Abstract
Proton pump inhibitors (PPIs) have become known for both their therapeutic effect and good safety profile. An application was submitted to the US Food and Drug Administration for approval of a reformulated PPI product that failed bioequivalence testing, but was submitted on the basis of the long history of PPI use as a “surrogate” for equivalence. This review evaluates the safety data for PPIs, discuss variability of pharmacokinetic parameters of PPIs in the reformulation setting, and potential implications of those changes for long‐term safety.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ While PPI efficacy is undeniable and their short‐term safety profile is well established, a number of safety concerns associated with long‐term PPI use have surfaced recently. One of them is an increased risk of fracture in chronic PPI users.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ The review addressed the question as to whether drugs with proven efficacy and good safety records such as PPIs should be subjected to the “one size fits all” rigorous BE criteria.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ The results demonstrate that the long‐term safety issues such as an increased fracture risk in PPI users cannot be excluded despite PPI's good safety profile and their presence on the market for decades. Importantly, the review provides an insight into the decision‐making process by the regulatory authorities that factor in different kinds of safety evidence in considering different kinds of regulatory action where the important considerations are given to the protection of the public health.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ By raising potential safety concerns accrued by the increased levels of the PPIs, the review supports the current BA/BE assessment paradigm as an appropriate safety measure in the postmarketing setting.
The pharmaceutical product lifecycle begins with regulatory approval and ends with its market discontinuation. During this time the product's inactive ingredients or method of manufacture may change and post‐approval reformulation may be needed. This situation is analogous to when a patent expires and other manufacturers develop generic versions. In both situations the reformulated product or a generic copy must undergo bioequivalence (BE) testing and meet the BE requirements to ensure therapeutic equivalence.
Bioequivalence means the absence of a greater‐than‐allowable difference between the systemic bioavailability of a test product and that of a reference product. What is an “allowable difference” has been debated in the medical‐scientific community ever since the original “Bioavailabity and Bioequivalence” regulations were first published in the Code of Federal Regulations in 1976. The US Food and Drug Administration (FDA) defines test and reference products to be bioequivalent if the rate and extent of absorption of the test drug do not show a significant difference from the rate and extent of absorption of the reference drug, when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions following a single dose in a suitable number of normal subjects. The confidence interval (CI) of the geometric means (log transformed) of the “test vs. reference” is evaluated using a two one‐sided t‐test (90% CIs) with a BE acceptance range of 80–125%. This definition of the BE has been termed “average BE.”1, 2
The regulations require that bioequivalence examine both the extent of absorption and the rate of absorption. While there is consensus regarding area under the curve (AUC) as the appropriate metric for the extent of absorption (i.e., bioavailability),3 there was, initially, disagreement on the decision criterion for “rate,” as it is a continually varying parameter. From a strict pharmacokinetic (PK) definition standpoint, “rate of absorption” is defined as the absorption rate constant (Ka), and is taught as such in courses on PKs. While algebraically true, Ka is also a sampling‐dependent parameter, in that the calculation of Ka is dependent on the number and timing of blood samples. Ka is by its very definition a variable parameter and is unsuitable for bioequivalence testing. Instead, it has been generally accepted that maximum concentration (Cmax), being the peak concentration produced after dosing, is a more appropriate parameter for assessing the rate of absorption, even though it is a hybrid parameter, in that it is affected by both the rate of absorption and the rate of elimination; it is also sampling‐dependent. Sampling‐dependent in that the timing of samples is also critical to its demonstration; however, it is less sensitive than Ka is in this regard.
Both AUC and Cmax (and any other secondary parameters that may be evaluated) must pass the same standard, the aforementioned log transformed 90% CI, in the United States. For drugs under a new drug application (NDA), the FDA looks at the totality of the data with regard to safety and experience with higher doses and has allowed products with adequate data to be approved with “modest deviations” from the 80–125 acceptance interval. It should be noted that the Office of Generic Drugs does not allow this degree of flexibility as the mandate, for there is one of “switchability” between manufacturers. The European Union, however, has adopted more flexible criteria in allowing the acceptance interval for Cmax to be widened based on drug characteristics.4
Proton‐pump inhibitors (PPIs) are a group of drugs that cause a pronounced reduction of gastric acid production through inactivation of the proton pump in the gastric wall. Launched in the late 1980s, PPIs have been on the market for nearly three decades. This class of medication has become known for both their excellent therapeutic effect and good safety profile,5 leading to increasingly common long‐term use that was not studied in the original NDA nor are reflected in the current package insert. The “branded” drug product that is the subject of this article has, during its life‐cycle, been reformulated more than once, and numerous generic equivalents have been developed and approved for use. To support this proposed change in the manufacturing process, the sponsor submitted data from an open‐label, randomized, single dose, 4 × 4 crossover BE study comparing the reformulated product to the reference product at two different strengths. The results showed that the mean ratio for Cmax values of the reformulated product were more than 50% higher than that of the corresponding reference formulation, with 90% CI exceeding the FDA BE limit of 80–125%. However, the AUC mean ratios and corresponding 90% CI are within the BE acceptance limit. As part of the application submitted to the FDA there was an explanation that AUC and not Cmax would be more closely aligned to efficacy for a PPI and as AUC has demonstrated equivalence, then the lack of Cmax BE was not significant.
The above‐described case raises the question as to whether drugs with proven efficacy and good safety records such as PPIs should be subjected to the “one size fits all” rigorous BE criteria or not. Specifically, it questions the clinical significance of Cmax with regard to therapeutic effect and whether its interpretation should be more flexible. This review is aimed to evaluate the safety data for PPIs, discuss PK parameters of PPIs in the reformulation setting, and potential implications of those changes for long‐term safety.
METHODS
Relevant material was obtained: i) by reviewing the publicly available FDA review documents (Drugs@FDA) for generic and brand name PPIs including both clinical and bioequivalence results; ii) by reviewing published literature using strategic search terms; and iii) by searching the FDA Adverse Event Reporting System (FAERS) and performing pharmacovigilance disproportionality analysis. The searches were limited to PPI products marketed in the United States.
The MEDLINE/PubMed, Google, Google Scholar searches ranged from September 1989 to June 2015. Figure 1 shows the flow chart diagram of the selection process of the studies included in the review. Both generic and brand name products were evaluated. Preliminary screening of the literature showed that most safety concerns encountered with PPI use fell into the following categories: those related to the direct effect of gastric acid suppression (vitamin B12, iron, magnesium, and calcium malabsorption leading to osteoporotic fracture, enteric infections including Clostridium difficile‐associated diarrhea); the physiological response to acid suppression (hypergastrinemia leading to increased cancer risk or hyperparathyroidism); and PK interaction with the metabolism of other medications (PPI and clopidogrel; PPI and methotrexate (MTX) interaction) resulting in an altered pharmacodynamic effect. Due to a high volume of data, the search was narrowed to studies that contained information on the long‐term use safety concerns associated with the direct effect of gastric acid suppression, specifically to the risk of fractures. The search terms included Prilosec, Prilosec OTC, Omeprazole, Nexium, Esomeprazole, Dexilant, Dexlansoprazole, Prevacid, Prevacid 24HR, Lansoprazole, Zegerid, Zegerid OTC, Omeprazole with Sodium bicarbonate, Protonix, Pantoprazole, AcipHex, and Rabeprazole (All these trade names are the property of their respective owners and/or companies. No endorsement is intended or implied.); Proton pump inhibitor(s), Fracture, Osteoporosis, Bone mineral density, Hypocalcaemia, Long‐term use, Chronic use, and Off‐label use.
Figure 1.
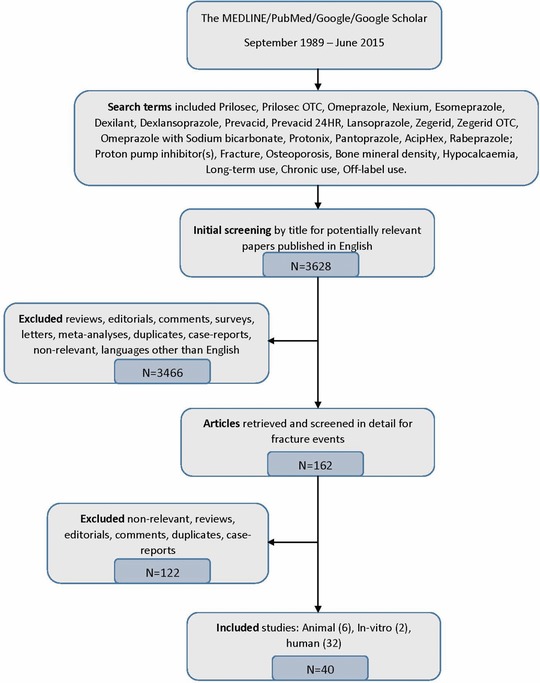
Flow chart diagram of studies selection process to include in the review.
The year of publication, the number of patients enrolled in the study, the strength of the association between fractures, and PPI formulation usage were documented and the results were tabulated.
Cumulative proportional reporting ratios (PRRs) for all PPI formulations marketed in the United States were calculated using an open FDA dynamic PRR software tool6, 7 to evaluate how PRR scores evolved over time. Input data were taken from the public release of the FAERS for spontaneous adverse reports of fractures from the first quarter of 2004 till June 2015. The potential signal criteria included the appearance of a minimum of three drug‐event reports of fracture, a cumulative PRR >2, and the crossing of a common boundary of statistical significance by chi‐squared (χ2) testing (χ2 (3.86 indicates a 95% CI, assuming 1 degree of freedom with the Yates correction)).8 The results were tabulated and transformed into a graph.
RESULTS
Review of the FDA source documents on BE studies confirmed that the estimated 90% CI for the ratio of geometric means of the primary PK parameters (AUC and Cmax) were totally within the BE limits of 80–125% for all generic PPIs marketed in the United States.
For two brand name products the estimated 90% CI for the ratio of geometric means of the AUC were within the BE limits, while the Cmax values deviated from the accepted BE range.9 The mean ratio for Cmax for Drug A (a delayed release (DR) capsule) was: 1.04, 90% CI (0.82–1.31%); and for Drug B (an immediate release (IR) capsule): 1.48, 90% CI (1.29–1.71%); and the Cmax for a higher strength DR capsule: 1.49, 90% CI (1.26–1.77%). In this case, as the comparison was that of a delayed release product to an immediate release product, a difference in absorption rate was to be expected, and the products were allowed onto the market with supporting in vivo clinical data. This specific exception to “rate equivalence” is specifically allowed for in the bioequivalence regulations (21 CFR 320.23 (a)(3)). The accepted BE interval for both AUC and Cmax parameters for all other brand PPI products was met.
As noted previously, the literature search retrieved 3,628 published articles: 40 of which were selected for being directly relevant to the main question (six animal, two in vitro, and 32 human studies).
Animal and in vitro studies10, 11, 12, 13, 14, 15, 16, 17 started to appear in the scientific literature in 1998 (Supplemental Material‐Table A). These studies were mostly conducted with omeprazole (the first approved PPI), and demonstrated that PPIs decreased calcium absorption in rats,10 delayed fracture healing in mice;11 decreased the differentiation and activation of osteoclasts;12, 13 decreased cell viability and function of human osteoclasts in vitro;14 produced a dose‐dependent decrease in bone resorption and reduction in bone formation, tibia calcium content, and serum tumor necrosis factor alpha (TNF‐α), and interleukin (IL)‐6.15 Different hypotheses to explain the effect of PPIs on bone remodeling were formulated.
Publications reporting human studies18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 started to appear in the scientific literature in 2005 (Supplemental Material‐Table B). The number of publications varied from one in 2005 to seven in 2013 and have increased over time. Figure 2 demonstrates the timeline of publication appearance in the scientific literature relevant to the year of the drug approval, with drugs approved for over‐the‐counter use being indicated above the line to distinguish them from the original NDA approvals. Only one of the 32 studies was a randomized control crossover trial;18 the rest were observational in nature. Two studies were short‐term and 30 were long‐term. While the randomized control trial was very short term (1 week), it nevertheless showed a significantly decreased fractional calcium absorption compared with placebo. Another short‐term study (12 weeks) in healthy adult males, aimed to evaluate the effect of PPIs on biochemical markers of calcium and bone metabolism, found no difference between the groups in levels of albumin, phosphate, calcium, ionized calcium PTH, 25‐OH‐vitamin D, osteocalcin, C‐terminal cross‐linked telopeptides of type I collagen before and after PPI treatment.19
Figure 2.
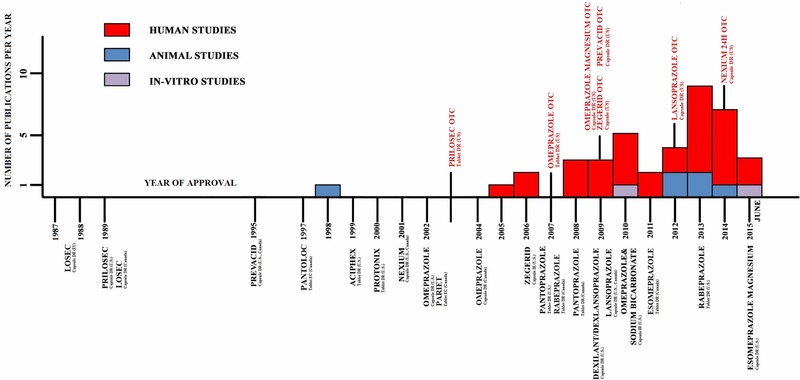
Timeline of the publication appearance in the literature relevant to PPI Formulation Approval.
Seven out of the 30 long‐term studies evaluated bone mineral density (BMD) in PPI users compared with nonusers. Four out of seven showed a decrease in BMD in PPI users compared with nonusers,20, 23, 24, 36 while three others21, 22, 49 showed no difference in BMD values. One of the studies that found a significant reduction in BMD in PPIs users20 compared the risk of fracture for different PPI products. The reduction of BMD was significantly higher in lansoprazole participants compared with those who received pantoprazole. The reduction in BMD was significant in esomeprazole participants for both the lumbar spine and the femoral neck, while those who received lansoprazole or pantroprazole treatment only showed a significant decrease in BMD in the lumbar spine. The majority of the studies, however, did not differentiate between formulations, making it impossible to evaluate fracture risk across different PPIs.
Twenty‐four studies evaluated the association between fracture risk and PPI intake. Twenty‐two out of 24 found a small statistically increased risk of fracture, with odds ratios ranging from 1–2. The increase in risk was found across different countries, study types (cohort and case‐control), and age groups ranging from 18 years old to the elderly. Some studies demonstrated that risk of fracture increased with age.36 However, the results were inconsistent across these studies for a dose‐ or a duration‐response, or time‐to‐onset of fracture. Not all subgroup analyses had statistically significant results. One cohort study of postmenopausal women25 found an association between regular PPI use and risk of fracture that increased with longer duration of PPI use. Many of the studies were population‐based and of large size. However, these studies were observational and prone to residual confounding. Some of the covariates had incomplete information and only one study26 validated electronic exposure and outcome data for accuracy of exposure or diagnosis. None of the studies accounted for over‐the‐counter (OTC) drug use for PPIs or concomitant drugs. Finally, none of the studies reported on fatalities associated with fractures.
Some plausible biologic mechanisms for increased fracture risk were proposed:
PPIs could affect osteoclasts by inhibiting the osteoclastic proton pump and impacts osteoclast bone remodeling by reducing bone absorption and thus reducing bone strength. A suppressive effect of omeprazole on bone resorption is through modulation of V‐type H+‐ATPase activity of osteoclast acid‐producing systems, which maintain bone turnover;
PPI induction of enterochromaffin‐like cells hyperplasia as a source of histamine, a possible mediator of bone loss in mastocytosis. Chronic PPI exposure leads to increased gastrin levels that result in stimulation of osteoclasts by histamine and blockade of osteoclast H1 receptors by H1RAs reducing bone resorption by mature osteoclasts;27
Without an appropriate acid environment, calcium may be retained in food, thereby reducing its absorption. Reduced calcium absorption may lead to compensatory secondary hyperparathyroidism, which may increase the rate of osteoclastic bone resorption. If PPIs inhibit the reabsorptive activity of osteoclasts, old bone cannot be replaced, predisposing patients to fractures;
PPIs’ suppression of gastric acid secretion interferes with (and lowers) folate and vitamin B12 absorption leading to alterations in homocysteine levels that may contribute to increased fracture risk;28
Dizziness and confusion, uncommon side effects of PPIs, may increase the likelihood of falls.29
The cumulative PRR statistical data on PPI associated fractures (drug‐event) are summarized in Table 1. In 2015 the PRR scores were as follows: Prilosec, PRR = 2.25 (95% CI, 1.75–2.90), Nexium, PRR = 2.20 (95% CI, 1.86–2.59), Dexilant, PRR = 2.25 (95% CI, 0.93–5.40), and Zegerid, PRR = 3.15 (95% CI, 0.79–12.56). The PRR for Zegerid was the highest; however, the number of drug‐event counts were low, and the 95% CIs included 1.0. Only two PPI products, Prilosec and Nexium, demonstrated PRRs slightly >2.0 over the years. The lower bound of the 95% CI for Prilosec crossed 1.0 in 2009; for Nexium in 2005, and remained above 1.0 since then. The number of drug‐event counts varied from 1.0 to 3.0, with occasional spikes up to 32 counts per marketing quarter. On these occasions the estimated 95% CI (lower and upper bounds) were higher than 1.0. Cumulative time‐trending PRR profiles for fracture for all PPIs tended to stabilize over increasing periods of time.
Table 1.
Ten‐year cumulative US FAERS reports of fracture associated with PPIs
|
Drug‐event counts | Drug counts | Event counts | Total counts | PRR (95% CI) | PRR (95% CI) |
|---|---|---|---|---|---|---|
| 2015–07 | 2015–07 | 2015–07 | 2015–07 | 2005–01 | 2015–07 | |
| Prilosec a | 61 | 34,053 | 3,510 | 4,370,123 | 2.29 (0.57–9.27) | 2.25 (1.75–2.90) |
| Omeprazole b | 130 | 105,128 | 3,510 | 4,370,123 | 1.24 (0.39–3.90) | 1.56 (1.31–1.86) |
| Nexium a | 146 | 84,955 | 3,510 | 4,370,123 | 2.40 (0.76–7.56) | 2.20 (1.86–2.59) |
| Esomeprazole b | N/A | N/A | ||||
| Dexilant a | 5 | 2,774 | 3,510 | 4,370,123 | N/A | 2.25 (0.93–5.40) |
| Dexlansoprazole b | ||||||
| Prevacid a | 56 | 43,985 | 3,510 | 4,370,123 | 2.05 (0.76–5.57) | 1.59 (1.22–2.08) |
| Lansoprazole b | 56 | 44,130 | 3,510 | 4,370,123 | N/A | 1.54 (1.18–2.02) |
| Zegerid a | 2 | 824 | 3,510 | 4,370,123 | N/A | 3.15 (0.79–12.56) |
| Omeprazole & Sodium Bicarbonate b | N/A | N/A | ||||
| Protonix a | 42 | 34,809 | 3,510 | 4,370,123 | 0.65 (0.09–4.67) | 1.57 (1.16–2.13) |
| Pantoprazole b | 62 | 53,103 | 3,510 | 4,370,123 | N/A | 1.56 (1.21–2.01) |
| AcipHex a | 13 | 12,346 | 3,510 | 4,370,123 | 0.99 (0.14–7.07) | 1.31 (0.76–2.26) |
| Rabeprazole b | 15 | 13,198 | 3,510 | 4,370,123 | N/A | 1.42 (0.85–2.35) |
Brand name producta and its generic equivalent.b N/A = not on the market yet or just approved or no data are available.
PRR statistics provides a very basic analysis that only accounts for statistical associations in the coreporting of drugs and suspected ADRs. It is entirely based on aggregate numbers of reports and disregards the strength of individual reports.
DISCUSSION
PPIs have been on the world market for almost three decades. While PPI efficacy is undeniable and their short‐term safety profile is well established, an alarming number of publications linking PPIs to several nutritional, metabolic, and infectious disorders have surfaced during the last decade. The usual adverse reporting pattern of a product is that healthcare professionals and patients are more inclined to report adverse reactions when the product is new and decreases over time. The life‐cycle adverse event (AE) reporting pattern of PPIs is somewhat unusual, in that the reporting of AEs, fractures in particular, started to emerge in the scientific literature only after the drug had been on the market for quite a while. This unusual reporting pattern may be indicative of long‐term safety issues that were not considered at the time of original approval, as years of continuous or semicontinuous use were not foreseen. Epidemiological data suggest a positive although weak association between PPI use and risk of fractures, with cumulative PRRs showing a marginal drug‐event association.
The most comprehensive explanation would be that in general PPIs are safe, but overprescribing and long‐term use have taken them outside of the study envelope that was the basis for approval. Indeed, PPIs are now some of the most commonly prescribed drugs in the world, with billions of dollars in annual sales.50, 51 Although the current PPI labeling includes recommendations to use the lowest PPI dose and shortest duration, while observing guidelines for patients at risk for osteoporosis‐related fractures, long‐term off‐label use is very common. Long‐term PPI usage rates increase with age.52 In a large population‐based cohort study, Lassen et al.53 showed that 83% of long‐term PPI users were >50 years old.
Other studies revealed that 65% of patients receiving long‐term acid‐suppressing treatment were age 60+ years and 25% were age 75+ years,54 and according to one study,55 a rate of >14% of long‐term PPI use was ascertained in the 65+‐year age group compared with under 2% in the 15–44‐year age group. Some studies showed that primary care physicians regard PPIs as very effective, and relatively safe, making withdrawal of long‐term PPIs difficult to achieve.56, 57, 58 Consumers also view PPIs as safe due to their OTC availability and widespread/pervasive marketing. Thus, overutilization due to perceived safety of PPIs, as well as the readiness of the physicians to prescribe acid suppressants as long‐term therapy, could explain the inconsistent but positive association between PPIs and the risk of fractures. Future studies evaluating the change in PPI prescription pattern over time (from 1989 to 2015) are needed to investigate long‐term prescribing as the cause of the association.
Prilosec and its generic copy omeprazole was referenced in the majority of studies of fracture risk. Yet it was not possible to link the specific PPI formulation to the fracture risk. However, given the strictures of the FDA BE testing paradigm that are utilized by both the new drug and generic drug review processes, this potential confounding issue is likely to be of minimal significance for comparisons between different products. The PRR values for Prilosec were slightly above 2.0, with a 95% CI lower bound above 1.0. The number of drug‐event counts for Prilosec was comparable to those of other PPIs, although occasional spikes in fracture occurrences were noted. These findings may indicate a marginal signal but should be evaluated with caution, as the PRR provides a very basic analysis that only accounts for statistical associations between a drug and an AE or a drug‐associated AE. Moreover, the analysis was performed based on the publicly available FAERS database that has numerous limitations, which makes it unfeasible to determine causality between PPI use and fractures. It should also be noted that the dynamic PRR data‐mining software searches for a specific preferred term (PT) such as “FRACTURE,” and not for all PTs that contain this word. The data extraction was also limited to data entered between 2004 and 2015. Nonetheless the dynamic PRR tool served our purpose well for evaluating how the safety signal evolved over the 10‐year period. The PRR scores for all PPIs remained consistently within a narrow range (1–2.25) for the extended period of time. Moreover, the PRR values for generic and brand name PPIs yielded a striking stability and similarity across the strata.
The original basis for the evaluation of the risk of long‐term use of PPIs and fracture risk came about due to a submitted reformulation of a marketed PPI where there was a significant deviation in the 90% CI. From a regulatory point of view, the FDA had only a few options:
-
1.
Reject the application as the sponsor has failed to demonstrate bioequivalence;
-
2.
Acknowledge the difference but determine that the product still has adequate safety and efficacy for the intended use, as described in the approved labeling.
In order to accept the second option the sponsor would have had to demonstrate not only the efficacy, which is conceded, but that the higher drug levels are safe. One approach to do so would be to examine if there was a higher dose approved or one that was clinically studied but not marketed, but for which there is a relevant and adequate body of safety data that can be evaluated. In the case of PPIs there is general acceptance that the use of these agents (under the conditions as described in the package insert) is safe; however, current medical practice has evolved since most of these were approved. In addition, as mentioned in this article, the safety concerns of PPIs have also evolved from acute concerns to chronic concerns that were not specifically considered or studied at the time of approval (i.e., the studies were of insufficient duration to assess these issues).
Finally, this seemingly simple question of equivalence is in itself wrapped up in other questions that cut to the core of being a regulator, where decisions reached for one product have far‐reaching implications for precedent and, in this example, include:
-
1.
What would be an appropriate body of information (population and duration) to assure the safe use of this new formulation, assuming the increased Cmax value?
-
2.If approved as a product replacement, how would the FDA regulate new generics to this PPI in light of the fact that there are current generics available that are equivalent to the “old” formulation?
-
a)Two reference products (old vs. new)
-
b)Interchangeability
-
c)Naming convention
-
a)
-
3.
How would labeling be constructed to make it clear that although the “new” product produces higher levels, that these levels in and of themselves have not been demonstrated to offer any efficacy advantages, despite the perception that higher levels are viewed as being more efficacious.
-
4.What would the implication be to the overall bioavailability/bioequivalence assessment paradigm, in that a product with such widely different performance was approved as a replacement product without new clinical trials?
-
a)Could products that were previously turned down by the agency challenge their nonapproval on the basis of the BE argument that they were the same or “better” than this one?
-
a)
4a is a particularly troubling issue for the FDA, as if approved without clinical trials it would be viewed as a precedent and could be used to unwittingly complicate a BE paradigm that has served the American public well over these last 40+ years. Complicating it is that one of the assurances of the in vivo bioavailability/bioequivalence testing paradigm is that there is a tight alignment between reformulations, generic, and reference comparisons, and other such performance questions.
Ultimately, the choice boils down, as is said by the FDA: “It is not what you or I believe, but what we can prove.” In this situation, as the levels are so markedly different between the two formulations, and in the absence of a study demonstrating that there is no safety concern accrued by these increased levels that the decision to not approve this reformulation was determined to be the correct one.
In the spirit of the aforementioned statement, our review could neither prove nor disprove the clinical significance of the Cmax bioinequivalence and the relationship between Cmax and the occurrence of fractures in PPI users. This is because this specific question outstrips the possibility of currently existing methodologies to give a quantitative precise answer for the increase in Cmax demonstrated by the BE studies given the retrospective nature of the question. There is a lack of direct evidence of harmful effects and only indirect observations of a positive but marginal association between PPI use and fracture risk. The subtle changes in nutritional status that may cause long‐term AEs are very difficult to detect, as these changes only become apparent over time.
While a small increase in Cmax may not be clinically evident, an increase of 50% could be, especially when the drug is used continuously for a long time. The magnitude of the risk for fractures with PPIs based on the current available data, if present, is relatively low. According to the estimation by Laine, it would translate into an additional 0.13% of the population over 50 years of age developing hip fractures annually.59 Whether a 50% increase in Cmax would translate into a greater proportion of fractures and other AEs in chronic PPI users, changing the safety signal from marginal to obvious, is not known and may remain unknown for a long time until a sufficient number of subjects accumulate who have been on continuous (or nearly continuous) exposure to PPIs for the required number of years to see such a risk mature.
In conclusion, the long‐term safety issues such as an increased fracture risk in PPI users cannot be excluded. Overprescribing and long‐term PPI use that was not considered in the original risk–benefit approval “metric” are plausible explanations, although no definite conclusion can be drawn from the studies conducted so far. Future studies evaluating the change in PPIs prescription pattern over time are needed. The ability to do these studies will be hampered by the expected exiting of the marketplace by innovator companies once marketing becomes economically less viable. In such situations, the lodging of the data in a university consortia or in a “safe harbor” may be necessary to ensure the ability of such long‐term evaluations to be made.
Supporting information
Supplemental Data‐Table A.‐Summary of the Animal Studies Reviewed
Supplemental Data‐Table B.‐Summary of the Human Studies Reviewed
Acknowledgments
The opinions expressed are those of the authors, and should not be interpreted as the position of the US Food and Drug Administration. This project was supported in part by an appointment to the research participation program at the Center of Drug Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the US Food and Drug Administration.
Author Contributions
E.D.B., E.D., P.B‐B., L.P.K., J.L., and C.M. wrote the article; E.D.B. designed the research; E.D. and P.S. performed the research; E.D.B., E.D., P.S., and J.L. analyzed the data.
Conflict of Interest
E.D. was an ORISE fellow. No financial disclosures. All other authors have no financial disclosures.
References
- 1. U.S. Food and Drug Administration . Statistical Approaches to Establishing Bioequivalence. (issued January 2001) http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdff Accessed 11 June 2015.
- 2. U.S. Food and Drug administration . Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs — General Considerations. Guidance for Industry. March 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389370.pdf. Accessed 11 June 2015.
- 3. Bois, F.Y. , Tozer, T.N. , Hauck, W.W. , Chen, M.L. , Patnaik, R. & Williams, R.L. Bioequivalence: Performance of several measures of extent of absorption. Pharm. Res. 11, 715–722 (1994). [DOI] [PubMed] [Google Scholar]
- 4. European Medicines Agency . Committee for medicinal products for human use. Guideline on the investigation of bioequivalence. London, 20 January 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf Accessed 26 June 2015.
- 5. Johnson, D.A. & Oldfield IV, E.C. Perspectives in clinical gastroenterology and hepatology. Clin. Gastroent. Hepatol. 11, 458–464 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Open FDA Dynamic PRR http://54.201.82.167/dynprr/ Accessed 02 July 2015.
- 7. EMEA . Eudravigilance Expert Working Group (EV‐EWG) . Guideline on the use of statistical signal detection methods in the Eudravigilance data analysis system. 2008 http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500011437.pdf Accessed 26 July 2015.
- 8. Evans, S.J. , Waller, P.C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486 (2001). [DOI] [PubMed] [Google Scholar]
- 9. U.S. Food and Drug Administration . Code of Federal Regulations. 21CFR320.23 (b). http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.23 Accessed 18 July 2015.
- 10. Chonan, O. , Takahashi, R. , Yasui, H. & Watanuki, M. Effect of L‐lactic acid on calcium absorption in rats fed omeprazole. J. Nutr. Sci. Vitaminol. 44, 473–481 (1998). [DOI] [PubMed] [Google Scholar]
- 11. Histing, T. , et al Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif. Tissue Int. 90, 507–514 (2012). [DOI] [PubMed] [Google Scholar]
- 12. Joo, M.K. , et al The effect of a proton pump inhibitor on bone metabolism in ovariectomized rats. Mol. Med. Rep. 7, 1267–1272 (2013). [DOI] [PubMed] [Google Scholar]
- 13. Hyun, J.J. , et al Effect of omeprazole on the expression of transcription factors in osteoclasts and osteoblasts. Int. J. Mol. Med. 26, 877–883 (2010). [DOI] [PubMed] [Google Scholar]
- 14. Prause, M. , Seeliger, C. , Unger, M. , Balmayor, E.R. , van Griensven, M. & Haug, A.T. Pantoprazole decreases cell viability and function of human osteoclasts in vitro. Mediat. Inflamm. 2015, 413097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasanin, A.H. Impact of omeprazole on bone remodeling in normal and ovariectomized Wistar rats. Eur. Rev. Med. Pharmacol. Sci. 18, 1948–1956 (2014). [PubMed] [Google Scholar]
- 16. Fossmark, R. , et al Decreased bone mineral density and reduced bone quality in H(+) /K(+) ATPase beta‐subunit deficient mice. J. Cell Biochem. 113, 141–147 (2012). [DOI] [PubMed] [Google Scholar]
- 17. Dobrowolski, P. , et al Can 2‐oxoglutarate prevent changes in bone evoked by omeprazole? Nutrition 29, 556–561 (2013). [DOI] [PubMed] [Google Scholar]
- 18. O'Connell, M.B. , Madden, D.M. , Murray, A.M. , Heaney, R.P. & Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am. J. Med. 118, 778–781 (2005). [DOI] [PubMed] [Google Scholar]
- 19. Sharara, A.I. , et al Proton pump inhibitors have no measurable effect on calcium and bone metabolism in healthy young males: A prospective matched controlled study. Metabolism 62, 518–526 (2013). [DOI] [PubMed] [Google Scholar]
- 20. Ozdil, K. , et al Bone density in proton pump inhibitors users: a prospective study. Rheumatol. Int. 33, 2255–2260 (2013). [DOI] [PubMed] [Google Scholar]
- 21. Targownik, L.E. , Lix, L.M. , Leung, S. , & Leslie, W.D. Proton‐pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 138, 896–904 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Targownik, L.E. , et al CaMos Research Group. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population‐based study (corrected) from the Canadian Multicentre Osteoporosis Study (CaMos). Am. J. Gastroenterol. 107, 1361–1369 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maggio, M. , et al Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 57, 437–442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirkpantur, A. , Altun, B. , Arici, M. & Turgan, C. Proton pump inhibitor omeprazole use is associated with low bone mineral density in maintenance haemodialysis patients. Int. J. Clin. Pract. 63, 261–268 (2009). [DOI] [PubMed] [Google Scholar]
- 25. Khalili, H. , Huang, E.S. , Jacobson, B.C. , Camargo, C.A. Jr , Feskanich, D. & Chan, A.T. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ 344, e372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cea Soriano, L. , Ruigómez, A. , Johansson, S. & García Rodríguez, L.A. Study of the association between hip fracture and acid‐suppressive drug use in a UK primary care setting. Pharmacotherapy 34, 570–581 (2014). [DOI] [PubMed] [Google Scholar]
- 27. Abrahamsen, B. & Vestergaard, P. Proton pump inhibitor use and fracture risk ‐ effect modification by histamine H1 receptor blockade. Observational case‐control study using National Prescription Data. Bone 57, 269–271 (2013). [DOI] [PubMed] [Google Scholar]
- 28. Fraser, L.‐A. , Leslie, W.D. , Targownik, L.E. , Papaioannou, A. , Adachi, J.D. & CaMos Research Group . The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos. Int. 24, 1161–1168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis, J.R. , et al Long‐term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study. J. Bone Miner. Res. 29, 2489–2497 (2014). [DOI] [PubMed] [Google Scholar]
- 30. Vestergaard, P. , Rejnmark, L. & Mosekilde, L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif. Tissue Int. 79, 76–83 (2006). [DOI] [PubMed] [Google Scholar]
- 31. Yang, Y.X. , Lewis, J.D. , Epstein, S. & Metz, D.C. Long‐term proton pump inhibitor therapy and risk of hip fracture. JAMA 296, 2947–2953 (2006). [DOI] [PubMed] [Google Scholar]
- 32. Yu, E.W. , et al Acid‐suppressive medications and risk of bone loss and fracture in older adults. Calcif. Tissue Int. 83, 251–259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Targownik, L.E. , Lix, L.M. , Metge, C.J. , Prior, H.J. , Leung, S. & Leslie, W.D. Use of proton pump inhibitors and risk of osteoporosis‐related fractures. CMAJ 179, 319–326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaye, J.A. & Jick, H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 28, 951–959 (2008). [DOI] [PubMed] [Google Scholar]
- 35. De Vries, F. , Cooper, A.L. , Cockle, S.M. , van Staa, T.P. & Cooper, C. Fracture risk in patients receiving acid‐ suppressant medication alone and in combination with bisphosphonates. Osteoporos. Int. 20, 1989–1998 (2009). [DOI] [PubMed] [Google Scholar]
- 36. Roux, C. , et al Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif. Tissue Int. 84, 13–19 (2009). [DOI] [PubMed] [Google Scholar]
- 37. Corley, D.A. , Kubo, A. , Zhao, W. & Quesenberry, C. Proton Pump Inhibitors and Histamine‐2 Receptor Antagonists are Associated with Hip Fractures among At‐Risk Patients. Gastroenterology 139, 93–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gray, S.L. , et al Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women. Arch. Intern. Med. 170, 765–771 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiu, H.F. , Huang, Y.W. , Chang, C.C. & Yang, C.Y. Use of proton pump inhibitors increased the risk of hip fracture: a population‐based case‐control study. Pharmacoepidemiol. Drug Saf. 19, 1131–1136 (2010). [DOI] [PubMed] [Google Scholar]
- 40. Pouwels, S. , et al Use of proton pump inhibitors and risk of hip/femur fracture: a population‐based case‐control study. Osteoporos. Int. 22, 903–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abrahamsen, B. , Eiken, P. & Eastell, R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch. Intern. Med. 171, 998–1004 (2011). [DOI] [PubMed] [Google Scholar]
- 42. Lee, J. , et al A population‐based case‐control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. Gastroenterology 48, 1016–1022 (2013). [DOI] [PubMed] [Google Scholar]
- 43. Reyes, C. , et al Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone 52, 557–561 (2013). [DOI] [PubMed] [Google Scholar]
- 44. Ding, J. , Heller, D.A. , Ahern, F.M. & Brown, T.V. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcif. Tissue Int. 94, 597–607 (2014). [DOI] [PubMed] [Google Scholar]
- 45. Adams, A.L. , Black, M.H. , Zhang, J.L. , Shi, J.M. & Jacobsen, S.J. Proton‐pump inhibitor use and hip fractures in men: a population‐based case‐control study. Ann. Epidemiol. 24, 286–290 (2014). [DOI] [PubMed] [Google Scholar]
- 46. Moberg, L.M.E. , Nilsson, P.M. , Samsioe, G. & Borgfeldt, C. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA study. Maturitas 78, 310–315 (2014). [DOI] [PubMed] [Google Scholar]
- 47. Prieto‐Alhambra, D. , et al Predictors of fracture while on treatment with oral bisphosphonates: a population‐based cohort study. J. Bone Miner. Res. 29, 268–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freedberg, D.E. , et al Use of proton pump inhibitors is associated with fractures in young adults: a population‐based study. Osteoporosis Int. 26, 2501–2507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solomon, D.H. , et al Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: A SWAN Cohort Study. J. Bone Miner. Res. 30, 232–239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peery, A.F. , et al Burden of gastrointestinal disease in the United States. Gastroenterology 143, 1179–1187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. AstraZeneca Annual Report and Form 20‐F Information 2013 . http://www.astrazeneca-annualreports.com/2013/_assets/pdfs/Strategic_report.pdf (accessed 2015 June 08).
- 52. Roberts, S.J. & Bateman, D.N. Prescribing of antacids and ulcer healing drugs in primary care in the north of England. Aliment. Pharmacol. Ther. 9, 137–143 (1995). [DOI] [PubMed] [Google Scholar]
- 53. Lassen, A. , Hallas, J. & Schaffalitzky De Muckadell, O.B. Use of antisecretory medication: a population‐based cohort study. Aliment. Pharmacol. Ther. 20, 577–583 (2004). [DOI] [PubMed] [Google Scholar]
- 54. Ryder, S.D. , O'Reilly, S. , Miller, R.J. , Ross, J. , Jacyna, M.R. & Levi, A.J. Long‐term acid suppressing treatment in general practice. BMJ 308, 827–830 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boutet, R. , Wilcock, M. & MacKenzie, I. Survey on repeat prescribing for acid suppression drugs in primary care in Cornwall and the Isles of Scilly. Aliment. Pharmacol. Ther. 13, 813–817 (1999). [DOI] [PubMed] [Google Scholar]
- 56. Pollock, K. , Grime, J. The cost and cost‐effectiveness of PPIs – GP perspectives and responses to a prescribing dilemma and their implications for the development of patient‐centered healthcare. Eur. J. Gen. Pract. 9, 126–133, 140 (2003). [DOI] [PubMed] [Google Scholar]
- 57. Martin, R.M. , Lim, A.G. , Kerry, S.M. & Hilton, S.R. Trends in prescribing H2‐receptor antagonists and proton pump inhibitors in primary care. Aliment. Pharmacol. Ther. 12, 797–805 (1998). [DOI] [PubMed] [Google Scholar]
- 58. Raghunath, A.S. & Hungin, A.P.S. Proton Pump inhibitors Understanding the prescribing behaviour of general practitioners. Gut 49, A1461 (2001). [Google Scholar]
- 59. Laine, L. Proton Pump Inhibitors and Bone Fractures? Am. J. Gastroenterol. 104, S21–S26 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data‐Table A.‐Summary of the Animal Studies Reviewed
Supplemental Data‐Table B.‐Summary of the Human Studies Reviewed


