM-7 contains 5 out of 7 amino acids that are homologous to bovine BCM-7 and is found in the highest concentrations in breast milk in the first days after parturition (35). Human BCM can also be considered A2-like, in that the amino acid at the equivalent position is Pro and not His.
Casein and opioid activity in vivo
Rodent studies have consistently reported opioid-mediated reduction in the rate of gastric emptying and increases in gastrointestinal transit time after the consumption of commercial casein (Supplemental Tables 5 and 6). In vivo assays in rodents showed that a casein meal slowed gastric emptying and increased gastrointestinal transit time, compared with a whey protein meal (10, 36). Similarly, in dogs, decreased intestinal motility and motor activity were observed with a casein-rich meal compared with a soy protein meal (37). Casein has also been shown to slow gastrointestinal transit time in young rats compared with hydrolyzed casein that has been predigested and which does not release BCMs (38). In each case, the effects of casein were reversed by naloxone.
In a direct comparison of A1 and A2 fed to Wistar rats, gastrointestinal transit time was significantly greater in the A1 group between 8 and 14 h postprandially (39). Co-administration of naloxone decreased gastrointestinal transit time significantly in the A1 diet group but not in the A2 diet group at 8 and 11 h, but not at 14 h.
There is also some evidence that BCM-7 may interact with κ-opioid receptors in the gut. When administered in combination with enterostatin, which acts on κ-opioid receptors, via a gastric cannula, BCM-7 partially reversed the decrease in fat intake from a high-fat diet, observed with enterostatin alone, but did not affect intake from a low-fat diet (40).
Casein and bowel irritation
The action of β-casein and BCMs on bowel irritation has been studied in rats, mice, and humans (Supplemental Tables 5 and 6). In a study in which mice were administered β-casein isolated from milk of A1/A1, A1/A2, or A2/A2 Indian Karan Fries cows, by gavage at a dose of 85 mg/d (or a saline control), there were increases in indicators of gut inflammation associated with both A1/A1 and A1/A2 compared with A2/A2 or control (41). Both A1/A1 and A1/A2 significantly increased myeloperoxidase activity in intestinal tissue (P < 0.01) and IL-4, IgE, IgG, IgG1, and IgG2a concentrations in intestinal fluid compared with both control and A2/A2 (P < 0.01). The ratio of IgG1 to IgG2a also increased significantly (P < 0.05) in the A1/A1 group compared with the control. There was no difference between the control and the A2/A2-fed groups for any of these variables. There were no significant changes in IgA concentrations in the intestinal fluid with any of the β-casein variants compared with control.
In the same study, all β-casein variants significantly increased monocyte chemotactic protein 1 (MCP-1) concentrations in intestinal fluid compared with control, although this effect was greatest for A1/A1 and A1/A2 than for A2/A2. On histologic examination, both A1/A1 and A1/A2 significantly increased the number of leukocytes in the intestinal villi compared with both control and A2/A2, whereas there was no difference between A2/A2 and controls. However, β-casein had no effect on the number of goblet cells observed in the intestinal villi or IgA+ plasma cells in ileum sections compared with controls. A1/A1 and A1/A2 also significantly increased Toll-like receptor (TLR) 4 expression compared with control and A2/A2, and A1/A1 also increased TLR-2 expression compared with control and both A1/A2 and A2/A2 (neither of which were significantly different from the control group).
In a second study by the same research group in which mice received BCM-7, BCM-5, or saline by gavage for 15 d, there were similar significant increases in indicators of inflammation compared with the saline control (42). The β-casein and BCM doses administered in both of these studies were standardized according to a body size–dose–translation formula (human to mouse) (43) and the release of BCM-7 from A1 (20). Myeloperoxidase activity in intestinal tissue and MCP-1, IL-4, and histamine concentrations in intestinal fluid all increased in mice treated with BCM-7 and BCM-5. The magnitudes of the effect produced on opioid-mediated markers of inflammation were consistent between A1/A1 and BCM-7 and BCM-5 (Table 3). In contrast, the magnitude of the non–opioid-mediated increase in MCP-1 observed with BCM-7 was approximately one-third of that with A1/A1. Total IgE concentrations in intestinal fluid were also increased and the effect of BCM-7 was greater than that of BCM-5. Total IgG, IgG1, and IgG2a concentrations in intestinal fluid also increased, as did the ratio of IgG1 to IgG2a. Histologic examination also showed increases in the number of leukocytes in the intestinal villi. The expression of TLR mRNA (TLR-2 and TLR-4) also increased significantly in intestinal extracts. As with the casein study, intestinal fluid IgA was not affected by either BCM-7 or BCM-5, nor were histologic observations of the number of goblet cells in the intestinal villi or IgA-positive plasma cells in ileum sections, compared with controls.
TABLE 3.
Effects of bovine β-casein variants or BCMs on markers of gastrointestinal inflammation in mice1
| Test material, % increase |
|||||
| Marker | A1/A12 | A1/A22 | A2/A22 | BCM-73 | BCM-53 |
| MPO | 204.2* | 43.54* | NS | 129.76** | 117.55** |
| MCP-1 | 95.83* | 79.16* | 42.05* | 33.38*** | 31.73*** |
| IL-4 | 255.12* | 277.41* | NS | 175.54** | 164** |
| IgA | NS | NS | NS | NS | NS |
| IgE | 50.67* | 46.75* | NS | 77.09** | 52.37** |
| IgG | 77.56*** | 24.09*** | NS | 42.13*** | 45.17*** |
| IgG1 | 145.65* | 82.6* | NS | 126.63*** | 159.78*** |
| IgG2a | 81.07* | 66.23* | NS | 77.39*** | 90.27*** |
| IgG1/IgG2a | 35.5*** | NS | NS | 26.87*** | 27.32*** |
| Leukocytes in intestinal villi | 178.51** | 159.5** | NS | 154.99** | 118.04** |
| TLR-2 expression | 349** | NS | NS | 160*** | 63*** |
| TLR-4 expression | 414** | 408** | NS | 381*** | 290*** |
Values are mean ± SEM percentage increases relative to control (n = 6/group). *P < 0.01; **P < 0.001; ***P < 0.05. A1, A1-type bovine β-casein; A2, A2-type bovine β-casein; BCM, β-casomorphin; MCP-1, monocyte chemoattractant protein 1; MPO, myeloperoxidase; TLR, Toll-like receptor.
Data from reference 41.
Data from reference 42.
In another recent rodent study in which rats were fed a diet containing A1 or A2, A1 but not A2 also resulted in an increase in a range of markers of intestinal inflammation (39). A 65% increase in colonic myeloperoxidase was observed in the rats that consumed A1 rather than A2 and this was inhibited by (−)naloxone. Jejunal dipeptidyl peptidase 4 (DPP-4) activity (the enzyme responsible for cleaving BCMs at the second proline on the opioid peptide chain) also increased by 40% and 37% in rats fed A1, with or without (−)naloxone, compared with A2. The lack of any effect of (−)naloxone indicates that the effect on DPP-4 activity does not involve a μ-opioid–mediated pathway. A similar increase was not observed for colonic DPP-4, for which there was no difference from the controls irrespective of the type of β-casein consumed. Histologic injury scores also tended to be higher in the A1-fed groups, although the difference was not significant.
Human studies
In young children, the consumption of cow milk or cow-milk–based formula has been shown to be associated with chronic constipation in some individuals (8, 9). In a study in children aged 11 mo to 6 y fed cow milk and with chronic constipation that had not responded to laxatives, symptoms were alleviated by replacement with soy milk alternatives in 44 of 65 individuals (i.e., 21 did not respond to the removal of cow milk) (5). In a follow-up double-blind, placebo-controlled trial in the 44 milk-sensitive individuals only, constipation returned within 5–10 d in all those given cow milk but in none of those given the soy milk control. Histologic evidence of gastrointestinal inflammation was also observed via biopsy samples in a subset of 26 of the 44 milk-sensitive children, characterized by infiltration by lymphocytes, particularly infiltration of the lamina propria by eosinophils and the presence of intraepithelial eosinophils in the crypts.
In a separate double-blind crossover study in cow-milk–drinking children, aged 18 mo to 12 y, with chronic constipation unresolved by laxatives, symptoms were relieved by substitution with soy milk (9 of 9) or by a non–cow-milk or soy-milk diet (8 of 9) over a 2-wk period (9). There was a significant difference in the incidence of resolution of constipation and the number of bowel movements during the cow-milk compared with the soy-milk stages of the study. Despite the presence of an identified potential for bias in the study because of organoleptic differences between cow milk and soy milk, such as taste, and a higher prevalence of dropout due to constipation of subjects who consumed the cow milk first, the results of this study in relation to a causal association between cow milk and constipation are consistent with those of the preceding study (8). A follow-up double-blind crossover side study that attempted to investigate the comparative effects of A1 and A2 on constipation by using commercially available milk failed to show a difference (9). However, the study design contained a potential for bias because neither the sources nor β-casein analyses of the commercially available test milks were reported. In light of these limitations, it is difficult to draw any conclusions from this study.
In a double-blind, randomized crossover study in 41 Australian adults, who consumed 750 mL milk/d containing either A1 or A2 exclusively for 2 wk separated by a 2-wk washout period, the stool consistency score, self-assessed by using the Bristol Stool Scale, was significantly higher with A1 than with A2 milk, indicating softer stools in the A1 group (41). The result was strongest for women alone. This difference in stool consistency was also evident within a subgroup who were self-identified as being milk-tolerant individuals (n = 28), but not for those who self-identified as being milk intolerant (n = 8), likely because of the smaller group number. There were no reported differences in bowel frequency between the A1 and A2 milk phases of this study, although there was considerable intragroup variation (A1: 0.43–3.6; A2: 0.3–4.5).
This study also reported a number of secondary correlations between the measured variables. At a whole-group level, there was a significant positive association between stool consistency and abdominal pain with the A1 diet (r = 0.52) but not with the A2 diet (r = −0.13). The difference between these 2 correlations (0.52 compared with −0.13) was also highly significant. For the A1 milk stage, there were also correlations between gastrointestinal inflammation, measured as fecal calprotectin, and self-recorded markers of digestive discomfort (e.g., bloating, abdominal pain, flatus, and voiding difficulty), either individually or when combined in a composite measure, for all participants, whereas during the A2 stage, there was no correlation with bloating or abdominal pain and the correlation with the composite measure was markedly weaker. There were significant correlations between gastrointestinal inflammation and both higher abdominal pain (r = 0.46) and higher bloating (r = 0.36) scores during the A1 phase but not during the A2 milk phase. The difference in the correlation measures between gut inflammation and both abdominal pain (A1 compared with A2: 0.46 compared with 0.03) and bloating (A1 compared with A2: 0.36 compared with 0.02) was also significant for the 2 study phases. There was no reported overall difference in fecal calprotectin between the A1 and A2 milk–drinking groups, and analyses from most participants were within the normal range (<50 mg/g). However, analyses from 5 individuals, who consumed A1 milk first, showed >50 mg/g for both A1 and A2 stages of the study and 3 other individuals had fecal calprotectin concentrations >100 mg/g when consuming A1 milk but <50 mg/g when consuming A2 milk.
In a further recent randomized, double-blind crossover study in 45 Han Chinese adults who self-identified as being milk intolerant (but without previous diagnosis of cause), the consumption of two 250-mL servings of A1/A2 (40:60 ratio) but not A2/A2 milk/d was associated with an increase in self-reported markers of digestive discomfort and stool frequency and softer stools, as measured by using the Bristol Stool Scale (11). Whole gastrointestinal transit time, as measured by using the OMOM Controllable Capsule Endoscope [Jianshan Science & Technology (Group) Co., Ltd., Chongqing, China], was significantly longer with A1/A2 milk than with A2/A2 milk (by 6.3 h; P < 0.0001), and was similar for colon transit time (by 6.6 h; P < 0.0001) but not for small bowel transit time (−0.20 h; P = 0.59). There were also significant increases in serum markers of inflammation, IL-4, IgG, IgE, and IgG1 associated with A1/A2 milk compared with A2/A2 milk. Within the trial, urinary galactose was used as a marker for the gastrointestinal presence of lactase. On that basis, approximately half of the participants were classified as being lactose intolerant. However, the effects of the A1/A2 mix compared with all A2 milks were found to be similar in both the presence and absence of the lactase marker, indicating that both groups were reacting to the A1.
Quality of the data
Much of the research relating to the release of BCMs from milk or casein has been undertaken within in vitro studies, which simulate gastrointestinal digestion, to find and characterize naturally occurring exorphins. These studies are generally performed with adequate controls. The potential for bias within individual studies is addressed in Supplemental Tables 2, 4, and 6. Accordingly, evidence on the in vitro release of peptides with μ-opioid activity, and differences therein in relation to A1 compared with A2, can be considered reliable. The in vivo evidence confirms these differences when bovine milk is consumed by animals and humans and shows peptide release at concentrations that have the potential to be of biological significance. Overall, there is a high degree of consistency in outcomes within the data reviewed.
The evidence for the gastrointestinal effects of A1 compared with A2 in animals comes from multiple well-controlled experiments. However, in vivo clinical data for humans are currently limited to 3 controlled, blinded, crossover pilot studies, one in children (9) and 2 in adults (44, 45). The 2 studies in adults drew on distinct populations: one being Australian, largely self-identified as milk tolerant, of mixed ethnicity but predominantly European, and the other being Han Chinese with self-identified milk intolerance. Accordingly, although the crossover designs show high levels of significance, caution is appropriate in relation to generalization to other populations.
Discussion
The evidence identified in this systematic review can be classified as a combination of conclusive (in which findings have been documented and found to be repeatable in different investigatory settings) and emerging (in which significant findings have been obtained, but where repeatability of findings in diverse research settings is required before the science can be regarded as settled). It is established that BCM-7 and related short BCMs (BCM-5, BCM-4, and BCM-3) are released by gastrointestinal digestion from the A1-type (but not the A2-type) β-caseins under in vitro conditions and in animals. Furthermore, these short BCMs exhibit direct μ-opioid receptor agonist activity both in vitro and in animal studies. In animals, there is consistent evidence that A1 slows gastrointestinal transit, and it is conclusive that this is opioid-mediated. A current clinical study also reported the release of pharmacologic quantities of BCM-7 in humans after the consumption of a quantity of bovine casein equivalent to 1 L of milk (28).
There is also consistent evidence in rodents that A1 and BCM-7 are proinflammatory and induce T cell–mediated immune response and emerging evidence of similar proinflammatory markers in humans. There is well-established previous evidence that endogenous μ-opioid agonists, such as β-endorphin, are released in the gut in response to chronic pain or injury and have signaling roles both neurologically and peripherally (46, 47). It is also well established that localized responses to endogenous opioid peptides in the gastrointestinal tract include alterations to gut motility and transit time (46, 48) and T cell–mediated inflammatory responses (49–51). Accordingly, the above effects from A1 and BCM-7 are consistent with a priori hypotheses.
Although the overall evidence for gastrointestinal effects from A1 and BCM-7 in animal trials and in vitro studies can be considered conclusive, the evidence from human clinical studies can be classed as emerging. Although there are strong theoretical grounds arising from the universality of μ-opioid receptors within mammalian gastrointestinal systems and the consistent evidence of β-casein effects on gastrointestinal transit to postulate that the gastrointestinal transit results from this specific milk-intolerant Han Chinese population will be repeatable with other population groups, such generalizability cannot be concluded without specific investigations thereof. It is also reasonable to postulate that longer gastrointestinal transit times may lead to increased effects of lactose fermentation and other dietary components, such as FODMAPS, which, together with genetic predisposition, may also be relevant in relation to clinical and subclinical outcomes, including both digestive discomfort and proinflammatory effects (3).
The 2 clinical studies in adult humans that investigated Bristol Stool Scale measures of fecal consistency both reported softer stools with A1 relative to A2, with one of these studies also showing softer stools associated with the A1 diet relative to a nondairy baseline diet. Although these results could be considered counterintuitive, given the delayed gastrointestinal transit and hence a perceived potential for possible constipation, they are consistent with the proinflammatory markers evident in both human and animal studies. However, given that these findings are counter to a conventional perception that longer transit will lead to constipation, further replication of these results is appropriate.
The published evidence in relation to A1 and gastrointestinal effects relates primarily to μ-opioid pathways. However, it is evident that A1 stimulates production of the enzyme DPP-4 in the rat jejunum through a nonopioid mechanism (39). It is also known that BCMs are 5-hydroxytryptamine2 (5HT2)-serotonin receptor antagonists through an unrelated mechanism (52–54). Furthermore, recent research has shown that A1 compared with A2 has implications for casein micelle structure and associated chaperone activity within the gastrointestinal system that is unrelated to opioid mechanisms (55). Accordingly, further research into these mechanisms within the human gastrointestinal system is warranted.
Within this study, we emphasize that our focus has been on a systematic analysis that draws on in vivo evidence—human and animal—supported by in vitro studies of underlying science. We wish to clearly distinguish between such a systematic study and a meta-analysis of human clinical studies, which would clearly be premature. We also acknowledge the likelihood that sensitivity to A1 may vary both across and within populations, with these issues yet to be elucidated.
In conclusion, it is evident that, under normal digestive conditions, the μ-opioid peptide BCM-7 is released from A1 but not from A2. There is extensive evidence from animal trials and emerging evidence in humans that this is associated with slower gastrointestinal transit and hence increased gastrointestinal transit times. It is also evident in animals and at least in some human population groups that the A1–derivative peptide BCM-7 is proinflammatory. The balance between the extent to which these effects are direct inflammatory responses to BCM-7, or indirect consequences of delayed transit influencing other biological processes, is yet to be elucidated. Although the current gastrointestinal evidence is strongly linked to BCM-7 and μ-opioid pathways, the possibility that some gastrointestinal effects involve nonopioid pathways is relevant. There is now a need for further clinical studies of A1 effects in a broad range of population groups (ages, ethnicities, and different genetic haplotypes) and dietary conditions.
Acknowledgments
We thank Oleg Sokolov for his advice in relation to the interaction of BCM-7 with the 5HT2-serotonin system. The authors’ responsibilities were as follows—SB-T: analyzed the data and had primary responsibility for the final content; SB-T and KW: designed and conducted the research; SB-T, KW, and KD: contributed to the writing of the manuscript; NK: reviewed the manuscript and provided relevant content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: A1, A1-type bovine β-casein; A2, A2-type bovine β-casein; BCM, β-casomorphin; DPP-4, dipeptidyl peptidase 4; EFSA, European Food Safety Authority; MCP-1, monocyte chemotactic protein 1; TLR, Toll-like receptor.
References
- 1.Australia New Zealand Food Standards Code. Schedule 6—required elements of a systematic review [Internet]. 2015. Australian Government Gazette No. FSC 96 of 10 April 2015. [cited 2017 Jun 21]. Available from: https://www.legislation.gov.au/Details/F2015L00476.
- 2.Kamiński S, Cieslińska A, Kostyra E. Polymorphism of bovine beta-casein and its potential effect on human health. J Appl Genet 2007;48:189–98. [DOI] [PubMed] [Google Scholar]
- 3.De Noni RJ, FitzGerald HJT, Korhonen Y, Le Roux CT, Livesey I, Thorsdottir D, Tomé RW. Scientific report of EFSA prepared by a DATEX working group on the potential health impact of β-casomorphins and related peptides. EFSA Sci Rep 2009;231:1–107. [Google Scholar]
- 4.Pal S, Woodford K, Kukuljan S, Ho S. Milk intolerance, beta-casein and lactose. Nutrients 2015;7:7285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truswell AS. The A2 milk case: a critical review. Eur J Clin Nutr 2005;59:623–31. [DOI] [PubMed] [Google Scholar]
- 6.Woodford KB. A critique of Truswell’s A2 milk review. Eur J Clin Nutr 2006;60:437–9. [DOI] [PubMed] [Google Scholar]
- 7.Allison AJ, Clarke AJ. Further research for consideration in “theA2 milk case”. Eur J Clin Nutr 2006;60:921–4. [DOI] [PubMed] [Google Scholar]
- 8.Chabance B, Marteau P, Rambaud JC, Migliore-Samour D, Boynard M, Perrotin P, Guillet R, Jollès P, Fiat AM. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 1998;80:155–65. [DOI] [PubMed] [Google Scholar]
- 9.Iacono G, Cavataio F, Montalto G, Florena A, Tumminello M, Soresi M, Notarbartolo A, Carroccio A. Intolerance of cow’s milk and chronic constipation in children. N Engl J Med 1998;339:1100–4. [DOI] [PubMed] [Google Scholar]
- 10.Crowley ET, Williams LT, Roberts TK, Dunstan RH, Jones PD. Does milk cause constipation? A crossover dietary trial. Nutrients 2013;5:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara H, Nishikawa H, Kiriyama S. Different effects of casein and soyabean protein on gastric emptying of protein and small intestinal transit after spontaneous feeding of diets in rats. Br J Nutr 1992;68:59–66. [DOI] [PubMed] [Google Scholar]
- 12.Brantl V, Teschemacher H. A material with opioid activity in bovine milk and milk products. Naunyn Schmiedebergs Arch Pharmacol 1979;306:301–4. [DOI] [PubMed] [Google Scholar]
- 13.Brantl V, Teschemacher H, Henschen A, Lottspeich F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers Z Physiol Chem 1979;360:1211–6. [DOI] [PubMed] [Google Scholar]
- 14.Henschen A, Lottspeich F, Brantl V, Teschemacher H. Novel opioid peptides derived from casein (beta-casomorphins). II. Structure of active components from bovine casein peptone. Hoppe Seylers Z Physiol Chem 1979;360:1217–24. [PubMed] [Google Scholar]
- 15.Lottspeich F, Henschen A, Brantl V, Teschemacher H. Novel opioid peptides derived from casein (beta-casomorphins). III. Synthetic peptides corresponding to components from bovine casein peptone. Hoppe Seylers Z Physiol Chem 1980;361:1835–9. [DOI] [PubMed] [Google Scholar]
- 16.Ribadeau Dumas B, Brignon G, Grosclaude F, Mercier J. Primary structure of bovine beta casein: complete sequence. Eur J Biochem 1972;25:505–14. [DOI] [PubMed] [Google Scholar]
- 17.Svedberg J, de Haas J, Leimenstoll G, Paul F, Teschemacher H. Demonstration of beta-casomorphin immunoreactive materials in in vitro digests of bovine milk and in small intestine contents after bovine milk ingestion in adult humans. Peptides 1985;6:825–30. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzer CE, Schops R, Reynell L, Ulbrich-Hofmann R, Neubert RH, Raith K. Peptic digestion of beta-casein: time course and fate of possible bioactive peptides. J Chromatogr A 2007;1166:108–15. [DOI] [PubMed] [Google Scholar]
- 19.Jinsmaa Y, Yoshikawa M. Enzymatic release of neocasomorphin and beta-casomorphin from bovine beta-casein. Peptides 1999;20:957–62. [DOI] [PubMed] [Google Scholar]
- 20.De Noni I. Release of b-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine b-casein variants and milk-based infant formulas. Food Chem 2008;110:897–903. [DOI] [PubMed] [Google Scholar]
- 21.Ul Haq MR, Kapila R, Kapila S. Release of beta-casomorphin-7/5 during simulated gastrointestinal digestion of milk beta-casein variants from Indian crossbred cattle (Karan Fries). Food Chem 2015;168:70–9. [DOI] [PubMed] [Google Scholar]
- 22.Cieslinska A, Kaminski S, Kostyra E, Sienkiewicz-Szlapka E. Beta-casomorphin-7 in raw and hydrolyzed milk derived from cows of alternative β-casein genotypes. Milchwissenschaft 2007;62:125–7. [Google Scholar]
- 23.Cieślińska A, Kostyra E, Kostyra H, Oleński K, Fiedorowicz E, Kamiński S. Milk from cows of different beta-casein genotypes as a source of beta-casomorphin-7. Int J Food Sci Nutr 2012;63:426–30. [DOI] [PubMed] [Google Scholar]
- 24.De Noni I, Cattaneo S. Occurrence of beta-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem 2010;2022:560–6. [Google Scholar]
- 25.Hellmig S, Von Schöning F, Gadow C, Katsoulis S, Hedderich J, Fölsch UR, Stüber E. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: influence of age, sex and body mass index. J Gastroenterol Hepatol 2006;21:1832–8. [DOI] [PubMed] [Google Scholar]
- 26.Meisel H. Chemical characterization and opioid activity of an exorphin isolated from in vivo digests of casein. FEBS Lett 1986;196:223–7. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Rosen CL, Chang KJ, Haddad GG. Plasma beta-casomorphin-7 immunoreactive peptide increases after milk intake in newborn but not in adult dogs. Pediatr Res 1989;26:34–8. [DOI] [PubMed] [Google Scholar]
- 28.Boutrou R, Gaudichon C, Dupont D, Jardin J, Airinei G, Marsset-Baglieri A, Benamouzig R, Tomé D, Leonil J. Sequential release of milk protein–derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr 2013;97:1314–23. [DOI] [PubMed] [Google Scholar]
- 29.Brantl V, Teschemacher H, Blasig J, Henschen A, Lottspeich F. Opioid activities of beta-casomorphins. Life Sci 1981;28:1903–9. [DOI] [PubMed] [Google Scholar]
- 30.Trompette A, Claustre J, Caillon F, Jourdan G, Chayvialle JA, Plaisancie P. Milk bioactive peptides and beta-casomorphins induce mucus release in rat jejunum. J Nutr 2003;133:3499–503. [DOI] [PubMed] [Google Scholar]
- 31.Dalziel JE, Spencer NJ, Dunstan KE, Lynch AT, Haggarty NW, Gopal PK, Roy NC. An in vitro rat model of colonic motility to determine the effect of beta-casomorphin-5 on propagating contractions. Food Funct 2014;5:2768–74. [DOI] [PubMed] [Google Scholar]
- 32.Claustre J, Toumi F, Trompette A, Jourdan G, Guignard H, Chayvialle JA, Plaisancié P. Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum. Am J Physiol Gastrointest Liver Physiol 2002;283:G521–8. [DOI] [PubMed] [Google Scholar]
- 33.Zoghbi S, Trompette A, Claustre J, El Homsi M, Garzon J, Jourdan G, Scoazec JY, Plaisancié P. Beta-casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am J Physiol Gastrointest Liver Physiol 2006;290:G1105–13. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi MS, Shah JS, Al-Mughairy S, Hodgson NW, Simms B, Trooskens GA, Van Criekinge W, Deth RC. Food-derived opioid peptides inhibit cysteine uptake with redox and epigenetic consequences. J Nutr Biochem 2014;25:1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarmolowska B, Sidor K, Iwan M, Bielikowicz K, Kaczmarski M, Kostyra E. Changes of beta-casomorphin content in human milk during lactation. Peptides 2007;28:1982–6. [DOI] [PubMed] [Google Scholar]
- 36.Daniel H, Vohwinkel M, Rehner G. Effect of casein and beta-casomorphins on gastrointestinal motility in rats. J Nutr 1990;120:252–7. [DOI] [PubMed] [Google Scholar]
- 37.Defilippi C, Gomez E, Charlin V, Silva C. Inhibition of small intestinal motility by casein: a role of beta casomorphins? Nutrition 1995;11:751–4. [PubMed] [Google Scholar]
- 38.Mihatsch WA, Franz AR, Kuhnt B, Hogel J, Pohlandt F. Hydrolysis of casein accelerates gastrointestinal transit via reduction of opioid receptor agonists released from casein in rats. Biol Neonate 2005;87:160–3. [DOI] [PubMed] [Google Scholar]
- 39.Barnett MP, McNabb WC, Roy NC, Woodford KB, Clarke AJ. Dietary A1 beta-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 beta-casein in Wistar rats. Int J Food Sci Nutr 2014;65:720–7. [DOI] [PubMed] [Google Scholar]
- 40.White CL, Bray GA, York DA. Intragastric beta-casomorphin(1–7) attenuates the suppression of fat intake by enterostatin. Peptides 2000;21:1377–81. [DOI] [PubMed] [Google Scholar]
- 41.Ul Haq MR, Kapila R, Sharma R, Saliganti V, Kapila S. Comparative evaluation of cow beta-casein variants (A1/A2) consumption on Th2-mediated inflammatory response in mouse gut. Eur J Nutr 2014;53:1039–49. [DOI] [PubMed] [Google Scholar]
- 42.Ul Haq MR, Kapila R, Saliganti V. Consumption of β-casomorphins-7/5 induce inflammatory immune response in mice gut through Th2 pathway. J Funct Foods 2014;8:150–60. [Google Scholar]
- 43.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008;22:659–61. [DOI] [PubMed] [Google Scholar]
- 44.Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr 2014;68:994–1000. [DOI] [PubMed] [Google Scholar]
- 45.Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J 2016;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 2009;155:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma-Gandhu M, Verdu EF, Bercik P, Blennerhassett PA, Al-Mutawaly N, Ghia JE, Collins SM. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut 2007;56:358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bianchi G, Ferretti P, Recchia M, Rocchetti M, Tavani A, Manara L. Morphine tissue levels and reduction of gastrointestinal transit in rats: correlation supports primary action site in the gut. Gastroenterology 1983;85:852–8. [PubMed] [Google Scholar]
- 49.Pol O, Sasaki M, Jimenez N, Dawson VL, Dawson TM, Puig MM. The involvement of nitric oxide in the enhanced expression of mu-opioid receptors during intestinal inflammation in mice. Br J Pharmacol 2005;145:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philippe D, Dubuquoy L, Groux H, Brun V, Chuoi-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 2003;111:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma-Gandhu M, Verdu EF, Cohen-Lyons D, Collins SM. Lymphocyte-mediated regulation of beta-endorphin in the myenteric plexus. Am J Physiol Gastrointest Liver Physiol 2007;292:G344–8. [DOI] [PubMed] [Google Scholar]
- 52.Sokolov OY, Kost NV, Zolotarev YA, Ryukert EN, Zozulya AA. Influence of human β-casomorphin-7 on specific binding of 3H-spiperone to the 5-HT2-receptors of rat brain frontal cortex. Protein Pept Lett 2006;13:169–70. [DOI] [PubMed] [Google Scholar]
- 53.Sokolov OY, Pryanikova NA, Kost NV, Zolotarev YA, Ryukert EN, Zozulya AA. Reactions between beta-casomorphins-7 and 5-HT2-serotonin receptors. Bull Exp Biol Med 2005;140:582–4. [DOI] [PubMed] [Google Scholar]
- 54.Zozulya AA, Meshavkin VK, Sokolov OY, Kost NV. Naloxone-reversible suppression of the behavioral manifestation of serotoninergic system hyperactivation by beta-casomorphins-7 in mice. Eksp Klin Farmakol 2009;72:3–5. [PubMed] [Google Scholar]
- 55.Raynes JK, Day L, Augustin MA, Carver JA. Structural differences between bovine A(1) and A(2) β-casein alter micelle self-assembly and influence molecular chaperone activity. J Dairy Sci 2015;98:2172–82. [DOI] [PubMed] [Google Scholar]