Abstract
The incidence of overweight and obesity has reached epidemic proportions, making the control of body weight and its complications a primary health problem. Diet has long played a first-line role in preventing and managing obesity. However, beyond the obvious strategy of restricting caloric intake, growing evidence supports the specific antiobesity effects of some food-derived components, particularly (poly)phenolic compounds. The relatively new rediscovery of active brown adipose tissue in adult humans has generated interest in this tissue as a novel and viable target for stimulating energy expenditure and controlling body weight by promoting energy dissipation. This review critically discusses the evidence supporting the concept that the antiobesity effects ascribed to (poly)phenols might be dependent on their capacity to promote energy dissipation by activating brown adipose tissue. Although discrepancies exist in the literature, most in vivo studies with rodents strongly support the role of some (poly)phenol classes, particularly flavan-3-ols and resveratrol, in promoting energy expenditure. Some human data currently are available and most are consistent with studies in rodents. Further investigation of effects in humans is warranted.
Keywords: brown adipose tissue, dietary (poly)phenols, energy expenditure, obesity, resveratrol, flavan-3-ols, uncoupled protein 1
Introduction
Obesity, particularly visceral obesity, is considered one of the main drivers of the metabolic syndrome. Individuals with a BMI (in kg/m2) >30 are classified as obese. By this criterion, the WHO estimates that ∼13% of the world’s adult population is obese, making obesity and its complications one of the most challenging public health problems (1).
To date, strategies aiming to control body weight have focused mainly on reducing caloric intake and preventing food absorption (1). The control of body weight depends on the tightly regulated equilibrium between energy intake and energy expenditure (EE) (2); therefore, approaches aimed to increase EE represent an alternative strategy to promote weight loss.
Energy dissipation through activation of brown adipose tissue (BAT) may be a promising target for obesity management. Accordingly, recent studies indicated the presence of metabolically active BAT in humans (3). The presence of human BAT negatively correlates with BMI, fat mass percentage, and plasma glucose (4–10). The capacity of BAT to influence EE relies on its unique ability to dissipate energy as heat and depends on the expression of uncoupling protein 1 (UCP1) in brown adipocytes. UCP1 uncouples the electron transport chain (ETC) from ATP synthesis, thus dissipating energy (11).
Several studies suggest that food-derived components, particularly (poly)phenols, may play a role in preventing and managing obesity and its comorbidities (12–14). Interestingly, some of the antiobesity and antidiabetic activities attributed to (poly)phenols have been associated with positive effects on EE (13, 14). This review critically evaluates the literature describing the possible role of (poly)phenols in the regulation of EE through BAT activation.
Current Status of Knowledge
Adrenergic stimulation of thermogenesis and involvement of UCP1
This section describes the intracellular cascade that norepinephrine (NE) causes in cells, triggering lipolysis, increasing mitochondrial respiration, and activating UCP1.
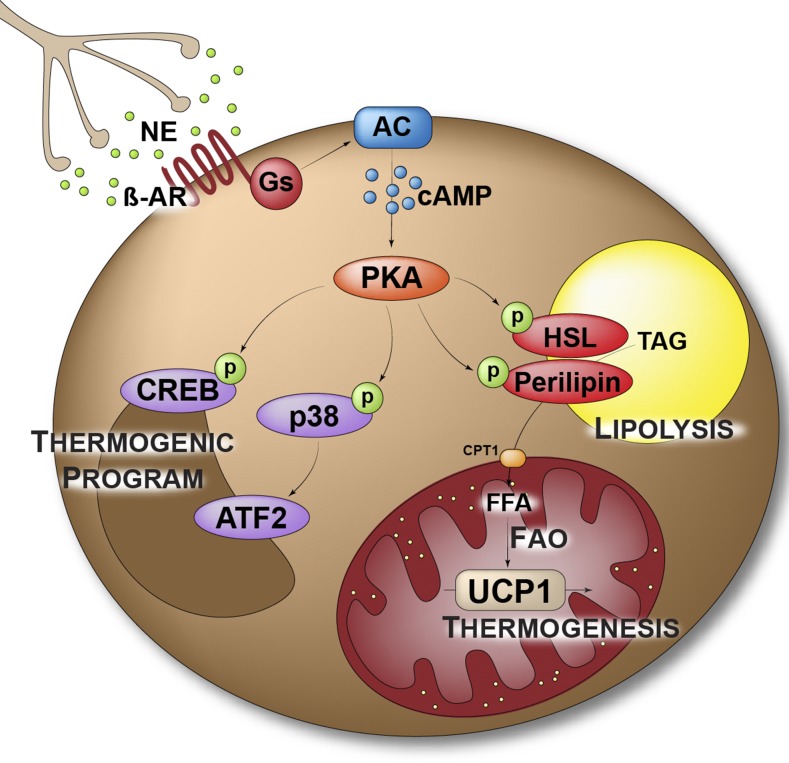
In conditions requiring an increase in body temperature, the sympathetic nervous system (SNS) releases NE close to postganglionic nerve endings in BAT, ensuring its activation (15). NE binds to the β3-adrenergic receptor (β3AR) present on the surface of brown adipocytes, promoting an intracellular signaling cascade as summarized in Figure 1. Activation of BAT requires the mobilization of energy through lipolysis. β3AR binding induces the production of cAMP by the adenylate cyclase. Increased intracellular concentrations of cAMP activate protein kinase A (PKA), which phosphorylates hormone-sensitive lipase (HSL) and perilipin (16) to promote TG hydrolysis. The released FFAs are then shuttled to mitochondria through carnitine palmitoyltransferase 1 (CPT1). In mitochondria, FFAs activate UCP1, and FA oxidation produces cofactors for the ETC (16, 17). UCP1 uses the proton gradient created by the ETC to produce heat and therefore dissipates energy.
FIGURE 1.
Adrenergic stimulation of thermogenesis. AC, adenylate cyclase; ATF2, activating transcription factor 2; CPT1, carnitine palmitoyltransferase 1; CREB, cAMP response element binding protein; FAO, fatty acid oxidation; Gs, Gs α subunit; HSL, hormone-sensitive lipase; NE, norepinephrine; p, phosphate group; PKA, protein kinase A; p38, p38 mitogen-activated protein kinase; TAG, triacylglycerol; UCP1, uncoupling protein 1; β-AR, β-adrenergic receptor.
In parallel with the direct activation of thermogenesis, NE stimulation leads to a transcriptional regulation of genes important for thermogenesis (i.e., induction of the “thermogenic program”). Indeed, activated PKA also phosphorylates and activates the transcription factor cAMP response element binding protein (CREB) and the p38 mitogen-activated protein kinase. In turn, the p38 mitogen-activated protein kinase phosphorylates transcription factors, such as activating transcription factor 2 or the transcriptional coactivator PPAR-γ coactivator-1α (PGC-1α) (16), to induce UCP1 expression. Moreover, phosphorylated CREB enhances transcription of type 2 iodothyronine deiodinase, which converts inactive tetraiodothyronine into triiodothyronine (T3), promoting T3 binding to its receptor. When the receptor does not bind T3, it acts as a UCP1 transcriptional repressor. Therefore, T3 indirectly increases UCP1 expression (18, 19).
Here, we underline the similar activity that NE exerts in white and brown adipocytes. Indeed, white adipose tissue (WAT) is also highly responsive to NE. Here, NE stimulates lipolysis through similar intracellular signaling events (17), thus promoting the release of FFAs that are used as energetic substrates in BAT to sustain thermogenesis. Moreover, a continued sympathetic tone in WAT also induces the recruitment of UCP1-expressing adipocytes, termed “beige” or “brite” (brown-in-white), particularly in subcutaneous and retroperitoneal depots (20). Several observations support a role for beige adipocytes in energy balance in rodents. Accordingly, loss of beige adipocytes is shown to cause obesity, whereas increased recruitment of beige adipocytes in the WAT can compensate a decrease in thermogenic activity of the BAT (21, 22). However, physiologic recruitment of UCP1 and adipocytes in human WAT is still highly debated and conflicting results have been reported (23–26).
Regulation of beige and brown adipogenesis
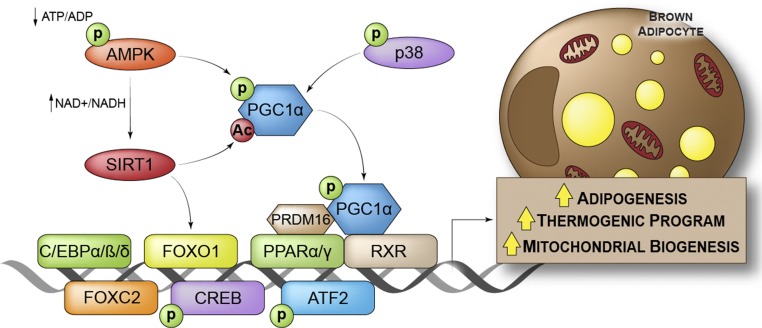
In adults, both WAT and BAT can expand in response to increased storage demand or chronic cold stress, respectively. Furthermore, as previously outlined, beige adipocytes can also be recruited in WAT depots in response to cold. This expansion relies on precursor cells with adipogenic potential. Although white and brown adipocytes fulfill different physiologic functions and display different embryonic origins, their terminal differentiation is controlled by the same transcriptional cascade (20). PPAR-γ and 3 CCAAT/enhancer-binding protein (C/EBP) family members (C/EBP-α, C/EBP-β, and C/EBP-δ) are the key transcription factors regulating both white and brown adipogenesis. However, additional specific factors such as PPAR-α, PGC-1α, PR domain zinc finger protein 16 (PRDM16), and forkhead box protein C2 (FOXC2) have been shown to specifically promote brown or beige adipogenesis (Figure 2) (20).
FIGURE 2.
Transcriptional regulation of beige and brown adipocytes. Ac, acetyl group; AMPK, 5′-AMP–activated protein kinase; ATF2, activating transcription factor 2; C/EBP α/β/δ, CCAAT/enhancer-binding protein α/β/δ CREB, cAMP response element binding protein; FOXC2, forkhead box protein C2; FOXO1, forkhead box protein O1; p, phosphate group; PGC-1α, PPAR-γ coactivator-1α PPAR-α/γ, peroxisome proliferator–activated receptor α/γ PRDM16, PR domain zinc finger protein 16; p38, p38 mitogen-activated protein kinase; RXR, retinoid X receptor; SIRT1, sirtuin 1.
PGC-1α drives mitochondrial biogenesis together with the thermogenic program in brown adipocytes and promotes oxidative metabolism in many cell types and organs. PGC-1α forms complexes with PPAR-α or PPAR-γ and the retinoid X receptor, which bind to a PPAR response element in the UCP1 promoter to activate its transcription. The PGC-1α/PPAR complex is also able to bind PRDM16, another cofactor specifically expressed in brown and beige adipocytes that stimulates the transcription of several genes involved in thermogenesis (including PGC-1α and UCP1) (Figure 2) (27). The transcriptional regulation of beige and brown adipogenesis was recently extensively reviewed (28).
A further level of regulation of brown or beige adipogenesis includes post-translation modification of transcription factors, whose activity can be modulated by covalent modifications. This mechanism is exemplified by the 5′-AMP–activated protein kinase (AMPK)–sirtuin 1 (SIRT1)–PGC-1α axis (Figure 2). The NAD-dependent protein deacetylase SIRT1 targets multiple transcription factors such as PGC-1α and forkhead box O1 (FOXO1), regulating oxidative metabolism and glucose homeostasis (29, 30). Moreover, SIRT1-mediated deacetylation of PPAR-γ favors beige adipocyte recruitment in WAT (31). AMPK is activated by phosphorylation and works as an energy sensor in cells. Indeed, the binding of AMP and ADP to AMPK enhances its activity and reduces its dephosphorylation. Activated AMPK in metabolic organs, including the liver, skeletal muscle, and WAT, inhibits anabolism and thus promotes catabolic processes. Notably, activated AMPK indirectly stimulates FA oxidation through phosphorylation of the acetyl-CoA carboxylase. Inactivation of the acetyl-CoA carboxylase by phosphorylation leads to a reduction in its product, malonyl-CoA (an inhibitor of CPT1), thus promoting FA transport in the mitochondria and its subsequent oxidation (32). Moreover, in skeletal muscle, AMPK activates SIRT1 through the modulation of NAD+ levels (33). In the same tissue, AMPK can also directly enhance PGC-1α activity by phosphorylation, thus increasing mitochondrial biogenesis (34). Evidence supports a similar role for the AMPK–SIRT1–PGC1α axis in brown and beige adipocytes. Accordingly, BAT activation is often associated with increased AMPK phosphorylation, both in vitro and in vivo (35, 36), and treatment with the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide increased WAT browning in mice (37). Despite the conflicting literature on this topic (38), Mottillo et al. (39) recently confirmed the importance of AMPK in BAT activation in mice, in which the deletion of AMPK specifically in adipocytes was associated with a reduction in thermogenesis.
Dietary (poly)phenols in the stimulation of EE
In this section, we critically assess the literature describing the role of some classes of (poly)phenols in the stimulation of EE (Figures 3 and 4). In vitro studies evaluating the effects of food extracts rather than specific molecules are not cited because of the difficulty in translating these results to either a nutritional or pharmacologic context.
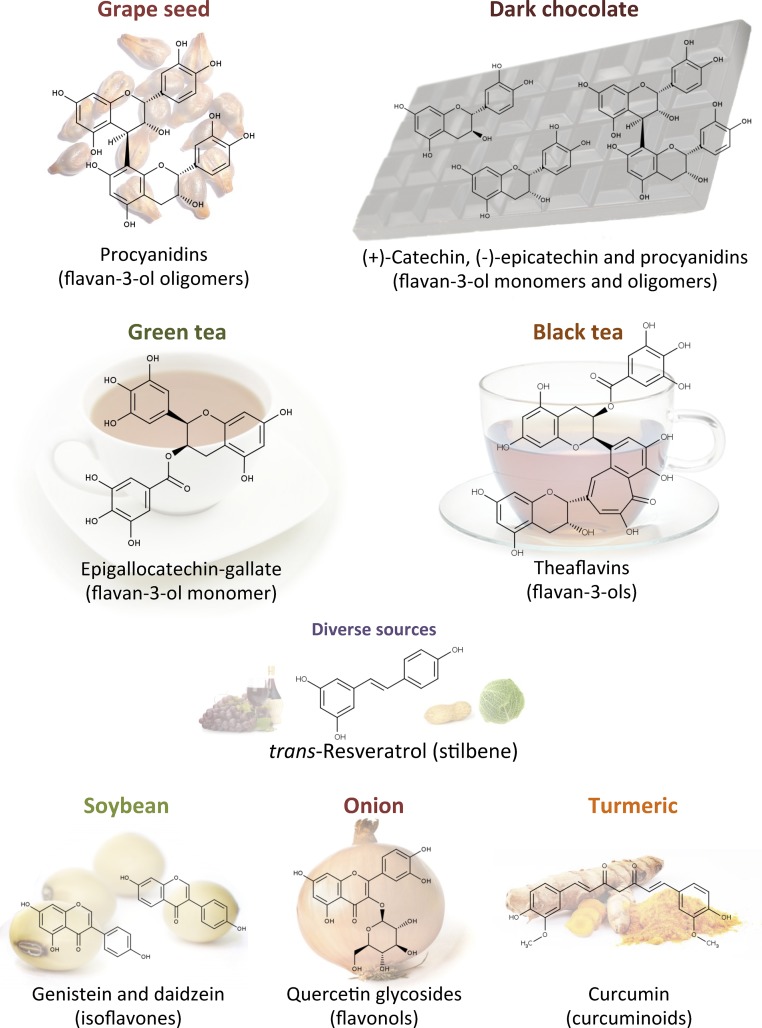
FIGURE 3.
Chemical structures and food sources of the (poly)phenols described as modulators of energy expenditure.
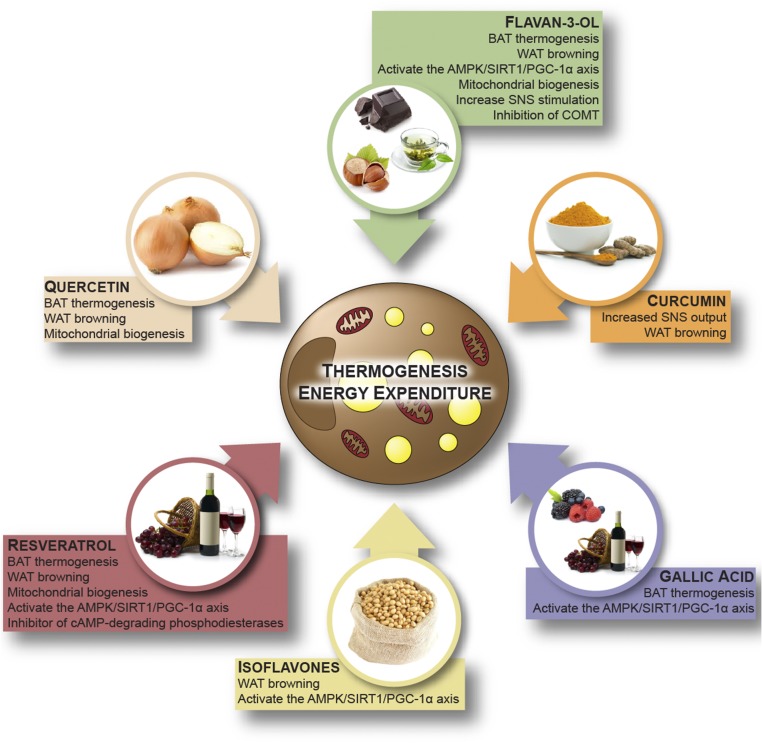
FIGURE 4.
Dietary (poly)phenols in the stimulation of energy expenditure. AMPK, 5′-AMP–activated protein kinase; BAT, brown adipose tissue; COMT, catechol-O-methyl transferase; PGC-1α, PPAR-γ coactivator-1α SIRT1, sirtuin 1; SNS, sympathetic nervous system; WAT, white adipose tissue.
Flavan-3-ols.
To our knowledge, flavan-3-ols are the most extensively consumed (poly)phenols in western populations (40). Their main sources are dark chocolate, green tea, berries, nuts, and red wine, all containing both flavan-3-ol oligomers (proanthocyanidins) and their monomeric forms (catechins) (Figure 3). Oligomeric flavan-3-ols are also highly present in grape seeds (41) (Figure 3). Consumption of foods rich in flavan-3-ols has been associated with positive effects in the framework of the metabolic syndrome (42–45). Moreover, in vivo evidence supporting a modulation of EE after flavan-3-ol consumption has been reported both in rodents and humans (46–51).
Pajuelo et al. (52) evaluated both chronic (25 or 50 mg/kg body weight for 21 d) and acute (250 mg/kg body weight) (53) effects of a grape seed proanthocyanidin extract (GSPE) in male rats, showing a direct effect on BAT. Chronic GSPE supplementation partially reversed BAT mitochondrial dysfunction attributable to diet-induced obesity evaluated as mitochondrial respiration capacity (52), whereas acute administration of GSPE stimulated the thermogenic program and positively modulated the activity of proteins involved in the citric acid cycle and ETC in BAT (53). In support of an effect of flavan-3-ols on EE, treatment with GSPE (500 mg/kg body weight for 7 d) increased EE and stimulated FA oxidation in aged male rats, as indicated by a reduction in the respiratory quotient (RQ). The treatment improved the oxidative capacity of subcutaneous WAT (increased expression of Hsl and Cpt-1) but the effects were lost in animals that received a higher dose of 1000 mg/kg body weight (46). However, this dose is massive, amply exceeding the mean dietary exposure to flavan-3-ols, which is estimated to be ∼300 mg/d (40).
A direct effect of flavan-3-ols on BAT was also observed in mice after chronic supplementation with cocoa procyanidins, which enhanced UCP1 expression (54–56). Moreover, dietary supplementation with 0.5% or 2% of cocoa procyanidins for 13 wk (corresponding to an extremely high dose) increased phosphorylation of AMPK in the liver, BAT, WAT, and skeletal muscle, reduced the incidence of obesity, and improved both fasting and postprandial hyperglycemia induced by a high-fat diet (HFD) (54). This treatment also improved glucose tolerance, as shown by a glucose tolerance test (54). Two-week treatment with 50 mg/kg body weight decreased RQ and increased mitochondrial biogenesis in both muscle and BAT (55). Kamio et al. (47) reported an increase in EE and increased Ucp1 and Pgc-1α gene expression in BAT after a single oral dose (10 mg/kg body weight) of the same cocoa extract. Interestingly, the effects were lost when mice were pretreated with β2AR and β3AR blockers, suggesting a direct effect of flavan-3-ol on the SNS. The same research group also compared the effect of a single dose (10 mg/kg) of the same flavan-3-ol fraction, containing a mixture of monomers and oligomers, with the equivalent dose of the monomer (−)-epicatechin. Mice that received (−)-epicatechin did not display any change in EE or UCP1 and PGC-1α expression in BAT (57). In contrast, Gutiérrez-Salmeán et al. (48) suggested that there is a positive effect of (−)-epicatechin on EE. In this study, HFD-fed mice that received 1 mg (−)-epicatechin/kg body weight for 2 wk displayed a decreased rate of weight gain, decreased hypertriglyceridemia, and increased expression of UCP1, PGC-1α, type 2 iodothyronine deiodinase, and SIRT1 in WAT and muscle compared with HFD-fed control mice.
Tea flavan-3-ol monomers.
Green tea contains high levels of flavan-3-ol monomers (Figure 3). In addition to (–)-epicatechin and (+)-catechin, green tea contains (epi)gallocatechin and 3-O-galloylated flavan-3-ols that do not occur in cocoa (41). Supplementation with green tea for 2 wk reduced body fat gain and increased EE and BAT protein content in HFD-fed male rats. These effects were prevented by the simultaneous administration of the βAR antagonist propranolol, suggesting a direct activation of the SNS (49), as observed by Kamio et al. (47) after administration of a flavan-3-ol fraction from cocoa. However, the (poly)phenol composition and caffeine content of the green tea were not evaluated, making the interpretation of results extremely difficult. In particular, caffeine represents an important factor to be considered, because a synergic action of green tea catechins and caffeine in enhancing SNS activity has been described (58).
Chronic treatment (16 wk) with pure (−)-epigallocatechin-3-gallate (EGCG) (0.32% diet) was reported to reduce body weight gain and improve insulin sensitivity in HFD-fed mice (59, 60). These effects were associated with increased expression of genes related to mitochondrial FA oxidation in skeletal muscle (59). In HFD-fed male mice, high doses of EGCG (0.5% and 1% diet supplementation, 4 wk) reduced body fat accumulation but did not affect UCP1 expression in brown fat (61). Moreover, acute oral administration of EGCG (500 mg/kg body weight) over 3 d did not affect body temperature and EE but resulted in an increased FA oxidation, as suggested by a reduced RQ during the night period (61).
Flavan-3-ols are oxidized during fermentation of green tea leaves, promoting the accumulation of theaflavins and thearubigins, which are found in high concentrations in fermented teas (41, 62) (Figure 3). Intake of oolong, pu-erh, and particularly black tea was shown to suppress adiposity and promote browning of mesenteric WAT in mice. These effects were concomitant with increased AMPK phosphorylation (63). Because the levels of theaflavins in black tea were higher compared with those in oolong and pu-erh tea, Yamashita et al. (63) speculated that these (poly)phenols could be partially responsible for the reported effects, despite their extremely low bioavailability. In accordance with this speculation, Kudo et al. (64) demonstrated an increase in EE in mice after a single oral dose of theaflavins, which was associated with increased gene expression of UCP1 and Pgc-1α in BAT.
Several clinical intervention studies suggest favorable effects of green tea in the control of body weight (65–69). Whether this activity depends on their flavan-3-ol content and whether flavan-3-ols are able to increase EE is not yet clear.
Gosselin and Haman (50) tested the effects of a green tea extract containing 1600 mg EGCG and 600 mg caffeine on nonshivering thermogenesis in response to 3-h exposure to cold in healthy male subjects. They reported an increase in EE and a reduction in shivering thermogenesis. However, to our knowledge, there appears to be no obvious way to fully discriminate between the contribution of caffeine and EGCG at this time. Dullo et al. (51) also demonstrated the thermogenic properties of EGCG in a study in which they administered 50 mg caffeine and 90 mg EGCG to healthy men, which significantly increased EE and urinary NE excretion while decreasing the RQ over a 24-h period. In contrast, the treatment with caffeine alone had no effect. According to a meta-analysis published by Hursel et al. (70) in 2011, both the mixture of catechin-caffeine and caffeine alone were able to increase EE in humans, but only the combination of catechins and caffeine resulted in enhanced FA oxidation. However, these beneficial effects remain controversial, and daily supplementation with green tea extract (1350 mg catechins including ≥560 mg EGCG and 280–450 mg caffeine) for 12 wk failed to increase EE and modulate body composition (71). Finally, in a recent study of healthy young women, Nirengi et al. (72) showed an increase in BAT density after participants consumed a beverage containing a mixture of 540 mg flavan-3-ol monomers [(+)-catechin, (−)-epicatechin, (+)-catechin-3-gallate, (+)-gallocatechin, (+)-gallocatechin-3-gallate, (−)-epicatechin-3-gallate, epigallocatechin, and (−)-EGCG], supporting the hypothesis that flavan-3-ol monomers may activate or increase BAT mass. However, the flavan-3-ol–rich beverage also contained a higher concentration of caffeine (80 mg) than the control drink (45.5 mg). Moreover, Nirengi et al. (72) did not report the exact (poly)phenol composition of the beverage.
In summary, despite some conflicting results, most of the available evidence supports a role for flavan-3-ols in the enhancement of EE, but the mechanisms involved in these effects are not yet fully understood. Some studies supported an activation of the AMPK–SIRT1–PGC-1α axis, but they did not clarify the mechanism. Moreover, a direct action on the SNS should be considered, because flavan-3-ol monomers are reported to inhibit catechol-O-methyltransferase (an enzyme that inactivates NE by methylation) (73), but this hypothesis has not yet been tested in vivo (Figure 4). These data highlight the need for further studies.
Resveratrol.
Resveratrol (3,5,4′-trihdroxystilbene) is a phenolic compound found at high concentrations in the woody root of the noxious weed Polygonum cuspidatum (Japanese knotweed or Mexican bamboo) and in trace amounts in dietary items such red wine, peanuts, berries, red cabbage, and spinach (Figure 3). Although resveratrol is present in food at extremely low concentrations compared with other (poly)phenols (41), interest in resveratrol’s bioactivity has increased exponentially in the past 2 decades, principally because of its remarkable effects on energy metabolism in mammals.
Studies with rodents demonstrated that resveratrol can exert beneficial effects on glucose homeostasis, reducing the effects of obesity, diabetes, and metabolic dysfunction (74–80). Moreover, several animal studies have pointed to the role of resveratrol in stimulating mitochondrial biogenesis and mitochondrial activity, both in the muscle (75, 81–83) and liver (74), owing to its capacity to activate the AMPK–SIRT1–PGC-1α axis (74, 75, 81–83). Given the important role of AMPK, SIRT1, and PGC-1α in the physiology of adipose tissue (38), it is not surprising that resveratrol may also affect body composition and EE.
High doses of resveratrol (∼400 mg/kg body weight) have been shown to reduce weight gain in HFD-fed mice (75, 76, 81), in association with decreases in visceral fat pad weight and smaller adipocytes in epididymal WAT (75, 76). Interestingly, Lagouge et al. (75) reported an increase in basal EE and improved cold tolerance in mice fed an HFD supplemented with resveratrol. These effects were combined with increased mitochondrial volume and mitochondrial DNA content, an increase in Sirt1 gene expression, a decrease in PGC-1α acetylation, and an increase in PGC-1α activity in BAT of mice treated with resveratrol for 15 wk (75). The effects of resveratrol on BAT metabolism are supported by other studies in which treatment of both mice (400 mg/kg body weight for 8 wk) and Sprague-Dawley rats (30 mg/kg body weight for 8 wk) significantly increased BAT Ucp1 and Sirt1 gene expression (84, 85). It is noteworthy that 8 wk of resveratrol treatment was also sufficient to increase BAT expression of bone morphogenetic protein 7 (84), which is shown to promote both brown and beige adipocyte development and activation (86–88).
In addition to the studies in rodents, positive effects of resveratrol on EE have been reported in nonhuman primate models of obesity (89, 90). A daily dose of 200 mg resveratrol/kg body weight for 4 wk (89) or 1 y (90) significantly increased the resting EE of male gray mouse lemurs. Furthermore, resveratrol supplementation for 2 y (80 and 480 mg/d for the first and second years, respectively) decreased adipocyte size and increased SIRT1 expression in visceral WAT from rhesus monkeys fed a high-fat and high-sugar diet (91).
Resveratrol is also reported to induce browning of WAT both in vivo (81, 92) and in vitro (92, 93), with the acquisition of a beige phenotype being dependent on AMPK phosphorylation (81, 92). This effect on WAT may contribute to the increase in EE after resveratrol consumption, as previously described.
Taken together, these results illustrate a clear role of resveratrol in brown fat differentiation, possibly via activation of the AMPK–SIRT1–PGC-1α axis (Figure 4). However, the mechanisms involved in this activation are still strongly debated. Some evidence supports a direct activation of SIRT1 by resveratrol (83, 94), whereas other data indicate an activation via AMPK. This second hypothesis is supported by the lack of effect of resveratrol in the absence of AMPK (81, 92). Resveratrol has also been shown to increase the NAD+:NADH ratio in an AMPK-dependent manner (81), which supports an indirect activation of SIRT1 (33). Furthermore, resveratrol’s activity as a competitive inhibitor of cAMP-degrading phosphodiesterases has also been proposed (95). The resulting elevated cAMP levels, beyond the stimulation of PKA, could lead to AMPK activation, an increased NAD+:NADH ratio, and subsequently increased SIRT1 activity (95).
Independent of the mechanisms involved, activation of the AMPK–SIRT1–PGC-1α axis by resveratrol leads to an increase in brown fat differentiation and activation in rodents. Whether resveratrol can exert the same effects in humans remains to be determined. To our knowledge, no effects of resveratrol on body weight have been reported in human trials (77, 82, 96–99), with the exception of one trial in which 3 mo of resveratrol supplementation (500 mg/d) administered to patients with metabolic syndrome led to a reduction in body weight, fat mass, and waist circumference compared with baseline values (100). In contrast, resveratrol (75 mg/d for 12 wk) did not change the resting metabolic rate, body composition, inflammatory markers or plasma lipids, insulin sensitivity, AMPK phosphorylation, and Sirt1 and Pgc-1α gene expression in skeletal muscle and adipose tissue in healthy postmenopausal women (96). Timmers et al. (82) reported activation of AMPK, increased SIRT1 and PGC-1α protein levels, and improved mitochondrial respiration in muscle after 30 d of resveratrol supplementation (150 mg/d) in obese but otherwise healthy male subjects. However, these effects were associated with a reduction in the sleeping metabolic rate and in postprandial EE, which arguably is in contrast with the effects described in mice. Nevertheless, the authors also reported beneficial effects on a more general metabolic profile (decreased circulating glucose and TGs and decreased systolic blood pressure), demonstrating resveratrol’s capacity to induce metabolic changes in obese humans. In line with this finding, the same concentration of resveratrol led to a decrease in adipocyte size in WAT of obese men, highlighting the beneficial effect of resveratrol supplementation in adipose tissue function (101).
In conclusion, the evidence produced to date is not sufficient to clearly define whether resveratrol can affect EE or body composition in humans. However, the studies in rodents herein reported strongly support a role for resveratrol in the control of EE, BAT activation, and WAT browning, underlining the potential of its supplementation for the management of obesity and related morbidities.
Other (poly)phenols.
To date, there are only a few reports supporting the role of other classes of (poly)phenols in EE (Figure 4). Both human and animal studies suggest a role of isoflavones in the control of body weight (102–106). Isoflavones are classified as phytoestrogens because of their structural similarity to estrogen. They are found almost exclusively in leguminous plants, with the highest concentrations occurring in soybean (41) (Figure 3). A study by Cederroth et al. (102), in which male mice received a soy-containing diet (198 parts per million of daidzein and 286 parts per million of genistein) for 3 wk, supports the possible implication of isoflavones in the regulation of EE. The treatment improved insulin sensitivity, reduced fat mass, and increased AMPK phosphorylation and the expression of genes implicated in FA oxidation, mitochondrial biogenesis, and the ETC in WAT.
Aziz et al. (107) investigated the cellular mechanisms involved in these effects and showed the browning effects of isoflavones on white adipocytes. Treatment of 3T3-L1 cell line of white preadipocytes during the differentiation process with 100 μM genistein increased both baseline oxygen consumption and maximal respiratory capacity. Moreover, UCP1 expression was increased through a mechanism involving SIRT1 but independent of genistein’s action on the estrogen receptor. However, the high concentrations used in this study did not take into account the low bioavailability and the extensive metabolism of this molecule in vivo (41).
Some studies support an effect of the flavonol quercetin on the control of EE. Flavonols are present in vegetables and fruits such as kale, berries, onion, broccoli, and tomato, principally as glycosides (41) (Figure 3). Lee et al. (25) evaluated the WAT browning effects of an onion-peel extract containing 6.8 mg quercetin/100 mg dry weight and 8.1 mg isoquercetin (quercetin-3-O-glucoside)/100 mg dry weight. Supplementation with 0.5% of onion-peel extract to HFD-fed mice (corresponding to ∼340 and 405 mg of quercetin and isoquercetin/kg of diet) for 8 wk induced browning of retroperitoneal and subcutaneous WAT. Aiming to identify the active compounds, the authors tested the capacity of both compounds (25–100 μM) to drive the differentiation of 3T3-L1 toward a brown phenotype. Only quercetin at the highest concentration positively modulated the expression of thermogenic genes, highlighting the importance of considering the extensive metabolism that flavonols undergo in vivo (41) for the identification of possible bioactive metabolites (108). In support of a browning effect of quercetin, the addition of 1 g rutin (quercetin-3-O-rutinoside)/L drinking water for 10 wk strongly influenced adipose tissue metabolism in both diet-induced and genetically obese mice. The treatment decreased body weight gain, improved glucose homeostasis, and increased body EE, while inducing the thermogenic program in subcutaneous WAT and in BAT (109). Henagan et al. (110) evaluated the effects of lower doses of both onion extract and pure quercetin supplementation. Dietary supplementation with either pure quercetin (17 mg/kg of diet) or an onion extract containing an equivalent amount of quercetin for a 9-wk period decreased fat mass and insulin resistance in HFD-fed mice. These effects were associated with increased EE, mitochondrial biogenesis, and skeletal muscle function; the latter was evaluated as incomplete palmitate oxidation assessed by measuring acid-soluble metabolite production in skeletal muscle homogenates (110). However, Henagan et al. (110) also reported enhanced physical activity after quercetin supplementation, which could have been responsible for the increased EE.
Doan et al. (111) investigated the metabolic effects of gallic acid in diet-induced obese mice. Gallic acid is a hydroxybenzoic acid that is present in substantial amounts in red wine, tea, and some berries, where it occurs principally as complex sugar esters (the gallotannins) (40) (Figure 3). Daily intraperitoneal administration of gallic acid (10 mg/kg body weight) for 9 wk improved glucose and insulin homeostasis and reduced body weight gain without affecting food intake. An effect on EE is supported by the increased expression of thermogenesis-related genes (Ucp1, Pgc-1α, and 3 βAr) in the BAT of treated mice. Moreover, these effects were associated with increased AMPK phosphorylation and SIRT1 and PGC-1α protein levels, suggesting that gallic acid plays a role in activation of the AMPK–SIRT1–PGC-1α axis (111).
Curcumin is a yellow pigment occurring in the spice turmeric (Curcuma longa) (Figure 3) and its sole dietary source is as a flavor ingredient of curries, which are popular in Indian cuisine. However, evidence is growing regarding a possible effect of curcumin on the stimulation of EE. Daily administration of 50 or 100 mg curcumin/kg body weight to mice for 50 d decreased body weight and fat mass and improved cold tolerance. Curcumin caused browning of subcutaneous WAT (induced mitochondrial biogenesis and expression of thermogenic genes). Moreover, these effects were associated with elevated levels of plasma NE, suggesting an involvement of the SNS (112). The browning effects of curcumin in vitro have also been confirmed. Curcumin (1–20 μM) stimulated mitochondrial biogenesis and the protein expression of thermogenic genes (Ucp1, Pgc-1α, Ppar-γ, and Prdm16) in both 3T3-L1 and primary adipocytes derived from subcutaneous WAT (113). However, further studies are needed to better understand the reported bioactivity, because the concentrations used are very high and largely nonphysiologic. The impact of dietary curcumin is likely to be virtually nonexistent, because it has very low in vivo bioavailability and daily intakes from curries for most populations will be very limited (112).
Conclusions
Changes in lifestyle and dietary habits in particular are crucial for preventing and managing obesity. Increasing evidence supports the role of diet in the control of body weight. Beyond calorie restriction, some food-derived components, particularly (poly)phenols, have been shown to exert antiobesity effects.
Obesity results from the imbalance between energy intake and EE; therefore, enhancing EE represents a promising solution to promote weight loss and reduce obesity. The discovery of functional BAT in humans paved the way for the development of antiobesity treatments aiming to increase EE. In vivo studies have demonstrated that the antiobesity effect of some classes of (poly)phenols, particularly flavan-3-ols and stilbenes, may be related to their capacity to enhance EE and activate BAT. This evidence supports the concept that some of the health benefits of these compounds might go beyond their widely studied anti-inflammatory or antioxidant effects. However, further work is needed to properly characterize the biologic effects of (poly)phenols in the framework of EE and BAT activation.
Discrepancies exist in the literature, which are likely attributable to a variation in doses, time of exposure, and (poly)phenol composition, especially when treatments with foods or mixtures (rather than single compounds) are considered. Furthermore, (poly)phenolic compounds undergo extensive metabolism in the gastrointestinal tract, with substantial interindividual variability. Moreover, translation from animals to humans should take into consideration the important differences in (poly)phenol metabolism that occur in the different species (114), which complicate comparisons of data sets. To explain the high variability between studies, further studies should aim to associate the biological effect of a particular set of (poly)phenolic compounds with their principal metabolites (i.e., the ones present in the circulatory system after consumption or administration). Moreover, this approach may allow the identification of the most likely metabolites responsible for the positive effects, whose bioactivity can be further evaluated in vitro.
Despite the highlighted limitations, studying the role of (poly)phenolics that occur widely in the human diet, such as flavan-3-ols, in the regulation of EE appears to be a very challenging but promising target and should be carefully pursued.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMPK, 5′-AMP–activated protein kinase; BAT, brown adipose tissue; C/EBP, CCAAT/enhancer-binding protein; CPT1, carnitine palmitoyltransferase 1; EE, energy expenditure; EGCG, epigallocatechin-3-gallate; ETC, electron transport chain; GSPE, grape seed proanthocyanidin extract; HFD, high-fat diet; NE, norepinephrine; PGC-1α, PPAR-γ coactivator-1α PKA, protein kinase A; PRDM16, PR domain zinc finger protein 16; RQ, respiratory quotient; SIRT1, sirtuin 1; SNS, sympathetic nervous system; T3, triiodothyronine; UCP1, uncoupling protein 1; WAT, white adipose tissue; β3AR, β3-adrenergic receptor.
References
- 1.Saltiel AR. New therapeutic approaches for the treatment of obesity. Sci Transl Med 2016;8:323rv2. [DOI] [PubMed] [Google Scholar]
- 2.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012;126:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293:E444–52. [DOI] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–8. [DOI] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, Li B, Wang W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One 2015;10:e0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 2010;59:1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011;96:192–9. [DOI] [PubMed] [Google Scholar]
- 10.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for body-weight control. Proc Nutr Soc 2009;68:401–7. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E; PREDIMED Investigators. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
- 13.Cherniack EP. Polyphenols: planting the seeds of treatment for the metabolic syndrome. Nutrition 2011;27:617–23. [DOI] [PubMed] [Google Scholar]
- 14.Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 2016;17:573–86. [DOI] [PubMed] [Google Scholar]
- 15.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 17.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab 2011;13:238–40. [DOI] [PubMed] [Google Scholar]
- 18.López M, Alvarez CV, Nogueiras R, Diéguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 2013;19:418–27. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–9. [DOI] [PubMed] [Google Scholar]
- 20.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature 2014;510:76–83. [DOI] [PubMed] [Google Scholar]
- 21.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013;495:379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern PA, Finlin BS, Zhu B, Rasouli N, McGehee RE Jr., Westgate PM, Dupont-Versteegden EE. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. J Clin Endocrinol Metab 2014;99:E2772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014;63:3686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, Jorgensen JA, Boekschoten MV, Hesselink MK, Havekes B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015;21:863–5. [DOI] [PubMed] [Google Scholar]
- 27.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 2016;17:480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 2005;280:20589–95. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–8. [DOI] [PubMed] [Google Scholar]
- 31.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012;150:620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci (Lond) 2013;124:491–507. [DOI] [PubMed] [Google Scholar]
- 33.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007;104:12017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulligan JD, Gonzalez AA, Stewart AM, Carey HV, Saupe KW. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol 2007;580:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 2005;48:2386–95. [DOI] [PubMed] [Google Scholar]
- 37.Vila-Bedmar R, Lorenzo M, Fernandez-Veledo S. Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 2010;151:980–92. [DOI] [PubMed] [Google Scholar]
- 38.van Dam AD, Kooijman S, Schilperoort M, Rensen PC, Boon MR. Regulation of brown fat by AMP-activated protein kinase. Trends Mol Med 2015;21:571–9. [DOI] [PubMed] [Google Scholar]
- 39.Mottillo EP, Desjardins EM, Crane JD, Smith BK, Green AE, Ducommun S, Henriksen TI, Rebalka IA, Razi A, Sakamoto K, et al. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab 2016;24:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanotti I, Dall’Asta M, Mena P, Mele L, Bruni R, Ray S, Del Rio D. Atheroprotective effects of (poly)phenols: a focus on cell cholesterol metabolism. Food Funct 2015;6:13–31. [DOI] [PubMed] [Google Scholar]
- 41.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res 2006;50:176–87. [DOI] [PubMed] [Google Scholar]
- 43.Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J Am Diet Assoc 2011;111:1173–81. [DOI] [PubMed] [Google Scholar]
- 44.Gu Y, Lambert JD. Modulation of metabolic syndrome-related inflammation by cocoa. Mol Nutr Food Res 2013;57:948–61. [DOI] [PubMed] [Google Scholar]
- 45.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr 2011;141:1982–8. [DOI] [PubMed] [Google Scholar]
- 46.Serrano J, Casanova-Martí À, Gual A, Pérez-Vendrell AM, Blay MT, Terra X, Ardévol A, Pinent M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur J Nutr 2017;56:1629–36. [DOI] [PubMed] [Google Scholar]
- 47.Kamio N, Suzuki T, Watanabe Y, Suhara Y, Osakabe N. A single oral dose of flavan-3-ols enhances energy expenditure by sympathetic nerve stimulation in mice. Free Radic Biol Med 2016;91:256–63. [DOI] [PubMed] [Google Scholar]
- 48.Gutiérrez-Salmeán G, Ortiz-Vilchis P, Vacaseydel CM, Garduño-Siciliano L, Chamorro-Cevallos G, Meaney E, Villafaña S, Villarreal F, Ceballos G, Ramírez-Sánchez I. Effects of (-)-epicatechin on a diet-induced rat model of cardiometabolic risk factors. Eur J Pharmacol 2014;728:24–30. [DOI] [PubMed] [Google Scholar]
- 49.Choo JJ. Green tea reduces body fat accretion caused by high-fat diet in rats through beta-adrenoceptor activation of thermogenesis in brown adipose tissue. J Nutr Biochem 2003;14:671–6. [DOI] [PubMed] [Google Scholar]
- 50.Gosselin C, Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr 2013;110:282–8. [DOI] [PubMed] [Google Scholar]
- 51.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 1999;70:1040–5. [DOI] [PubMed] [Google Scholar]
- 52.Pajuelo D, Quesada H, Diaz S, Fernandez-Iglesias A, Arola-Arnal A, Blade C, Salvado J, Arola L. Chronic dietary supplementation of proanthocyanidins corrects the mitochondrial dysfunction of brown adipose tissue caused by diet-induced obesity in Wistar rats. Br J Nutr 2012;107:170–8. [DOI] [PubMed] [Google Scholar]
- 53.Pajuelo D, Diaz S, Quesada H, Fernandez-Iglesias A, Mulero M, Arola-Arnal A, Salvado MJ, Blade C, Arola L. Acute administration of grape seed proanthocyanidin extract modulates energetic metabolism in skeletal muscle and BAT mitochondria. J Agric Food Chem 2011;59:4279–87. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys 2012;527:95–104. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe N, Inagawa K, Shibata M, Osakabe N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids Health Dis 2014;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osakabe N, Hoshi J, Kudo N, Shibata M. The flavan-3-ol fraction of cocoa powder suppressed changes associated with early-stage metabolic syndrome in high-fat diet-fed rats. Life Sci 2014;114:51–6. [DOI] [PubMed] [Google Scholar]
- 57.Matsumura Y, Nakagawa Y, Mikome K, Yamamoto H, Osakabe N. Enhancement of energy expenditure following a single oral dose of flavan-3-ols associated with an increase in catecholamine secretion. PLoS One 2014;9:e112180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
- 59.Sae-Tan S, Grove KA, Kennett MJ, Lambert JD. (-)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct 2011;2:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr 2008;138:1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 2005;29:615–23. [DOI] [PubMed] [Google Scholar]
- 62.Del Rio D, Stewart AJ, Mullen W, Burns J, Lean ME, Brighenti F, Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric Food Chem 2004;52:2807–15. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita Y, Wang L, Wang L, Tanaka Y, Zhang T, Ashida H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct 2014;5:2420–9. [DOI] [PubMed] [Google Scholar]
- 64.Kudo N, Arai Y, Suhara Y, Ishii T, Nakayama T, Osakabe N. A single oral administration of theaflavins increases energy expenditure and the expression of metabolic genes. PLoS One 2015;10:e0137809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr 2005;81:122–9. [DOI] [PubMed] [Google Scholar]
- 66.Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity (Silver Spring) 2008;16:1338–48. [DOI] [PubMed] [Google Scholar]
- 67.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, Jones F, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr 2009;139:264–70. [DOI] [PubMed] [Google Scholar]
- 68.Cardoso GA, Salgado JM, Cesar Mde C, Donado-Pestana CM. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. J Med Food 2013;16:120–7. [DOI] [PubMed] [Google Scholar]
- 69.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr 2010;29:31–40. [DOI] [PubMed] [Google Scholar]
- 70.Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W, Westerterp-Plantenga MS. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev 2011;12:e573–81. [DOI] [PubMed] [Google Scholar]
- 71.Janssens PL, Hursel R, Westerterp-Plantenga MS. Long-term green tea extract supplementation does not affect fat absorption, resting energy expenditure, and body composition in adults. J Nutr 2015;145:864–70. [DOI] [PubMed] [Google Scholar]
- 72.Nirengi S, Amagasa S, Homma T, Yoneshiro T, Matsumiya S, Kurosawa Y, Sakane N, Ebi K, Saito M, Hamaoka T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. Springerplus 2016;5:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol 2005;69:1523–31. [DOI] [PubMed] [Google Scholar]
- 74.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–22. [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 2011;81:1343–51. [DOI] [PubMed] [Google Scholar]
- 77.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 2012;32:537–41. [DOI] [PubMed] [Google Scholar]
- 78.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009;77:1053–63. [DOI] [PubMed] [Google Scholar]
- 79.Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism 2012;61:424–33. [DOI] [PubMed] [Google Scholar]
- 80.Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012;61:1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010;59:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 2012;15:675–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, de Paula AM, Coimbra CC, Santos SH. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr 2014;53:1503–10. [DOI] [PubMed] [Google Scholar]
- 85.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem 2013;141:1530–5. [DOI] [PubMed] [Google Scholar]
- 86.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008;454:1000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid Redox Signal 2013;19:243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA 2011;108:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dal-Pan A, Terrien J, Pifferi F, Botalla R, Hardy I, Marchal J, Zahariev A, Chery I, Zizzari P, Perret M, et al. Caloric restriction or resveratrol supplementation and ageing in a non-human primate: first-year outcome of the RESTRIKAL study in Microcebus murinus. Age (Dordr) 2011;33:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab 2013;18:533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes (Lond) 2015;39:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and retinol-binding protein 4 expression in white adipocytes. J Nutr Biochem 2011;22:828–34. [DOI] [PubMed] [Google Scholar]
- 94.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003;425:191–6. [DOI] [PubMed] [Google Scholar]
- 95.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012;148:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab 2012;16:658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci 2012;67:1307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013;62:1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Movahed A, Nabipour I, Lieben Louis X, Thandapilly SJ, Yu L, Kalantarhormozi M, Rekabpour SJ, Netticadan T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Alternat Med 2013;2013:851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Méndez-del Villar M, González-Ortiz M, Martínez-Abundis E, Pérez-Rubio KG, Lizárraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2014;12:497–501. [DOI] [PubMed] [Google Scholar]
- 101.Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Muller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 2014;38:470–3. [DOI] [PubMed] [Google Scholar]
- 102.Cederroth CR, Vinciguerra M, Gjinovci A, Kuhne F, Klein M, Cederroth M, Caille D, Suter M, Neumann D, James RW, et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes 2008;57:1176–85. [DOI] [PubMed] [Google Scholar]
- 103.Bu L, Setchell KD, Lephart ED. Influences of dietary soy isoflavones on metabolism but not nociception and stress hormone responses in ovariectomized female rats. Reprod Biol Endocrinol 2005;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lephart ED, Setchell KD, Handa RJ, Lund TD. Behavioral effects of endocrine-disrupting substances: phytoestrogens. ILAR J 2004;45:443–54. [DOI] [PubMed] [Google Scholar]
- 105.Allison DB, Gadbury G, Schwartz LG, Murugesan R, Kraker JL, Heshka S, Fontaine KR, Heymsfield SB. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. Eur J Clin Nutr 2003;57:514–22. [DOI] [PubMed] [Google Scholar]
- 106.Li Z, Hong K, Saltsman P, DeShields S, Bellman M, Thames G, Liu Y, Wang HJ, Elashoff R, Heber D. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr 2005;59:411–8. [DOI] [PubMed] [Google Scholar]
- 107.Aziz SA, Wakeling LA, Miwa S, Alberdi G, Hesketh JE, Ford D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol Nutr Food Res 2017;61:1600574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem 2017;42:62–71. [DOI] [PubMed] [Google Scholar]
- 109.Yuan X, Wei G, You Y, Huang Y, Lee HJ, Dong M, Lin J, Hu T, Zhang H, Zhang C, et al. Rutin ameliorates obesity through brown fat activation. FASEB J 2017;31:333–45. [DOI] [PubMed] [Google Scholar]
- 110.Henagan TM, Cefalu WT, Ribnicky DM, Noland RC, Dunville K, Campbell WW, Stewart LK, Forney LA, Gettys TW, Chang JS, et al. In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity. Genes Nutr 2015;10:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doan KV, Ko CM, Kinyua AW, Yang DJ, Choi YH, Oh IY, Nguyen NM, Ko A, Choi JW, Jeong Y, et al. Gallic acid regulates body weight and glucose homeostasis through AMPK activation. Endocrinology 2015;156:157–68. [DOI] [PubMed] [Google Scholar]
- 112.Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, Wei M, Jiang Y, Zhang Y, Wang L, et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun 2015;466:247–53. [DOI] [PubMed] [Google Scholar]
- 113.Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3–L1 and primary white adipocytes. J Nutr Biochem 2016;27:193–202. [DOI] [PubMed] [Google Scholar]
- 114.Ottaviani JI, Borges G, Momma TY, Spencer JP, Keen CL, Crozier A, Schroeter H. The metabolome of [2-(14)C](-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci Rep 2016;6:29034. [DOI] [PMC free article] [PubMed] [Google Scholar]