Abstract
Allergenic pollen is produced by the flowers of a number of trees, grasses and weeds found throughout the UK. Exposure to such pollen grains can exacerbate pollen-related asthma and allergenic conditions such as allergic rhinitis (hay fever). Maps showing the location of these allergenic taxa have many applications: they can be used to provide advice on risk assessments; combined with health data to inform research on health impacts such as respiratory hospital admissions; combined with weather data to improve pollen forecasting systems; or as inputs to pollen emission models. In this study we present 1 km resolution maps of 12 taxa of trees, grass and weeds found in the UK. We have selected the main species recorded by the UK pollen network. The taxa mapped in this study were: Alnus (alder), Fraxinus (ash), Betula (birch), Corylus (hazel), Quercus (oak), Pinus (pine) and Salix (willow), Poaceae (grass), Artemisia (mugwort), Plantago (plantain), Rumex (dock, sorrels) and Urtica (nettle). We also focus on one high population centre and present maps showing local level detail around the city of London. Our results show the different geographical distributions of the 12 taxa of trees, weeds and grass, which can be used to study plants in the UK associated with allergy and allergic asthma. These maps have been produced in order to study environmental exposure and human health, although there are many possible applications. This novel method not only provides maps of many different plant types, but also at high resolution across regions of the UK, and we uniquely present 12 key plant taxa using a consistent methodology. To consider the impact on human health due to exposure of the pollen grains, it is important to consider the timing of pollen release, and its dispersal, as well as the effect on air quality, which is also discussed here.
Keywords: Aeroallergen, Allergenic pollen, Human health, Land cover, Source map, Species distribution
Graphical Abstract
Highlights
-
•
12 key allergenic vegetation types mapped across the UK at 1 km resolution
-
•
Method combines data from the atmosphere, biosphere, and anthroposphere.
-
•
Different geographical distributions of 12 trees, weeds and grass
-
•
Maps can be used to study UK plants associated with allergy and allergic asthma.
-
•
London results show local level detail around the city, relevant for human exposure.
1. Introduction
Allergenic pollen is produced by a number of trees, grasses and weeds found throughout the United Kingdom (UK). Exposure to such pollen grains can result in exacerbation of pollen-related asthma and allergenic conditions such as allergic rhinitis (pollenosis or hay fever) (Greiner et al., 2012). With the total effect of future environmental changes on pollen production and spread being highly uncertain (Osborne and Eggen, 2014), there is a need for detailed and robust pollen-related health impact information. Highly detailed maps of locations of allergenic plants in the UK, as presented here, are an important part of this as they provide the spatial detail required for impact assessments. Such maps will be highly useful for the next generation co-exposure modelling system currently under development for the UK area. This system is based on an extension of WRF-Chem model (e.g. Grell et al., 2011) and is designed for handling both chemical air pollutants (e.g. Werner et al., 2016, Werner et al., 2017) and bioaerosols (e.g. pollen and spores) at the species level (Skjøth et al., 2015a) where both chemical air pollutants and bioaerosols directly interact with and feedbacks to atmospheric physics.
The UK has one of the highest prevalence of doctor diagnosed asthma affecting around 10% of the adult population (Netuveli et al., 2005). Approximately 80% of people with asthma also have a pollen allergy (Asthma UK, 2017). The NHS Choice website states that over 10 million people have hay fever in England (NHS Choices, 2017). Physician-based diagnosis of allergic rhinitis was 13.2% (95% CI 11.6–14.9) in the UK in 2001 (Bauchau and Durham, 2004).
Detailed maps of allergenic pollen producing taxa, in combination with pollen forecasts and calendars, can help sufferers to manage their condition by reducing their exposure. Asthma and allergic rhinitis significantly reduce quality of life and have a large economic impact (Bousquet et al., 2001). As such, it is a significant environmental health issue.
Our method is novel as it not only provides maps of many different plant types, but also at high resolution across regions of the UK. These maps fill a need for detailed vegetation mapping of allergenic plant species across a whole country, to improve understanding of relationships between allergenic pollen exposure and human health outcomes. With an initial assessment of data availability and expertise on plant types and land cover, the method presented here could be duplicated for other locations around the world.
In this paper we present detailed maps of location of different taxa monitored by the UK pollen network that are associated with allergy and allergic asthma. These have been made to study environmental exposure and human health, although these new vegetation maps have many possible applications as outlined in the following section.
1.1. Purpose of this work and wider context
Vegetation maps have many applications worldwide: they can be used to provide advice on risk assessments, e.g. on invasive species (for example Csornai et al., 2011); combined with health data to inform research on health impacts such as respiratory hospital admissions caused by exposure of environmental aeroallergens (Bousquet et al., 2007, Newson et al., 2014); combined with weather data to improve pollen forecasting systems (e.g. Zink et al., 2012); or as inputs to dedicated pollen emission models (Zink et al., 2013).
The vegetation maps presented here have been developed to study human exposure to allergenic pollen, and the effect on human health. They can be combined with hospital admissions data for asthma to study the impact of different allergenic taxa in the UK. By producing detailed maps of the location of allergenic pollen producing plants for the UK it may be possible to identify the taxa that increase risk of higher hospital admissions for asthma in that particular region. This level of detail could help with the accurate measurement of health impacts as well as monitoring for climate impacts through changes in vegetation distribution, and changes to pollen allergenicity.
Vegetation mapping of plants with allergenic pollen may also help affected individuals with self-management of their allergy or asthma. Once linked with health effects, another application of these maps will be to provide advice for vegetation management practices. These practices can include the choice of tree species, sex of tree for planting, and grass cutting regimes to limit exposure to the most allergenic pollen.
Detailed maps of the location of pollen producing plants are also key to a future pollen forecasting system. Maps can be coupled with key weather variables such as wind direction and speed, precipitation, humidity and temperature, and both phenological and dispersion models, to predict the emission timing, amount (Skjøth et al., 2012) and dispersion of pollen grains, to provide more spatially and temporally resolved pollen forecasts for the UK, compared to the existing regression based approaches for trees (Adams-Groom et al., 2002), grasses (e.g. Smith and Emberlin, 2005, Smith and Emberlin, 2006) and weeds.
1.2. Health impacts of allergenic pollen
Exposure to allergenic pollen from certain trees, grasses and weeds is associated with a range of health effects, including allergic rhinitis (hay fever), exacerbation of asthma in susceptible individuals, and atopic dermatitis (eczema) (Cecchi, 2012). These pollen grains are produced in the flowers of angiosperm plants (e.g. most deciduous trees, weeds and grasses), and in the pollen cones of gymnosperm plants (e.g. conifer trees), and the timing of their release varies depending on the species and environmental conditions. Susceptible individuals can be sensitive to pollen from one or more different type of trees, grasses or weeds. Estimates of the levels of allergies towards environmental aeroallergens (pollen, spores and cat/dog/house dust mites) among patients range typically from up to 30%−50% in Europe (Newson et al., 2014), where the largest fractions of sensitisations towards specific pollen typically are against grasses and trees of the Betulaceae family with clinically relevant sensitisation rates for grasses exceeding 50% for both UK and Denmark (e.g. Burbach et al., 2009).
1.3. Background to allergenic vegetation mapping
Here we outline methods used in the literature to produce spatial maps detailing the location of vegetation that emits allergenic pollen, often referred to as pollen source maps, or inventories (Skjøth et al., 2012). ‘Bottom up’ and ‘top down’ approaches to creating pollen source location maps are outlined in Skjøth et al. (2012). ‘Bottom up’ techniques start with the location and amount of the pollutant — they combine land cover maps with regional scale statistics, for example as used in Skjøth et al. (2008). Availability of these regional scale statistics is what limits this approach, for example statistical information on the regional abundance of weeds and certain taxa is largely not available. Many sources exist which refer to the presence or absence of a particular plant type or species, but not their abundance, and these rarely cover a whole area, for example the size of the UK. ‘Top down’ inventories start with a measured quantity and work backwards using models to calculate the distribution. They use station based pollen monitoring observations along with land cover maps, for example Skjøth et al. (2012) which uses data from the European Aeroallergen Network (EAN). Geographic and temporal coverage of monitoring stations are however limiting factors of ‘top down’ methodologies. Existing pollen mapping includes tree source maps at 50 km × 50 km resolution (Skjøth et al., 2008) and Ambrosia (ragweed) source inventories for different European locations (Karrer et al., 2015, Skjøth et al., 2010, Thibaudon et al., 2014). Statistical mapping of trees over Europe at 1 km × 1 km resolution is presented in Brus et al. (2011). Skjøth et al. (2015b) used back-trajectories of pollen observations to create footprint areas of Alnus (alder) and Betula (birch) in Worcester. Remote sensing can also be used such as Skjøth et al. (2013) who created a grass pollen inventory in Aarhus (Denmark) using GIS and remote sensing.
The quality of the mapping is dependent on resolution and taxa specificity of available input datasets. High resolution inputs can provide a challenge for handling large volumes of data, and lower resolution inputs may be easier to obtain, but can miss important spatial detail.
In this paper we present a novel set of maps of key plants associated with allergy and allergic asthma in the UK. They are provided at higher resolution coverage than currently available elsewhere in the literature, use a state-of-the-art tree dataset (Bluesky, National Tree Map, 2015) for highly accurate tree locations, and uniquely present 12 key plant taxa (monitored by the UK pollen network), using a consistent methodology.
2. Materials and methods
2.1. Selection of allergenic vegetation
Tree and weed taxa and grasses were selected which were monitored by the UK pollen network (run by the Met Office (Met Office, 2017b) ), where the majority are considered medium to highly allergenic, and for which datasets could be produced covering Great Britain. As these include either species i.e. Acer pseudoplatanus (sycamore) or genera i.e. Alnus (alder), for clarity in the following text we will refer to all as ‘taxa’.
A summary of the availability of tree taxa information present in the Forestry Commission's National Forest Inventory (Forestry Commission, Great Britain, 2011), and the Trees in Towns II report (Britt and Johnston, 2008) — both of which were used in this study is given in Table 1. The seven tree taxa which had data availability that were selected to be studied in this paper, are indicated in bold font in Table 1. Note that the seven selected tree taxa include those which were reported to be most allergenic by the review by de Weger et al. (2012) — Corylus (hazel), Alnus (alder), Betula (birch) and Quercus (oak) — excluding Platanus (plane) and those allergenic but not prevalent in the UK, Olea (olive) and Cupressaceae (cypress).
Table 1.
Summary of the availability of data for tree genera or species — collectively referred to in the text as taxa — from the Forestry Commission National Forest Inventory (NFI) report (Forestry Commission, Great Britain, 2011) and the Trees in Towns II report (Britt and Johnston, 2008). The tree taxa mapped in this study were chosen as those which were monitored as part of the UK pollen network and appeared in both the NFI and Trees in Towns II data, highlighted in the table in bold font. ‘*’ denotes where specific species are recorded: Picea (spruce) — sitchensis (sitka spruce), abies (Norway spruce); Pinus (pine) — sylvestris (Scots pine), nigra (Corsican pine), contorta (lodgepole pine).
| Tree genera or |
Common |
Broadleaf |
Pollen |
NFI |
Trees in |
|---|---|---|---|---|---|
| species — taxa | name | or conifer | network | Towns II | |
| Acer pseudoplatanus | Sycamore | Broadleaf | — | ||
| Alnus | Alder | Broadleaf | |||
| Betula | Birch | Broadleaf | |||
| Castanea | Chestnut | Broadleaf | — | — | |
| Corylusavellana | Common hazel | Broadleaf | |||
| Crataegus | Hawthorn | Broadleaf | — | ||
| Eucalyptus | Gum | Broadleaf | — | — | |
| Fagus | Beech | Broadleaf | — | ||
| Fraxinus | Ash | Broadleaf | |||
| Larix | Larch | Conifer | — | — | |
| Picea* | Spruce | Conifer | — | ||
| Pinus* | Pine | Conifer | |||
| Platanus | Plane | Broadleaf | — | ||
| Populus | Poplar | Broadleaf | — | ||
| Pseudotsuga | Douglas fir | Conifer | — | — | |
| Quercus | Oak | Broadleaf | |||
| Salix | Willow | Broadleaf | |||
| Taxus | Yew | Conifer | — | ||
| Tilia | Lime | Broadleaf | — | ||
| Ulmus | Elm | Broadleaf | — |
All grasses, Poaceae, were considered together as the current UK pollen monitoring observations are unable to delineate between the various species, also very little is known about which species of grass are most allergenic in the UK. Note that, in Europe, grass pollen is the most important pollen allergen due to both its large distribution in Europe (Skjøth et al., 2012) and its recorded high sensitisation rates among patients (Burbach et al., 2009).
The final group of taxa looked at fall into the category of ‘weeds'. The National Pollen and Aerobiological Research Unit (University of Worcester) identifies five plants in this category, all of which are monitored in the UK as part of the pollen network: Rumex (dock, sorrels), Artemisia (mugwort), Urtica (nettle), Plantago (plantain) and Brassica napus (oil seed rape). Oil seed rape is planted in the UK for agriculture, and due to a lack of detailed mapping information on the spatial abundance of it in the UK, we omitted this plant from this study.
Summarising, the 12 selected taxa from the UK pollen network mapped in this study were as follows. Trees: Alnus (alder), Fraxinus (ash), Betula (birch), Corylus (hazel), Quercus (oak), Pinus (pine) and Salix (willow). Grass: Poaceae (bulk grass, not species specific). Weeds: Artemisia (mugwort), Plantago (plantain), Rumex (dock, sorrels) and Urtica (nettle).
2.2. Grass mapping methodology
The Centre for Ecology and Hydrology (CEH) Land Cover Map 2007 (hereafter LCM 2007) is a 25 m resolution raster map of Great Britain which shows land cover using 23 different classifications (Morton et al., 2011). This was used to create the grass map in this study. Land cover classes where grasses are predominantly found were selected. The six grassland classes used are shown in Table 2. Note that some other categories — namely, Arable, Urban and Suburban (classes 3, 22 and 23 respectively) are likely to contain some grass, but due to a lack of detailed spatial information these areas have not been included. These were not selected for this study. See Centre for Ecology & Hydrology, UK (2011) for full description of the land cover classes and their habitats. Raster masks of the selected categories were made and applied using a script written in the programming language Python (Python Software Foundation, 2017). This resulted in a 25 m raster map showing only grassland areas. The grass map was then regridded to 1 km resolution and converted to percentage cover, using the spatial analyst toolbox in software ArcGIS Desktop version 10 (ESRI, 2017).
Table 2.
LCM 2007 classes selected as grassland, along with their classification number in the LCM 2007 dataset.
| LCM 2007 class | Class number |
|---|---|
| Improved grassland | 4 |
| Rough grassland | 5 |
| Neutral grassland | 6 |
| Calcareous grassland | 7 |
| Acid grassland | 8 |
| Heather grassland | 11 |
2.3. Weed mapping methodology
Statistical information on the regional abundance of allergenic weeds is not consistently available across the UK. Hence, we have developed a method based on that used by Karrer et al., 2015, Kaye and Burgess, 2013, Skjøth et al., 2010, Thibaudon et al., 2014 and Thibaudon et al. (2014) which uses land cover map classes and input of expert plant knowledge to filter for habitat selection. Here we used expert elicitation to determine the suitability of a habitat (land cover class) for each weed. The type of expert elicitation used was ‘behavioural aggregation’ — group decision making — where experts pooled knowledge (O’Hagan et al., 2006) to reach a consensus.
Three experts with documented specialist knowledge on the selected weeds were chosen and each provided their individual independent assessment of ‘habitat suitability’ and percentage cover of the weeds in each land class category of the LCM 2007 dataset. For each taxon, each expert scored each land class 0,1 or 2 — 0 indicated that the habitat was not suitable for this taxon, 1 that the habitat was mildly suitable, and 2 that it was a largely suitable habitat. Using this ‘habitat suitability’ indicator as a guide, the experts then individually and independently assigned an estimate of ‘fractional cover’ for that taxon in that habitat. For example, for a habitat suitability of ‘2’ (largely suitable habitat) of Plantago in land class ‘urban’, each expert estimated a typical area coverage in that land area of Plantago. Following a similar approach to the ‘Delphi method’ (O’Hagan et al., 2006), each expert first provided their own independent assessment. This was then supplied to all the other experts in the group, and the experts were given the chance to update their assessment. The experts were also asked to make any relevant notes regarding land classes and these taxa, in order to capture any key aspects to take into account with the mapping. After having the independent assessments from each expert, the experts then collaboratively reached a final value for the percentage cover estimates (‘behavioural aggregation’). The experts collectively discussed each weed taxon and came to a joint agreement on value for each percentage cover in each of the land classes. Values of percentage cover for typical 25 m × 25 m grid box of each land class ranged between: mugwort 0–0.1%; plantain 0–5.0%; Rumex 0–2.0%; nettle 0–1.0%.
A 25 m percentage cover map was created for each of the four weeds, by using ‘reclassify’ tool in ArcGIS to apply taxa coverage to different land classifications. The 25 m map was then aggregated to 1 km using the same technique used for grass, as described above.
Note that there are differing numbers of taxon in each genus and that each of these will vary in terms of their relative abundance by land class. This will not overly affect the outcomes as most of the species are either rare or not present in the UK. For example, there are 57 species of Artemisia in the north temperate regions (Grewling et al., 2012), but only A. vulgaris (mugwort), A. campestris (field wormwood) and A. absinthium (wormwood) are widespread throughout Europe. The online Atlas of the British and Irish flora lists 10 different Artemisia species, but only wormwood and mugwort have widespread records. A consequence is that the vast majority of pollen related to a specific genera will originate from a few common species.
2.4. Tree mapping methodology
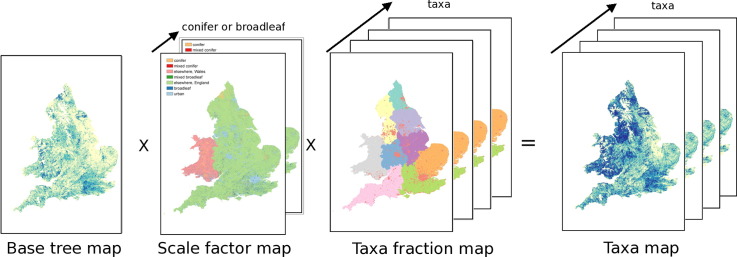
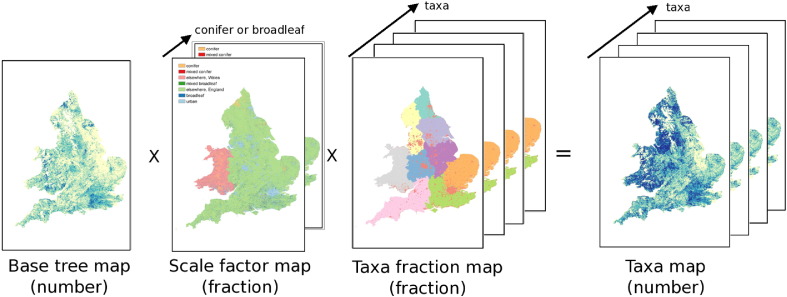
A high resolution tree map was used to build a base map of all tree locations. Scaling factors were created which accounted for the proportion of broadleaf and conifer trees at different locations. Finally, regional taxa fractions were applied. The final taxa maps were created by combining all of these data. This combination can be described in the following way to calculate the number of trees of a given taxon in a grid box:
| (1) |
where Tall is the total number of all trees in a grid box, αtaxon is the fraction of that taxon found in that grid box (a function of region/urban area), and β is the scaling factor for broadleaf/conifer abundance in a given land type (a function of region and also different for broadleaf/conifer taxa).
The method used to assign each of these terms is described in detail below.
2.4.1. Base map, Tall
The Bluesky International, National Tree Map (Bluesky, National Tree Map (2015), hereafter NTM) is a dataset built from high resolution aerial photography and remote sensing, and contains the location, height and canopy/crown extents for every single tree which is over 3 m in height. NTM does not contain any species-level or taxon information for the trees. It covers England and Wales in extent. The dataset is provided as a vector GIS dataset, with height and area attributes for each tree. The number of trees (count) in 25 m × 25 m grid cells was calculated from the NTM dataset using a Python script, with the grid aligned to that of the Forestry Commission dataset. This map formed a ‘base map’ which showed the locations of all trees (over 3 m), to which taxon fractions would be applied. This base map provides the value of Tall in Eq. (1), the total number of all trees in a given grid box.
2.4.2. Taxon fractions, αtaxon
Regional measures of taxa totals in Great Britain are presented by the Forestry Commission for broadleaved trees (Brewer, 2013) and coniferous trees (Forestry Commission, Great Britain, 2012). To calculate taxa fractions in forests for the broadleaved taxa, data from Number of trees by principal broadleaved species for National Forest Inventory regionsBrewer (2013, Table A.7) were used to calculate the proportion of each taxon as a fraction of total number of ‘all broadleaves'. A measure of the number of trees per taxon per region was not available for coniferous taxa, and so taxon fractions for pine were instead calculated using the timber volume measure. Data from Standing coniferous timber volume by principal species for National Forest Inventory regionsForestry Commission, Great Britain (2012, Table 9) were used to calculate the proportion of pine as fraction of total volume of ‘all conifers' in forests. In urban areas, tree taxa fractions were calculated using Trees in Towns II report (Britt and Johnston, 2008, Fig. 2.18), a large study of urban trees carried out in towns with populations over 3000. The distribution of taxa in towns differed sufficiently from the forest data that we used these taxa fractions in urban areas. Note that Corylus (hazel) was not included in this Fig. 2.18, and so a fraction for hazel was obtained from Britt and Johnston (2008, Table 2.8), other fractions calculated from Fig. 2.18. The remaining areas — those which were neither forest, nor towns — were grouped as the ‘elsewhere’ category, and in these areas the Forestry Commission taxa fractions, as calculated for forests were used. We considered Countryside Survey data (Maskell et al., 2013) which contained some data on some of the key allergenic taxa in non-forested countryside areas. However, it did not have sufficient information for us to calculate taxa fractions1. Table 3 presents a summary of the sources of taxa fraction data for broadleaf and conifer trees in different land types (forests, urban, elsewhere). Also shown is spatial variation (final column) of these taxa data — which is regional for all but the urban data.
Table 3.
Source of taxa fraction data for broadleaf and conifer trees in different land types (forests, urban, elsewhere). The final column outlines the spatial variation of these taxa data. ‘Forestry Commission 2013’ denotes data from Brewer (2013, Table A.7) — fractions were calculated per taxon as fraction of number of ‘all broadleaves'. ‘Forestry Commission 2012’ denotes data from Forestry Commission, Great Britain (2012, Table 9) — fractions were calculated per taxon, as a fraction of total volume of ‘all conifers'. In urban areas, tree taxa fractions were calculated using Trees in Towns II report Britt and Johnston (2008, Fig. 2.18). N.B. Corylus (hazel) was not included in Fig. 2.18, and so a taxa fraction for hazel was obtained from Britt and Johnston (2008, Table 2.8). Taxa fractions calculated in urban areas are given as a fraction of total number of all trees.
| Tree type | Location | Taxa fraction source | Spatial variation |
|---|---|---|---|
| Broadleaf | Forests | Forestry Commission 2013 | Regional |
| Conifer | Forests | Forestry Commission 2012 | Regional |
| All | Urban | Trees in Towns II | None |
| Broadleaf | Elsewhere | Forestry Commission 2013 | Regional |
| Conifer | Elsewhere | Forestry Commission 2012 | Regional |
The values of taxon fraction described above provide the value of αtaxon in Eq. (1), the fraction of a given taxon in a grid box.
2.4.3. Scaling factor, β
A ‘scaling factor’ was necessary to account for the fact that many of the taxa fractions were available as a proportion of either all broadleaves or all coniferous taxa, and not as a proportion of total number of trees. Differing proportions of broadleaf and conifer trees are found in different land types and forest types, and so these classifications were used to scale the tree map in different areas, depending on whether the tree type being mapped was classed as broadleaved (alder, ash, birch, hazel, oak and willow) or coniferous (pine). The scaling for broadleaved or coniferous trees in different land types is outlined below.
Forest boundaries from Forestry Commission National Forest Inventory (NFI) map (25 m × 25 m resolution; Forestry Commission, Great Britain, 2011) were used to define forested areas. Four NFI interpreted forest types were used: broadleaf; mixed mainly broadleaf; mixed mainly conifer; conifer. Table 4 shows the NFI interpreted forest types, the range of broadleaf and conifer trees expected in each (as defined by Forestry Commission, Great Britain, 2011) and the scale factors, β used for each, for broadleaf and conifer trees.
Table 4.
National Forest Inventory interpreted forest types and range of areas covered by broadleaf and conifer trees in these categories (‘broadleaf cover’, ‘conifer cover’). Note that forest type ‘mixed (b)’ denotes a forest with mixture of broadleaf and conifer trees, but with majority broadleaf. Similarly ‘mixed (c)’ contains mixed trees, mainly conifer. Also shown is the scale factor β assigned to each forest/land type, and value of this scale factor for broadleaf and conifer trees (final two columns). The taxa fraction in urban areas is calculated as a proportion of all trees, and so no scaling factor is needed (i.e. a multiplicative scaling factor of value 1 was used). Land types ‘elsewhere (E)’ and ‘elsewhere (W)’ denote whether the ‘elsewhere’ (non-forest, non-urban) area was in England or in Wales respectively, as differing broadleaf/conifer proportions were used.
| Land type | Broadleaf cover | Conifer cover | Scale factor | Broadleaf | Conifer |
|---|---|---|---|---|---|
| Forest: | |||||
| Broadleaf | >80% | <20% | βb | 0.9 | 0.1 |
| Mixed (b) | 50%−80% | 20%−50% | βmb | 0.65 | 0.35 |
| Mixed (c) | 20%−50% | 50%−80% | βmc | 0.35 | 0.65 |
| Conifer | <20% | >80% | βc | 0.1 | 0.9 |
| Urban | − | − | βu | 1 | 1 |
| Elsewhere (E) | 71% | 29% | βe | 0.71 | 0.29 |
| Elsewhere (W) | 48% | 52% | βe | 0.48 | 0.52 |
LCM 2007 ‘urban’ and ‘suburban’ classes were used to define urban areas. They were selected to be continuous areas ≥50 ha in size, with populations ≥3000 (selected using Office of National Statistics (Office of National Statistics, 2017) population dataset), not defined as forest in the NFI forestry dataset, and not a thin motorway or airport type infrastructure. Because the Trees In Towns II report used for taxa data included urban parks (for example Regent Park, London) these were included in the urban category here, by including any areas which were completely surrounded by urban/suburban land classes. In Trees In Towns II, each taxa is given as a proportion of all trees, and so no scaling factor is needed (i.e. a multiplicative scaling factor of value 1 was used), as shown in Table 4.
The remaining areas — those which were neither forest, nor towns — were grouped as the ‘elsewhere’ category. A calculation of broadleaf and conifer proportions was carried out, using data from Forestry Commission, Great Britain (2011, Table 3). Values of total area (ha) of broadleaf and conifer trees were used and a relative proportion of each calculated from this. Because of the regional differences seen in the forestry data, the highest resolution data available were used for this — in this case England and Wales (rather than GB as a whole). The result was 71% broadleaf and 29% conifer in England, and 48% broadleaf and 52% conifer in Wales. These values were used as the scaling factor, β for ‘elsewhere’ areas, as shown in the last two rows of Table 4.
2.4.4. Combining to create final maps
For each of the individual tree taxa, the base map, scaling factor and taxa fractions were combined as illustrated in Fig. 1. Note that our tree maps cover England and Wales only because of the data availability of Bluesky NTM. This figure demonstrates the spatial variation in each of the values of the components of Eq. (1) — with the scale factor being a function of land type (i.e. type of forest, and variation in ‘elsewhere’ category in England and Wales) and the proportions of broadleaf/conifers expected there, and the taxa fraction being a function of region and urban areas. The base tree map has a lot of spatial structure, and is in units of number of trees per grid box, the same units as the resulting taxon map. These maps were then regridded to 1 km ×1 km resolution, for comparison with other data and be more manageable in size.
Fig. 1.
Diagram illustrating method used to create tree taxa maps.
2.5. Alternative datasets and areas
In this study we used datasets which are specific to the UK, or parts of it. In order to apply our methodology to different geographical areas, one must identify suitable input datasets. We recommend that one assesses the relevant merits of land use datasets (land classifications) in the desired area of study. Things to consider when selecting a land use dataset would be accuracy, age, resolution and coverage of the dataset, as well as any specific merits/caveats of each dataset which might relate to the specific type of vegetation being studied. Some examples of large land use datasets are Corine (Corine, 2017, 100 m resolution, covering most of Europe;), ESA Landcover map (ESA, 2017, 300 m resolution, global coverage), IGBP (2017), and land cover data from satellite observations such as SPOT VEGETATION (Spot Vegetation, 2017) and MODIS Vegetation Continuous Fields (2017). Similarly, one must assess the availability and quality of forestry or tree information, and also that of taxon information provided at a regional (or higher) level. One such dataset which is available over Europe is the European Forest Inventory (EFI) dataset (Schelhaas et al., 2006) which has taxa level maps at 1 km resolution. In this study we considered the EFI but found that the EFI total tree map (all taxa) differed significantly from the Bluesky International NTM dataset on tree location, which we believed to be a more accurate indication of tree location due to its methodology. For this reason we used just the NTM dataset in further analysis. Thus caution would be urged if using EFI in place of a higher resolution and more accurate tree dataset (e.g. up-to-date aerial or satellite based dataset with a common methodology across the dataset), as it will affect the quality of the resulting maps.
We note that without access to such a high resolution dataset such as the Bluesky International NTM dataset on tree location, this will have an impact on the quality of the resulting maps. One must consider changing the resolution of the final maps with respect to the quality of the input maps, and not provide higher resolution maps than the data reasonably allow. This judgement must be made by those familiar with land cover and vegetation datasets and their strengths and weaknesses, and considering what the final maps will be used for.
For this reason we have not produced tree taxon maps in Scotland where the Bluesky International NTM maps were not available. This decision was made because we wanted to use a consistent methodology and produce consistent output such that the provided taxon maps could be used for human health studies.
3. Results
Here we present 1 km gridded maps of the 12 selected taxa.
3.1. Grass map
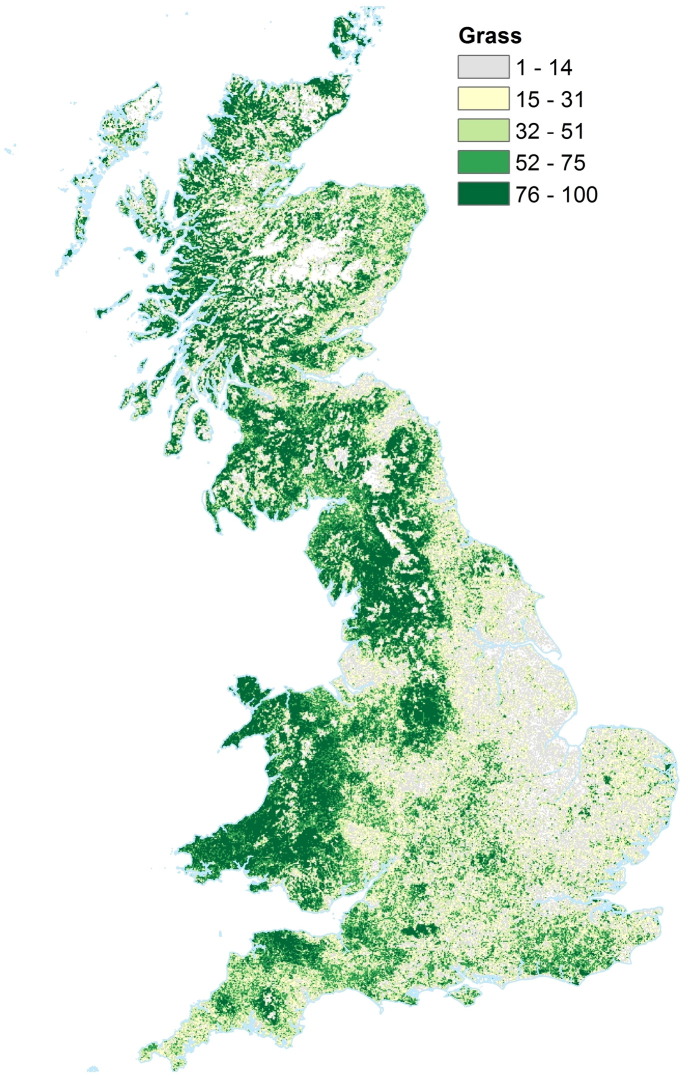
Fig. 2 shows the resulting Poaceae (grass) location map (percentage coverage), gridded at 1 km resolution, for Great Britain. The colourbar shows equal sized quantiles. An overall pattern of higher percentage cover of grass in the northern and western regions of Great Britain, and lower overall cover to the south east can be seen. High mountainous regions in Scotland show lower levels of grass cover than their surrounding lower lying areas. The eastern central area of England has the lowest percentage grass coverage, while Wales and north west England, and western Scotland have the highest density of grass coverage. Note that these maps show the vegetation location of grass, and not of the pollen concentration, we discuss how different factors affect the spread of pollen grains in Section 4.
Fig. 2.
Map showing location of Poaceae (grass), in units of percentage cover per 1 km ×1 km grid box, across Great Britain. Colour ranges have been set to 5 equal sized quantiles (i.e. each contains 20% of data). Image Crown Copyright, 2016, The Met Office. Based on digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH).
3.2. Weed maps
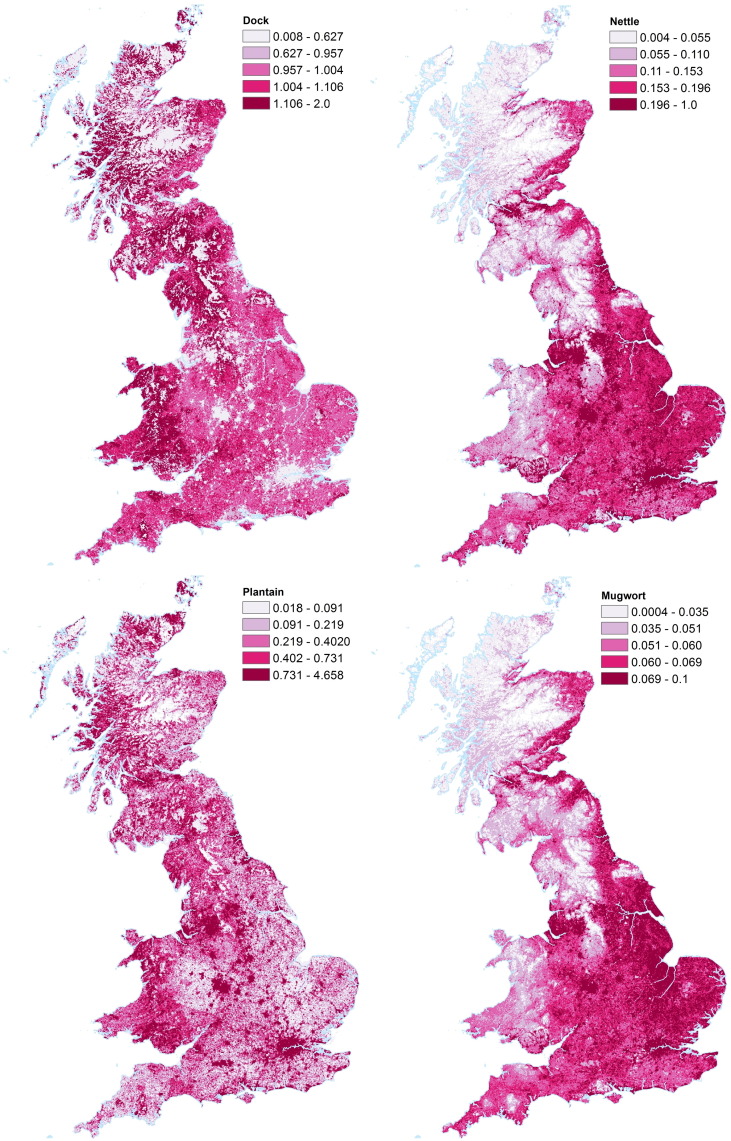
Fig. 3 shows the location maps (percentage coverage) for the four selected weed taxa, gridded at 1 km resolution, for Great Britain with quantile based colourbars, allowing a comparison of the relative locations of each taxon across Great Britain. Note that the total quantities are different for each weed taxon. The same figure without quantile-based colourbars are shown in the Supplementary materials, Fig. A.8. Dock (Rumex, includes dock and sorrels) is fairly evenly distributed across Great Britain, with no overall north/south or east/west trend. It is not abundant in urban areas, or in high altitudes, and these areas can be seen in white and grey on the map. Nettle distribution displays a generally lower value of percentage cover than dock, and shows a north/west-south/east trend, with higher abundance in southern and eastern Great Britain, largely a result of lower cover at higher altitude. Unlike dock, nettle has its highest coverage in urban areas. Plantain is relatively evenly distributed across the whole of Great Britain, with the highest areas of coverage in urban areas. Mugwort has much lower general levels of coverage, reaching a maximum of only 0.1%. Mugwort has a similar distribution to nettle, being found mostly in the south and eastern areas of Great Britain, and in urban areas.
Fig. 3.
Maps showing location of four allergenic weeds in Great Britain, in units of percentage cover per 1 km ×1 km grid box. Top left: percentage cover of Rumex (dock, sorrels); Top right: percentage cover of Urtica (nettle); Bottom left: percentage cover of Plantago (plantain); Bottom right: percentage cover of Artemisia (mugwort). Colour ranges have been set to 5 equal sized quantiles (i.e. each contains 20% of data). Note that absolute values for each taxon are different, see ranges on each colourbar. Images Crown Copyright, 2016, The Met Office. Based on digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH).
3.3. Tree maps
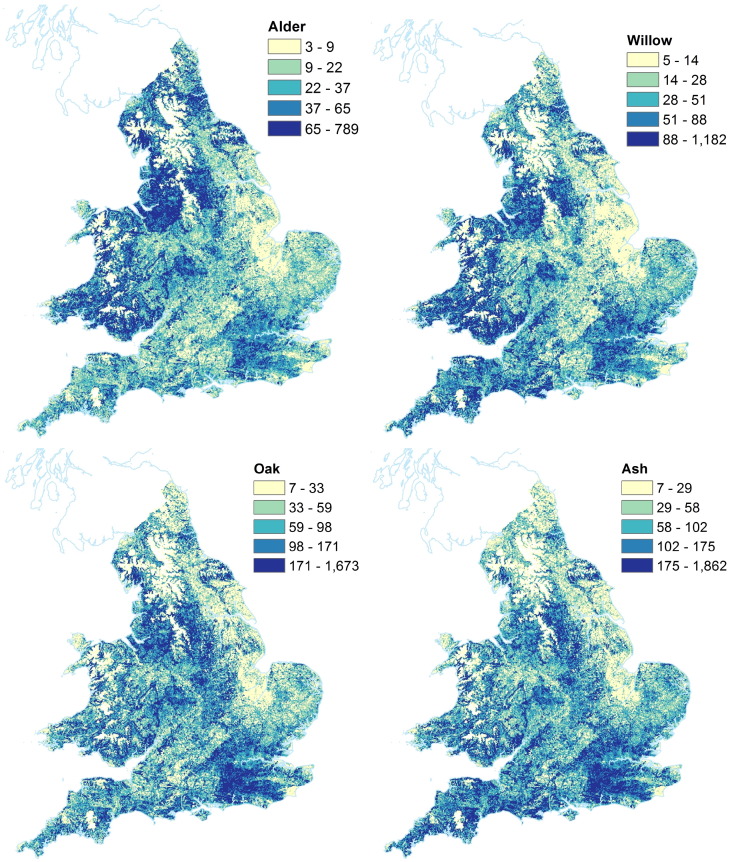
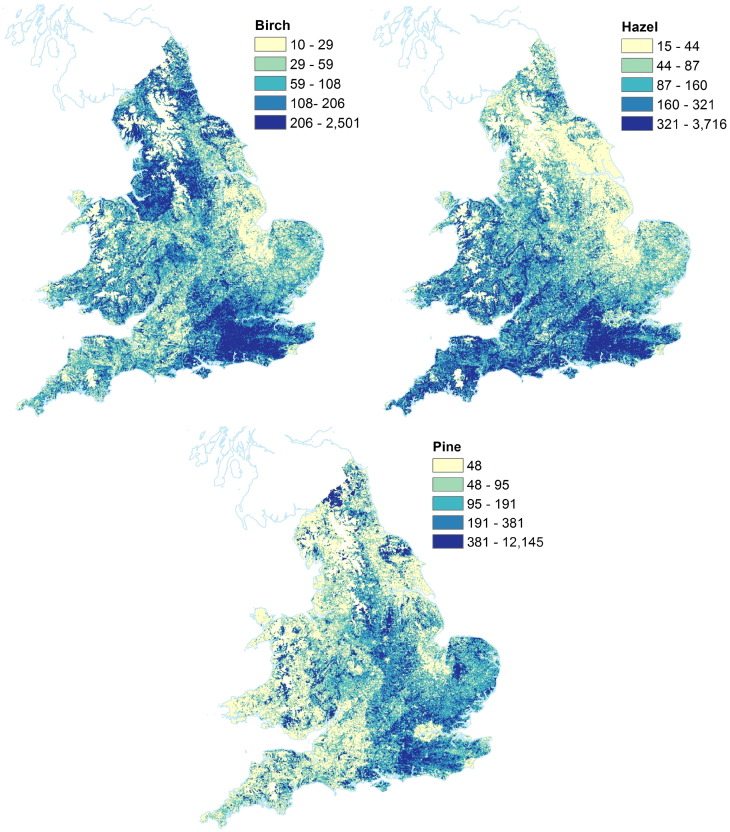
Tree taxon plots for the 7 tree taxa are shown in Figs. 4 and 5. Units are in number of trees per 1 km ×1 km grid square. These figures have quantile based colourbars, allowing a comparison of the relative locations of each taxon in England and Wales. Note that the total quantities are different for each tree taxon. The same figures without quantile-based colourbars are shown in the Supplementary materials Figs. A.9 and A.10. Of the broadleaf trees, alder and willow have the lowest number of trees, followed by oak and ash. Birch and hazel both have higher abundance in terms of number of trees. Pine is the only conifer tree mapped in this study, and has much higher number of trees than the broadleaves mapped here. In England, pine is the main conifer taxon found, and it is very abundant. The pine map shows a different pattern of distribution to that of the broadleaves, notably a lack of pine in greater London is seen, and less pine trees in Wales and western England. The highest concentrations of hazel are found in the south east of England, and the lowest values in the eastern area of England. Birch also has peaks in the south east of England. The distributions of oak and ash are very similar to each other; while both the alder and willow maps show a peak in western areas of England and Wales, as well as the south east of England. The results here are not directly comparable with those in Skjøth et al. (2008), because our maps are of much higher resolution, and include all trees — not only those in forests.
Fig. 4.
Maps showing location of tree taxa in England and Wales. Units are number of trees per 1 km ×1 km grid square. Colour ranges have been set to 5 equal sized quantiles (i.e. each contains 20% of data). Note that absolute values for each taxon are different, see ranges on each colourbar. Tree locations of Top left Alnus (alder); Top right Salix (willow); Bottom left Quercus (oak); Bottom right Fraxinus (ash). Images Crown Copyright, 2016, The Met Office. Based on data licensed from National Tree Map, Copyright Bluesky International Limited, 2016; digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH); and contains, or is based on, information supplied by the Forestry Commission.
Fig. 5.
Maps showing location of tree taxa in England and Wales. Units are number of trees per 1 km ×1 km grid square. Colour ranges have been set to 5 equal sized quantiles (i.e. each contains 20% of data). Note that absolute values for each taxon are different, see ranges on each colourbar. Tree locations of Top left Betula (birch); Top right Corylus (hazel); Bottom Pinus (pine). Images Crown Copyright, 2016, The Met Office. Based on data licensed from National Tree Map, Copyright Bluesky International Limited, 2016; digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH); and contains, or is based on, information supplied by the Forestry Commission.
3.4. Focus on high population centre
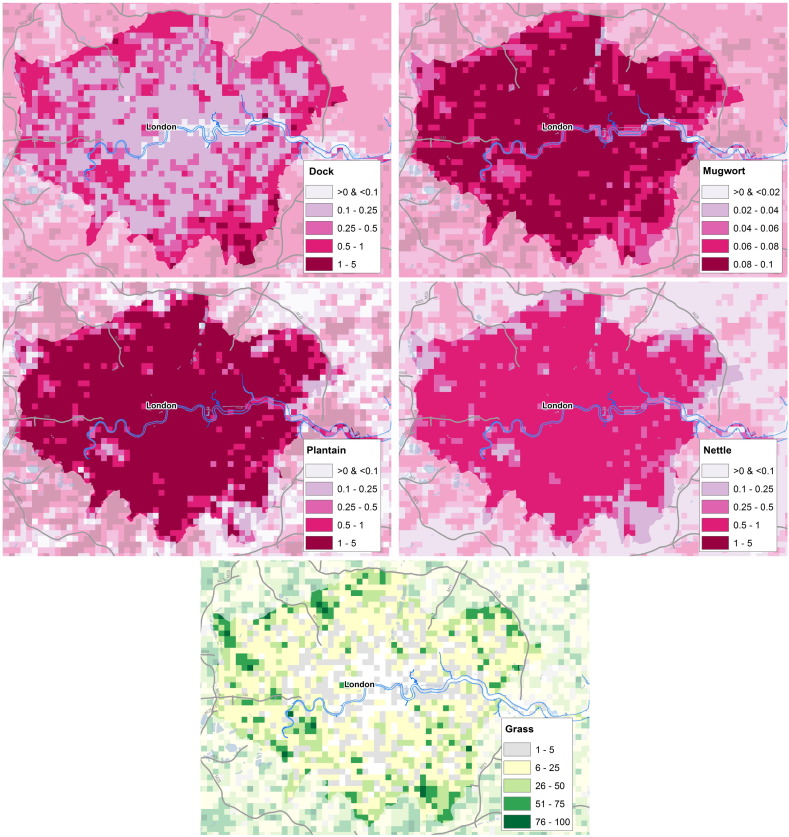
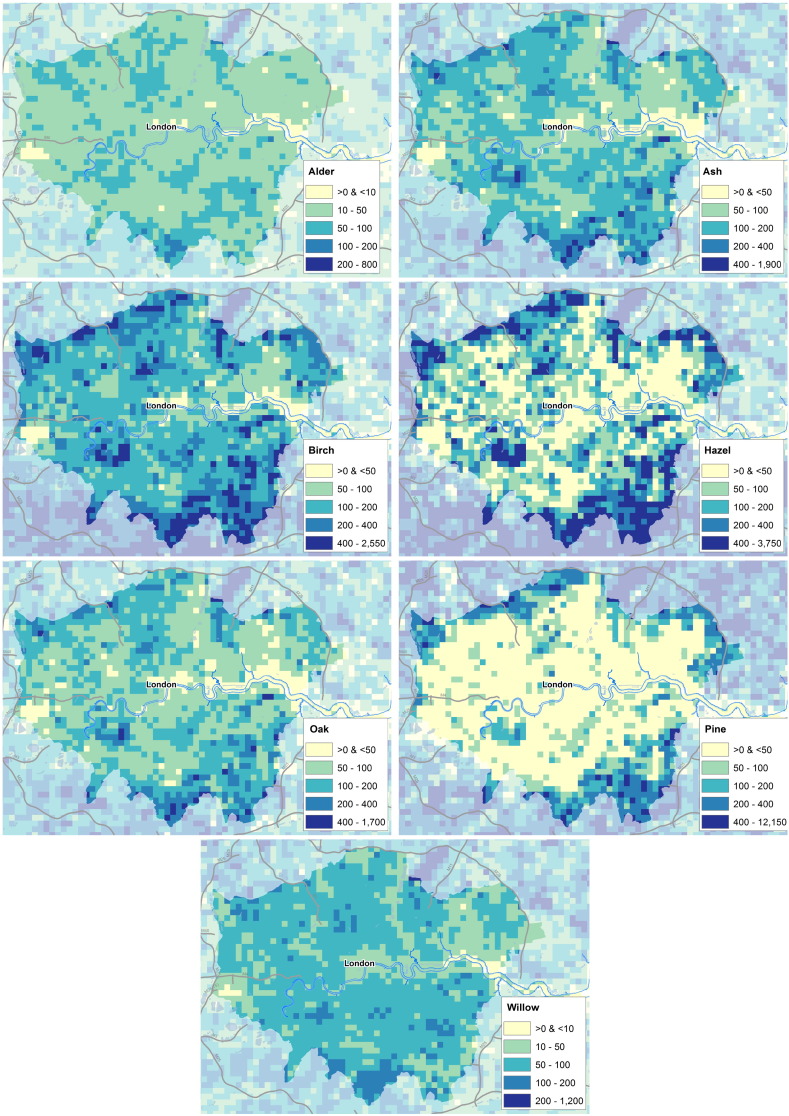
Maps showing the allergenic plant locations across areas of high population are valuable because the large number of people living in these cities means that many are exposed to pollen from these plants. Fig. 6 shows the percentage cover of the four weed taxa, and grass, across greater London. Fig. 7 presents the percentage cover of the 7 allergenic trees in this same area.
Fig. 6.
Maps showing location of weed taxa and grass in greater London, UK. Top left: percentage cover of Rumex (dock, sorrels); Top right: percentage cover of Artemisia (mugwort); Centre left: percentage cover of Plantago (plantain); Centre right: percentage cover of Urtica (nettle); Bottom: percentage cover of Poaceae (grass). Images Crown Copyright, 2016, The Met Office. Based on digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH); and contains OS data Crown copyright (2016).
Fig. 7.
Maps showing location of tree taxa in greater London, UK. Units are number of trees per 1 km ×1 km grid square. Top left: percentage cover of Alnus (alder); Top right: percentage cover of Fraxinus (ash); Second row, left: percentage cover of Betula (birch); right: percentage cover of Corylus (hazel); Third row, left: percentage cover of Quercus (oak); right: percentage cover of Pinus (pine); Bottom: percentage cover of Salix (willow). Images Crown Copyright, 2016, The Met Office. Based on data licensed from National Tree Map, Copyright Bluesky International Limited, 2016; digital spatial data licensed from the Centre for Ecology & Hydrology, copyright NERC (CEH); contains, or is based on, information supplied by the Forestry Commission; and contains OS data Crown copyright (2016).
The weed location maps in London show that dock is not nearly as abundant in the central area, while the other weeds are more highly concentrated in the city. The grass map shows that there is less grass in central London, with mixed values in the surrounding area. The tree maps show more variation between taxa. Notably pine stands out as there is very little pine found in London, and while much higher values are seen in the surrounding area. Willow and alder, are similar again, with fairly even levels of number of these taxa across the area. In the pine, hazel, birch and oak maps, a peak can be seen south of the river just west of the centre of the map. This is a large green space in the city (Richmond Park), containing many trees, and it shows up in these maps as an area with higher density of these taxa.
4. Discussion
These maps of key allergenic tree, grass and weed taxa detail where these plants are located in the UK. They show the different geographical distributions of the 12 taxa of trees, weeds and grass, and can be used to study plants in the UK that are associated with allergy and allergic asthma. These have been made to study environmental exposure and human health, although these new vegetation maps have many possible applications. Because the taxa mapped here are all monitored by the UK pollen network, this would also allow further studies to compare to observational pollen count data.
This novel method not only provides maps of many different plant types, but also at high resolution across England and Wales, and Great Britain, and uniquely presents 12 key plant taxa using a consistent methodology.
We note that certain taxa are considered more important aeroallergens than others mapped in this study. We highlight that the review by de Weger et al. (2012) found the following of our selected taxa highly allergenic: Corylus (hazel), Alnus (alder), Betula (birch), Platanus (plane) and Quercus (oak), Poaceae (bulk grass, not species specific), Artemisia (mugwort). Of the remaining taxa that we have mapped, we note that Pinus (pine) is not considered to be allergenic. Neither is Urtica (nettle), although it has pollen which cannot be distinguished microscopically from Parietaria judaica (de Weger et al., 2012) which is a potential aeroallergen and is frequently recorded in southern Britain. Thus care would need to be taken if comparing these maps to observational pollen counts that Parietaria judaica pollen grains were not being misclassified as Urtica (nettle). Plantago (plantain) is thought to be only a minor cause of hay fever in Europe and has some cross-reactivity with grass pollen (de Weger et al., 2012). Similarly, Rumex (dock, sorrels) is not considered to be important aeroallergen. Salix (willow) is both wind pollinated and insect pollinated, it has minimal importance as an aeroallergen, with the airborne pollen loads often low.
We also note that some sites in the pollen network does also keep a watch out for other pollen taxa/species including Ambrosia (ragweed), due to it being very highly allergenic (Dahl et al., 1999). However, it is not a native plant nor currently commonly found in the UK, although it is highly invasive and there is a risk it may spread to the UK in the future with changes to the climate and land use (Hamaoui-Laguel et al., 2015, Pashley et al., 2015, Smith et al., 2013, Ziska et al., 2011). As with other pollen, ragweed pollen has been shown to be transported long distances in the wind, and so areas with high concentrations of ragweed pollen in the air are more extensive than those where most ragweed plants are located (de Weger et al., 2016, Grewling et al., 2016, Prank et al., 2013).
These maps fill a need for spatially detailed vegetation mapping of allergenic plants across a country, the first maps of their kind in the UK. Moreover, the method presented here could be duplicated for other locations around the world, with an initial assessment of data availability and expertise on plant types and land cover.
4.1. Uncertainty and comparisons with other results
Validation of inventories is generally not possible as they must include all data before they can be considered inventories. Common practice is therefore to discuss the main elements of uncertainty and compare the inventory results with relevant data including previous inventories (e.g. Oderbolz et al., 2013, Simpson et al., 1999, Skjøth et al., 2008). We have followed the same procedure by including these aspects in the following two main subsections:
4.1.1. Uncertainty with respect to the input data
The Land Cover Map 2007 dataset, and the National Forestry Inventory (NFI) dataset are both widely used environmental datasets, appearing in many other peer reviewed studies. Nevertheless they contain some limitations that will affect some of the species. The LCM2007 dataset is a remote sensing product with 20–30 m resolution used as input from several satellites such as Landsat and SPOT. A consequence is smaller units will not be mapped. This is however of minor importance for most of the weeds as we have applied an ecosystem approach combined with expert estimates to estimate the abundance of all weeds. However it will have some impact on Artemisia as this species is often found along roads and rivers (e.g. Essl et al., 2009). This weed will therefore have a larger uncertainty than the others. LCM will also have an impact on the mapping of grass areas in urban areas as grass ecosystems often are associated with road sides (Skjøth et al., 2013), that cannot be mapped using the LCM2007. Furthermore, it is known that grasses are also found in croplands under rotation (e.g. timothy and rye grass) that were not included in this study. These areas are found most abundantly in the eastern parts of England, where the mapping suggests to contain low amounts of grass areas. The Trees in Towns II dataset was used for urban taxa fractions and is the result of a very thorough study of urban trees across many towns and cities. However the information was only available as one nation-wide statistical data set and public data for London suggests, that not all tree species are uniformly distributed within the towns. However a UK study comparing the UK towns Torbay, Wrexham, Glasgow and Edinburgh (Rumble et al., 2015) suggests that tree species distribution within the towns is relatively similar, most likely caused by a similar planting practice and land use type and, to a smaller degree, climate. This indicates that urban data in this study are relatively robust, while in-town variations as seen over London are more uncertain. Finally, the Bluesky NTM datasets high resolution (individual tree level resolution) and mapping methodology means that we have confidence in the gridded tree count we created from it. Bluesky quote that “Independent verification shows that NTM identifies at least 95% of tree canopy”. This suggests that the loss of tree information is below 5% nationally as well as within each 1 km grid cell. This accuracy is much higher than other more coarse data satellite based landcover products (e.g. LCM2007, Corine Land Cover (CLC2000) or Globcover), that have shown to vary with with up to 50% in tree cover and sometimes contain a direct misplacement of the tree resources (Skjøth et al., 2015b), CLC2000, which is much lower. Hazel is in this respect a special case. Hazel is often found as as a bush or shrub in urban gardens and as an important part in hedgerows. Neither of these sources can be expected to be mapped by the Bluesky data set due to the 3 m limitation. Despite this limitation, the application of the Bluesky data set has added a unique accuracy to the tree resource, the urban areas in particular as the urban tree resource has never been mapped before within an entire country.
4.1.2. Uncertainty with respect to species information and inventory method
The regional-level species information is what is likely to cause the largest uncertainties in our maps, here divided into 13 different regions. These large geographical regions are not uniform in their distribution of species, but the information is provided at this coarse scale, and as such this limits the quality of the species-level information within these regions. Although the high-resolution tree database does mean that the locations of trees within these regions is highly accurate, the exact distribution of the species on a fine scale is less certain. Previous inventories of tree species information over the UK included up to 65 regions (Skjøth et al., 2008) showing that distribution of important species like birch is highly uneven in the UK with hotspots found in a few regions in South England (Surrey), central England (west Midlands) and North England (e.g Cumbria), respectively. A consequence is that some regions in our inventory will then have a lower quantity of birches than reality, while other regions will have a large quantity than reality. However comparing with the maps in Skjøth et al. (2008) this seems to be mainly an issue for birch causing additional uncertainties with more than a factor of two, while oak, and alders have a much more uniform distribution, thereby not being affected by this uncertainty. Outside the main forests oak and ash trees are known to completely dominate the small woodlands. A consequence as that our mapping will underestimate the abundance of these two species in forest sparse regions such as the eastern parts of England, while the more forest dense regions will not be affected. With respect to total numbers within all of UK it has previously been estimated that this error is only going to have a minor importance for oak (Skjøth et al., 2015b), while the impact on ash remains to be investigated. The use of national specialist in assessing weed abundance has with success been used for ragweed throughout Europe (Karrer et al., 2015, Thibaudon et al., 2014). Here this approach has been expanded to cover several specialists, each with their independent assessment. The main uncertainty in this element is likely to be the number of specialists, where a larger number of specialists could provide a more detailed picture. This is also related to the input data, as abundance of some species are related to land use (e.g. nettle and Artemisia) such as fertilization. The LCM2007 does not include land use but only land cover. Including land use could therefore improve the information for weeds and at the same time potentially also improve the information on grasses.
The maps over London for alder, ash, birch, hazel, oak, pine and willow (Fig. 7) appear to be almost 100% spatially correlated. This is related to the fact that the two main data sets for the urban mapping are the urban tree statistics (where there is only one set of nation-wide numbers) and GPS coordinates for trees that do not distinguish between broadleaves/conifers or or individual tree species. A comparison with the public road trees data set of London that contain 716 406 tree locations provides the following information (alder=10 325, ash=40 870, birch=34 839, hazel=5597, oak=36 853, pine=6399, willow=9102, other=572 421), which provides a different overall distribution than Fig. 7 (e.g. oak being more abundant than birch). This suggests that the urban maps for individual cities or towns should be treated with caution while the overall national distribution according to Rumble et al. (2015) must be assumed to be alright. Improvements in this area could be obtained with nation-wide high resolution satellite products that can distinguish between broadleaved and conifers and with town specific tree species information. Some of the allergenic species found in this inventory (e.g. hazel and mugwort) have never been mapped before. Comparison with previous studies is therefore not possible. However a comparison of the mapping of oak, alder, birch and ash with studies by Skjøth et al. (2015b) and Maskell et al. (2013) suggests that the patterns found throughout UK in this study are similar to previous studies. This suggests that hazel, despite the limitations on not mapping hedgerows and shrubs, will have similar quality as the other trees, thereby being unique not only for UK but the first of its kind in Europe.
4.2. Relevance to public health
The maps presented here do not provide a forecast of pollen in the air at any one time, or human exposure, but do provide the most likely locations of grass, tree and weed taxa that release allergenic pollen in Great Britain. The allergenic taxa maps could provide information to local authorities and healthcare practitioners. They could be a useful risk assessment tool to inform treatment or self-management of patients with long-term health conditions caused by pollen allergy.
Hay fever and pollen-related asthma are only invoked by airborne pollen from allergenic plant taxa. To consider the impact on human health due to exposure of the pollen grains, one must consider the timing of the pollen release, and its dispersal. Along with the potential impact of climate change, air quality, changing allergenicity of pollen, and possible mitigation options and their impact on public health, we discuss these important considerations in the following sections.
4.2.1. Timing of release of pollen grains
A summary of the timing of peak pollen emissions (month of peak emissions) and emission strength categorised by peak pollen count (number of grains per day) are given in Table 5. Results were calculated as averages of the day of the year of peak emissions (presented by month) and peak pollen count (presented as categories) for each of the species/taxa and across 9 sites in the UK pollen sampling network for the period 2006–2015. The categories for peak pollen count (in units of number of grains per day) are as follows: Low = 0–10; Low-Med = 10–50; Med = 50–100; Med-High = 100–300; High = 300–500. The sites span a wide spatial distribution (from south to north - Plymouth, Isle of Wight, London Islington, Cardiff, Worcester, Cambridge, Leicester, Belfast and Invergowrie). As there were many missing data, the years and sites used for these calculations are listed for each species/taxa. Note that for Corylus avellana and Alnus only the Worcester data were used as this was the only site with data available before March, when pollen emissions for these species are likely in many locations. Note that sensitivity tests on a range of different time periods have been made and the same general trends were evident.
Table 5.
A summary of the timing of peak pollen emissions for each of the species/taxa and across 9 sites in the UK pollen sampling network for the period 2006–2015. Month of peak emissions gives the timing of peak pollen emissions; while emission strength is categorised* by peak pollen count (number of grains per day). As there were many missing data, the years and sites used for these calculations are listed for each species/taxa. The sites span a wide spatial distribution (from south to north - PlymouthP, Isle of WightIoW, London IslingtonLI, CardiffCf, WorcesterW, CambridgeCb, LeicesterL, BelfastB, InvergowrieIg). *Based on peak daily pollen count (in units of number of grains per day): Low = 0–10, Low-Med = 10–50, Med = 50–100, Med-High = 100–300, High = 300–500.
| Taxa or species | Common name | Years and sites with data available | Month of peak emissions | Emissions strength* |
|---|---|---|---|---|
| Corylus avellana | Common hazel | 2006–12W | February | Med |
| Alnus | Alder | 2006–12W | February/March | Med-High |
| Betula | Birch | 2006–15P,IoW,Cf,Ig | April | High |
| 2006–12W | ||||
| 2006–11LI | ||||
| 2006, 2008, 2011–15L | ||||
| 2006–8, 2012B | ||||
| 2006–14Cb | ||||
| Fraxinus | Ash | 2006-15P,IoW,Cf,Ig | April | Med-High |
| 2006–12W | ||||
| 2006–11LI | ||||
| 2006, 2008, 2011–15L | ||||
| 2006–9, 2012B | ||||
| 2006–14Cb | ||||
| Salix | Willow | 2006–15P,IoW,Cf,Ig | April | Low-Med |
| 2006–12W | ||||
| 2006–11LI | ||||
| 2008, 2011–15L | ||||
| 2006–9, 2012B | ||||
| 2006–14Cb | ||||
| Pinus | Pine | 2008L | May | Med-High |
| Quercus | Oak | 2006–15P,IoW,Cf,Ig | May | Med-High |
| 2006–12W | ||||
| 2006–11LI | ||||
| 2006, 2008, 2011–15L | ||||
| 2006–9, 2011–12B | ||||
| 2006–14Cb | ||||
| Poaceae | Grass | 2006-15P,IoW,Cf,Ig,W | June | Med-High |
| 2006–11LI | ||||
| 2006, 2008–15L | ||||
| 2006–9, 2011–12B | ||||
| 2006–14Cb | ||||
| Rumex | Dock, sorrel | 2008L | June | Low-Med |
| Urtica | Nettle | 2006–15P,IoW,Cf,Ig | June/July | High |
| 2006–12W | ||||
| 2006–11LI | ||||
| 2006, 2008, 2011–15L | ||||
| 2006–9, 2011–12B | ||||
| 2006–14Cb | ||||
| Plantago | Plantain | 2008L | June | Low |
| Artemisia | Mugwort | 2006–15P,IoW | July | Low |
| 2009, 2011–15Cf | ||||
| 2007, 2009, 2015Ig | ||||
| 2006–12W | ||||
| 2006–11LI | ||||
| 2006, 2008, 2011–15L | ||||
| 2006–7, 2009, 2011B | ||||
| 2006–14Cb |
Pollen grains contain the male reproductive cells of seed plants, and the timing of their development and release varies depending on the taxon and environmental conditions, particularly meteorology (Emberlin et al., 2007). The production of pollen is dependent not only on the current meteorological conditions (including day length, temperature, precipitation, and wind speed/direction), but also on the conditions and water availability experienced in the year prior during which pollen is formed (Emberlin et al., 2007). Land management is also critical, for example if fields are cut or grazed by animals then the potential to release pollen is significantly affected. Any changes in these conditions affect the phenology of the tree and thus the timing of the onset of pollen release, the total volume of pollen produced, and the length of the flowering season (Dahl et al., 2012). Typically in the UK, the pollen season for tree taxa ranges from January to April for early flowering taxa such as alder and hazel, to mid-March to end of July for the later taxa, including oak and pine. Grass flowering occurs generally between May and early-September, coincident with many taxa of weeds with allergenic pollen, see Worcester University (2017). Depending on the taxon, pollen grains are dispersed by wind, animals or water.
4.2.2. Dispersion of pollen grains
Dispersion of pollen grains once they are released from a plant is dependent on many factors, predominantly meteorological. Land management is also important, for example hay cutting can shed substantial amounts of pollen in an acute manner. Pollen can be transported large distances from its source (Sofiev et al., 2012), with the main factors in their dispersion being wind speed and direction, as well as precipitation (which brings down the pollen to the ground). The distance that a pollen grain travels depends on the taxon of plant, as each of the pollen grains are different sizes and shapes and so have different aerodynamic properties; and also on the release height of the grain (for example tree pollen are released higher from the ground and are more likely to be transported long-range). Pollen may travel some distance, and the concentration of pollen (particles per m3) is not just a localised phenomenon; it varies on a fairly broad geographical scale. Pollen grain counts have been found to correlate across distances of 20 km (Erbas et al., 2007) and 41 km (Pashley et al., 2009), with the potential for pollen to travel much further (Skjøth et al., 2007), indeed across the globe (Sofiev et al., 2012).
4.2.3. Effect of climate change
The local effect of climate change on pollen production, release timing, transport and deposition is highly complex, and its impact on pollen allergies is very uncertain (Osborne and Eggen, 2014). There is some evidence that climate change may result in earlier seasonal appearance of respiratory symptoms and longer duration of exposure to pollen (Fitter and Fitter, 2002, Vardoulakis and Heaviside, 2012). Across the Northern Hemisphere, early onset of spring has been measured, with some tree taxa releasing pollen earlier (Beggs, 2004, Emberlin et al., 2002, Emberlin et al., 1997, Frei, 1998) and cases of lengthened pollen season (Ziska et al., 2011). Further warming of global temperature and increases in atmospheric CO2 concentration may lead to great pollen release through increased plant productivity, unless the plants are limited by other factors such as water stress. Failure to frost means that the pollen season may be prolonged (Gezon et al., 2016). Climate change may also affect the allergenicity of pollen for some taxa (Cecchi et al., 2010). Furthermore, the effect of climate change on the frequency or severity of thunderstorms, and thus the health impact of thunderstorm asthma (Elliot et al., 2013) (where it is thought pollen plays a role) under a future climate is very uncertain.
4.2.4. Interaction with air quality
People who live in urban areas have been shown to be more affected by pollen allergies (asthma and allergic rhinitis) than those who live in rural areas (D’Amato et al., 2007, Ehrenstein et al., 2000, Riedler et al., 2001). Urban environments, with high levels of vehicle emissions have been shown to coincide with increased pollen-induced respiratory allergies. While it has been known for some time that exposure to air pollution prior to pollen exposure can exacerbate symptoms and lower the threshold of pollen required to trigger symptoms in allergy sufferers (Emberlin, 1998, Molfino et al., 1991), further research is required to characterise the interaction between chemical air pollutants and pollen. For example, to fully understand and quantify the effect of exposure to both allergenic pollen and pollutants, the effect on both the allergenicity (such as increased allergenicity of pollen which had been exposed to NO2 found by Cuinica et al. (2013) ) and volume of pollen grains released under increased air pollution must be determined. The health impacts of all these factors, in high co-exposure areas need to be considered. Co-exposure of pollen and air pollutants (namely NO2, SO2 and particulate matter PM2.5 and PM10 and ozone) is currently an active area of research (Mücke et al., 2014, Ørby et al., 2015); allergens from birch pollen have been found bound to particulate matter, PM10 which means the pollen allergen can travel into the lower respiratory tract (Suring et al., 2016). Vardoulakis et al. (2015) discuss how a range of health risks from indoor pollutants, including pollen, might change under a changing climate. When considering the public health relevance of these taxa maps, it is important to note the interaction between pollen and air quality, as well as the spatial variability of air pollution (e.g. see Defra/Met Office air quality maps (Defra, 2017, Met Office, 2017a)).
4.2.5. Changing allergenicity
The allergen content of pollen has also been shown to vary throughout the season and across regions. Buters et al. (2015) found that grass pollen allergen release (potency) varied significantly, and that differences were found per location in Europe, and time in the pollen season. They concluded that although pollen count is a proxy for exposure, it is not a measure of the actual allergens in the air.
4.2.6. Mitigation and adaptation
To mitigate the health effects of pollen from allergenic trees in urban areas, adaptation measures have been suggested. Policies to oversee planting of allergenic taxa in urban areas, e.g. Platanus (London plane), and the use of an allergenicity scale in the US (Seitz and Francisco Escobedo, 2009) to select suitable taxa for urban planting have been proposed. Cecchi et al., 2010, D’Amato, 2011 recommend planting non-allergenic trees such as Palmaceae (palm) and Ulmaceae (elm), avoiding Cupressaceae (cyprus), Betulaceae (birch) and Oleaceae (olive). Cryptomeria japonica (Sugi, or Japanese cedars) were planted extensively in Japan, Asia and North America after the second world war. These highly allergenic trees have had a large health effect on populations (Okuda, 2003, Yamada et al., 2014). Furthermore, the current practice of the planting of solely male trees in urban areas in order to reduce street litter from seeds and fruit increases the total pollen load, and so consideration of reduction of pollen exposure through female tree planting could potentially mitigate effects. The services and disservices that a particular tree taxon and canopy design may provide must be considered by local decision makers (Salmond et al., 2016).
Other vegetation management schemes such as grass cutting regimes where grass is cut before it flowers could be used in areas where these maps show that there is a high coverage of grass close to high population centres. It should be noted, however, that if plants are routinely managed to maintain vegetative phases of growth and restrict flowering there will be consequent negative impacts on biodiversity.
5. Conclusions
In this paper we present a methodology to produce location maps of selected trees, grass and weeds, to study plant taxa in the UK that are associated with allergy and allergic asthma. The taxa and species used in this study are all monitored as part of the UK pollen monitoring network. These have been made in order to study environmental exposure and human health, although these new vegetation maps have many possible applications.
The novel method used in this study uniquely maps 12 key plant taxa using a consistent, reproducible methodology, covering more plant types, and higher spatial resolution across England and Wales, and Great Britain, than currently available elsewhere.
To create these maps, several different observational and modelled datasets were combined. These include land use datasets and tree taxa databases including those from the Forestry Commission and the National Tree Map from Bluesky International. A framework was designed to combine these datasets allowing integration of existing UK land use and vegetation datasets with the taxa data. We presented 1 km resolution maps of 12 taxa of trees, grass and weeds found in the UK, and plots showing local level detail around the city of London.
The next step of this work is to combine these taxa maps with health data including emergency hospital admissions for asthma to study the impact of different taxa in the UK. The maps could also be combined with population data in a risk assessment framework. This will provide valuable information on which taxa in the UK have the most significant health impact for asthma. Furthermore, in the future these maps could also form part of a UK pollen forecast model, to provide detailed taxon-specific, localised information to the public.
Acknowledgements
Funding was provided by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Environmental Change and Health at the London School of Hygiene and Tropical Medicine in partnership with Public Health England, and in collaboration with the University of Exeter, University College London, and the Met Office. Additional financial support was provided by the Joint DECC/Defra Met Office Hadley Centre Climate Programme (GA01101). McInnes and Skjøth have both been directly supported by NERC since March 2016, grant ID: NE/N002431/1, (Acronym PollerGEN and Skjøth by European Commission since 2014, Project ID CIG631745, Acronym SUPREME). Observed pollen data for Table 5 were collected by the following sites and we thank all the individual collectors at each site for this: Belfast, Cambridge, Cardiff, Invergowrie, Isle of Wight, Leicester, London Islington, Plymouth and Worcester. We also thank the Midlands Asthma and Allergy Research Association (MAARA) as they support the collection of aerobiological data at Leicester; and the Met Office who have operated the UK pollen network since 2011.
The authors thank: Phil Bentley for contribution to scripting in python for tree and grass mapping analysis, and big data handling; Neil Kaye for contribution to tree mapping work and expertise in ArcGIS; Nick Morgan and Nick Dobson for their help with ArcGIS and data management; Yolanda Clewlow head of health research and services at the Met Office, for managing the UK pollen network and establishing relationships with external health colleagues; Ben Ditchburn (Forestry Commission) for initial discussions on tree mapping and forestry datasets. Furthermore, the authors warmly thank the anonymous reviewer for their constructive comments which have improved the paper.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Editor: D. Barcelo
Footnotes
Although the Countryside Survey had estimates of areas covered by the above taxa, and upper and lower confidence limits on those, it does not provide, as the Trees in Towns did, either an ‘everything else’ category for trees beyond the ‘principal species', nor does it give the total amount of coverage of all trees, so it is not possible to calculate accurate taxa fractions.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2017.04.136.
Appendix A. Supplementary data
Supplementary figures.
References
- Adams-Groom B., Emberlin J., Corden J., Millington W., Mullins J. Predicting the start of the birch pollen season at London, Derby and Cardiff, United Kingdom, using a multiple regression model, based on data from 1987 to 1997. Aerobiologia. 2002;18(2):117–123. [Google Scholar]
- Asthma U.K. 2017. Asthma UK, last accessed 06/03/2017.https://www.asthma.org.uk/advice/triggers/pollen/ [Google Scholar]
- Bauchau V., Durham S. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir. J. 2004;24(5):758–764. doi: 10.1183/09031936.04.00013904. http://erj.ersjournals.com/content/24/5/758 [DOI] [PubMed] [Google Scholar]
- Beggs P.J. Impacts of climate change on aeroallergens: past and future. Clin. Exp. Allergy. 2004;34(10):1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- Bluesky, National Tree Map . 2015. Bluesky National Tree Map.http://www.bluesky-world.com/#!national-tree-map/c1pqz Accessed: 25/01/2016. [Google Scholar]
- Bousquet J., Van Cauwenberge P., Khaltaev N., Aria Workshop Group. World Health Orgnisation Allergic rhinitis and its impact on asthma. J. Allergy Clin. Immunol. 2001;108(Suppl.5):147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- Bousquet P.-J., Chinn S., Janson C., Kogevinas M., Burney P., Jarvis D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62(3):301–309. doi: 10.1111/j.1398-9995.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- Brewer A. Tech. rep. Forestry Commission, Great Britain; 2013. NFI Preliminary Report NFI Preliminary Estimates of Quantities of Broadleaved species in British Woodlands, with Special Focus on Ash. [Google Scholar]
- Britt C., Johnston M. Research for Amenity Trees. Department for Communities and Local Government; 2008. Trees in Towns II, A New Survey of Urban Trees in England and their Condition and Management. [Google Scholar]
- Brus D.J., Hengeveld G.M., Walvoort D.J.J., Goedhart P.W., Heidema a. H., Nabuurs G.J., Gunia K. Statistical mapping of tree species over Europe. Eur. J. For. Res. 2011;131(1):145–157. http://link.springer.com/10.1007/s10342-011-0513-5 Apr. [Google Scholar]
- Burbach G.J., Heinzerling L.M., Edenharter G., Bachert C., Bindslev-Jensen C., Bonini S., Bousquet J., Bousquet-Rouanet L., Bousquet P.J., Bresciani M., Bruno A., Canonica G.W., Darsow U., Demoly P., Durham S., Fokkens W.J., Giavi S., Gjomarkaj M., Gramiccioni C., Haahtela T., Kowalski M.L., Magyar P., Muraközi G., Orosz M., Papadopoulos N.G., Röhnelt C., Stingl G., Todo-Bom A., Von Mutius E., Wiesner A., Wöhrl S., Zuberbier T. GA2LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64(10):1507–1515. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- Buters J., Prank M., Sofiev M., Pusch G., Albertini R., Annesi-Maesano I., Antunes C., Behrendt H., Berger U., Brandao R., Celenk S., Galan C., Grewling L., Jackowiak B., Kennedy R., Rantio-Lehtimaki A., Reese G., Sauliene I., Smith M., Thibaudon M., Weber B., Cecchi L. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season. J. Allergy Clin. Immunol. 2015;136(1):87–95. doi: 10.1016/j.jaci.2015.01.049. http://www.sciencedirect.com/science/article/pii/S0091674915004121 e6. [DOI] [PubMed] [Google Scholar]
- Cecchi L. Introduction. In: Sofiev M., Bergmann K.-C., editors. Allergenic pollen: a review of the production, release, distribution and health impacts. Springer; 2012. pp. 1–7. [Google Scholar]
- Cecchi L., D’Amato G., Ayres J.G., Galan C., Forastiere F., Forsberg B., Gerritsen J., Nunes C., Behrendt H., Akdis C., Dahl R., Annesi-Maesano I. Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy. 2010;65(9):1073–1081. doi: 10.1111/j.1398-9995.2010.02423.x. [DOI] [PubMed] [Google Scholar]
- Centre for Ecology & Hydrology, UK . Tech. rep. NERC/Centre for Ecology & Hydrology; 2011. Land Cover Map 2007 Dataset Documentation, Countryside Survey Technical Report. July. [Google Scholar]
- Corine . 2017. Corine, last accessed 06/03/2017.http://land.copernicus.eu/pan-european/corine-land-cover [Google Scholar]
- Csornai G., Mikus G., Nádor G., Hubik I., László I., Suba Z. The first seven years of the remote sensing based ragweed monitoring and control system. EARSeL eProceedings. 2011;10(2):110–118. [Google Scholar]
- Cuinica L.G., Abreu I., Esteves da Silva J. Effect of air pollutant NO2 on Betula pendula, Ostrya carpinifolia and Carpinus betulus pollen fertility and human allergenicity. Environ. Pollut. (Barking, Essex : 1987) 2013;186C(2):50–55. doi: 10.1016/j.envpol.2013.12.001. http://www.ncbi.nlm.nih.gov/pubmed/24361564 Dec. [DOI] [PubMed] [Google Scholar]
- Dahl Å., Galan C., Hajkova L., Pauling A., Sikoparija B., Smith M., Vokou D. The onset, course and intensity of the pollen season. In: Sofiev M., Bergmann K.-C., editors. Allergenic Pollen: A Review of the Production, Release, Distribution and Health Impacts. Springer; 2012. pp. 29–70. Ch. 3. [Google Scholar]
- Dahl Å., Strandhede S.-O., Wihl J.-Å. Ragweed - An allergy risk in Sweden? Aerobiologia. 1999;15(4):293–297. [Google Scholar]
- D’Amato G. Effects of climatic changes and urban air pollution on the rising trends of respiratory allergy and asthma. Multidiscip. Respir. Med. 2011;6(1):1–10. doi: 10.1186/2049-6958-6-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G., Cecchi L., Bonini S., Nunes C., Annesi-Maesano I., Behrendt H., Liccardi G., Popov T., Van Cauwenberge P. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62(9):976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- de Weger L., Bergmann K.-C., Rantio-Lehtimäki A., Dahl Å., Buters J., Déchamp C., Belmonte J., Thibaudon M., Cecchi L., Besancenot J.-P., Galán C., Waisel Y. The onset, course and intensity of the pollen season. In: Sofiev M., Bergmann K.-C., editors. Allergenic Pollen: A Review of the Production, Release, Distribution and Health Impacts. Springer; 2012. pp. 161–216. Ch. 6. [Google Scholar]
- de Weger L.A., Pashley C.H., Šikoparija B., Skjøth C.A., Kasprzyk I., Grewling Ł., Thibaudon M., Magyar D., Smith M. The long distance transport of airborne Ambrosia pollen to the UK and the Netherlands from Central and south Europe. Int. J. Biometeorol. 2016;60(12):1829–1839. doi: 10.1007/s00484-016-1170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defra . 2017. Defra daily air quality index.http://uk-air.defra.gov.uk/air-pollution/daqi last accessed 06/03/2017. [Google Scholar]
- Ehrenstein V., Mutius V., Illi, Baumann, Bhm, Kries V. Reduced risk of hay fever and asthma among children of farmers. Clin. Exp. Allergy. 2000;30(2):187–193. doi: 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- Elliot A.J., Hughes H.E., Hughes T.C., Locker T.E., Brown R., Sarran C., Clewlow Y., Murray V., Bone A., Catchpole M., McCloskey B., Smith G.E. The impact of thunderstorm asthma on emergency department attendances across London during July 2013. Emerg. Med. J. 2013 doi: 10.1136/emermed-2013-203122. http://emj.bmj.com/content/early/2013/10/08/emermed-2013-203122.abstract Oct emermed–2013-203122- [DOI] [PubMed] [Google Scholar]
- Emberlin J. vol. 8, 53. 1998. The effects of air pollution on allergenic pollen; pp. 164–167. [Google Scholar]
- Emberlin J., Detandt M., Gehrig R., Jaeger S., Nolard N., Rantio-Lehtimäki A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int. J. Biometeorol. 2002;46(4):159–170. doi: 10.1007/s00484-002-0139-x. http://www.ncbi.nlm.nih.gov/pubmed/12242471 Sep. [DOI] [PubMed] [Google Scholar]
- Emberlin J., Mullins J., Corden J., Millington W., Brooke M., Savage M., Jones S. The trend to earlier birch pollen seasons in the U.K.: a biotic response to changes in weather conditions? Grana. 1997;36(1):29–33. Jan. [Google Scholar]
- Emberlin J., Smith M., Close R., Adams-Groom B. Changes in the pollen seasons of the early flowering trees Alnus spp. and Corylus spp. in Worcester, United Kingdom, 1996-2005. Int. J. Biometeorol. 2007;51(3):181–191. doi: 10.1007/s00484-006-0059-2. http://www.ncbi.nlm.nih.gov/pubmed/17024396 Jan. [DOI] [PubMed] [Google Scholar]
- Erbas B., Chang J.-H., Dharmage S., Ong E.K., Hyndman R., Newbigin E., Abramson M. Do levels of airborne grass pollen influence asthma hospital admissions? Clin. Exp. Allergy. 2007;37(11):1641–1647. doi: 10.1111/j.1365-2222.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- ESA . 2017. ESA Landcover map.http://www.esa-landcover-cci.org/ last accessed 06/03/2017. [Google Scholar]
- ESRI . 2017. ESRI, Redlands, US.http://www.esri.com/software/arcgis last accessed 06/03/2017. [Google Scholar]
- Essl F., Dullinger S., Kleinbauer I. Changes in the spatio-temporal patterns and habitat preferences of Ambrosia artemisiifolia during invasion of Austria. Preslia. 2009;81:119–133. [Google Scholar]
- Fitter A.H., Fitter R.S.R. Rapid changes in flowering time in British plants. Science. 2002;296(5573):1689–1692. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Forestry Commission, Great Britain . Tech. rep. 2011. NFI 2011 woodland map GB. [Google Scholar]
- Forestry Commission, Great Britain . Tech. rep. Forestry Commission, Great Britain; 2012. Standing Timber Volume for Coniferous Trees in Britain: National Forest Inventory Report. [Google Scholar]
- Frei T. The effects of climate change in Switzerland 1969–1996 on airborne pollen quantities from hazel, birch and grass. Grana. 1998;37(3):172–179. [Google Scholar]
- Gezon Z.J., Inouye D.W., Irwin R.E. Phenological change in a spring ephemeral: implications for pollination and plant reproduction. Glob. Chang. Biol. 2016 doi: 10.1111/gcb.13209. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Greiner A.N., Hellings P.W., Rotiroti G., Scadding G.K. Allergic rhinitis. Lancet. 2012;378(9809):2112–2122. doi: 10.1016/S0140-6736(11)60130-X. http://www.sciencedirect.com/science/article/pii/S014067361160130X [DOI] [PubMed] [Google Scholar]
- Grell G., Freitas S.R., Stuefer M., Fast J. Inclusion of biomass burning in WRF-Chem: impact of wildfires on weather forecasts. Atmos. Chem. Phys. 2011;11(11):5289–5303. http://www.atmos-chem-phys.net/11/5289/2011/ [Google Scholar]
- Grewling Ł., Bogawski P., Jenerowicz D., Czarnecka-Operacz M., Šikoparija B., Skjøth C.A., Smith M. Mesoscale atmospheric transport of ragweed pollen allergens from infected to uninfected areas. Int. J. Biometeorol. 2016:1–8. doi: 10.1007/s00484-016-1139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewling Ł., Šikoparija B., Skjøth C., Radišić P., Apatini D., Magyar D., Páldy A., Yankova R., Sommer J., Kasprzyk I., Myszkowska D., Uruska A., Zimny M., Puc M., Jäger S., Smith M. Variation in Artemisia pollen seasons in Central and Eastern Europe. Agric. For. Meteorol. 2012;160:48–59. http://www.sciencedirect.com/science/article/pii/S0168192312000937 [Google Scholar]
- Hamaoui-Laguel L., Vautard R., Liu L., Solmon F., Viovy N., Khvorostyanov D., Essl F., Chuine I., Colette A., Semenov M.A., Schaffhauser A., Storkey J., Thibaudon M., Epstein M.M. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Chang. 2015;5(8):766–771. [Google Scholar]
- IGBP . 2017. IGBP, last accessed 06/03/2017.http://glcf.umd.edu/data/lc/ [Google Scholar]
- Karrer G., Skjøth C., Šikoparija B., Smith M., Berger U., Essl F. Ragweed (Ambrosia) pollen source inventory for Austria. Sci. Total Environ. 2015;523:120–128. doi: 10.1016/j.scitotenv.2015.03.108. http://www.sciencedirect.com/science/article/pii/S0048969715003927 [DOI] [PubMed] [Google Scholar]
- Kaye N., Burgess P. Tech. rep. Met Office Hadley Centre; 2013. A proposed methodology for quantifying and mapping habitat resilience in devon.http://www.metoffice.gov.uk/hadobs/climviz/Culm/ [Google Scholar]
- Maskell L., Henrys P., Norton L., Smart S., Wood C. Tech. rep. 2013. Distribution of Ash trees (Fraxinus excelsior) in Countryside Survey data. January. [Google Scholar]
- Met Office . 2017. Met Office Air Quality.http://www.metoffice.gov.uk/guide/weather/air-quality last accessed 06/03/2017. [Google Scholar]
- Met Office . 2017. Met Office Pollen Forecast.http://www.metoffice.gov.uk/health/public/pollen-forecast last accessed 06/03/2017. [Google Scholar]
- MODIS Vegetation Continuous Fields . 2017. MODIS Vegetation Continuous Fields.http://glcf.umd.edu/data/vcf/ last accessed 06/03/2017. [Google Scholar]
- Molfino N., Wright S., Katz I., Tarlo S., Silverman F., McClean P., Slutsky A., Zamel N., Szalai J., Raizenne M. Originally published as volume 2, issue 8761 effect of low concentrations of ozone on inhaled allergen responses in asthmatic subjects. Lancet. 1991;338(8761):199–203. doi: 10.1016/0140-6736(91)90346-q. http://www.sciencedirect.com/science/article/pii/014067369190346Q [DOI] [PubMed] [Google Scholar]
- Morton D., Rowland C., Wood C., Meek L., Marston C., Smith G., Wadsworth R., Simpson I. Tech. rep. NERC/Centre for Ecology & Hydrology; 2011. Final Report for LCM2007 - The New UK Land Cover Map. Countryside Survey Technical Report no 11/07.http://nora.nerc.ac.uk/14854/ July. [Google Scholar]
- Mücke H.-G., Wagener S., Werchan M., Bergmann K.-C. Measurements of particulate matter and pollen in the city of Berlin. Urban Clim. 2014;10:621–629. http://www.sciencedirect.com/science/article/pii/S2212095514000236 Part 4. [Google Scholar]
- Netuveli G., Hurwitz B., Levy M., Fletcher M., Barnes G., Durham S.R., Sheikh A. Ethnic variations in UK asthma frequency, morbidity, and health-service use: a systematic review and meta-analysis. Lancet. 2005;365(9456):312–317. doi: 10.1016/S0140-6736(05)17785-X. [DOI] [PubMed] [Google Scholar]
- Newson R.B., van Ree R., Forsberg B., Janson C., Lötvall J., Dahlén S.-E., Toskala E.M., Bælum J., Brożek G.M., Kasper L., Kowalski M.L., Howarth P.H., Fokkens W.J., Bachert C., Keil T., Krämer U., Bislimovska J., Gjomarkaj M., Loureiro C., Burney P.G.J., Jarvis D. Geographical variation in the prevalence of sensitization to common aeroallergens in adults: the GA2LEN survey. Allergy. 2014;69(5):643–651. doi: 10.1111/all.12397. [DOI] [PubMed] [Google Scholar]
- NHS Choices . 2017. NHS Choices, hay-fever.http://www.nhs.uk/conditions/Hay-fever/Pages/Introduction.aspx last accessed 06/03/2017. [Google Scholar]
- Oderbolz D.C., Aksoyoglu S., Keller J., Barmpadimos I., Steinbrecher R., Skjøth C.A., Plaß-Dülmer C., Prévôt A.S.H. A comprehensive emission inventory of biogenic volatile organic compounds in Europe: improved seasonality and land-cover. Atmos. Chem. Phys. 2013;13(4):1689–1712. http://www.atmos-chem-phys.net/13/1689/2013/ [Google Scholar]
- Office of National Statistics . 2017. Office of National Statistics.https://www.ons.gov.uk/ last accessed 06/03/2017. [Google Scholar]
- O’Hagan A., Buck C.E., Daneshkhah A., Eiser J.R., Garthwaite P.H., Jenkinson D.J., Oakley J.E., Rakow T. John Wiley & Sons, Ltd; 2006. Uncertain Judgements: Eliciting Experts' Probabilities. [Google Scholar]
- Okuda M. Epidemiology of Japanese cedar pollinosis throughout Japan. Ann. Allergy Asthma Immunol. 2003;91(3):288–296. doi: 10.1016/S1081-1206(10)63532-6. http://www.sciencedirect.com/science/article/pii/S1081120610635326 [DOI] [PubMed] [Google Scholar]
- Ørby P., Peel R., Skjøth C., Schlünssen V., Bønløkke J., Ellermann T., Brændholt A., Sigsgaard T., Hertel O. An assessment of the potential for co-exposure to allergenic pollen and air pollution in Copenhagen, Denmark. Urban Climate. 2015 http://www.sciencedirect.com/science/article/pii/S2212095514001102 Jan. [Google Scholar]
- Osborne N., Eggen B. Living with Environmental Change, LWEC, NERC; 2014. Pollen and asthma: impacts of anthropogenic climate change. [Google Scholar]
- Pashley C., Fairs A., Edwards R., Bailey J., Corden J., Wardlaw A. Reproducibility between counts of airborne allergenic pollen from two cities in the east Midlands, UK. Aerobiologia. 2009;25(4):249–263. [Google Scholar]
- Pashley C.H., Satchwell J., Edwards R.E. Ragweed pollen: is climate change creating a new aeroallergen problem in the UK? Clin. Exp. Allergy. 2015;45(7):1262–1265. doi: 10.1111/cea.12572. [DOI] [PubMed] [Google Scholar]
- Prank M., Chapman D.S., Bullock J.M., Belmonte J., Berger U., Dahl Å., Jager S., Kovtunenko I., Magyar D., Niemela S., Rantio-Lehtimaki A., Rodinkova V., Sauliene I., Severova E., Sikoparija B., Sofiev M. An operational model for forecasting ragweed pollen release and dispersion in Europe. Agric. For. Meteorol. 2013;182-183:43–53. http://www.sciencedirect.com/science/article/pii/S0168192313002104 [Google Scholar]
- Python Software Foundation . 2017. Python Software Foundation.https://www.python.org/ last accessed 06/03/2017. [Google Scholar]
- Riedler J., Braun-Fahrlnder C., Eder W., Schreuer M., Waser M., Maisch S., Carr D., Schierl R., Nowak D., von Mutius E. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–1133. doi: 10.1016/S0140-6736(01)06252-3. http://www.sciencedirect.com/science/article/pii/S0140673601062523 [DOI] [PubMed] [Google Scholar]
- Rumble H., Rogers K., Doick K., Hutchings T. Institute of Chartered Foresters; 2015. A comparison of urban tree populations in four UK towns and cities; pp. 181–195. [Google Scholar]
- Salmond J.A., Tadaki M., Vardoulakis S., Arbuthnott K., Coutts A., Demuzere M., Dirks K.N., Heaviside C., Lim S., Macintyre H., McInnes R.N., Wheeler B.W. Health and climate related ecosystem services provided by street trees in the urban environment. Environ. Health. 2016;15(Suppl 1):36. doi: 10.1186/s12940-016-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelhaas M., Varis S., Schuck A., Nabuurs G. Tech. rep. European Forest Institute, Joensuu, Finland; 2006. Efiscen inventory database.http://www.efi.int/portal/virtual_library/databases/efiscen/ [Google Scholar]
- Seitz J., Francisco Escobedo F. Tech. rep., This document is FOR 206, one of a series of the School of Forest Resources and Conservation Department, UF/IFAS Extension. 2009. Urban Trees and Allergies in North Florida.http://edis.ifas.ufl.edu (accessed 24 April 2015) [Google Scholar]
- Simpson D., Winiwarter W., Brjesson G., Cinderby S., Ferreiro A., Guenther A., Hewitt C.N., Janson R., Khalil M.A.K., Owen S., Pierce T.E., Puxbaum H., Shearer M., Skiba U., Steinbrecher R., Tarrasn L., quist M.G. Inventorying emissions from nature in Europe. J. Geophys. Res. Atmos. 1999;104(D7):8113–8152. [Google Scholar]
- Skjøth C.A., Baker P., Sadys M., Adams-Groom B. Pollen from alder (Alnus sp.), birch (Betula sp.) and oak (Quercus sp.) in the {UK} originate from small woodlands. Urban Clim. 2015;14, Part 3:414–428. http://www.sciencedirect.com/science/article/pii/S2212095514000716 New Sensing Technologies and Methods for Air Pollution Monitoring. [Google Scholar]
- Skjøth C.A., Bilinska D., Werner M., Adams-Groom B., Drzeniecka-Osiadacz A. Footprint areas of pollen from alder (Alnus) and birch (Betula) in the UK (Worcester) and Poland (Wroclaw) during 2005-2014. Acta Agrobot. 2015;68:315–324. Dec. [Google Scholar]
- Skjøth C.A., Geels C., Hvidberg M., Hertel O., Brandt J., Frohn L.M., Hansen K.M., Hedegaard G.B., Christensen J.H., Moseholm L. An inventory of tree species in Europe-an essential data input for air pollution modelling. Ecol. Model. 2008;217(3-4):292–304. [Google Scholar]
- Skjøth C.A., Ørby P.V., Becker T., Geels C., Schlünssen V., Sigsgaard T., Bønløkke J.H., Sommer J., Søgaard P., Hertel O. Identifying urban sources as cause of elevated grass pollen concentrations using GIS and remote sensing. Biogeosciences. 2013;10(1):541–554. http://www.biogeosciences.net/10/541/2013/ [Google Scholar]
- Skjøth C.A., Sikoparija B., Jager S., EAN-Network . Pollen sources. In: Sofiev M., Bergmann K.-C., editors. Allergenic pollen: a review of the production, release, distribution and health impacts. Springer; 2012. pp. 9–27. [Google Scholar]
- Skjøth C. a., Smith M., Šikoparija B., Stach A., Myszkowska D., Kasprzyk I., Radišić P., Stjepanović B., Hrga I., Apatini D. A method for producing airborne pollen source inventories: an example of Ambrosia (ragweed) on the Pannonian Plain. Agric. For. Meteorol. 2010;150(9):1203–1210. http://linkinghub.elsevier.com/retrieve/pii/S0168192310001292 Aug. [Google Scholar]
- Skjøth C.A., Sommer J., Stach A., Smith M., Brandt J. The long-range transport of birch (Betula) pollen from Poland and Germany causes significant pre-season concentrations in Denmark. Clin. Exp. Allergy. 2007;37(8):1204–1212. doi: 10.1111/j.1365-2222.2007.02771.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Cecchi L., Skjøth C., Karrer G., Åikoparija B. Common ragweed: a threat to environmental health in Europe. Environ. Int. 2013;61:115–126. doi: 10.1016/j.envint.2013.08.005. http://www.sciencedirect.com/science/article/pii/S0160412013001682 [DOI] [PubMed] [Google Scholar]
- Smith M., Emberlin J. Constructing a 7-day ahead forecast model for grass pollen at north London, United Kingdom. Clin. Exp. Allergy. 2005;35(10):1400–1406. doi: 10.1111/j.1365-2222.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Emberlin J. A 30-day-ahead forecast model for grass pollen in north London, United Kingdom. Int. J. Biometeorol. 2006;50(4):233. doi: 10.1007/s00484-005-0010-y. [DOI] [PubMed] [Google Scholar]
- Sofiev M., Belmonte J., Gehrig R., Izquierdo R., Smith M., Dahl A., Siljamo P. Airborne pollen transport. In: Sofiev M., Bergmann K.-C., editors. Allergenic pollen: a review of the production, release, distribution and health impacts. Springer; 2012. pp. 127–159. Ch. 5. [Google Scholar]
- Spot Vegetation . 2017. Spot Vegetation.http://www.vgt.vito.be/pages/VegetationProgramme/generaldescription.htm last accessed 06/03/2017. [Google Scholar]
- Suring K., Bach S., Bossmann K., Wolter E., Neumann A., Straff W., Hflich C. PM10 contains particle-bound allergens: dust analysis by flow cytometry. Environ. Techn. Innovation. 2016;5:60–66. http://www.sciencedirect.com/science/article/pii/S2352186416300049 [Google Scholar]
- Thibaudon M., Šikoparija B., Oliver G., Smith M., Skjøth C.A. Ragweed pollen source inventory for France – the second largest centre of Ambrosia in Europe. Atmos. Environ. 2014;83:62–71. http://www.sciencedirect.com/science/article/pii/S1352231013008108 Feb. [Google Scholar]
- Vardoulakis S., Dimitroulopoulou C., Thornes J., Lai K.-M., Taylor J., Myers I., Heaviside C., Mavrogianni A., Shrubsole C., Chalabi Z., Davies M., Wilkinson P. Impact of climate change on the domestic indoor environment and associated health risks in the UK. Environ. Int. 2015;85:299–313. doi: 10.1016/j.envint.2015.09.010. http://www.sciencedirect.com/science/article/pii/S0160412015300507 [DOI] [PubMed] [Google Scholar]
- Vardoulakis S., Heaviside C. Tech. rep. Health Protection Agency; 2012. Health effects of climate change in the UK. [Google Scholar]
- Werner M., Geels C., Kryza M., Skjøth C.A. Springer International Publishing; 2016. Using a dynamical approach for implementing Ammonia emissions into WRF-Chem over Europe; pp. 345–350. [Google Scholar]
- Werner M., Kryza M., Geels C., Ellermann T., Skjøth C. Ammonia concentrations over Europe–application of the WRF-Chem model supported with dynamic emission. Pol. J. Environ. Stud. 2017;26(3):1–19. [Google Scholar]
- Worcester University . 2017. Worcester University pollen calendar.http://www.worcester.ac.uk/pdfs/pollen-calendar.pdf last accessed 06/03/2017. [Google Scholar]
- Yamada T., Saito H., Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J. Allergy Clin. Immunol. 2014;133(3) doi: 10.1016/j.jaci.2013.11.002. http://www.sciencedirect.com/science/article/pii/S0091674913017119 632–639 e5. [DOI] [PubMed] [Google Scholar]
- Zink K., Pauling A., Rotach M.W., Vogel H., Kaufmann P., Clot B. EMPOL 1.0: a new parameterization of pollen emission in numerical weather prediction models. Geosci. Model Dev. 2013;6(6):1961–1975. http://www.geosci-model-dev.net/6/1961/2013/ [Google Scholar]
- Zink K., Vogel H., Vogel B., Magyar D., Kottmeier C. Modeling the dispersion of Ambrosia artemisiifolia l. pollen with the model system COSMO-ART. Int. J. Biometeorol. 2012;56(4):669–680. doi: 10.1007/s00484-011-0468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska L., Knowlton K., Rogers C., Dalan D., Tierney N., Elder M.A., Filley W., Shropshire J., Ford L.B., Hedberg C., Fleetwood P., Hovanky K.T., Kavanaugh T., Fulford G., Vrtis R.F., Patz J.A., Portnoy J., Coates F., Bielory L., Frenz D. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc. Natl. Acad. Sci. U. S. A. 2011;108(10):4248–4251. doi: 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.