Abstract
Swordtails and platyfish of the genus Xiphophorus are valuable models for biomedical research and are also commercially raised as ornamental fish valued by aquarists. While research use and commercial interest increases yearly in these fish, cryopreservation of sperm is unexplored in this genus. Xiphophorus are live-bearing fishes characterized by small body sizes, limited sperm volumes, and internal fertilization, an atypical reproductive mode for fish. These attributes make research involving cryopreservation of Xiphophorus germplasm challenging. To explore methods for sperm cryopreservation, this study evaluated the effect of different loading volumes of sperm suspension in 0.25-ml French straws, different dilution ratios of sperm to extender, an osmolality range of extender without cryoprotectant and with dimethyl sulfoxide (DMSO) as cryoprotectant, and short-term storage at room temperature and 4 °C after thawing. No significant difference in sperm motility due to straw loading volume was observed after thawing. Sperm motility was observed to decrease with increasing dilution. The osmolality of Hanks’ balanced salt solution (HBSS) without cryoprotectant in which the highest sperm motility (67%) was observed was 320 ± 3 mOsm/kg, which was also the osmolality of X. helleri blood plasma. When cryopreserved with 10% DMSO, however, the highest motilities within 10 min after thawing were observed with HBSS in the range of 240–300 mOsm/kg. Sperm suspended in HBSS at 320 mOsm/kg with a dilution factor of 100 maintained motility for 24 h at room temperature, but persisted for 10 days when stored at 4 °C. These results provided the first evidence that cryopreservation may be applied to conservation of genetic resources in live-bearing fishes.
Keywords: Cryopreservation, Xiphophorus helleri, Sperm, Germplasm repositories, Osmotic pressure
1. Introduction
Species of live-bearing fishes of the genus Xiphophorus can be hybridized and are widely used in diverse areas of contemporary scientific research, including evolution [1,2], sex determination [3–5], endocrinology [6,7], ethology and behavioral ecology [8–11], toxicology [12,13], parasitology [14,15], immunology [16,17], and cancer genetics [18–20]. In the 1920s, Myron Gordon published a report showing that hybrids between the platyfish Xiphophorus maculatus and the green swordtail Xiphophorus helleri developed cancers virtually identical to malignant melanomas in man (for review see [20]). This animal model was one of the first to prove that certain cancers were inherited diseases. Building on this finding, Dr. Gordon established the Xiphophorus Genetic Stock Center in 1939 at the American Museum of Natural History in New York (USA). This Stock Center continues to provide pedigreed Xiphophorus fishes to research laboratories and commercial interests worldwide (see the website www.xiphophorus.org).
In addition to their value as experimental models for biomedical research, swordtails and platyfish are also valued as ornamental fish because of vibrant body coloration and a long sword-like tail. In addition, deforestation and urban expansion in Mexico and South America are resulting in loss of Xiphophorus habitat. Current stocks held in captivity may no longer exist in their natural habitats. To keep up with the demand for new varieties, scientists and commercial farmers constantly develop new strains [21]. The greatest increase in the number of varieties of swordtails began after 1985 with the development of approximately one new strain per year [21]. Given the increasing interest in Xiphophorus fishes it is unfortunate that early attempts at cryopreservation of Xiphophorus sperm, using protocols optimized for non-live-bearing aquaria fish, have not been successful (Walter, unpublished data). Thus, more than 22 species and more than 67 pedigreed lines of Xiphophorus are currently maintained only as live animal stocks, perpetuated by labor-intensive breeding regimes. As new species are identified, new stock lines developed, and rare chromosomal conditions delineated, it becomes increasingly important to cryogenically preserve Xiphophorus sperm samples in the long-term.
Despite the study of sperm cryopreservation in some 200 species of freshwater and marine fishes [22,23], sperm cryopreservation is essentially unexplored in Xiphophorus or in live-bearing fishes as a group. The sperm of Xiphophorus fishes are different in structure (e.g. head shape) and physiology (e.g. energy metabolism) from the sperm of oviparous fishes. Sperm of internally fertilizing species possess atypical features such as well-developed mitochondrial sheaths in the midpiece of spermatozoa [24] and glycolytic activity comparable to that of mammalian sperm [25]. These may be adaptations for movement or long-term survival in the female reproductive tract, and suggest physiological differences from the sperm of externally fertilizing fishes sufficient to necessitate development of specialized techniques for sperm handling, refrigerated storage, and cryopreservation.
Development of sperm cryopreservation protocols for Xiphophorus is also complicated by their small body sizes [26,27] and limited sperm volume [28,29]. Work with live-bearers is further hampered because specialized devices and technical skill are required to inseminate females, and embryonic development in the female is not easily monitored [30]. Once inseminated, female Xiphophorus may store sperm for months or years and release broods about every 30 days [31]. The present study was designed to identify basic parameters for sperm collection and processing, and to develop practical techniques for cryopreservation of sperm for members of the genus Xiphophorus. Presented herein are results using the green swordtail, X. helleri to examine the basic parameters involved in developing a successful cryopreservation protocol for this species. Our areas of investigation included: relationships of body length, body weight and testis weight to sperm density, osmolality of blood plasma and sperm motility; the effect of loading volume, dilution, and cryprotectant on sperm viability, and finally, the effect of temperature of sperm storage on sperm motility after thawing.
2. Materials and methods
2.1. Sperm collection
A total of 45 male X. helleri were shipped weekly by overnight delivery from the Xiphophorus Genetic Stock Center (XGSC) of Texas State University (San Marcos, TX, USA) to the Aquaculture Research Station of the Louisiana State University Agricultural Center in November and December of 2002. The fish were anesthetized in 0.01% MS-222 for ~2 min, and their standard lengths and wet weights were measured. Blood samples were collected in micro-hematocrit tubes with an internal diameter of 0.5–0.6 mm (VWR Scientific, Niles, IL, USA) by severing of the tail. The samples were pooled because of the small volumes available from each fish. After centrifugation at 10,000 rpm for 10 min, six 10-μl replicates were obtained from the pooled blood samples from 25 fish. Osmotic pressure of the blood plasma was measured by vapor pressure osmometry (model 5500, Wescor Inc., Logan, UT, USA).
Sperm were collected by surgical removal of the testis. Adherent tissue was dissected away and testes were placed in tared resealable plastic bags (NASCO whirl-pak, MBCOCT, New Haven, CT, USA) and weighed. Hanks’ balanced salt solution (HBSS) [32] was added before squeezing of the testis to release sperm. Dilutions with HBSS were based on the testis weight. Except for the experiment used to evaluate the effect of sperm dilution with HBSS on sperm motility, the ratio of testis to HBSS (mass:volume) was always 1:100. Based on our preliminary (unpublished) experiments, HBSS at 300 mOsm/kg was used for sperm suspension after collection. Within this manuscript, HBSS at specific osmolalities such as 300 mOsm/kg are abbreviated as HBSS 300. Sperm numbers per testis were obtained from the average of duplicate counts using a hemocytometer (with a dilution factor of 101).
2.2. Motility estimation
A 5-μl aliquot was removed from each sample of sperm suspended in HBSS (without freezing) or sperm suspended in HBSS and DMSO (after thawing) and placed on a glass microscope slide to estimate motility. Sperm motility was estimated visually at 200× magnification using dark-field microscopy (Optiphot 2, Nikon Inc., Garden City, NY, USA) and was expressed as the percentage of cells (in increments of 5%) actively moving in a forward direction [33]. Sperm vibrating in place were not considered to be motile. Sperm of X. helleri were found in this study to be atypical in comparison to other teleost fishes. The sperm were motile upon collection before dilution and sustained motility long after suspension in HBSS, and therefore activating solutions were not necessary for motility estimates.
2.3. Cryopreservation
Aliquots of sperm suspended in HBSS 300 were stored in 1.5-ml centrifuge tubes at 4 °C for 10–30 min, and were mixed with equal volumes of freshly prepared HBSS–DMSO solution. Each sperm–HBSS–DMSO aliquot was drawn into a 0.25-ml French straw (IMV International Corporation, Minneapolis, MN, USA) and held (equilibrated) for 10 min at room temperature. After equilibration, the straws were cooled in a controlled-rate freezer (Kryo 10 Series II; Planer Products, Sunbury-on-Thames, UK) at a rate of 45 °C per min from 5 to −80 °C. The straws were transferred to a liquid nitrogen storage dewar (−196 °C) after the temperature reached −80 °C. After a minimum of 4 h, the straws were thawed for 7 s in a 40 °C water bath (Model 1141, VWR Scientific). The present study used HBSS as the extender and 10% DMSO as cryoprotectant.
2.4. Evaluation of sperm loading volume in 0.25-ml straws
To maximize the ability to evaluate treatment effects with small volumes of sperm, the loading volume in 0.25-ml straws was evaluated with sperm from five male X. helleri. Sperm–HBSS–DMSO suspensions were prepared for each fish, drawn into straws in volumes of 30, 60, 100, and 200 μl, and frozen. The motility of each sample was estimated immediately after thawing.
2.5. Evaluation of dilution ratio of sperm to extender–cryoprotectant
To maximize the sperm volume for subsequent experiments, the ratio of sperm to HBSS 300 was evaluated for refrigerated storage at dilution ratios of 1:50, 1:100, 1:200, 1:400, 1:800, and 1:1600 with sperm samples from eight males, and sperm samples from six males were evaluated for cryopreservation. The sample volume of sperm–HBSS–DMSO chosen for this experiment in 0.25-ml straws was 80 μl. Motility was estimated before adding cryoprotectant and after thawing.
2.6. Evaluation of osmolality on sperm storage without cryoprotectant
Sperm from three males were used in this experiment. Sperm–extender suspensions were prepared from each fish by adding HBSS 300 at a ratio of sperm to HBSS of 1:50, and were divided into 14 sub-samples. Each sub-sample was mixed with a different volume of HBSS 800 and distilled water to prepare sperm–HBSS mixtures with final osmolalities of 110, 140, 170, 200, 230, 260, 290, 320, 350, 380, 410, 440, 470, and 500 mOsm/kg. The final ratio of sperm to HBSS was 1:100, except for the sperm suspensions at the two lowest osmolalities 110 mOsm/kg (1:162) and 140 mOsm/kg (1:120) due to the limitation of the initial dilution with HBSS 300. The sperm–HBSS mixtures were stored in a refrigerator at 4 °C, and motility was estimated after 15 min, 5 h, 1, 2, 3, 4, 6, 8, and 10 days. The sperm–HBSS mixtures with an osmolality of 320 mOsm/kg had three samples. One sample was stored at 4 °C and the other two were stored at room temperature (22–26 °C). Sperm motility of samples stored at room temperature was estimated after 10 min, 2, 5, 10, 24, and 36 h.
2.7. Evaluation of osmolality on sperm storage after thawing
Sperm from eight males were used in this experiment, of which four males provided sperm for the first trial with osmotic pressures of HBSS at 210, 240, 270, 300, 330, 360, and 390 mOsm/kg, and the other four were used for the second trial with osmotic pressures of HBSS at 100, 150, 200, 250, 300, 350, 400, 450, 500, and 550 mOsm/kg. Sperm–extender suspensions were prepared for each sample with HBSS 300 at a volume ratio of sperm to HBSS of 1:50, and were divided into sub-samples for different trials. Each sub-sample was mixed with a different volume of HBSS 800, distilled water, and DMSO to obtain the final osmolalities of sperm–HBSS–DMSO mixtures indicated above, while maintaining the final concentration of DMSO at 10%. The final volume ratio of sperm to HBSS–DMSO was 1:100 for all samples except for the sperm–HBSS–DMSO mixtures at 100 mOsm/kg (1:183) and 150 mOsm/kg (1:110), due to the limitation of the initial dilution with HBSS 300. After thawing, samples were stored at 4 °C and post-thaw motility was estimated at 10 min, 12, 24, 36, 60, and 84 h.
2.8. Data analysis
One-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) procedure was used to test for differences (P = 0.05) among results for sperm loading volume in 0.25-ml straws and among volume ratios of milt to extender–cryoprotectant. Curvilinear and simple linear regression (SLR) were used to estimate the relationships of testis weight to sperm per testes, body weight to testes weight, and standard length to body weight. The general linear model (GLM) repeated measure analysis was used to test for differences (P = 0.05) among results for the osmolality on sperm storage without cryoprotectant and after thawing. Results were presented as mean ± S.D. Data for sperm motility were arcsine transformed prior to analysis when heterogeneity of variance occurred. The software used was SPSS 10.0 for Windows, 1999.
3. Results
3.1. Basic parameters
The sizes of the male X. helleri used in this study were 3.20 ± 0.25 cm (n = 45) for standard length and 630 ± 168 mg (n = 45) for body wet weight. The standard length was positively (P < 0.0001) related to the body wet weight (Fig. 1a). The average testis wet weight was 9.18 ± 5.49 mg (n = 34), and no significant relationship was found between body wet weight and testis weight (Fig. 1b). There were approximately 4.39 ± 4.24 × 107 sperm cells per testis (n = 12), and sperm density was 5.38 ± 2.20 × 109 cell/g of testis. There was a significant (P < 0.0001) polynomial relationship between testis weight and sperm per testis (Fig. 1c). Osmotic pressure of the blood plasma was 320 ± 3 mOsm/kg (n = 6).
Fig. 1.
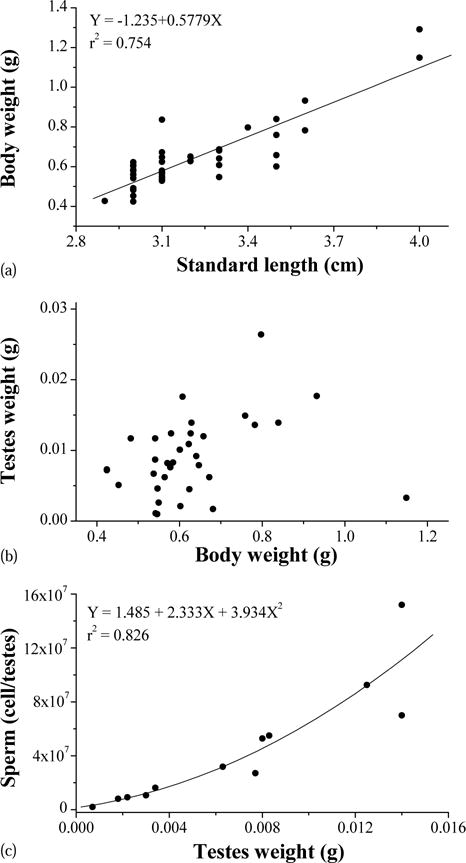
Relationships between standard length and body wet weight (a), body wet weight and testes weight (b), and testes weight and sperm number (c) of Xiphophorus helleri.
3.2. Evaluation of sperm loading volume in 0.25-ml straws
No significant difference was found for sperm motility among the loading volumes in 0.25-ml straws (Table 1). However, one straw containing 30 μl of sperm suspension failed to provide sufficient volume for motility estimation after thawing because the cotton end of the straw absorbed most of the sample. Post-thaw motilities ranged from 10 to 30% in this experiment and averaged 19 ± 7%.
Table 1.
Motility after thawing (%) of sperm from Xiphophorus helleri when suspended in Hanks’ balanced salt solution (HBSS) at 300 mOsm/kg and loaded in 0.25-ml French straws in different volumes
| Fish number | Volume (μl)
|
|||
|---|---|---|---|---|
| 30 | 60 | 100 | 200 | |
| 1 | – | 10 | 10 | 10 |
| 2 | 10 | 20 | 25 | 25 |
| 3 | 30 | 15 | 20 | 20 |
| 4 | 15 | 15 | 15 | 15 |
| 5 | 20 | 30 | 25 | 25 |
| Mean ± S.D. | 19 ± 9 | 18 ± 8 | 19 ± 7 | 19 ± 7 |
3.3. Evaluation of dilution ratio of sperm to extender–cryoprotectant
Before freezing and without cryoprotectant, the highest motility (43 ± 17%) was found with the volume ratio (sperm:extender) of 1:50 and the lowest motility (1 ± 0%) was at 1:1600 (Fig. 2). No significant difference was observed among the volume ratios within the grouping (Tukey’s HSD) of sperm to extender of 1:50, 1:100, and 1:200 or the grouping of 1:400, 1:800, and 1:1600 although a significant difference was found between these two groupings. After the addition of DMSO and freezing, motility decreased in all treatment groups, but variations among them were less similar than those before freezing (Fig. 2). The highest observed post-thaw motility (16 ± 10%) was found with the volume ratio of 1:50 and the lowest (1 ± 0%) was at 1:1600. In this experiment, the highest post-thaw motility was 25% at a volume ratio of 1:50, and motilities for samples with volume ratios of 1:50 and 1:100 ranged from 2 to 25%.
Fig. 2.
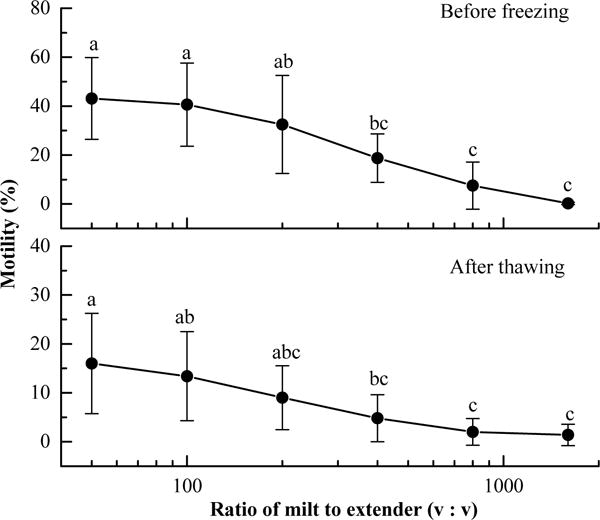
Motility of sperm (mean ± S.D.) from Xiphophorus helleri before freezing and after thawing in six different ratios of milt to extender–cryoprotectant (HBSS–DMSO): 1:50, 1:100, 1:200, 1:400, 1:800, and 1:1600. Values sharing supercript letters within a panel were not significantly different.
3.4. Evaluation of osmolality on sperm storage without cryoprotectant
After storage at 4 °C for 15 min, the motility of sperm suspended in HBSS at different osmolalities varied from 0 to 80%. The lowest average motility (1 ± 1%) was found in HBSS at the lowest osmolality (110 mOsm/kg). The motility increased with osmolality of HBSS and the highest (67 ± 8%) was found with osmolalities of 320–380 mOsm/kg (Fig. 3). Higher motilities were observed with sperm in HBSS above 380 mOsm/kg than below 320 mOsm/kg, although the standard deviations were larger for the sperm above 380 mOsm/kg. With prolonged storage time, the motility of sperm above 320 mOsm/kg declined faster than that of sperm below 320 mOsm/kg. After 4 days storage, the motility of sperm above 320 mOsm/kg was lower than that of sperm below 320 mOsm/kg. Eight days later, bacteria were observed in some samples such as those at 200 and 380 mOsm/kg (no formal identification of bacteria was attempted in this study). Sperm in HBSS at 320 mOsm/kg consistently showed the highest motility throughout the experiment except for at the eighth day. However, GLM repeated measures analysis showed that there was no difference (P = 0.315) among the sperm motilities in the samples with osmolalities of 230–440 mOsm/kg, although a difference (P = 0.040) was found among those at 200–470 mOsm/kg, indicating sperm of X. helleri has a capability to adapt a wide range of osmotic pressures.
Fig. 3.
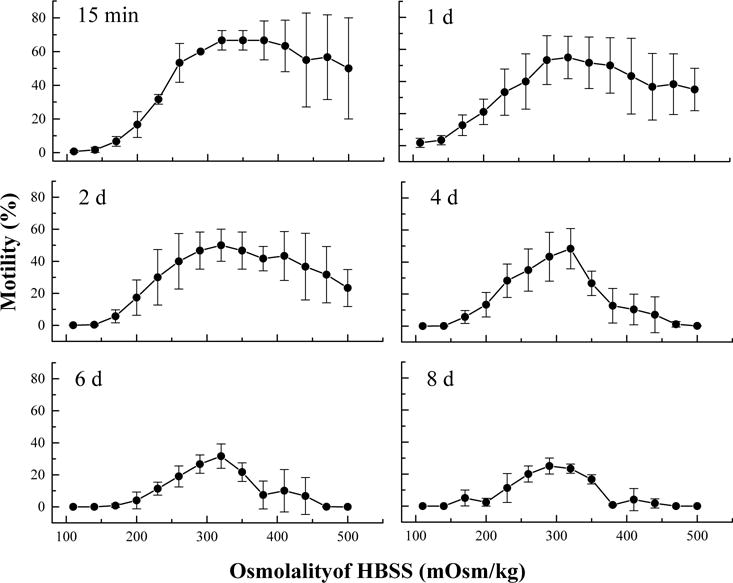
Motility of sperm (mean ± S.D.) from Xiphophorus helleri when suspended in Hanks’ balanced salt solution at 14 different osmolalities and stored refrigerated at 4 °C for 8 days.
When the sperm of X. helleri were suspended in HBSS at 320 mOsm/kg, they retained motility for more than 24 h at room temperature (22–26 °C) and for more than 10 days at 4 °C (Fig. 4). At room temperature, bacteria were observed at 10 h, but at 4 °C bacterial growth was not apparent until after 8 days. Motility of samples decreased rapidly after observation of bacterial growth. At room temperature, motility of sperm decreased to 40 ± 10% in the first 10 h. At 4 °C, motility decreased to 48% over 4 days, and sperm retained 17 ± 8% motility after 10 days of storage.
Fig. 4.
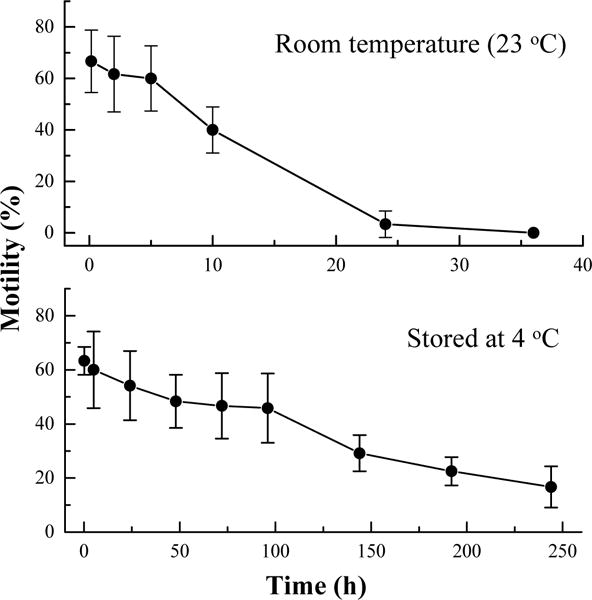
Motility of sperm (mean ± S.D.) from Xiphophorus helleri when suspended in Hanks’ balanced salt solution (HBSS) at 320 mOsm/kg and stored at room temperature for 36 h (upper panel) and at 4 °C for 10 days (lower panel).
3.5. Evaluation of osmolality on sperm storage after thawing
In the first trial, 10 min after thawing, the highest motility (29 ± 8%) was found for sperm suspended in HBSS at 240 and 270 mOsm/kg. Motility of sperm at 210 mOsm/kg was higher than that at 300–390 mOsm/kg (Fig. 5). Twelve hours after thawing, motility of sperm in HBSS 270 declined to 9 ± 8%, and the highest motility (19 ± 13%) was found for HBSS 240. Twenty-four hours later, the highest motility was at HBSS 210, and sperm motility in all samples declined below 10%. Although some sperm retained motility at 84 h after thawing when stored at 4 °C, only minimal motility (<1%) was found for samples at 210 and 240 mOsm/kg. In this trial, all samples were found to be motile within 10 min after thawing and the highest motility (40%) was found for samples at 210, 240, and 270 mOsm/kg. General linear model repeated measures analysis detected differences among motilities in the samples with osmolalities of 210–390 mOsm/kg (P = 0.034), 240–360 mOsm/kg (P = 0.042), and of 240–390 mOsm/kg (P = 0.022), but no difference was found among those of 240–330 mOsm/kg (P = 0.081) or of 210–360 mOsm/kg (P = 0.072) (Table 2), indicating that osmolalities of greater than 360 mOsm/kg would yield lower motility than would an osmolality less than 240 mOsm/kg.
Fig. 5.
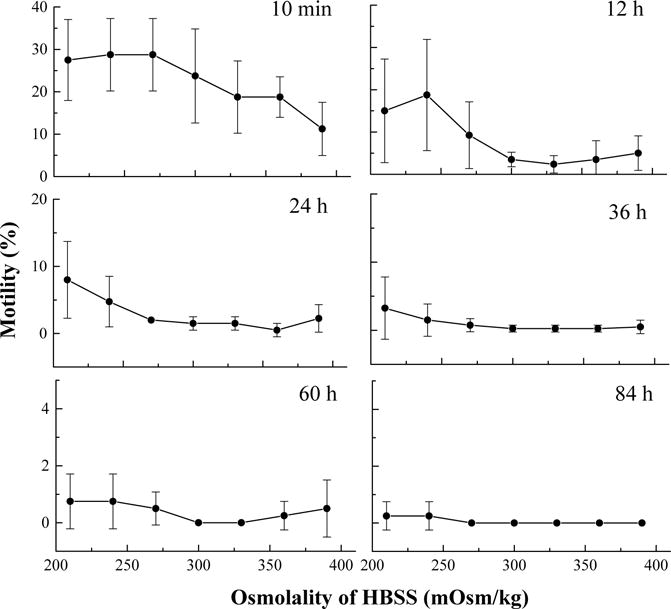
Motility after thawing of sperm (mean ± S.D.) from Xiphophorus helleri when suspended in Hanks’ balanced salt solution (HBSS) at seven different osmolalities ranging from 210 to 390 mOsm/kg and stored at 4 °C for 84 h.
Table 2.
Statistical results (Tukey’s HSD) of significance groupings of motility after thawing of sperm from Xiphophorus helleri when suspended in Hanks’ balanced salt solution (HBSS) at seven different osmolalities ranging from 210 to 390 mOsm/kg and stored at 4 °C for 84 h
| Osmolality of HBSS | Grouping |
|---|---|
| 210 | AB |
| 240 | A |
| 270 | AB |
| 300 | AB |
| 330 | AB |
| 360 | B |
| 390 | B |
In the second trial, the highest motility at 10 min after thawing was found within the range of 200–400 mOsm/kg, with the highest motility (28 ± 13%) at 300 mOsm/kg (Fig. 6). At 12 h after thawing, the motility of sperm suspended in HBSS 300 declined to 7 ± 6%, and the highest motility was found at HBSS 250 (15 ± 10%), and HBSS 200 (15 ± 5%). Similar to the results of the first trial, the motility in all samples declined below 10% in 24 h after thawing, but the highest motility was in HBSS 250. At 60 h after thawing, the highest motility (<1%) was found in HBSS 200. No sample was found to be motile at 84 h after thawing. In this trial, no motile sperm were found in samples suspended in HBSS 100, and the highest motility (40%) 10 min after thawing was found in samples between 250 and 300 mOsm/kg. General linear model repeated measures analysis detectecd a difference (P = 0.005) among the sperm motilities in the samples with osmolalities of 150–450 mOsm/kg, but no difference was found among those of 200–400 mOsm/kg (P = 0.280).
Fig. 6.
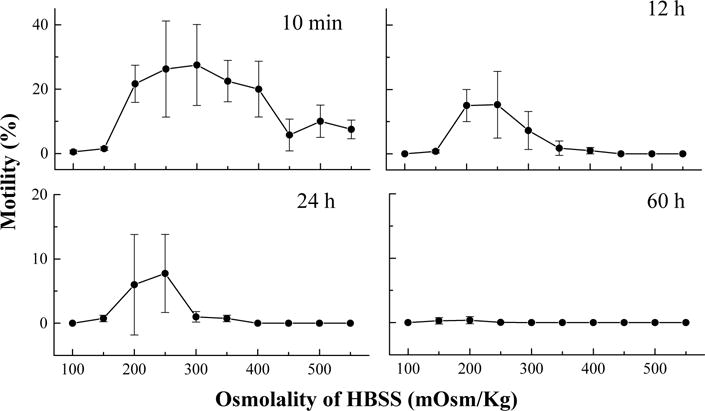
Motility after thawing of sperm (mean ± S.D.) from Xiphophorus helleri when suspended in Hanks’ balanced salt solution (HBSS) at 10 different osmolalities ranging from 100 to 500 mOsm/kg and stored at 4 °C for 60 h.
4. Discussion
The mature X. helleri males used in the present study were <3.5 cm long and weighed <650 mg. The average testis weight of these fish was 9.2 mg. If we assume a specific gravity of 1.0 for the testis and assume that the entire volume of the testis is filled with sperm, these male X. helleri could produce a maximum volume of 9.2 μl of sperm. Because the volume of the smallest standard straw used for cryogenic storage is 0.25 ml, and given that there are numerous variables involved in research trials of sperm cryopreservation, the use of this 9.2-μl volume of milt to evaluate cryopreservation becomes a serious practical question. The loading volumes tested in this study for 0.25-ml straws did not affect the motility of X. helleri sperm after thawing. However, as the cotton plug of the straw absorbed part of the sample, and some volume was necessary for motility estimation after thawing, a minimum of 80 μl of sperm suspension in each 0.25-ml straw was chosen for further experiments in the present study.
Dilution of samples to increase volume could reduce sperm motility by removing protective components of seminal plasma, as suggested for mammals and other fishes [34–36]. In this study, the sperm motility of X. helleri declined with dilution, although the motility among dilutions of 1:50, 1:100, and 1:200 was not significantly different before freezing or after thawing. For sperm cryopreservation of aquatic animals, optimal dilution generally is in a range of 2- to 10-fold [33,37–40]. However, sperm of X. helleri diluted 100-fold in HBSS at 320 mOsm/kg maintained motility at room temperature with a 27% loss over 10 h. If a dilution factor of 1:100 were chosen, 920 μl of sperm suspension could be obtained given a testis weight of 9.2 mg. Dividing this into 80-μl aliquots, a maximum of 11 0.25-ml straws could be prepared from a single male X. helleri, which would be sufficient to perform replication for factors to be studied for the development of sperm cyropreservation techniques for this fish.
Previous studies have shown that the osmotic pressure of the extender can be important in the storage and activation of sperm from aquatic species (e.g. [24,41]). For marine fishes, sperm motility generally increases with osmolality of the extender solution [27,42–44]. In contrast, for freshwater fishes, sperm activation is triggered by a decrease in osmotic pressure or by changes in the concentration of specific ions [23,38,41,45]. Sperm from X. helleri showed the highest motility in HBSS, with an osmolality of around 320 mOsm/kg, which agreed with the osmolality of the blood plasma. Immediately after sperm were released from the testis, motility was higher in HBSS above 320 mOsm/kg than in HBSS below 320 mOsm/kg, although the results reversed after 4 days of storage at 4 °C.
The genus Xiphophorus is characterized by internal fertilization, in which males produce sperm in packages (spermatophores) and females store sperm in the receptaculum seminis or “Delle” of the genital tract for as long as several months after insemination [21,26,46,47]. Inside the sperm package or within the female genital tract, the sperm seem to not move freely. Therefore, sperm of X. helleri may simply be activated by release from the sperm package or the genital tract, but not by variation of osmotic pressure or ions, as in fish with external fertilization [48–52]. However, a suitable osmolality may be important to retain ions or sugars at specific concentrations within the sperm; this might explain why the sperm of X. helleri can retain motility in HBSS at 320 mOsm/kg for 10 days. The role of specific ions, sugars, or other chemicals in the maintenance of vitality of Xiphophorus sperm requires further study.
After the addition of DMSO and thawing, the osmolality of HBSS yielding the highest sperm motility was lower than that without DMSO. The addition of 10% DMSO to HBSS 270 would raise the osmolality of sperm suspension to 1568 mOsm/kg [32]. This may explain the apparent discrepancy in osmolalities that yielded the highest motility before freezing and after thawing. Considering that artificial insemination and internal fertilization would likely require a minimum of several hours, HBSS in the range of 200–250 mOsm/kg would be more appropriate for sperm suspension when using DMSO as a cryoprotectant.
Sperm of X. helleri diluted 100-fold in HBSS at 320 mOsm/kg maintained continuous motility for 10 h at room temperature and as long as 10 days at 4 °C. This is in stark contrast to sperm of fishes that have external fertilization. In those fishes, motility is elicited with an activating solution, and the duration of active sperm motility is typically brief, usually lasting no longer than 1–2 min [32,37,53–55]. Thus, storage studies in non-live-bearing fishes represent retention of the capacity for sperm activation, while maintenance of continuous motility characterized the sperm of X. helleri. The extended motility of Xiphophorus sperm is distinct in fishes, and would seem to reflect the requirements for prolonged activity and survival of sperm in the female reproductive tract. This atypical reproductive mode also suggests that motile sperm would be more likely to have fertilization success after thawing than would non-motile sperm, indicating the value of motility data as a sperm quality indicator for these preliminary experiments.
To our knowledge, this is the first report of sperm cryopreservation in a live-bearing fish. This study provides useful protocols for the development of sperm cryopreservation for other live-bearing fishes. Overall, the highest post-thaw motility occurred when sperm were suspended in HBSS at 240–300 mOsm/kg with 10% DMSO, equilibrated for 10 min in 80-μl aliquots in 0.25-ml French straws, cooled at 45 °C per min from 5 to −80 °C before plunging into liquid nitrogen, and thawed at 40 °C in a water bath for 7 s. However, the small body size of Xiphophorus females also limits the volume of sperm suspension that can be injected for fertilization with artificial insemination. Future research should address practical problems such as increasing post-thaw motility, lengthening motility duration after thawing, reducing cryoprotectant toxicity, and optimizing sperm concentrations. Of special importance will be development of standardized assays to evaluate fertilization and early development in live-bearers.
Acknowledgments
We thank L. Hazlewood and R. Bowers of the Xiphophorus Genetic Stock Center for providing fish, S. Kazianis for advice and discussion, and R. Smeal for administrative help. This work was supported by USPHS grants, RR-17072 from the National Center for Research Resources and CA-75137 from the National Cancer Institute, with additional support provided by the Roy F. and Joanne Cole Mitte Foundation and the U.S. Department of Agriculture. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 03-11-1468.
References
- 1.Meyer A. The evolution of sexually selected traits in male swordtail fishes (Xiphophorus : Poeciliidae) Heredity. 1997:329–37. [Google Scholar]
- 2.Meyer A, Morrissey JM, Schartl M. Recurrent origin of a sexually selected trait in Xiphophorus fishes inferred from a molecular phylogeny. Nature. 1994;368:539–42. doi: 10.1038/368539a0. [DOI] [PubMed] [Google Scholar]
- 3.Kallman KD. The sex determining mechanism of the poeciliid fish, Xiphophorus montezumae, and the genetic control of the sexual maturation process and adult size. Copeia. 1983;3:755–69. [Google Scholar]
- 4.Kallman KD. A new look at sex determination in Poeciliid fishes. In: Turner BJ, editor. Evolutionary genetics of fishes. New York: Plenum Publishing Corporation; 1984. pp. 95–171. [Google Scholar]
- 5.Tiersch TR, Chandler RW, Kallman KD, Wachtel SS. Estimation of nuclear DNA content by flow cytometry in fishes of the genus Xiphophorus. Comp Biochem Physiol B. 1989;94:465–8. doi: 10.1016/0305-0491(89)90182-x. [DOI] [PubMed] [Google Scholar]
- 6.Breuckmann A, Paris F, Schreibman Martin P, Bluem V. Immunoreactive gonadotropin-releasing hormone (GnRH) in the brain and pituitary of adult and juvenile swordtails (Xiphophorus helleri, Teleostei, Poeciliidae) J Morphol. 1996:55–67. doi: 10.1002/(SICI)1097-4687(199610)230:1<55::AID-JMOR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Flynn KM, Schreibman MP, Yablonsky-Alter E, Banerjee SP. Sexually dimorphic development and binding characteristics of NMDA receptors in the brain of the platyfish. Gen Comp Endocrinol. 1999;115:282–91. doi: 10.1006/gcen.1999.7317. [DOI] [PubMed] [Google Scholar]
- 8.Beaugrand JP, Goulet C. Distinguishing kinds of prior dominance and subordination experiences in males of green swordtail fish (Xiphophorus helleri) Behav Processes. 2000;50:131–42. doi: 10.1016/s0376-6357(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 9.Hoefler CD, Morris MR. A technique for the temporary application and augmentation of pigment patterns in fish. Ethology. 1999:431–38. [Google Scholar]
- 10.Rosenthal GG, Evans CS. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc Natl Acad Sci USA. 1998;95:4431–6. doi: 10.1073/pnas.95.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trainor BC, Basolo AL. An evaluation of video playback using Xiphophorus helleri. Anim Behav. 2000;59:83. doi: 10.1006/anbe.1999.1289. [DOI] [PubMed] [Google Scholar]
- 12.De Wolf W, Seinen W, Hermens JLM. N-Acetyltransferase activity in rainbow trout liver and in vitro biotransformation of chlorinated anilines and benzenes in fish. Xenobiotica. 1993:1045–56. doi: 10.3109/00498259309057042. [DOI] [PubMed] [Google Scholar]
- 13.Gandzyura VP, Ignatyuk AA. The effect of lead and ammonium ions on bioproductional parameters of fishes offspring [in Russian, we referred to the English abstract] Gidrobiol Zh/Hydrobiol J. 1998:85–90. [Google Scholar]
- 14.Dove AD. Richness patterns in the parasite communities of exotic poeciliid fishes. Parasitology. 2000;120:609–23. doi: 10.1017/s0031182099005958. [DOI] [PubMed] [Google Scholar]
- 15.Schmahl G, Schmidt H, Ritter G. The control of ichthyophthiriasis by a medicated food containing quinine: efficacy tests and ultrastructure investigations. Parasitol Res. 1996;82:697–705. doi: 10.1007/s004360050188. [DOI] [PubMed] [Google Scholar]
- 16.Hogarth PJ. Protection of the developing foetus from immunological attack in Xiphophorus helleri. J Reprod Fertil. 1970;23:532. doi: 10.1530/jrf.0.0230532. [DOI] [PubMed] [Google Scholar]
- 17.McConnell TJ, Godwin UB, Norton SF, Nairn RS, Kazianis S, Morizot DC. Identification and mapping of two divergent, unlinked major histocompatibility complex class II B genes in Xiphophorus fishes. Genetics. 1998;149:1921–34. doi: 10.1093/genetics/149.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schartl M. Platyfish and swordtails: a genetic system for the analysis of molecular mechanisms in tumor formation. Trends Genet. 1995;11:185–9. doi: 10.1016/S0168-9525(00)89041-1. [DOI] [PubMed] [Google Scholar]
- 19.Nairn RS, Kazianis S, McEntire BB, Della CL, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proc Natl Acad Sci USA. 1996:13042–47. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. J Inst Lab Anim Res. 2001;42(4):299–322. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- 21.Tamaru CS, Cole B, Bailey R, Brown C, Ako H. A manual for commercial production of the swordtail, Xiphophorus helleri. Honolulu: 2001. (CTSA Publication Number 128). ( http://www.soest.hawaii.edu/SEAGRANT) [Google Scholar]
- 22.Rana KJ. Cryopreservation of fish spermatozoa. Cryopreservation and freeze–drying protocols. Methods Mol Biol. 1995;38:151–65. doi: 10.1385/0-89603-296-5:151. [DOI] [PubMed] [Google Scholar]
- 23.Tiersch TR, Wayman WR, Figiel CR, Gorman OT, Williamson JH, Carnichael GJ. Field collection, handling, and storage of sperm of the endangered razorback sucker. N Am J Fish Manage. 1997;17:167–73. [Google Scholar]
- 24.Stoss J. Fish gamete preservation and spermatozoan physiology. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish physiology, vol. 9, part B, behavior and fertility control. San Diego: Academic Press; 1983. pp. 305–50. [Google Scholar]
- 25.Gardiner DM. Cyclic changes in fine structure of the epithelium lining the ovary of the viviparous teleost, Cymatogaster aggregata (Perciformes: Embiotocidae) J Morphol. 1978;156:367–79. doi: 10.1002/jmor.1051560304. [DOI] [PubMed] [Google Scholar]
- 26.Kallman KD. The platyfish, Xiphophorus maculatus. In: King RC, editor. Handbook of genetics. New York: Plenum Publishing Corporation; 1975. pp. 81–132. [Google Scholar]
- 27.Takai H, Morisawa M. Change in intracellular K+ concentration caused by external osmolality change regulates sperm motility of marine and freshwater teleosts. J Cell Sci. 1995;108:1175–81. doi: 10.1242/jcs.108.3.1175. [DOI] [PubMed] [Google Scholar]
- 28.Tiersch TR. Introduction. In: Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. Baton Rouge: World Aquaculture Society; 2000. pp. xix–xxvi. [Google Scholar]
- 29.Knapp WE. Foreword. In: Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. Baton Rouge: World Aquaculture Society; 2000. pp. xvii–xviii. [Google Scholar]
- 30.Tiersch TR. Cryopreservation in aquarium fishes. Mar Biotechnol. 2001;3:212–23. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- 31.Gordon M. Hereditary basis of melanosis in hybrid fishes. Am J Cancer. 1931;15:1495–523. [Google Scholar]
- 32.Tiersch TR, Goudie CA, Carmichael GJ. Cryopreservation of channel catfish sperm: storage in cryoprotectants, fertilization trials, and growth of channel catfish produced with cryopreserved sperm. Trans Am Fish Soc. 1994;123:580–6. [Google Scholar]
- 33.Paniagua-Chavez CG, Tiersch TR. Laboratory studies of cryopreservation of sperm and trochophore larvae of the eastern oyster. Cryobiology. 2001;43:211–23. doi: 10.1006/cryo.2001.2346. [DOI] [PubMed] [Google Scholar]
- 34.Harrison RAP, Dott HM, Foster GC. Effect of ionic strength, serum albumin and other macromolecules on the maintenance of motility and the surface of mammalian spermatozoa in a simple medium. J Reprod Fertil. 1978;52:65–73. doi: 10.1530/jrf.0.0520065. [DOI] [PubMed] [Google Scholar]
- 35.Billard R. Effects of coelomic and seminal fluids and various saline diluents on the fertilizing ability of spermatozoa in the rainbow trout, Salmo gairdneri. J Reprod Fertil. 1983;68:77–84. doi: 10.1530/jrf.0.0680077. [DOI] [PubMed] [Google Scholar]
- 36.Scott AP, Baynes SM. A review of the biology, handling and storage of salmonid spermatozoa. J Fish Biol. 1980;17:707–39. [Google Scholar]
- 37.Harvey B, Kelley RN, Ashwood-Smith MJ. Cryopreservation of zebra fish spermatozoa using methanol. Can J Zool. 1982;60:1867–70. [Google Scholar]
- 38.Bates MC, Wayman WR, Tiersch TR. Effect of osmotic pressure on the activation and storage of channel catfish sperm. Trans Am Fish Soc. 1996;125:798–802. [Google Scholar]
- 39.Wayman WR, Thomas RG, Tiersch TR. Refrigerated storage and cryopreservation of sperm of red drum, Sciaenops ocellatus L. Aquacult Res. 1998;29:267–73. [Google Scholar]
- 40.Paniagua-Chavez CG, Supan J, Buchanan J, Tiersch TR. Settlement and growth of eastern oysters produced from cryopreserved larvae. Cryo Lett. 1998;19:283–92. [Google Scholar]
- 41.Lin F, Dabrwoski K. Characteristics of muskellunge spermatozoa 2: effects of ions and osmolality on sperm motility. Trans Am Fish Soc. 1996;125:195–202. [Google Scholar]
- 42.Palmer PJ. Chilled storage of pikey bream (Acanthopagrus berda) sperm and activation in different salinities. Asian Fish Sci. 1994;7:35–40. [Google Scholar]
- 43.Wayman WR, Thomas RG, Tiersch TR. Cryopreservation of sperm of spotted seatrout (Cynoscion nebulosus) Gulf Res Rep. 1996;9:183–8. [Google Scholar]
- 44.Dong Q, Eudeline B, Allen JR, Tiersch TR. Factors affecting sperm motility of tetraploid pacific oysters. J Shellfish Res. 2002;21:719–23. [Google Scholar]
- 45.Tan-Fermin JD, Miura T, Adachi S, Yamauchi K. Seminal plasma composition, sperm motility and milt dilution in the Asian catfish Clarias marcrocephalus (Gunther) Aquaculture. 1999;171:323–38. [Google Scholar]
- 46.Paaben U, Paris F, Schlierenkamp H, Blum V. Sperm storage and fertilization in Xiphophorus helleri. Proc CEBAS Workshops. 1996b:17–24. [Google Scholar]
- 47.Paris F, Paassen U, Bluem V. Sperm storage of the female swordtail (Xiphophorus helleri) Verh Ges Ichthyol. 1998;1:157–65. [Google Scholar]
- 48.Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J Exp Biol. 1983;107:105–13. doi: 10.1242/jeb.107.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K. Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Biol. 1983;107:95–103. doi: 10.1242/jeb.107.1.95. [DOI] [PubMed] [Google Scholar]
- 50.Mims SD. Evaluation of activator solutions, motility duration, and short-term storage of paddlefish spermatozoa. J World Aquacult Soc. 1991;22:224–9. [Google Scholar]
- 51.Cosson MP, Billard R, Letellier L. Rise of internal Ca2+ accompanies the initiation of trout sperm motility. Cell Motility Cytoskel. 1989;14:424–34. [Google Scholar]
- 52.Thorogood J, Blackshaw A. Factors affecting the activation, motility, and cryopreservation of the spermatozoa of the yellowfin bream, Acanthopagrus australis (Guûther) Aquacult Fish Manage. 1992;23:337–44. [Google Scholar]
- 53.Ginzburg AS. Fertilization in fishes and the problem of polyspermy. Israel: Keter Press; 1972. [Google Scholar]
- 54.Suquet M, Omnes MH, Normant Y, Fauvel C. Assessment of sperm concentration and motility in turbot (Scophthalmus maximus) Aquaculture. 1992;101:177–85. [Google Scholar]
- 55.Billard R, Cosson MP. Some problems related to the assessment of sperm motility in freshwater fish. J Exp Zool. 1992;261:122–31. [Google Scholar]


