Abstract
Soy may be a suitable food for anti-obesity efforts because of its high protein and isoflavone content. We conducted this meta-analysis to evaluate potential effects of soy and soy isoflavones on weight, waist circumference, and fat mass. PubMed, MEDLINE, Scopus, EMBASE, and Cochrane databases were searched. Twenty-four trials with soy and 17 trials with isoflavones passed the eligibility stage. According to the results, soy showed no overall statistically significant effect on weight, waist circumference, or fat mass, but a significant increasing effect on weight was observed in some circumstances: for instance, in obese subjects [mean difference (MD): 0.80 kg; 95% CI: 0.15, 1.45 kg; P = 0.02], with ingestions of ≥40 g soy protein/d (MD: 0.94 kg; 95% CI: 0.11, 1.77 kg; P = 0.03), with short-term applications (1–3 mo) (MD: 0.45 kg; 95% CI: 0.05, 0.86 kg; P = 0.03), and when soy was compared with meat (MD: 0.36 kg; 95% CI: 0.09, 0.64 kg; P = 0.03) and whey protein (MD: 1.53 kg; 95% CI: 0.10, 2.96 kg; P = 0.04). In contrast to the effects of soy on weight, soy significantly decreased waist circumference in older ages (MD: −0.36 cm; 95% CI: −0.71, −0.01 cm; P = 0.04), in women (MD: −0.32 cm; 95% CI: −0.57, −0.08 cm; P = 0.01), and at doses of <40 g soy protein/d (MD: −0.31 cm; 95% CI: −0.57, −0.05 cm; P = 0.02). Isoflavone studies, conducted only in women, showed that isoflavones may reduce body mass index (BMI; in kg/m2) (MD: −0.26; 95% CI: −0.55, 0.04; P = 0.085), especially in dosages <100 mg/d (MD: −0.48; 95% CI: −0.90, −0.06; P = 0.02) and in intervention periods of 2–6 mo (MD: −0.28; 95% CI: −0.56, 0.00; P = 0.053), but no effect was observed in higher doses or longer intervention periods. Also, a trend for reduced BMI after consumption of isoflavones was observed in Caucasians (MD: −0.35; 95% CI: −0.74, 0.04; P = 0.08). Overall, results showed that, although soy is the major source of isoflavones, soy and isoflavones may have different impacts on weight status.
Keywords: soy, isoflavones, weight, waist circumference, fat mass
Introduction
Soy has been approved as a health-promoting food because of its biological effects in prevention of metabolic disorders, such as hyperlipidemia, cardiovascular diseases, and type 2 diabetes (1). These beneficial effects are supposed to be exerted by constituents such as unsaturated fats, fiber, and the high content of protein and isoflavones (2).
Both animal and human studies have shown the effect of dietary soy protein on weight control and prevention of obesity (3). Inhibition of hepatic lipogenic enzymes and FA synthesis, simulation of muscle FA oxidation, elevation of plasma concentration of adiponectin, and increased fecal TG excretion are some of the mechanisms proposed for the anti-obesity effect of soy proteins (3). In a number of clinical trials, the effect of soy consumption on weight and other obesity-related variables has been examined. However, no meta-analysis on the issue has yet been published to our knowledge. In the current work, we collected the available data from controlled clinical trials examining the effect of soy or soy isoflavones on weight or BMI (in kg/m2), waist circumference, and fat mass in healthy adults aged ≥18 y. There is a somewhat similar meta-analysis by Zhang et al. (4) who found beneficial effects of soy isoflavones on body weight and glucose metabolism in non-Asian postmenopausal women. The ways this analysis differentiates from the previous one are the main subject, which is soy, the lack of limitation on study subjects (both sexes, all adulthood ages), and the separate meta-analyses on soy and soy isoflavones.
Methods
This systematic review and meta-analysis was performed in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses at all stages of design, implementation, and reporting (5).
Search
We searched PubMed (beginning 1950), MEDLINE (beginning 1946), Scopus (beginning 1966), EMBASE (beginning 1947), and Cochrane (beginning 1992) databases from the earliest online available date through August 2016 to find controlled clinical trials examining the effect of soy or isoflavones on weight, BMI, waist circumference, fat mass, and abdominal fat mass in healthy adults aged ≥18 y. We included isoflavone reports because soy contains considerable amounts of isoflavones, which are phytochemicals that have known biological effects on weight and metabolic pathways.
Search terms were as follows: soy, isoflavones, weight, BMI, waist circumference, obesity, overweight, obese, adiposity, adipose, abdominal circumference, and fat mass. In the PubMed search, soy, isoflavones, body weight, obesity, overweight, adiposity, BMI, and abdominal fat were used as MeSH terms. One investigator searched the literature, and 2 independent investigators screened titles and abstracts and assessed full texts for qualifying eligibility. There was little disagreement between the 2 investigators in the screening and eligibility steps, and differences were resolved through scrutinized reassessment. The search was limited to adults. No restriction on language was applied.
Eligibility criteria
Clinical trials needed to be controlled and randomized, but blindness was not a requirement because in some studies soy was administered in the form of soybean or soy-containing foods, where blindness was not possible. Subjects were apparently healthy and normal weight, overweight, and obese, but underweight subjects were excluded. Trials examining diseases were not included, apart from those on hyperlipidemia or nonalcoholic fatty liver disease because such conditions do not have an impact on weight (obesity is a causative factor of both, but neither one affects weight).
There was no restriction on the type of study (parallel or crossover), the type of treatment (diet or pure compounds), or length of intervention, and studies were included if a full description of the intervention and control treatments, including diets, the inclusion and exclusion criteria, and sufficient information on the study outcomes, was reported and confounding factors were appropriately controlled. Studies were included if sufficient information on weight, BMI, waist circumference, or fat mass was reported. Trials were excluded for the following reasons: the dose of treatment was not reported; the study included mixed diets where the amount of soy in the diet was not determined and controlled; an incomparable control was used or isocaloric diets were not used for treatment and control groups; weight change was prevented during the intervention; there was a great difference in the calcium content of the treatments; soy ingredients such as β-conglycinin, soy polysaccharides, and soy oil were used (but soy protein was allowed); soy varieties other than regular soy, such as black soy, or tofu or fermented soy products were used; and the number of participants in the control and treatment arms was not clearly reported. Repeated publications and reports with insufficient information for obtaining mean and SD (or SE) were also excluded. For isoflavones studies, trials were excluded if the isoflavones used were products of other plant sources or if the trials used single isoflavone compounds, such as daidzein and genistein.
Statistical analysis
The mean difference (MD) and SD of the difference between baseline and postintervention for control and intervention groups (or control and intervention conditions in crossover studies) were used to calculate pooled overall effects. The SD of the MD was calculated by using the following equation:
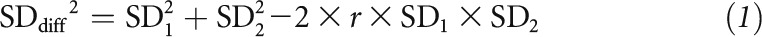 |
Where SDdiff is the standard deviation of the mean, SD1 and SD2 are SDs of baseline and postintervention values, and r (r = 0.5) is a coefficient for the correlation between baseline and postintervention values (6).
In trials in which SEs were reported, the SD was calculated from the SE multiplied by square root of the sample size:
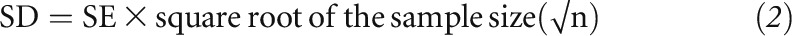 |
Statistical heterogeneity was assessed with the I2 test by using random inverse-variance heterogeneity (6). I2 values >50% were considered moderate heterogeneity. To delineate the sources of heterogeneity, the subgroup analysis was performed based on the BMI of participants [in normal (BMI <25), overweight (BMI = 25–29.9), and obese (BMI ≥30) categories (7)], age [younger ages (<50 y), young and old ages (e.g., 20–60 y), and older ages (>45–50 y) for soy trials; premenopausal, perimenopausal, and postmenopausal women for isoflavone trials], sex (men, women, and both), ethnicity (Caucasians compared with Asians), treatment dose (<40 compared with ≥40 g soy protein/d; <100 compared with ≥100 mg isoflavones/d), intervention period (1–3 compared with 4–24 mo for soy protein; 2–6 compared with 10–12 mo for isoflavones), and the type of control group (meat, whey protein, casein or whole milk, and usual diets and cereals). The basis of categorization of the treatment dose and intervention length for the subgroup analysis was the median of each classification. For isoflavones, the subgroup analysis for the type of control and sex was not performed because in all trials a nonactive placebo was used for the control group and the participants in all studies were women. Publication bias was determined by visual evaluation of funnel plots and Egger’s and Begg’s tests (8). STATA software version 12.0 (StataCorp) was used for data analysis.
Results
Search results
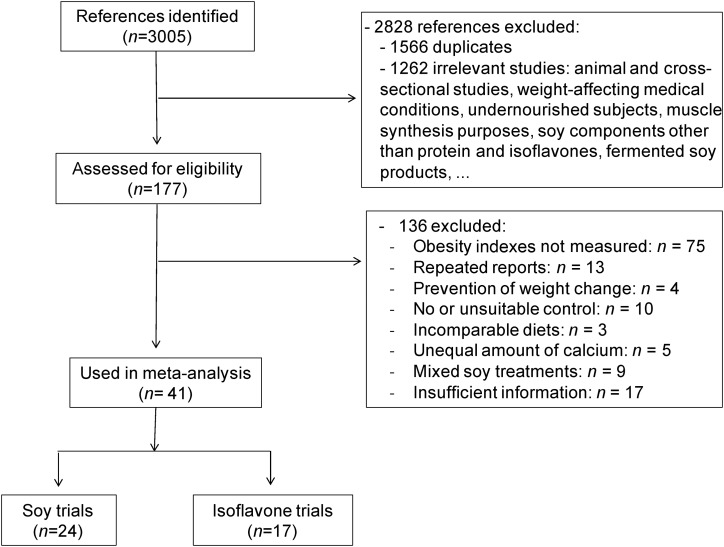
After the search of the 5 databases, 3005 publications were identified, of which 2828 were excluded based on the title and abstract (1566 duplicates and 1262 irrelevant studies) (Figure 1). Full texts of 195 reports were assessed for eligibility, of which 157 citations were excluded for various reasons, such as no reporting on obesity-related anthropometric measures, an inappropriate control group, the prevention of weight change during the intervention, use of soy mixed with other weight-affecting compounds, and insufficient information. Finally, 41 clinical trials were selected for the meta-analysis, 24 trials with soy (4 from 2 publications) and 17 trials with isoflavones (2 from 1 publication).
FIGURE 1.
Summary of the screening and selection process of trials included in the meta-analysis of the effect of soy and soy isoflavones on obesity-related anthropometric measures.
A total of 1634 and 1113 subjects were entered in the meta-analyses of soy and isoflavones, respectively. In all of these studies, soy or isoflavones were administered in specified and designated ways regarding both the dose and the nature of implementation while controlling for calories, protein, and other important weight-affecting compounds. All of the trials had a parallel design except for one crossover (with 2 treatment arms) (9) among soy trials and another (10) among isoflavone trials. Table 1 presents a summary of the studies included.
TABLE 1.
Characteristics of the clinical trials included in the meta-analysis of the effect of soy and soy isoflavones on obesity-related anthropometric measures
| First author, year (reference) | Subjects | Age, y | Subjects, n, sex | Study design | Duration | Intervention | Control | Soy protein, g/d, or isoflavones, mg/d | Outcome |
| Soy | |||||||||
| Arjmandi et al., 2005 (11) | Postmenopausal | 50–651 | 62, F | Parallel | 12 mo | Soy protein–based products | Non-soy foods | 25 | Weight, BMI |
| Azadbakht et al., 2007 (9a) | Postmenopausal | — | 42, F | Crossover | 8 wk | Soy nuts | Red meat | 30 | Weight, waist circumference |
| Azadbakht et al., 2007 (9b) | Postmenopausal | — | 42, F | Crossover | 8 wk | Soy protein | Red meat | 30 | Weight, waist circumference |
| Baer et al., 2011 (12) | Overweight and obese | 40–60 | 48, M/F | Parallel | 23 wk | Soy protein isolate | Whey protein | 56 | Weight, waist circumference, fat mass |
| Beavers et al., 2015 (13) | Obese | 60–79 | 24, M/F | Parallel | 12 wk | Soy protein–based meals | Whey- and egg-based meals | 44 | Weight, BMI, waist circumference, fat mass |
| Berger et al., 2014 (14) | Healthy | 18–19 | 120, F | Parallel | 16 wk | Soy protein meal shake | Casein meal shake | 20 | Weight, waist circumference, fat mass |
| Chiechi et al., 2002 (15) | Postmenopausal | 39–60 | 67, F | Parallel | 6 mo | Soy products | Usual diet | ∼30 | Weight, BMI |
| Christie et al., 2010 (16) | Postmenopausal | 45–60 | 33, F | Parallel | 3 mo | Soy protein shakes | Casein shakes | 20 | Fat mass |
| Faghih et al., 2011 (17) | Overweight and obese premenopausal | 20–50 | 43, F | Parallel | 8 wk | Calcium-fortified soy milk | Calcium supplement | 25 | Weight, BMI, waist circumference, fat mass |
| Kani et al., 2014 (18) | Non-alcoholic fatty liver disease | 48.9 ± 3.62 | 30, M/F | Parallel | 8 wk | Soy nuts | Red meat | 10 | Weight, BMI |
| Liao et al., 2007 (19) | Obese | 20–60 | 30, M/F | Parallel | 8 wk | Soy protein | Usual diet | 45 | Weight, BMI, waist circumference |
| Liu et al., 2010 (20a) | Postmenopausal | 48–70 | 120, F | Parallel | 6 mo | Soy protein + isoflavones | Milk protein | 15 | Weight, BMI, waist circumference, fat mass |
| Liu et al., 2010 (20b) | Postmenopausal | 48–70 | 120, F | Parallel | 6 mo | Soy protein + isoflavones | Milk protein + isoflavones | 15 | Weight, BMI, waist circumference, fat mass |
| Liu et al., 2013 (21) | Postmenopausal | 48–65 | 180, F | Parallel | 6 mo | Whole soy flour | Milk powder | 12.8 | Weight, BMI, waist circumference, fat mass |
| Ma et al., 2011 (22) | Hypercholesterolemic | 25–70 | 90, M/F | Parallel | 8 wk | Soy protein isolate | Milk protein | 18 | Weight, BMI |
| Maesta et al., 2007 (23) | Postmenopausal | 45–70 | 21, F | Parallel | 16 wk | Soy protein | Maltodextrin | 25 | BMI, waist circumference |
| Maskarinec et al., 2004 (24) | Premenopausal | 43 ± 3 | 220, F | Parallel | 2 y | Soy products | Usual diet | ∼60 | Weight, BMI |
| Moeller et al., 2003 (25) | Perimenopausal | 42–62 | 45, F | Parallel | 24 wk | Soy protein | Whey protein | 40 | Weight, fat mass |
| Moriguchi et al., 2008 (26) | High cardiovascular risk | 45–60 | 37, F | Parallel | 12 wk | Whole soy cell juice in peach juice | Peach juice | 7.5 | Weight, BMI |
| Noroozi et al., 2011 (27) | Hyperlipidemic | 25–65 | 52, M/F | Parallel | 4 wk | Soy protein | Usual diet | 30 | Weight, BMI, waist circumference |
| Simão et al., 2014 (28) | Metabolic syndrome | 47.9 ± 10 | 30, F | Parallel | 3 mo | Toasted ground soybean | Usual diet | 13 | BMI, waist circumference |
| St-Onge et al., 2007 (29) | Overweight | 25–49 | 47, F | Parallel | 12 wk | Soy-containing foods | Usual diet | 25 | Weight, waist circumference, fat mass |
| Tahavorgar et al., 2014 (30) | Overweight and obese | 30–65 | 45, M | Parallel | 12 wk | Soy protein isolate | Whey protein | 54 | Weight, BMI, waist circumference |
| Yamashita et al., 1998 (31) | Overweight and obese | 30–61 | 36, F | Parallel | 16 wk | Soy-containing foods | Lean meat | 116 | Weight, BMI, waist circumference |
| Isoflavones | |||||||||
| Aubertin-Leheudre et al., 2007 (32) | Obese postmenopausal | 50–70 | 50, F | Parallel | 12 mo | Soy isoflavones | Placebo | 70 | Weight, BMI, fat mass |
| Cheng et al., 2015 (33) | Postmenopausal | 45–65 | 82, F | Parallel | 12 mo | Soy isoflavones | Placebo | 300 | Weight, BMI |
| Choquette et al., 2011 (34) | Overweight and obese postmenopausal | 50–70 | 45, F | Parallel | 6 mo | Soy isoflavones | Cellulose | 70 | Weight, BMI, waist circumference, fat mass |
| Colacurci et al., 2005 (35) | Postmenopausal | 50–60 | 57, F | Parallel | 6 mo | Soy isoflavones | Placebo | 60 | BMI |
| Delmanto et al., 2013 (36) | Postmenopausal | ≥45 | 66, F | Parallel | 10 mo | Soy isoflavones | Placebo | 100 | BMI, waist circumference |
| Garrido et al., 2006 (37) | Postmenopausal | 45–60 | 29, F | Parallel | 12 wk | Soy isoflavones | Placebo | 100 | Weight, BMI |
| Han et al., 2002 (38) | Postmenopausal | 45–55 | 80, F | Parallel | 4 mo | Soy isoflavones | Placebo | 33.3 | BMI |
| Lebon et al., 2014 (39) | Postmenopausal | 50–70 | 34, F | Parallel | 6 mo | Soy isoflavones | Cellulose | 70 | Weight, BMI, waist circumference, fat mass |
| Liu et al., 2010 (20) | Postmenopausal | 48–70 | 120, F | Parallel | 6 mo | Milk protein + isoflavones | Milk protein | 100 | Weight, BMI, waist circumference, fat mass |
| Llaneza et al., 2011 (40) | Obese postmenopausal | 50–64 | 70, F | Parallel | 6 mo | Soy isoflavones | Usual diet | 80 | BMI, waist circumference |
| Maskarinec et al., 2003 (41) | Premenopausal | 35–46 | 30, F | Parallel | 12 mo | Soy isoflavones | Placebo | 100 | Weight, BMI |
| Maskarinec et al., 2009 (42a) | Postmenopausal | 40–60 | 106, F | Parallel | 2 y | Soy isoflavones | Placebo | 80 | BMI |
| Maskarinec et al., 2009 (42b) | Postmenopausal | 40–60 | 120, F | Parallel | 2 y | Soy isoflavones | Placebo | 120 | BMI |
| Moeller et al., 2003 (25) | Perimenopausal | 42–62 | 48, F | Parallel | 24 wk | Soy protein-rich isoflavones | Soy protein-poor isoflavones | 76 | Weight, fat mass |
| Mori et al., 2004 (43) | Perimenopausal | 40–60 | 70, F | Parallel | 24 wk | Soy isoflavones | Placebo | 100 | BMI, fat mass |
| Weickert et al., 2006 (10) | Postmenopausal | 59 ± 6 | 68, F | Crossover | 8 wk | Soy isoflavone–enriched cereal bars | Placebo cereal bars | 50 | BMI |
| Wu et al., 2006 (44) | Postmenopausal | 45–60 | 66, F | Parallel | 6 mo | Isoflavones | Dextrin | 75 | Weight, BMI, fat mass |
Range (all such values).
Mean ± SD (all such values).
Soy publications mostly used either whole soy (9, 15, 17, 18, 21, 24, 26, 28, 31) or isoflavone-containing soy protein (9, 11, 13, 14, 16, 19, 20, 23, 25, 27, 29). Only a few studies (12, 20, 22, 30) used isoflavone-free soy protein isolate. However, subgroup analysis did not show a noticeable difference between the results of studies with isoflavone-containing compared with isoflavone-free soy treatments.
Of the soy trials, 7 trials were performed on normal-weight (14, 19–22, 26), 7 on overweight (9, 11, 15, 18, 23, 24), and 10 on obese individuals (12, 13, 16, 17, 19, 27–31). Of the isoflavone trials, 8 were performed on normal-weight (10, 20, 25, 33, 38, 41, 43, 44), 6 on overweight (35–37, 39, 42), and 3 on obese individuals (32, 34, 40). One study from soy trials was conducted on men (30–65 y), 6 studies included both men and women (ranging in age from 20 to 79 y), and 17 studies included only women (ages 18–70 y). However, all isoflavone studies (17 studies) were conducted on women (ages 35–70 y). The amounts of the administered soy protein and isoflavones ranged from 7.5 to 116 g and 33.3 to 300 mg, respectively. The length of interventions was also quite variable, ranging from 4 wk to 2 y for soy trials and from 8 wk to 2 y for isoflavones. Twenty-one, 17, 16, and 10 of 24 soy publications measured weight, BMI, waist circumference, and fat mass, respectively. The corresponding numbers for the isoflavone publications were 8, 17, 5, and 7, respectively.
Effect of soy on weight
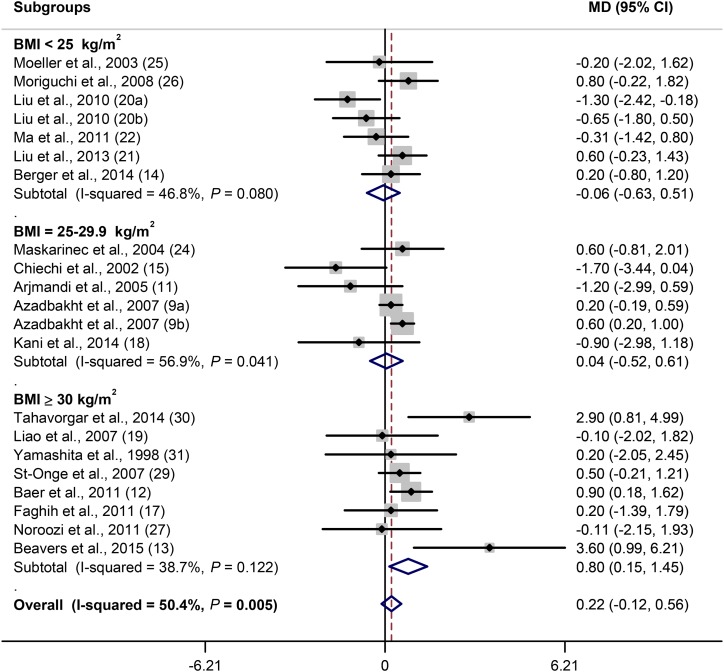
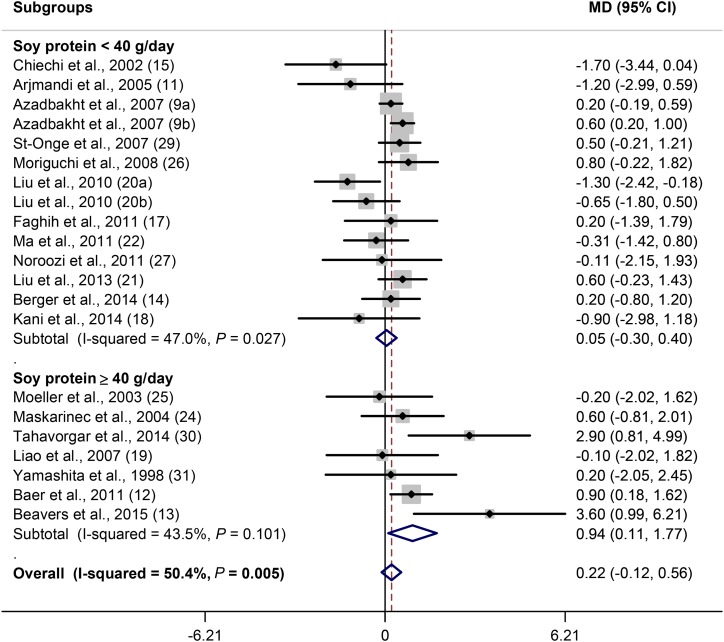
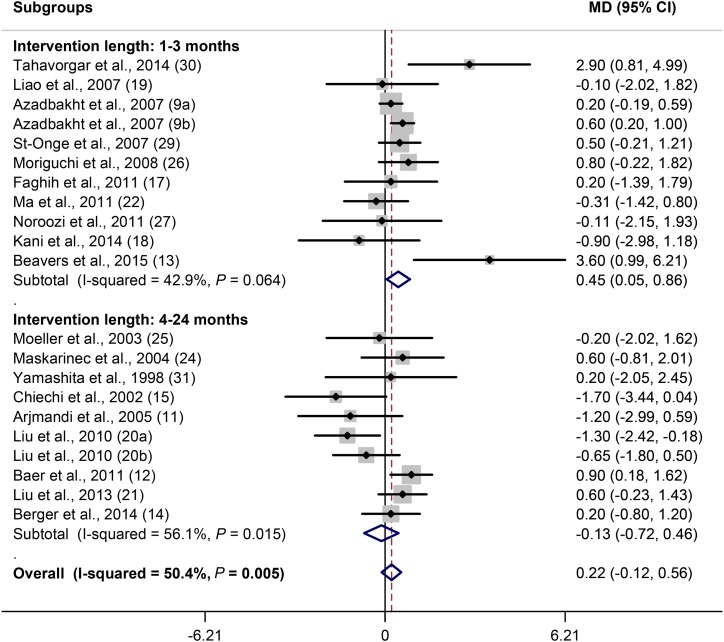
As stated, 21 of 24 soy trials evaluated the effect of soy consumption on weight. Because the number of trials that examined the effect of soy on weight was more than those that assessed BMI (21 compared with 17), the effect of soy consumption on weight was evaluated. There was no overall effect of soy consumption on weight [MD (soy minus control): 0.22 kg; 95% CI: −0.12, 0.56 kg; P = 0.2]. There was a moderate heterogeneity (I2 = 50.4%; P = 0.005) between studies. Subgroup analysis based on subjects’ BMI values revealed no effect of soy on weight of normal-weight and overweight subjects, but there was a significant weight increase in obese subjects (P = 0.02) (Figure 2). Also, a slightly obesogenic effect of soy was observed in younger ages (<50 y) (P = 0.1) (Supplemental Figure 1A). Sex did not show a significant impact on the association, but the only trial on men showed a significant obesogenic effect (Supplemental Figure 1B). Soy protein in amounts of <40 g/d had no effect on weight, but in doses of ≥40 g/d it increased weight (P = 0.03) (Figure 3). Regarding intervention length, weight increased during 1–3 mo of soy consumption (P = 0.03), but there was no effect during 4–24 mo of consumption (Figure 4). A significant positive effect of soy on weight was observed when soy was compared with meat (P = 0.03) and whey protein (P = 0.04) but not when compared with casein or whole milk and usual diet and cereals (Supplemental Figure 1C). Subgroup analysis based on ethnicity did not show a particular effect (data not shown).
FIGURE 2.
Forest plot of clinical trials examining the effect of soy on body weight (kilograms) with subgroup analysis based on BMI status of participants. Data are expressed as MDs between treatment and control groups with 95% CIs. Estimates were pooled by using the random-effects, inverse-variance model. MD, mean difference.
FIGURE 3.
Forest plot of clinical trials examining the effect of soy on body weight (kilograms) with subgroup analysis based on dosage. Data are presented as MDs between treatment and control groups with 95% CIs. MD, mean difference.
FIGURE 4.
Forest plot of clinical trials examining the effect of soy on body weight (kilograms) with subgroup analysis based on intervention length. Data are presented as MDs between treatment and control groups with 95% CIs. MD, mean difference.
Effect of soy on waist circumference
Sixteen trials evaluated the effect of soy consumption on waist circumference. There was no significant overall effect; the overall pooled estimated MD was 0.40 cm (95% CI: −0.42, 1.22 cm) with substantial heterogeneity between trials (I2 = 91.9%; P < 0.0001) (Table 2). Subgroup analysis revealed that BMI, age, sex, treatment dose, and the type of control affect the effect of soy on waist circumference (Table 2). For overweight subjects, those in older ages (>50 y), and women; in a treatment dose of <40 g/d; and in comparison with meat, soy may decrease waist circumference.
TABLE 2.
Subgroup analysis of the effect of soy consumption on waist circumference in the meta-analysis of the effect of soy and soy isoflavones on obesity-related anthropometric measures1
| Subgroup categorization | Studies, n | Waist circumference mean difference (95% CI), cm | P | Heterogeneity, % |
| BMI, kg/m2 | ||||
| <25 | 4 | −0.28 (−1.07, 0.51) | 0.5 | 63.8 |
| 25–29.9 | 3 | −0.34 (−0.61, −0.07) | 0.01 | 0 |
| ≥30 | 9 | 0.93 (−0.90, 2.75) | 0.3 | 93.9 |
| Age | ||||
| Younger | 3 | −0.22 (−0.72, 0.29) | 0.4 | 0 |
| Middle | 5 | 2.26 (−0.37, 4.89) | 0.09 | 93.7 |
| Older | 8 | −0.38 (−0.71, −0.04) | 0.03 | 24.1 |
| Sex | ||||
| Men | 1 | 7.40 (6.08, 8.72) | <0.001 | — |
| Men and women | 4 | 0.95 (−0.58, 2.49) | 0.2 | 67.1 |
| Women | 11 | −0.32 (−0.57, −0.08) | 0.01 | 8.6 |
| Ethnicity | ||||
| Caucasian | 7 | 0.17 (−1.04, 1.39) | 0.8 | 84.4 |
| Asian | 9 | 0.58 (−0.57, 1.73) | 0.3 | 94.2 |
| Treatment dose, g soy protein/d | ||||
| <40 | 11 | −0.31 (−0.57, −0.05) | 0.02 | 15.4 |
| ≥40 | 5 | 1.98 (−0.81, 4.77) | 0.2 | 94.1 |
| Intervention length, mo | ||||
| 1–3 | 9 | 0.58 (−0.63, 1.79) | 0.3 | 93.9 |
| 4–24 | 7 | 0.18 (−0.94, 1.30) | 0.7 | 86.5 |
| Control type | ||||
| Meat | 3 | −0.34 (−0.61, −0.07) | 0.01 | 0 |
| Whey protein | 6 | 3.09 (−0.92, 7.10) | 0.1 | 96.3 |
| Casein/whole-milk protein | 3 | −0.28 (−1.07, 0.51) | 0.5 | 63.8 |
| Usual diet/cereals | 4 | −0.33 (−0.89, 0.22) | 0.2 | 0 |
| Overall estimates | 16 | 0.40 (−0.42, 1.22) | 0.3 | 91.9 |
Mean differences and SDs of control and intervention groups were used to calculate pooled overall effects. Statistical heterogeneity was assessed with the I2 test by using random inverse-variance heterogeneity.
Effect of soy on fat mass
Ten trials examined the effect of soy consumption on fat mass. There was no significant overall pooled effect (MD: 0.21 kg; 95% CI: −0.31, 0.73 kg) with a moderate heterogeneity (I2 = 50.1%; P = 0.035). Because of the low number of studies in this group, no specific effect from soy on fat mass was observed in any of the aforementioned subgroups.
Effect of isoflavones on BMI
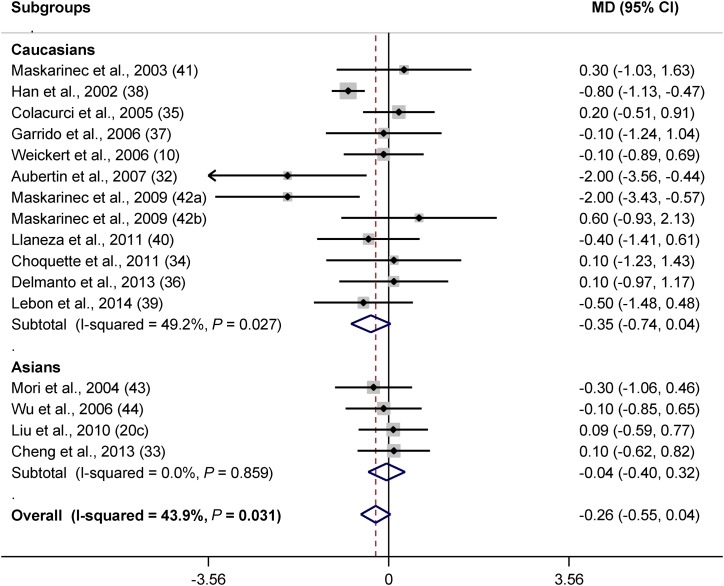
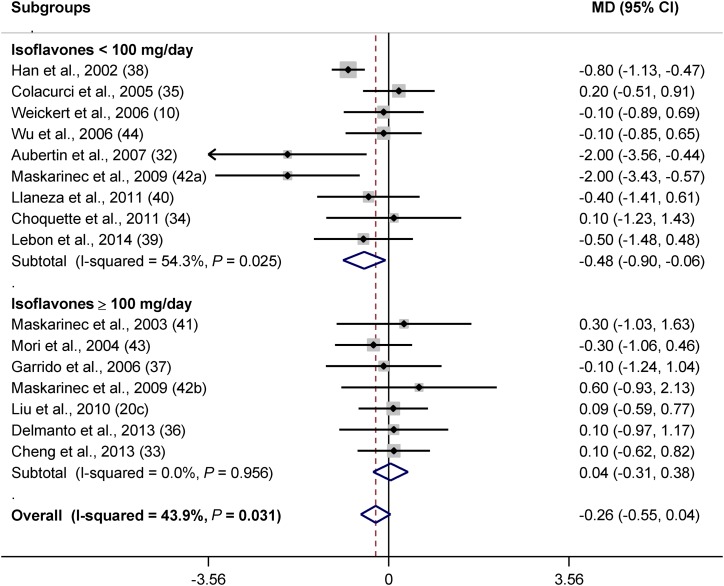
The number of trials that examined the effect of isoflavones on BMI was higher than those on weight (16 compared with 9). Therefore, for the analysis of isoflavone studies, BMI was assessed instead of weight. Evaluating the effect of isoflavones on BMI revealed that isoflavones tended to decrease BMI (MD: −0.26; 95% CI: −0.55, 0.04; P = 0.085). Subgroup analysis did not show a significant effect in any of the subcategories of BMI (Supplemental Figure 2A). Menopausal stage may have an impact because in postmenopausal women isoflavones almost significantly decreased BMI (P = 0.1) (Supplemental Figure 2B). Ethnicity was also effective because Caucasians benefited more from isoflavones (P = 0.08) (Figure 5). Lower doses (P = 0.02) (Figure 6) and shorter intervention lengths (P = 0.053) (Supplemental Figure 2C) were more effective in the prevention of obesity by isoflavones.
FIGURE 5.
Forest plot of clinical trials examining the effect of isoflavones on BMI (in kg/m2) with subgroup analysis based on ethnicity. Data are presented as MDs with 95% CIs. MD, mean difference.
FIGURE 6.
Forest plot of clinical trials examining the effect of isoflavones on BMI (in kg/m2) with subgroup analysis based on isoflavones dosage. Data are presented as MDs with 95% CIs. MD, mean difference.
Effect of isoflavones on waist circumference and fat mass
Five trials reported on the effect of isoflavones on waist circumference; no effect was observed (MD: −0.13 cm; 95% CI: −1.06, 0.79 cm; I2 = 41.8%). Seven trials assessed the effect of isoflavones on fat mass; yet, no significant effect was observed in overall pooled estimates (MD: −0.36 kg; 95% CI: −0.83, 0.12 kg; I2 = 0.0%).
Publication bias
No publication bias was detected by Begg’s and Egger’s tests in any of the evaluated outcomes in either soy or isoflavones trials.
Discussion
Numerous animal and human studies have shown the beneficial effect of soy on weight reduction (3). The anti-obesity effect of soy is partly attributed to its high protein content (45). High-protein diets have been effective in ad libitum food consumption in weight maintenance and reduction (46, 47). Nevertheless, the results of this meta-analysis showed no statistically significant overall effect of soy on weight, waist circumference, or fat mass. However, a statistically significant obesogenic effect of soy was observed in obese subjects (BMI ≥30), in short-term applications (1–3 mo), in consumptions of ≥40 g soy protein/d, and in comparisons with meat and whey protein but not milk or usual diets.
The diverse impact of soy on obese and nonobese individuals may be explained by the fact that obese individuals have less control over their food intake than normal-weight subjects (48, 49). Normal-weight individuals compensate the extra energy of soy consumption by reducing the energy obtained from other food items, whereas obese individuals are less likely to do so (50). In line with this explanation, consuming soy protein in quantities of ≥40 g/d led to increased body weight whereas lower amounts exhibited no effect.
The lack of the obesogenic effect in long-term interventions is probably the result of poor adherence to the treatment in long-term interventions, a fact that has been highlighted in previous investigations in which short-term interventions proved to be more efficacious than long-term treatments (51, 52). The results of this meta-analysis also suggest that the effect of soy is not increased with cumulative doses, which are usually obtained during long-term interventions.
Although no significant effect of soy on weight was observed in comparison with usual diet or casein, soy increased weight compared with whey and meat. Because the comparison of soy with usual diets revealed no overall effect on weight, the difference in the impact of soy and whey proteins on weight seems to result from the anti-obesity effect of whey rather than the obesogenic effect of soy. The anti-obesity effect of whey has been reported numerous times and is likely due to its potential to induce satiety. The consumption of whey reduces appetite and hunger more than the consumption of casein or soy (30, 53). In free-calorie diets, the consumption of whey protein by overweight and obese individuals has reduced weight, waist circumference, and fat mass whereas soy protein either increased or had an impact on these characteristics (12, 30). Soy was also more obesogenic than meat. Studies examining the anti-obesogenic effect of meat are scarce, and no study has yet investigated the possible underlying mechanisms. However, essential amino acids, such as lysine, leucine, isoleucine, tryptophan, and threonine, which are abundant in animal proteins, including meats, might be involved in regulating body weight through the suppression of appetite (53). Special amino acids may also stimulate production of hormones that influence metabolism (54).
Contrary to the above-mentioned obesogenic potential of soy on weight, isoflavones almost significantly (P = 0.085) reduced BMI, especially in doses of <100 mg/d and intervention lengths of 2–6 mo but not in higher doses or longer intervention lengths. Isoflavones also caused a nearly significant reduced BMI in Caucasians compared with Asians, which is suggested to be the result of a higher frequency of UDP-glucuronosyltransferase 1A3 alleles, which produce flavonoid metabolites with high-biological activity (55).
Isoflavones belong to flavonoids, a broad family of phytochemicals with extensive metabolic effects (56). Many animal and human studies have highlighted the anti-obesity potential of flavonoids (57), including isoflavones (58). Inhibition of lipogenesis and increased FA β-oxidation, which lead to the reduction of body fat depots, have been found to be mechanisms of isoflavones’ action against obesity (56, 58). Isoflavones are also categorized as phytoestrogens, a group of compounds that, because of their similarity in the structure to 17-β-estradiol, are able to bind to estrogen receptors, mimicking estrogen activity (59).
A majority of studies on isoflavones were conducted in postmenopausal women; only 2 trials included pre- and perimenopausal women. Therefore, it is hard to distinguish whether these beneficial effects of isoflavones were the consequence of isoflavone consumption, the impact of postmenopausal status, or both. In fact, because of low amounts of estrogen, postmenopausal women may benefit the most from consumption of isoflavones. A part of the anti-obesity effects of isoflavones is likely exerted through their binding to estrogen receptors. Estrogens are known to have anti-obesity effects of regulating food intake and energy expenditure, as well as preventing fat accumulation in adipose tissue (60). It has been reported that isoflavones interact with estrogen receptors in adrenal medullary cells, where they stimulate catecholamine synthesis (61). The anti-obesity potential of isoflavones is clarified more when we note that catecholamine release is associated with higher systemic energy expenditure (62). However, the catecholamine-stimulating effect of isoflavones is dose dependent; low doses stimulate catecholamine secretion whereas high doses inhibit it (61). This mechanism may explain the dose-dependent effect of isoflavones on BMI in this meta-analysis in which low doses exhibited a nearly significant negative effect on BMI, and high doses (≥100 mg/d) appeared to have no effect. This dose-dependent effect has also been reported for the lipid-lowering and satiating potential of soy protein in that low doses (<30 g/d) showed a dose-response lipid-lowering effect (63), whereas higher doses revealed no effect of either lipid-lowering (64) or satiating (53) potential.
Soy seemed to have a sex-specific effect on weight in this meta-analysis. This sex-dependent effect may be the result of faster pharmacokinetics and higher excretion rates of isoflavones in men compared with women (65–67). In fact, it may be the higher and longer-lasting plasma isoflavone concentrations in women that are responsible for the anti-obesity effect in this sex. In men, however, because of faster excretion rates, higher concentrations may be needed to exert the same biological effects (58).
Age also seemed to be an important factor in the effect of soy and isoflavones on weight; a relatively obesogenic effect of soy (P = 0.1) was observed in younger subjects (<50 y), and a relatively anti-obesity effect of isoflavones (P = 0.1) was seen in older subjects (postmenopausal). It seems that a sort of resistance to the obesogenic effect of soy and a kind of sensitivity to the anti-obesity effect of isoflavones exists in older subjects. In this regard, Fujimoto et al. (68) reported that equol production in the intestine is increased by age. In their study, subjects aged 10–40 y had lower equol serum concentrations than individuals in ages 40–60 y. Equol is produced by intestinal microflora from daidzein, one of the main isoflavones in soy, and like isoflavones, it binds to estrogen receptors (68). With consideration of the above-mentioned anti-obesity effects of estrogen (60), the role of equol in the age-dependent anti-obesity effect of isoflavones is more clarified.
In some subgroups, soy had an opposite effect on weight and waist circumference. For instance, although doses of ≥40 g soy protein/d increased weight, quantities of <40 g soy protein/d decreased waist circumference. Such an opposite effect of soy on weight and waist circumference may have resulted from the aforementioned sex-specific effect of soy. In fact, only 3 of 7 studies that reported increased weight after the consumption of soy and examined the effect of ≥40 g soy protein/d were conducted on women, whereas 10 trials of 11 trials that observed a reduction in waist circumference after ingestion of <40 g soy protein/d were conducted on women, further emphasizing the impact of sex on the effect of soy in prevention or intensification of obesity. Overall, sex seemed to influence many associations especially in subgroups with low number of studies.
Soy is the richest dietary source of isoflavones (69). Many health benefits of soy have been attributed to its high isoflavone content (1). Therefore, soy is supposed to have the same impact on obesity as isoflavones. However, this meta-analysis showed that soy and soy isoflavones have opposite effects on obesity. Because subgroup analysis of soy publications showed BMI, dosage, the length of treatment, and sex as important determining factors for the effect of soy on obesity indexes, the difference in these variables might have caused such differences between soy and isoflavone results. Hence, these variables were compared between soy and isoflavone studies. The participants’ mean BMI (28.8 compared with 26.4), mean dose of isoflavones (78.3 ± 42.8 mg/d compared with 77.8 ± 24.4 mg/d), and mean intervention lengths (5 mo compared with 8 mo) seemed to be comparable in the soy and isoflavone studies. However, the sex of participants remarkably differed between the 2 set of studies because in a number of soy investigations both sexes participated, whereas isoflavone studies were conducted only on women. Subgroup analysis based on sex revealed that women benefited more from the anti-obesity effect of soy and isoflavones. In soy trials, for instance, studies that included only men or both men and women showed a rather obesogenic effect of soy on weight, waist circumference, and fat mass, whereas trials with only women showed a neutral or an anti-obesity effect. Likewise, in trials that reported reduced waist circumference after the consumption of <40 g soy protein/d (P = 0.02), the anti-obesity effect of soy may be in part affected by the sex of the participants because participants of all but one of the studies were women.
In this meta-analysis, we observed a high degree of heterogeneity between studies that was not completely resolved by subgroup analysis based on BMI, age, sex, ethnicity, treatment dosage, intervention duration, and the type of placebo. Other sources of the heterogeneity may be the difference in the experimental design of the studies. Also, the low number of studies in some subgroups hindered making firm and powerful conclusions in a few cases. In some soy trials, a wide subject age range made age categorization for subgroup analysis hard and relatively imprecise, and so studies that target specific age categories and more importantly trials that compare adults and in particular women in young compared with old ages are warranted. The lack of data for the effect of isoflavones on men and on women of younger ages also leaves a gap in our knowledge that needs to be closed by future investigations. Because isoflavones likely act as phytoestrogens, the response to isoflavones probably differs in postmenopausal compared with premenopausal women. To our knowledge, this was the first meta-analysis on the effect of soy consumption on obesity-related anthropometric measures. The strength of this meta-analysis was the inclusion of studies on soy and soy isoflavones, which enabled us to make a comparison between the effects of soy and soy isoflavones on obesity-related anthropometric measures and helped in better interpretation of the results.
Conclusions
Overall, the results of this meta-analysis show that, although soy is the main source of isoflavones, soy and isoflavones may have different impacts on weight status. Although isoflavones demonstrate anti-obesity properties, soy consumption may actually increase weight in some groups, for instance, in obese subjects and when consumed in high quantities. The anti-obesity effect of isoflavones did not depend on participants’ BMI but was more pronounced in low dosages and short intervention lengths. However, because all isoflavone trials were conducted in women the actual comparison between the anti-obesity effects of soy and isoflavones cannot be performed unless the anti-obesity effect of isoflavones is tested in men as well. Future clinical trials are needed to compare the effects of soy and isoflavones on obesity-related variables and to examine specific differences between the 2 sexes. The existence of nonsignificant trends in some subgroups also calls for examining the impact of age, sex, and ethnicity on the effect of soy and isoflavones on obesity indexes.
Acknowledgments
We thank Mohammad Salehi-Marzijarani from Research and Computer Center at Shiraz University of Medical Sciences for statistical assistance on this work. We also thank Elisabeth Baker for her assistance in English-language editing. The authors’ responsibilities were as follows—MA: was responsible for all aspects of the paper; and all authors: read and approved the final version of the manuscript.
References
- 1.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr 2008;138:1244S–9S. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006;113:1034–44. [DOI] [PubMed] [Google Scholar]
- 3.Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci 2007;4:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YB, Chen WH, Guo JJ, Fu ZH, Yi C, Zhang M, Na XL. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women–a meta-analysis. Nutrition 2013;29:8–14. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated 2011 Mar] [Internet]. [cited 2015 Oct 28]. Available from: http://handbook.cochrane.org/index.htm#part_2_general_methods_for_cochrane_reviews.htm.
- 7.Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes 1985;9:147–53. [PubMed] [Google Scholar]
- 8.Sedgwick P. What is publication bias in a meta-analysis? BMJ 2015;351:h4419. [DOI] [PubMed] [Google Scholar]
- 9.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr 2007;85:735–41. [DOI] [PubMed] [Google Scholar]
- 10.Weickert MO, Reimann M, Otto B, Hall WL, Vafeiadou K, Hallund J, Ferrari M, Talbot D, Branca F, Bügel S, et al. . Soy isoflavones increase preprandial peptide YY (PYY), but have no effect on ghrelin and body weight in healthy postmenopausal women. J Negat Results Biomed 2006;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arjmandi BH, Lucas EA, Khalil DA, Devareddy L, Smith BJ, McDonald J, Arquitt AB, Payton ME, Mason C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr J 2005;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr 2011;141:1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beavers KM, Gordon MM, Easter L, Beavers DP, Hairston KG, Nicklas BJ, Vitolins MZ. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study. J Nutr Health Aging 2015;19:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger PK, Principe JL, Laing EM, Henley EC, Pollock NK, Taylor RG, Blair RM, Baile CA, Hall DB, Lewis RD. Weight gain in college females is not prevented by isoflavone-rich soy protein: a randomized controlled trial. Nutr Res 2014;34:66–73. [DOI] [PubMed] [Google Scholar]
- 15.Chiechi LM, Secreto G, Vimercati A, Greco P, Venturelli E, Pansini F, Fanelli M, Loizzi P, Selvaggi L. The effects of a soy rich diet on serum lipids: the Menfis randomized trial. Maturitas 2002;41:97–104. [DOI] [PubMed] [Google Scholar]
- 16.Christie DR, Grant J, Darnell BE, Chapman VR, Gastaldelli A, Sites CK. Metabolic effects of soy supplementation in postmenopausal Caucasian and African American women: a randomized, placebo-controlled trial. Am J Obstet Gynecol 2010;203:153.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faghih Sh, Abadi AR, Hedayati M, Kimiagar SM. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr Metab Cardiovasc Dis 2011;21:499–503. [DOI] [PubMed] [Google Scholar]
- 18.Kani AH, Alavian SM, Esmaillzadeh A, Adibi P, Azadbakht L. Effects of a novel therapeutic diet on liver enzymes and coagulating factors in patients with non-alcoholic fatty liver disease: a parallel randomized trial. Nutrition 2014;30:814–21. [DOI] [PubMed] [Google Scholar]
- 19.Liao FH, Shieh MJ, Yang SC, Lin SH, Chien YW. Effectiveness of a soy-based compared with a traditional low-calorie diet on weight loss and lipid levels in overweight adults. Nutrition 2007;23:551–6. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZM, Ho SC, Chen YM, Ho YP. A mild favorable effect of soy protein with isoflavones on body composition–a 6-month double-blind randomized placebo-controlled trial among Chinese postmenopausal women. Int J Obes (Lond) 2010;34:309–18. [DOI] [PubMed] [Google Scholar]
- 21.Liu ZM, Ho SC, Chen YM, Woo J. A six-month randomized controlled trial of whole soy and isoflavones daidzein on body composition in equol-producing postmenopausal women with prehypertension. J Obes 2013;2013:359763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma L, Grann K, Li M, Jiang Z. A pilot study to evaluate the effect of soy isolate protein on the serum lipid profile and other potential cardiovascular risk markers in moderately hypercholesterolemic Chinese adults. Ecol Food Nutr 2011;50:473–85. [DOI] [PubMed] [Google Scholar]
- 23.Maesta N, Nahas EA, Nahas-Neto J, Orsatti FL, Fernandes CE, Traiman P, Burini RC. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas 2007;56:350–8. [DOI] [PubMed] [Google Scholar]
- 24.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr 2004;134:3089–94. [DOI] [PubMed] [Google Scholar]
- 25.Moeller LE, Peterson CT, Hanson KB, Dent SB, Lewis DS, King DS, Alekel DL. Isoflavone-rich soy protein prevents loss of hip lean mass but does not prevent the shift in regional fat distribution in perimenopausal women. Menopause 2003;10:322–31. [DOI] [PubMed] [Google Scholar]
- 26.Moriguchi EH, Yamori Y, Mori M, Sagara M, Mori H, Sakuma T, Ishikawa PM, Moriguchi Y. New beverage for cardiovascular health, proposal based on oriental and occidental food culture from a world-wide epidemiological study. Geriatr Gerontol Int 2008;8:S3–7. [Google Scholar]
- 27.Noroozi M, Zavoshy R, Jahanihashemi H. The effects of low calorie diet with soy protein on cardiovascular risk factors in hyperlipidemic patients. Pak J Biol Sci 2011;14:282–7. [DOI] [PubMed] [Google Scholar]
- 28.Simão AN, Lozovoy MA, Dichi I. Effect of soy product kinako and fish oil on serum lipids and glucose metabolism in women with metabolic syndrome. Nutrition 2014;30:112–5. [DOI] [PubMed] [Google Scholar]
- 29.St-Onge MP, Claps N, Wolper C, Heymsfield SB. Supplementation with soy-protein-rich foods does not enhance weight loss. J Am Diet Assoc 2007;107:500–5. [DOI] [PubMed] [Google Scholar]
- 30.Tahavorgar A, Vafa M, Shidfar F, Gohari M, Heydari I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr Res 2014;34:856–61. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T, Sasahara T, Pomeroy SE, Collier G, Nestel PJ. Arterial compliance, blood pressure, plasma leptin, and plasma lipids in women are improved with weight reduction equally with a meat-based diet and a plant-based diet. Metabolism 1998;47:1308–14. [DOI] [PubMed] [Google Scholar]
- 32.Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause 2007;14:624–9. [DOI] [PubMed] [Google Scholar]
- 33.Cheng WC, Lo SC, Tsai KS, Tu ST, Wu JS, Chang CI, Chen CL, Shaw NS, Peng HY, Wang SY, et al. . Effects of high-dose phytoestrogens on circulating cellular microparticles and coagulation function in postmenopausal women. J Formos Med Assoc 2015;114:710–6. [DOI] [PubMed] [Google Scholar]
- 34.Choquette S, Riesco É, Cormier É, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr 2011;105:1199–209. [DOI] [PubMed] [Google Scholar]
- 35.Colacurci N, Chiàntera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiàntera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause 2005;12:299–307. [DOI] [PubMed] [Google Scholar]
- 36.Delmanto A, Nahas-Neto J, Traiman P, Uemura G, Pessoa EC, Nahas EA. Effects of soy isoflavones on mammographic density and breast parenchyma in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Menopause 2013;20:1049–54. [DOI] [PubMed] [Google Scholar]
- 37.Garrido A, De la Maza MP, Hirsch S, Valladares L. Soy isoflavones affect platelet thromboxane A2 receptor density but not plasma lipids in menopausal women. Maturitas 2006;54:270–6. [DOI] [PubMed] [Google Scholar]
- 38.Han KK, Soares JM Jr., Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol 2002;99:389–94. [DOI] [PubMed] [Google Scholar]
- 39.Lebon J, Riesco E, Tessier D, Dionne IJ. Additive effects of isoflavones and exercise training on inflammatory cytokines and body composition in overweight and obese postmenopausal women: a randomized controlled trial. Menopause 2014;21:869–75. [DOI] [PubMed] [Google Scholar]
- 40.Llaneza P, González C, Fernandez-Iñarrea J, Alonso A, Diaz F, Arnott I, Ferrer-Barriendos J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011;18:245–50. [DOI] [PubMed] [Google Scholar]
- 41.Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev 2003;12:165–9. [DOI] [PubMed] [Google Scholar]
- 42.Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, Murray MJ, Young RL, Wong WW. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr 2009;139:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori M, Aizawa T, Tokoro M, Miki T, Yamori Y. Soy isoflavone tablets reduce osteoporosis risk factors and obesity in middle-aged Japanesewomen. Clin Exp Pharmacol Physiol 2004;31 Suppl 2:S39–41. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, Fuku N, Teramoto T, Okuhira T, Ueno T, et al. . Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism 2006;55:423–33. [DOI] [PubMed] [Google Scholar]
- 45.Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000;16:349–54. [DOI] [PubMed] [Google Scholar]
- 46.Astrup A, Raben A, Geiker N. The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes (Lond) 2015;39:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101:1320S–9S. [DOI] [PubMed] [Google Scholar]
- 48.Campos-Uscanga Y, Gutiérrez-Ospina G, Morales-Romero J, Romo-González T. Self-regulation of eating and physical activity is lower in obese female college students as compared to their normal weight counterparts. Eat Weight Disord 2017;2:311–9. [DOI] [PubMed] [Google Scholar]
- 49.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 2010;52:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–73. [DOI] [PubMed] [Google Scholar]
- 51.Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009;63:1008–15. [DOI] [PubMed] [Google Scholar]
- 52.Barone Gibbs B, Kinzel LS, Pettee Gabriel K, Chang YF, Kuller LH. Short- and long-term eating habit modification predicts weight change in overweight, postmenopausal women: results from the WOMAN study. J Acad Nutr Diet 2012;112:1347–55, 1355.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav 2009;96:675–82. [DOI] [PubMed] [Google Scholar]
- 54.Modlinger RS, Schonmuller JM, Arora SP. Adrenocorticotropin release by tryptophan in man. J Clin Endocrinol Metab 1980;50:360–3. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Chen S, Li X, Wang X, Zeng S. Genetic variants of human UGT1A3: functional characterization and frequency distribution in a Chinese Han population. Drug Metab Dispos 2006;34:1462–7. [DOI] [PubMed] [Google Scholar]
- 56.Akhlaghi M. Non-alcoholic fatty liver disease: beneficial effects of flavonoids. Phytother Res 2016;30:1559–71. [DOI] [PubMed] [Google Scholar]
- 57.Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, Yang GM, Choi HY, Cho SG. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci 2016;17:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ørgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 2008;233:1066–80. [DOI] [PubMed] [Google Scholar]
- 59.Rietjens IM, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol 2017;174:1263–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ajdžanović V, Medigović I, Živanović J, Mojić M, Milošević V. Membrane steroid receptor-mediated action of soy isoflavones: tip of the iceberg. J Membr Biol 2015;248:1–6. [DOI] [PubMed] [Google Scholar]
- 62.Matsumura Y, Nakagawa Y, Mikome K, Yamamoto H, Osakabe N. Enhancement of energy expenditure following a single oral dose of flavan-3-ols associated with an increase in catecholamine secretion. PLoS One 2014;9:e112180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Høie LH, Graubaum HJ, Harde A, Gruenwald J, Wernecke KD. Lipid-lowering effect of 2 dosages of a soy protein supplement in hypercholesterolemia. Adv Ther 2005;22:175–86. [DOI] [PubMed] [Google Scholar]
- 64.Tonstad S, Smerud K, Høie L. A comparison of the effects of 2 doses of soy protein or casein on serum lipids, serum lipoproteins, and plasma total homocysteine in hypercholesterolemic subjects. Am J Clin Nutr 2002;76:78–84. [DOI] [PubMed] [Google Scholar]
- 65.Soukup ST, Helppi J, Müller DR, Zierau O, Watzl B, Vollmer G, Diel P, Bub A, Kulling SE. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: a cross-species and sex comparison. Arch Toxicol 2016;90:1335–47. [DOI] [PubMed] [Google Scholar]
- 66.Lu LJ, Anderson KE. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr 1998;68:1500S–4S. [DOI] [PubMed] [Google Scholar]
- 67.Slikker W Jr., Scallet AC, Doerge DR, Ferguson SA. Gender-based differences in rats after chronic dietary exposure to genistein. Int J Toxicol 2001;20:175–9. [DOI] [PubMed] [Google Scholar]
- 68.Fujimoto K, Tanaka M, Hirao Y, Nagata Y, Mori M, Miyanaga N, Akaza H, Kim WJ. Age-stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis 2008;11:252–7. [DOI] [PubMed] [Google Scholar]
- 69.Mulligan AA, Kuhnle GG, Lentjes MA, van Scheltinga V, Powell NA, McTaggart A, Bhaniani A, Khaw KT. Intakes and sources of isoflavones, lignans, enterolignans, coumestrol and soya-containing foods in the Norfolk arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk), from 7 d food diaries, using a newly updated database. Public Health Nutr 2013;16:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]