Abstract
Introduction
Pre -surgical measurement of supraspinatus muscle extensibility would be important for rotator cuff repair. The purpose of the present study was to explore the potential feasibility of a shear wave ultrasound electrograph (SWE) based method, combined with B-mode ultrasound, to non-invasively measure in vivo stiffness of supraspinatus muscle, and thus obtaining the key information on supraspinatus muscle extensibility.
Materials and Methods
Our investigation consisted of 2 steps. First, we evaluated orientation of the supraspinatus muscle fiber on cadaveric shoulders without rotator cuff tear in order to optimize the ultrasound probe positions for SWE imaging. Second, we investigated the feasibility of quantifying the normal supraspinatus muscle stiffness by SWE in vivo.
Results
The supraspinatus muscle was divided into four anatomical regions, namely anterior superficial (AS), posterior superficial (PS), anterior deep (AD) and posterior deep (PD) regions. SWE was evaluated at each of these regions. SWE stiffness on AD, AS, PD, and PS were measured as 40.0±12.4, 34.0±9.9, 32.7±12.7, 39.1±15.7 kPa, respectively.
Conclusions
SWE combined with B-Mode ultrasound image may be a feasible method to quantify local tissue stiffness of the rotator cuff muscles.
Keywords: rotator cuff, muscle, tendon, ultrasound, shear wave elastography, imaging, supraspinatus
INTRODUCTION
Rotator cuff tear is one of the most common causes of shoulder pain and dysfunction, especially in the middle-aged or elder population (Minagawa et al., 2013; Yamamoto et al., 2010). Acute tears can usually be readily mobilized and repaired; however, management becomes more challenging in the chronic setting. In the chronic condition, Safran et al. demonstrated that the detached rotator cuff becomes stiffer and requires a large passive load to bring torn tendons together to repair it (Safran et al., 2005). Because of these large tensile forces, the repaired tendon may re-tear, which is estimated to occur in 40% to 70% of cases (Bartl et al., 2012; Khazzam et al., 2012; Kim et al., 2012; Miller et al., 2011; Zumstein et al., 2008). In part, large or massive tears may be difficult to repair not only because of their size, but also because of the histological and ultrastructural changes which occur in the tendon and muscles (Matthews et al., 2006). These changes include development of fatty degeneration (Goutallier et al., 1994; Goutallier et al., 2003; Goutallier et al., 2005; Itoigawa et al., 2011), alteration of the pennation angle (Meyer et al., 2006; Zuo et al., 2012), a decreased number of sarcomeres (Firat and Turker, 2012; Tomioka et al., 2009), and fibrosis; often these changes lead to changes in stiffness of the muscle (Liu et al., 2011; Steinbacher et al., 2010). Therefore, detecting the passive stiffness of the rotator cuff muscles in patients with cuff tears is clinically important because the knowledge about the rotator cuff muscle passive stiffness can help determining the optimal surgical procedure such as anatomical repair, versus tendon grafting options, as well as predicting the prognosis such as the chance of retear and functional recovery.
Therefore, it is clinically significant to develop a non-invasive tool for acquiring the information on passive stiffness of rotator cuff muscles for the sake of better pre-surgical planning. We aim to achieve this goal by adopting a novel ultrasound based technology termed Shear Wave Elastography (SWE, which provides quantitative in vivo measurements of tissue stiffness by evaluating shear wave propagation speed, and is inherently related to tissue mechanical properties (Bercoff et al., 2004; Chen et al., 2009a; Chen et al., 2009b; Mariappan et al., 2010; Sarvazyan et al., 1998). We propose this approach, when combined with B-Mode ultrasound imaging, to quantify structural properties which will then be used to measure the passive stiffness, and consequently, the extensibility of supraspinatus muscles.
The purpose of this preliminary study was, as a very first step towards the above goal, to explore the method to measure stiffness of normal rotator cuff muscle, in particular, supraspinatus, using SWE and B-mode ultrasound. Our hypothesis was that SWE image is able to quantify stiffness of rotator cuff muscles. Our investigation consisted of 2 steps. First, we evaluated orientation of the supraspinatus muscle fiber on cadaveric shoulders without rotator cuff tear in order to optimize the ultrasound probe positions for SWE imaging. Second, we investigated the feasibility of quantifying the normal supraspinatus muscle stiffness by SWE in vivo.
MATERIALS AND METHODS
In vitro Experiments
Specimen Preparation
Three fresh-frozen shoulders with no rotator cuff tear (age 63, 78, and 59 years) were used. The frozen shoulders were thawed overnight at room temperature for experimentation. For first specimen (right shoulder), the subcutaneous soft tissues were removed except for the rotator cuff muscles, and the supraspinatus muscle was observed grossly. The other specimens (right and left shoulders) were dissected after measurement of SWE and B-mode ultrasound.
Gross anatomy
Superficial muscle fiber and intramuscular tendon on supraspinatus muscle of one specimen were observed from superior view. The scapular spine and acromion were preserved in order to properly identify the location of the area in the supraspinatus muscle to be evaluated by ultrasound. After observation of the superficial muscle, the superficial portion of the muscle was removed until the intramuscular tendon was exposed. On the other specimen s, the supraspinatus muscle was cut between anterior and posterior muscles to observe the muscle fiber on coronal section, and compared to the ultrasound images.
Orienting the Ultrasound images
The first step was to determine the proper orientation of the ultrasound probe in relation to the muscle fiber architecture. According to previous studies, the supraspinatus muscle consists of anterior and posterior regions with distinct muscle fiber architectures (Kim et al., 2010; Kim et al., 2007). Therefore, we hypothesized that the intramuscular tendon could be used as a landmark for dividing the supraspinatus muscle into two primary regions: the anterior region and the posterior region. To identify the proper orientation of the intramuscular tendon, the ultrasound probe was placed perpendicularly to skin surface of the middle of the spine of scapula, in the mediolateral direction, and between clavicle and scapula spine in the anteroposterior direction. This position was defined as Position A (Fig 1C black box). The ultrasound probe was then shifted parallel 1 cm anteriorly (Fig 1C red box) and 1 cm posteriorly (Fig 1C gray box) to investigate if intramuscular tendon was observed on those images on the anterior or posterior muscle regions or not. On the other hand, the probe was then rotated from Position A either clockwise (Fig 2A, white box) or counter -clockwise (Fig 2A, yellow box) until the muscle fiber displayed in the B-mode ultrasound screen reached its maximal length. At such position, the ultrasound probe was considered aligned with the intramuscular tendon orientation. The anterior region and the posterior region were further divided into the superficial region and the deep region bounded by intramuscular tendon, with superficial region being the one superior to the intramuscular tendon in the B-mode ultrasound image and the deep region being the one inferior to the intramuscular tendon in the B-mode ultrasound image. SWE images were then collected for each “quadrant”: that is, anterior superficial, anterior deep, posterior superficial, and posterior deep. When collecting SWE images in each quadrant, orientation of the ultrasound probe was fine tuned by inclining sagittally within ±20° until the muscle fiber length in the B-mode image of that quadrant reached maximal length. At such orientation, the ultrasound transducer was considered represent the optimal projection of the muscle fibers in that quadrant. SWE images were then collected at such final orientation.
Fig. 1.
(Blue arrow: intramuscular tendon) A: Gross anatomy (coronal section) of right supraspinatus muscle, posterior view (Yellow box: center of supraspinatus muscle, and the same region as ultrasound image of B). B: Ultrasound image on which intramuscular tendon was observed when the probe was, perpendicularly to skin, positioned on the middle of the spine of scapula in mediolateral direction, and between clavicle and scapula spine in anteroposterior direction. C: Right cadaveric shoulder, superior view. Black box means the probe positioning where intramuscular tendon was observed when the probe was, perpendicularly to skin, positioned on the middle of the spine of scapula in mediolateral direction, and between clavicle and scapula spine in anteroposterior direction (Position A). Red and gray boxes show the probe positioning 1 cm anterior and posterior from black box (Position A), respectively. D: Ultrasound image on 1 cm anterior. The intramuscular tendon disappeared. E: Ultrasound image on 1 cm posterior. The intramuscular tendon disappeared. F: When the probe was inclined by more than approximately 20° to anterior direction, or approximately 20° to posterior direction from Position A, the intramuscular tendon also vanished in all ultrasound images.
Fig. 2.
A: Right cadaveric shoulder, superior view. Black box shows the probe orientation where intramuscular tendon was observed when the probe was, perpendicularly to skin, positioned on the middle of the spine of scapula in mediolateral direction, and between clavicle and scapula spine in anteroposterior direction (Position A). The probe positioning was rotated centering around the intramuscular tendon to anterior direction (white box), or posterior direction (yellow box) from Position A. The muscle fiber orientation within each quadrant (AS or PS) was determined by rotating the ultrasound probe orientation until the muscle fiber displayed in the B-mode ultrasound screen reached its maximal length. B: Gross anatomy of right supraspinatus muscle, superior views (Blue arrow: intramuscular tendon, SSP: supraspinatus). The probe positioning of AS or PS meant anterior or posterior muscle fibers. C: Ultrasound images for anterior superficial muscle. (Red arrow: muscle fiber, Blue arrow: Intramuscular tendon) D: Ultrasound images for posterior superficial muscle. E: Ultrasound images for anterior deep muscle. F: Ultrasound images for posterior deep muscle. The muscle fibers mostly run parallel on each images and observed from origin of muscle to intramuscular tendon on allquadrants (C, D, E, F).
In vivo Experiments
Participant
Three subjects (age 37, 38, and 37 years) participated in this study. They had no history or findings of shoulder pain, and did not have rotator cuff tear on ultrasound images. The B-mode ultrasound and SWE were performed together on the subjects (two right and one left shoulders). The right supraspinatus was scanned with the arm resting on side and supported by the chair armrest. This study was approved by our institutional review board and informed consent was obtained.
Muscle Material Properties by Shear Wave Elastography
In both the in vivo and in vitro experiment, to assess the material properties of the muscle (along muscle fiber direction), shear wave speed maps were acquired with SWE on an Aixplorer® ultrasound scanner using an SL 10-2 linear array transducer (Supersonic Imagine, Aix-en-Provence, France). Alignment of the ultrasound probe with the muscle fiber orientation was achieved using the method described in the above sections. For each ultrasound measure, shear waves were generated in the supraspinatus via an unfocused ultrasound “push” beam transmitted by 16 elements of the transducer. The same transducer then detected propagation of the shear waves for 20 ms at a frame rate of 7.4 kHz within a small 2-D region of interest (ROI) to one side of the push beam. The ROI was selected mid-depth. The shear wave propagation velocity within the ROI was monitored and recorded for later analysis to calculate the shear modulus.
RESULTS
In vitro Experiments
Gross anatomy
The superficial muscle fiber consisted of anterior (AS) and posterior (PS) muscles, and pennately ran from lateral to medial in the center of intramuscular tendon (Fig 3A). The deep muscle fiber consisted of anterior (AD) and posterior (PD) muscles, and pennately ran the same as the superficial fiber (Fig 3B). However, the pennation angles of muscle fiber to intramuscular tendon were different on both anterior and posterior muscles between the superficial and deep muscles (Fig 3A, B). The intramuscular tendon was also observed on coronal section from posterior view (Fig 1A).
Fig. 3.
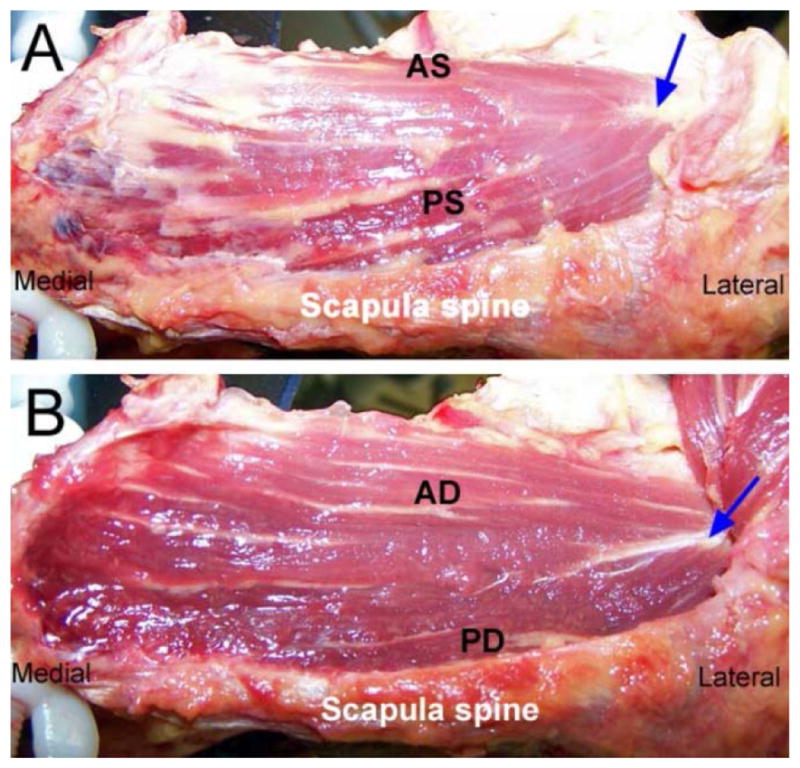
Gross anatomy of right supraspinatus muscle, superior views (Blue arrow: intramuscular tendon). A: Superficial supraspinatus muscle (AS: anterior superficial region, PS: posterior superficial region). B: Deep supraspinatus muscle (AD: anterior deep region, PD: posterior deep region).
Ultrasound images
The intramuscular tendon was identified on ultrasound image on Position A (Fig 1B). Area in this ultrasound image was grossly the same as the area inspected in coronal sectioned gross anatomy (Fig 1A, B, yellow box). When the probe positioning was deviated 1 cm anterior or posterior from Position A, the intramuscular tendon was not seen on the both images (Fig 1D, E), as expected. Furthermore, when the probe was inclined by more than approximately 20° to anterior direction, or approximately 20° to posterior direction from Position A, the intramuscular tendon also vanished in all ultrasound images (Fig 1F), suggesting that it was off the view from such scan angle.
The muscle fiber orientation within each quadrant (AS, PS, AD, and PD) was determined by inclining the ultrasound probe orientation until the muscle fiber displayed in the B-mode ultrasound screen reached its maximal length. The muscle fibers mostly ran parallel and observed from origin of muscle to intramuscular tendon on all quadrants (Fig 2C, D, E, F). On the gross anatomy, probe positioning of AS or PS meant runs of anterior or posterior muscle fibers, respectively (Fig 2A, B). The SWE stiffness of each muscle quadrant could be then measured by the Aixplorer® machine. The mean SWE stiffness on AD, AS, PD, and PS were 59.8±3.5, 56.2±5.0, 57.1±12.0, 57.0±7.4 kPa, respectively.
In vivo Experiments
Muscle Material Properties by Shear Wave Elastography
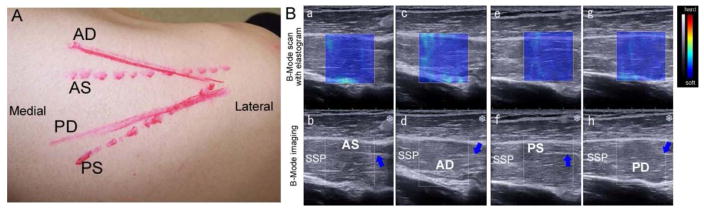
Similar to the in vitro experiment, the muscle fibers and intramuscular tendon on all quadrants (AS, PS, AD, and PD) were indentified on the B-mode ultrasound images (Fig 4b, d, f, g) on all subjects. The muscle fibers mostly ran parallel and observed from origin of muscle to intramuscular tendon on each image. The probe positioning drawn on all subject’s skins to indicate the angle of each muscle fiber was different among 4 quadrants (Fig 4A). The mean SWE stiffness of each muscle quadrant under such orientation on all subjects could be then measured by the Aixplorer® machine and reported (Fig 4B). The mean SWE stiffness on AD, AS, PD, and PS were 40.0±12.4, 34.0±9.9, 32.7±12.7, 39.1±15.7 kPa, respectively. The side-to-side comparison was not part of the purpose of this study, thus will be considered in the future.
Fig. 4.
A: The probe’s positions drawn on the skin of right shoulder B: Ultrasound imaging of human supraspinatus. B-Mode scan with overlaid elastogram (a, c, e, g) and B-Mode imaging (b, d, f, h). a, c, g, and f are same sections as b, d, f, and h, respectively. a and b are ultrasound imaging for anterior superficial muscle. c and d are ultrasound imaging for anterior deep muscle. e and f are ultrasound imaging for posterior superficial muscle. g and h are ultrasound imaging for posterior deep muscle. (AS: superficial region of anterior supraspinatus, SSP: supraspinatus, Blue arrow: intramuscular tendon, AD: deep region of anterior supraspinatus, PS: superficial region of posterior supraspinatus, PD: deep region of posterior supraspinatus).
DISCUSSION
The goal of this study was to investigate the potential of SWE imaging in the quantification of rotator cuff muscle passive stiffness. Previous studies suggested that the supraspinatus muscle consists of several regions with distinct muscle fiber architectures (Kim et al., 2010; Kim et al., 2007). Congruent with those, our gross anatomy and ultrasound images indicated that the muscle stiffness can be measured by scanning four regions, namely, of AS, AD, PS, and PD, as bounded by intramuscular tendon. In order to have adequate shear wave propagation, the ultrasound transducer needs to be parallel to the muscle fiber to be able to quantify muscle stiffness by SWE (Eby et al., 2013). Previous studies have evaluated the supraspinatus tendon and muscle stiffness using SWE (Arda et al., 2011; Plagou et al., 2011). However, a detailed probe orientation on the muscle has not been described. In the present study, within each region (quadrant), the muscle fibers ran approximately parallel to each image, making it feasible to quantify the shear stiffness along the muscle.
While our preliminary study measured the local muscle tissue stiffness in each of the four quadrants, clinically it would be even more ideal to predict the extensibility of the muscle as a whole based on the stiffness measurements of each of its subzones. Although not included in the present study, we are currently developing an algorithm to calculate the whole muscle extensibility based on summation of weighted contributions of the four quadrants. Contribution of each quadrant to the whole muscle extensibility would be a function of pennation angle, physical cross-sectional area (PCSA), average fiber length, and the SWE stiffness measurement in the quadrant. We anticipate pennation angle, PCSA and average fiber length are measurable from B-mode ultrasound images, a hypothesis to be examined in our next future study.
There are two limitations in the present study: First, as a preliminary study, its sample size was very small. Future investigations aimed at confirming the value of SWE and B-mode ultrasound in this application will be necessary with a significantly larger number of subjects. Second, this preliminary study only assessed the normal subjects instead of rotator cuff muscles with tendon rupture. Whether the pennation angle and muscle architecture of rotator cuff muscle in patients with torn tendon would be different from that of the normal subjects or not, as well as whether such difference (if any) would affect the SWE stiffness measurements, remains to be examined in future studies (Meyer et al., 2006; Zuo et al., 2012).
CONCLUSIONS
Based on the preliminary findings in the present study suggests SWE when combined with B-Mode ultrasound image is a feasible method to quantify local tissue stiffness of the rotator cuff muscles.
Acknowledgments
Contract grant sponsors: Mayo Foundation, Alumni Scholarship from Juntendo University School of Medicine. This study was partially funded by a grant awarded by NIH, R21 AR65550-01. The authors wish to acknowledge the generosity of the donors and families for their great gift to our Anatomical Bequest Program, which made the in vitro portion of this research possible.
Footnotes
All research performed at Mayo Clinic, Rochester, MN USA
CONFLICT OF INTEREST
The authors have no conflict of interest relationships to report.
References
- Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Ajr. 2011;197:532–536. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- Bartl C, Kouloumentas P, Holzapfel K, Eichhorn S, Wortler K, Imhoff A, Salzmann GM. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6:1–8. doi: 10.4103/0973-6042.94304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- Chen S, Urban MW, Pislaru C, Kinnick R, Greenleaf JF. Liver elasticity and viscosity quantification using shearwave dispersion ultrasound vibrometry (SDUV) Conf Proc IEEE Eng Med Biol Soc. 2009a;2009:2252–2255. doi: 10.1109/IEMBS.2009.5334992. [DOI] [PubMed] [Google Scholar]
- Chen S, Urban MW, Pislaru C, Kinnick R, Zheng Y, Yao A, Greenleaf JF. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans Ultrason Ferroelectr Freq Control. 2009b;56:55–62. doi: 10.1109/TUFFC.2009.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat T, Turker T. Is the long sarcomere length responsible for non-traumatic supraspinatus tendinopathy? Potential novel pathophysiology and implications for physiotherapy. Pathophysiology. 2012;19:179–183. doi: 10.1016/j.pathophys.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre-and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- Goutallier D, Postel JM, Van Driessche S, Voisin MC. Histological lesions of supraspinatus tendons in full thickness tears of the rotator cuff. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:109–113. doi: 10.1016/s0035-1040(05)84287-4. [DOI] [PubMed] [Google Scholar]
- Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011;29:861–866. doi: 10.1002/jor.21317. [DOI] [PubMed] [Google Scholar]
- Khazzam M, Kuhn JE, Mulligan E, Abboud JA, Baumgarten KM, Brophy RH, Jones GL, Miller B, Smith M, Wright RW. Magnetic resonance imaging identification of rotator cuff retears after repair: interobserver and intraobserver agreement. Am J Sports Med. 2012;40:1722–1727. doi: 10.1177/0363546512449424. [DOI] [PubMed] [Google Scholar]
- Kim S, Bleakney R, Boynton E, Ravichandiran K, Rindlisbacher T, McKee N, Agur A. Investigation of the static and dynamic musculotendinous architecture of supraspinatus. Clin Anat. 2010;23:48–55. doi: 10.1002/ca.20896. [DOI] [PubMed] [Google Scholar]
- Kim SY, Bleakney RR, Rindlisbacher T, Ravichandiran K, Rosser BWC, Boynton E. Musculotendinous architecture of pathological supraspinatus: A pilot in vivo ultrasonography study. Clin Anat. 2012;26:228–235. doi: 10.1002/ca.22065. [DOI] [PubMed] [Google Scholar]
- Kim SY, Boynton EL, Ravichandiran K, Fung LY, Bleakney R, Agur AM. Three-dimensional study of the musculotendinous architecture of supraspinatus and its functional correlations. Clin Anat. 2007;20:648–655. doi: 10.1002/ca.20469. [DOI] [PubMed] [Google Scholar]
- Liu X, Manzano G, Kim HT, Feeley BT. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29:588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23:497–511. doi: 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- Meyer D, Lajtai G, Von Rechenberg B, Pfirrmann C, Gerber C. Tendon retracts more than muscle in experimental chronic tears of the rotator cuff. J Bone Joint Surg Br. 2006;88:1533–1538. doi: 10.1302/0301-620X.88B11.17791. [DOI] [PubMed] [Google Scholar]
- Miller BS, Downie BK, Kohen RB, Kijek T, Lesniak B, Jacobson JA, Hughes RE, Carpenter JE. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39:2064–2070. doi: 10.1177/0363546511413372. [DOI] [PubMed] [Google Scholar]
- Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, Kijima H, Itoi E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop. 2013;10:8–12. doi: 10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagou A, Zoumpoulis PS, Leli D, Ruci J, Fandridis E, Gerostathopoulus N. Ultrasound Imaging and Elastography of the Rotator Cuff Muscles: Elasticity Measurements of the Supraspinatus and Infraspinatus Muscles Using Shear Wave Elastography Ultrasound. Med Biol. 2011;37:S140–S140. [Google Scholar]
- Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005;87:2662–2670. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- Steinbacher P, Tauber M, Kogler S, Stoiber W, Resch H, Sanger AM. Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue Cell. 2010;42:37–41. doi: 10.1016/j.tice.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Tomioka T, Minagawa H, Kijima H, Yamamoto N, Abe H, Maesani M, Kikuchi K, Shimada Y, Itoi E. Sarcomere length of torn rotator cuff muscle. J Shoulder Elbow Surg. 2009;18:955–959. doi: 10.1016/j.jse.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]
- Zuo J, Sano H, Itoi E. Changes in pennation angle in rotator cuff muscles with torn tendons. J Orthop Sci. 2012;17:58–63. doi: 10.1007/s00776-011-0176-6. [DOI] [PubMed] [Google Scholar]