Abstract
Objectives
To assess if in chronic venous insufficiency, there is a seasonal variation of cytokines levels which could explain the typical worsening of symptoms during Spring and Summer.
Participants
From 193 chronic venous insufficiency patients, we selected 32 patients in clinical stage C2–C3 of the Clinical–Etiology–Anatomy–Pathophysiology classification.
Design
A prospective, comparative and blinded cytokines assessment in two different seasons.
Setting
We sorted patients by two homogenous groups, 17 Autumn Group and 15 Spring Group. A complete clinical and haemodynamic assessment and laboratory analysis of 22 circulating cytokines were performed on each patient.
Main outcome measures
Circulating cytokines levels assessment.
Results
The two groups resulted homogenous for age, gender, clinical class, and haemodynamic parameters. Comparing cytokines expressions in Autumn Group vs. Spring Group, we found a significant difference of 11 out of 22 circulating cytokines (p < 0.05). Particularly Eotaxin, Interleukin-8, Monocyte Chemoattractant Protein-1, Tumour Necrosis Factor-α and Vascular Endothelial Growth Factor were increased in Autumn compared to the Control Group (p < 0.001); while significantly reduced in Spring, within the normal range (p, not significant).
Conclusions
Symptoms of chronic venous insufficiency are self-reported by patients more intense during warm seasons. Surprisingly, in our study, cytokines levels were significantly higher during Autumn and downregulated in Spring. These variations show for the first time the presence of a ‘Calendar of Cytokines’ in chronic venous insufficiency, which needs to be further investigated.
Keywords: Cytokines, chronic venous insufficiency, seasonality, inflammation, vitamin D
Introduction
Ethio-pathogenesis of chronic venous disease (CVD) is not fully understood. The pathophysiology is dominated by lower limbs venous hypertension. In most cases, it is caused by reflux through incompetent valves, condition that can be originated from a primary valvular failure or secondary to parietal dilation.1
It is well known how in course of CVD the inflammatory process is dominated by the so-called white cell trapping phenomenon.2 On the endothelium side, inflammation is characterized by a cytokine cascade with activation of matrix metallo proteinases and sustained remodelling of the valves and venous wall.3 However, the effective contribution of haemodynamics to the inflammatory phenotype of the endothelium is unknown. In vitro investigations, aimed to understand the contribution of flow to atherosclerosis, have already demonstrated the direct relationship between haemodynamic forces and endothelial expression,4 while a laminar flow is associated with low inflamed vessel walls,5 an oscillatory flow is linked to a pro-inflammatory endothelial lining.6
Reflux in the veins of the lower limbs is a perfect example of oscillatory flow.1,7 There is an upward component at muscular systole followed by a reverse flow wave at muscular diastole (Figure 1).
Figure 1.
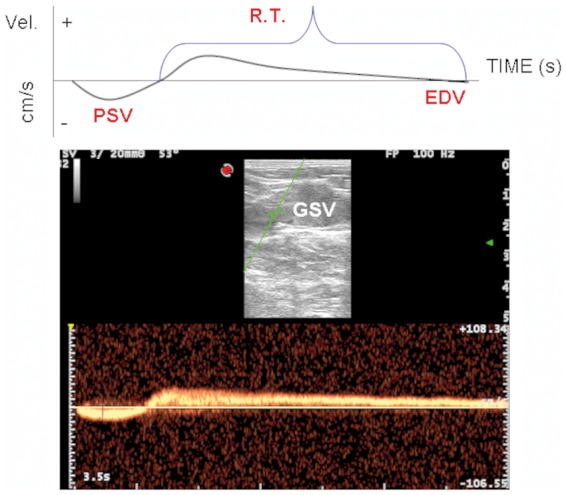
The classic oscillatory flow of venous reflux, with bi-directional positive and negative components are depicted. Top: exemplification of the parameter assessed, PSV, EDV, and RT. Bottom: duplex scanning of the GSV 15 cm below the junction, longitudinal access, where the parameters were assessed. PSV: peak systolic velocity; EDV: end diastolic velocity; RT: reflux time; GSV: great saphenous vein.
Chronic venous insufficiency (CVI) is a very spread disease.8 In all cases, the symptoms of affected patients are always reported as the most frequent during the warm seasons, Spring and Summer in our latitudes, consequently leading to a greater demand for treatments and access to the health system.9,10
The aim of this study is to verify if there is a chrono-biological variation of the circulating cytokines, related to endothelial function in CVI patients, in the different seasons of the year, and in case of variations, verify if this behaviour can be related to the clinical aspects of the disease.
Materials and methods
Patients’ population and samples collection
From a cohort of 193 patients affected by primary CVD, we selected patients for the present study according to the following inclusion criteria:
Primary CVD
Clinical–Etiology–Anatomy–Pathophysiology (CEAP) clinical class ranging from 2 to 3
Reflux confined to the GSV territory
Age 18–65 years old
BMI ≤ 28
Willing to participate to the study
Exclusion criteria were the followings:
Absence of concomitant acute and chronic inflammatory diseases
Steroids and immunosuppressant therapy
Presence of active or healed Venous Leg Ulcer
Smoking
Absence of significant comorbidities affecting the cardiovascular, hepatic, renal and nervous apparatus
Concomitant reflux in the SSV, deep venous system, pelvic veins, contralateral limb
Surgical, compressive, sclerotherapic, pharmacological treatment of CVI in the last 12 months
Thirty-two patients fulfilled the inclusion and exclusion criteria and entered the study. They were therefore sorted them by two homogenous groups, depending on the time of year when they have been subjected to surgical treatment in the Day Surgery:
Autumn Group (October to December) consists of 17 subjects (11 women and 6 men) with a mean age of 54.5 ± 12 years;
Spring Group (April to June) consists of 15 subjects (10 women and 5 men) with a mean age of 49.7 ± 13.4 years.
All the patients underwent an echo-colour-Doppler (ECD) investigation (ESAOTE My-Lab 70, Esaote Genoa, Italy), in standing position with complete scanning of the great saphenous vein (GSV) and small saphenous vein (SSV) systems, including junctions and tributaries. In addition, the main trunk of the deep venous system and the perforators were completely examined. Calf muscular pump was elicited by manual squeezing, considering as reflux the detection of a reverse flow lasting more than 0.5 s in all the examined segments.11
Moreover, at 15 cm distal to the sapheno-femoral junction, the following haemodynamic parameters were assessed into the GSV: peak systolic velocity (PSV), end diastolic velocity (EDV) and reflux time (RT) (Figure 1).
Blood samples were collected from the patients arm before the surgical treatment before entering the operating room. The samples were immediately sent to the biological laboratory for centrifugation and plasma isolation.
Surgery consisted in CHIVA conservative haemodynamic correction.12
Analysis of cytokines and chemokines in plasma samples
Plasma samples were frozen and thawed only once before performing the MILLIPLEX MAP Human Cytokine/Chemokine Panel (Merck Millipore, Billerica, MA), a bead-based multiplex immunoassay, which allows the simultaneous quantification of the following 29 human cytokines: Interleukin-1α (IL-1α), IL-1β, Interlukin-1 receptor antagonist (IL-1 ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, Interlukin-12 protein 40 (IL-12(p40)), Interlukin-12 protein 70 (IL-12(p70)), IL-13, IL-15, IL-17 A, Epidermal Growth Factor (EGF), Eotaxin, Granulocyte Colony Stimulating Factor (G-CSF), Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), Interferon-α2 (IFN-α2), IFN-γ, Interferon gamma-induced Protein 10 (IP-10), Monocyte Chemoattractant Protein-1 (MCP-1), Macrophage Inflammatory Protein-1α (MIP-1α), MIP-1β, Tumour Necrosis Factor-α (TNF-α), TNF-β and Vascular Endothelial Growth Factor (VEGF). Moreover, a custom made MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel (Merck Millipore) was used to quantify the cytokines Platelet Derived Growth Factor-AB/BB (PDGF-AB/BB) and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES). Samples were processed in duplicate following the manufacturer’s recommended protocols and read on a MAGPIX instrument equipped with the MILLIPLEX-Analyst Software using a five-parameter nonlinear regression formula to compute sample concentrations from the standard curves.
Statistical analysis
The data were reported as mean ± standard deviation. The results were compared by using Student’s t-test for paired data and Wilcoxon test for unpaired data, when appropriate. Spearman’s correlation coefficient was calculated to identify correlation between haemodynamic and lab parameters. Statistical significance was defined as p < 0.05. All statistical analyses were performed with GraphPad Instat software (San Diego, CA).
Control group
We used as Control Group healthy subjects previously assessed and also described in Literature from our Center. These subjects have been assessed throughout the entire solar year and were matched for age and gender with the two patients groups.13,14
Results
Haemodynamic results
Clinically, the two groups of patients resulted totally homogenous for age, gender, clinical class and all the haemodynamic parameters. We calculated the significance values, comparing the values of haemodynamic parameters collected in the Autumn Group with the values of haemodynamic parameters collected in the Spring group: none of the results reached statistical significance values as shown in Table 1.
Table 1.
Analysis of all the haemodynamic parameters calculated in the two patient groups.
| Haemodynamics parameters | Mean ± SD Autumn | Median Autumn | Mean ± SD Spring | Median Spring | P value Significative < 0.05 |
|---|---|---|---|---|---|
| PSV (cm/s) | 35.70 ± 17.3 | 34 | 44.46 ± 12.01 | 45 | 0.10 |
| EDV (cm/s) | 13.1 ± 7.75 | 12 | 15.66 ± 9.88 | 11 | 0.42 |
| RI | 1.39 ± 0.17 | 1.4 | 1.36 ± 0.84 | 1.39 | 0.63 |
| RT (s) | 2.94 ± 0.72 | 2.9 | 3.08 ± 0.84 | 3.2 | 0.63 |
SD: standard deviation; PSV: peak systolic velocity; EDV: end diastole velocity; RI: resistance index; RT: reflux time.
Correlation between circulating levels of cytokines/chemokines in the two groups
In Table 2, the change of levels of cytokines of the two groups of patients is given, respectively, and together to the control group and the level of significance as well.
Table 2.
Analysis of all cytokines/chemokines in plasma samples calculated in the two patient groups. Statistical analysis between the two groups and the control group, respectively.
| Cytokine/ chemokine | Detection range | Mean ± SD Control group | Mean ± SD Autumn group | Mean ± SD Spring group | P value Autumn vs. Spring | P value Autumn vs. control | P value Spring vs. control |
|---|---|---|---|---|---|---|---|
| EGF | 3.21 | 40 ± 23.30 | 135.47 ± 158.02 | 28.60 ± 34.56 | 0.013 | <0.0001 | 0.002 |
| EOTAXIN | 3.20 | 54.90 ± 26.00 | 282.63 ± 144.58 | 60.11 ± 22.04 | 0.001 | <0.0001 | 0.358 |
| G-CSF | 3.24 | 17.9 ± 6.8 | 28.41 ± 19.41 | 18.40 ± 8.12 | 0.059 | <0.0001 | 0.735 |
| GM-CSF | 1.58 | 4.9 ± 1.9 | 5.22 ± 2.52 | 3.40 ± 1.59 | 0.015 | 0.477 | <0.001 |
| IFN-α2 | 0.72 | 12.00 ± 8.3 | 12.93 ± 8.95 | 9.80 ± 4.96 | 0.209 | 0.641 | 0.226 |
| IFN-γ | 1.37 | 3.7 ± 1.9 | 10.99 ± 16.30 | 4.68 ± 8.86 | 0.168 | <0.001 | 0.024 |
| IL-12(p70) | 1.6 | 3.8 ± 2.00 | 4.93 ± 6.45 | 2.89 ± 1.36 | 0.250 | 0.028 | 0.070 |
| IL-13 | 0.82 | <OOR | 3.03 ± 2.50 | 5.17 ± 4.16 | 0.477 | NA | NA |
| IL-15 | 1.06 | <OOR | 1.40 ± 1.73 | 0.99 ± 1.78 | 0.231 | NA | NA |
| IL-17a | 1.28 | <OOR | 4.55 ± 5.11 | 4.72 ± 4.03 | 0.955 | NA | NA |
| IL-1ra | 1.8 | 20.3 ± 10.00 | 18.31 ± 15.28 | 13.25 ± 6.13 | 0.21 | 0.413 | 0.001 |
| IL-6 | 0.5 | <OOR | 2.36 ± 1.09 | 1.37 ± 0.64 | 0.120 | NA | NA |
| IL-7 | 0.4 | 2.9 ± 1.3 | 2.90 ± 2.47 | 1.82 ± 0.83 | 0.101 | 0.976 | <0.001 |
| IL-8 | 1.51 | 2.8 ± 1.8 | 17.81 ± 24.67 | 3.58 ± 1.73 | 0.028 | <0.001 | 0.163 |
| IP-10 | 1.9 | 203.5 ± 67.8 | 336.82 ± 161.09 | 290.57 ± 161.34 | 0.385 | <0.001 | <0.001 |
| MCP-1 | 4.00 | 162.50 ± 56.00 | 294.39 ± 139.94 | 166.05 ± 70.40 | 0.002 | <0.001 | 0.802 |
| MIP-1a | 0.9 | 3.6 ± 0.40 | 19.74 ± 13.72 | 6.96 ± 3.42 | 0.010 | <0.001 | <0.001 |
| MIP-1b | 8.3 | 20.20 ± 6.80 | 45.87 ± 28.44 | 16.57 ± 7.80 | <0.001 | <0.001 | 0.014 |
| TNF-α | 0.6 | 3.8 ± 1.6 | 6.77 ± 2.92 | 4.22 ± 1.74 | 0.003 | <0.001 | 0.223 |
| VEGF | 3.00 | 34.80 ± 17.00 | 85.51 ± 77.51 | 47.92 ± 43.80 | 0.033 | <0.001 | 0.399 |
| PDGF – AB | 8.6 | 5476.00 ± 5027.00 | 12929.47 ± 13811.65 | 2336 ± 1767.50 | 0.006 | <0.001 | 0.004 |
| RANTES | 2.2 | 16126.00 ± 15567 | 664473.47 ± 66995.02 | 3779.80 ± 3516.87 | 0.001 | <0.001 | <0.001 |
All cytokine values are expressed in pg/ml. <OOR (Out of (below) detection range) means the values are lower than instrumentally detectable. The statistical significance (grey box) is p < 0.05 values. NA (not available) is meant when the statistical evaluation was not executable.
The first and most important finding is that 11 out of 22 cytokines show a statistically significant difference between the group and the Autumn Spring: EGF, Eotaxin, GM-CSF, IL-8, MCP-1, MIP-1 a, MIP-1 b, TNF-α, VEGF, PDGF-BB and RANTES. All of them are known to be pro-inflammatory cytokines.
In particular observation, among the cytokines statistically different between the two groups of patients, different trends are of particular interest results compared with the values assumed in control group.13,14
The most interesting and significant among these was found to be shown by five cytokines (Eotaxin, IL-8, MCP-1, TNF-α, VEGF), which express a value of highly elevated inflammation in the autumn well above the control group levels; while they fall within the normal range during the Spring period, with a value of p never significant, marking of a lower inflammation state:
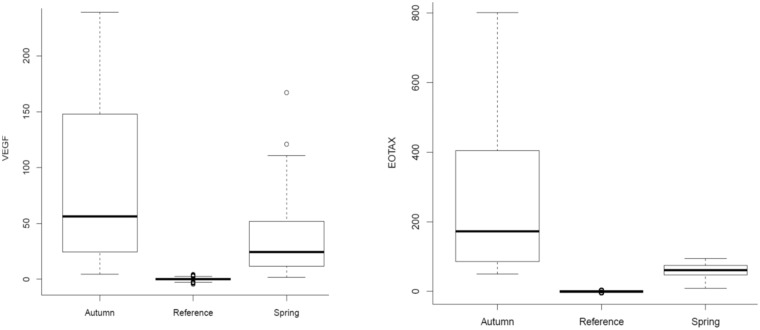
Eotaxin (Figure 2) is 282.63 ± 144.58 pg/ml in the Autumn period (p < 0.001), while in Spring, the values drop to 60.11 ± 4.22 pg/ml (p = 0.358);
Interleukin 8 in the group presents Autumn 17.81 ± 24.67 pg/ml (p < 0.001 compared to control) sagging 3.58 ± 1.73 pg/ml in Spring (p = 0.163);
MCP-1 in the Autumn assumes a value of 294.39 ± 139.94 pg/mL (p < 0.001 compared to control), while in Spring, the values of 166.05 ± 70.40 pg/ml (p = 0.802);
TNF-α in Autumn Group has a value of 6.77 ± 2.92 pg/ml (p < 0.001 compared to control), while Spring has a value of 4.22 ± 1.74 pg/ml (p = 0.223);
Vascular Endothelial Growth Factor (Figure 2) has a value in the Group Autumn equal to 85.51 ± 77.51 pg/ml (p < 0.001 compared to the control value) that falls in the Spring group at 47.92 ± 43.80 pg/ml (p = 0.399).
Figure 2.
Eotaxin and VEGF in pg/ml values, measured in the plasma of patients, in comparison of the Autumn Group (Autumn), the Spring Group (Spring) and the values taken as Control Group (Reference). VEGF: Vascular Endothelial Growth Factor.
Finally, the completion of the statistical analysis of laboratory results, two cytokines have a parallel and significant trend, that appears to be always significantly higher during the year than the control values, while not exhibit any statistically significant difference in the relationship between both Autumn and Spring:
IP10 in Autumn Group assumes the values of 336.82 ± 161.09 (p < 0.001 compared to control), while in the Spring Group assumes a value of 290.57 ± 161.34 pg/ml (p < 0.001).
INF-γ shows the values of 10.99 ± 16.30 pg/ml in Autumn (p < 0.001 compared to control) and values of 4.68 ± 8.86 pg/ml (p = 0.024) in Spring.
Discussion
The main finding of this study is represented by a circa-annual oscillation of endothelial cytokines in patients affected by CVI. This novel finding is apparently in contrast with the clinical observation of symptoms worsening during the warm season, since the inflammatory status herein assessed is greater during autumn.
The clinical interest at the base of this study was created by the well-known series of symptoms reported by patients suffering from CVI and the absence of a complete clinical and biological evaluation, so far.
From our study, it appears that symptoms are not related to the inflammatory status expressed by a deep analysis of inflammatory cytokines. This suggests us to investigate the levels also in Summer and in winter in order to have a more comprehensive chrono-biological assessment. Our finding also rises the hypothesis that symptoms of CVI are not completely related to the levels of circulating cytokines.
The CVI complications are closely related to endothelial inflammation, which lead to stimulation of an inflammatory biosignaling,5 that we can dose through the circulating inflammatory cytokines.
To prevent other inflammatory events may invalidate the result of the data, through very strict inclusion and exclusion criteria, we excluded all patients who had at the time of measurement of blood parameters other acute or chronic inflammatory disorders. At the same time, to avoid inflammatory phenomena relative to an advanced state of chronic disease, we excluded all patients to a state of advanced disease, retaining only the cases that were comprised between C2 and C3 according to the CEAP classification.
In doing so, from an initial population of 193 patients, we recorded a significant decrease in our study population, but making it more reliable. Analysing the 22 inflammatory cytokines, 11 out of 22 present a statistically difference between the measurement of the Group Autumn (October to December) and the one from the Spring Group (April to June).
Especially five inflammation proteins present a very consistent pattern. Indeed they seem to have a strong seasonality, capable of being significantly above the reference values in Autumn Group, thereby justifying an increased inflammatory value, while reducing up to re-enter within the normal values, in the Spring group. This biological rhythm was not linked to any surgical, compressive or pharmacological treatment. More specifically:
Eotaxin (EOTAX) is a protein with a powerful action towards eosinophils and Th2. Moreover, it plays an important role in ischemia-induced remodelling of vessel walls15;
IL-8, neutrophil chemotactic main factor, plays a key role in promoting angiogenesis and is frequently increased in the presence of oxidative stress phenomena16;
MCP-1 is a cytokine present in the plasma membrane of endothelial cells, with the function to recruit monocytes, memory T cells and dendritic cells in the sites of inflammation produced by a tissue injury or infection17;
TNF is one of the main factors of the inflammatory cascade. It is very present in macrophages and T lymphocytes, inducing chemotaxis of leukocytes and their adhesion to the vascular endothelium, which is the main mechanism of each local inflammatory complex18;
VEGF is the Vascular Endothelial Growth Factor. It plays a crucial and specific role in angiogenesis by regulating proliferation, migration, permeability and survival of endothelial cells derived from veins, arteries and lymphatic vessels. The expression levels are regulated by the oxygen tension in the tissues, as the hypoxia conditions there is an increase in the release, while on the contrary, the normoxia induces a down-regulation of VEGF production and a regression of the formation of vessels.19
The accurate analysis of these results, confirms strong inflammatory vascular changes with the changing seasons throughout the year, suggests resolutely the presence of a protective factor active in the Spring and Summer months, able to inhibit the inflammatory vascular endothelium carried out by these important cytokines.
This factor cannot be related to haemodynamic parameters, because there is no evidence of significant difference compared with the two seasons of the year.
Indeed, the RT, which is considered as the main diagnostic element of CVI, tends to have a slight improvement in the autumn than the hottest period, where instead worsens.
Therefore, the haemodynamic changes and pathological oscillatory flows may be the primary cause of inflammation of the endothelium, but not the factor that protective modulation is present in the Spring.
At the moment in the database of this first study, there are no data in our possession to explain the interesting trends reported by inflammatory cytokines, capable of open up a completely new point of view from the first aim of the study, where we all expected a higher inflammatory state in Spring, strongly connected to higher symptoms.
However, in literature, there are several traces which can be speculatively related to our novel and surprising findings.
For instance, Vitamin D is one of the major actors that presents a chrono-biological characterization, in fact, the crucial role of activation of the molecule can be attributed to sun exposure.20
It is of course significantly shortened in the autumn period, compared to Spring, has the function of allowing the skin to the irradiation of 7-dehydrocholesterol and the formation of cholecalciferol by isomerization.
Our speculative hypothesis could be therefore supported by the action of vitamin D and its narrow clinical and biological correlation with cytokines observed in particular. The Vitamin D, in fact, in addition to the adjustment of the phosphocalcium mechanism, presents a general level a role of suppression of all inflammatory cytokines.21
Among its many actions and functions is a potent modulator of the natural and specific immune system, going to act also on the same T lymphocytes, Th2, T-reg together with dendritic cells and macrophages,22 just as seen for MCP-1, IL-8 TNF-α.
At the same time, there are widespread actions of vitamin D within the context of immune diseases, infectious and autoimmune. These include the emblematic case of multiple sclerosis is,23 capable of a real protective role offered by sun exposure, or the latitudinal difference, compared to the onset of the disease. By the same token, other diseases in immune regulation such as inflammatory bowel diseases,24 vitamin D is a powerful factor in down-regulation of the inflammatory response.
Finally, also in vascular applications, vitamin D plays a crucial role and the arterial side.25 It is now a fact that a deficit of 1.25 dihydroxycholecalciferol, the active form of vitamin D3, exposes patients to an atheromatous process,26 with a mechanism that follows the main actions performed by VEGF.
There are still very few studies however that they correlate vitamin D with the venous endothelium effects, but there is scientific evidence of the protective role against the damage induced by oxidative27 stress and apoptosis. It appears that at the endothelial level, Vitamin D is capable of reducing the gene expression related to apoptosis, the maintenance of mitochondrial function, the activation of protein kinases involved in the mechanisms of survival and the production of nitric oxide.28
On a complete speculative basis, our suggestions and findings seem to be furthermore sustained by vast clinical evaluations. Unlike the reported symptoms, more aggressive in the warmer period, there are many evidences that highlight a greater presence of the complications of CVI in the winter months.
For example, venous ulcers (stage C6 CEAP classification) seem to have a significant increase of their appearance during the winter and autumn months in our latitudes.29 At the same time, Dentali et al.30 confirmed in an impressive systematic review on a base of more than 35,000 patients, a significantly increased risk of TEP during the winter months, and especially during the month of January.
Conclusions
For the first time, it is brought to light a sort of ‘Calendar of Cytokines’, including endothelial inflammation. These assumptions may provide an explanation, through accurate in vivo measurements, of the substantial seasonal difference comparing the typical symptom manifestations of venous disease and local complications closely related to CVI.
In a particular way, this work is established as one of the first prospective studies realized up to now to assess the presence of a factor capable of modulating the venous vascular endothelial inflammation, suggesting a potential role attributed to Vitamin D.
Limits of the study and future perspectives
Limitation of this study is the small population, all collected at the same latitude. At the same time, the absence of analysis during the entire period of the year due to organization aspects and the absence of a vitamin D blood analysis or administration is the lacking point that needs recognition.
Future perspectives must be a wider population in number of patients to be analysed in different groups at different latitudes, a 12-month covering and adding the assessment and the administration of Vitamin D at specified dose.
Final aims will be the definition of a possible protective factor and the drawing of a therapeutic protocol to prevent life-threatening complications in CVD patients.
Acknowledgements
This research has been submitted to John Dawson Medical Student Prize, sponsored by the Royal Society of Medicine – Section of Surgery. It was selected among the finalists works and presented as ‘Oral Presentation’ by the student Paolo Spath, University of Ferrara in London on 15 October 2014.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Italian Ministry of Education, University and Research (MIUR Programme PRIN 2010-2011), Grant no. 2010XE5L2R.
Ethical approval
The study was approved by the Ferrara University-Hospital Ethical Committee.
Guarantor
Paolo Zamboni
Contributorship
P Spath: Literature review, data collection, data interpretation, design, wrote and revised the manuscript. V Tisato: performed the cytokines assessment and data interpretation, read and approved the manuscript. S Gianesini: data collection and data interpretation, read and approved the manuscript. M Tessari: data collection and data interpretation, read and approved the manuscript. E Menegatti: data collection and data interpretation, read and approved the manuscript. R Manfredini: data interpretation, read and approved the manuscript. S Occhionorelli: data interpretation, read and approved the manuscript. P Secchiero: data interpretation and source of financial support, read and approved the manuscript. P Zamboni: concept and design, revised the manuscript, data interpretation and source of financial support.
References
- 1.Bergan JJ, Schmid-Schonbein GW, Smith PD, et al. Chronic venous disease. N Engl J Med 2006; 355: 488–498. [DOI] [PubMed] [Google Scholar]
- 2.Coleridge S, Thomas P, Scurr JH, et al. Causes of venous ulceration: a new hypothesis. Br Med J (Clin Res Ed) 1988; 296: 1726–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisato V, Zauli G, Voltan R, et al. Endothelial cells obtained from patients affected by chronic venous disease exhibit a pro-inflammatory phenotype. PLoS One 2012; 7: e39543–e39543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 2005; 38: 1949–1971. [DOI] [PubMed] [Google Scholar]
- 5.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 1998; 18: 677–685. [DOI] [PubMed] [Google Scholar]
- 6.Hsiai TK, Cho SK, Wong PK, et al. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J 2003; 17: 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower extremity veins. J Vasc Surg 2003; 38: 793–798. [DOI] [PubMed] [Google Scholar]
- 8.Meissner MH, Gloviczki P, Bergan J, et al. Primary chronic venous disorders. J Vasc Surg 2007; 46 Suppl S: 54S–67S. [DOI] [PubMed] [Google Scholar]
- 9.Vasquez MA, Munschauer CE. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebology 2008; 23: 259–275. [DOI] [PubMed] [Google Scholar]
- 10.Cook TA, Michaels JA, Galland RB. Varicose vein clinics: modelling the effects of seasonal variation in referrals. J R Coll Surg Edinb 1997; 42: 400–402. [PubMed] [Google Scholar]
- 11.Zamboni P, Cisno C, Marchetti F, et al. Reflux elimination without any ablation or disconnection of the saphenous vein: A haemodynamic model for venous surgery. Eur J Vasc Endovasc Surg 2001; 21: 361–369. [DOI] [PubMed] [Google Scholar]
- 12.Bellmunt-Montoya S, Escribano JM, Dilme J, et al. CHIVA method for the treatment of chronic venous insufficiency. Cochrane Database Syst Rev 2015(6, CD009648–CD009648. (Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voltan R, Zauli G, Rizzo P, et al. In vitro endothelial cell proliferation assay reveals distinct levels of proangiogenic cytokines characterizing sera of healthy subjects and of patients with heart failure. Mediators Inflamm 2014; 2014: 257081–257081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisato V, Zauli G, Rimondi E, et al. Inhibitory effect of natural anti-inflammatory compounds on cytokines released by chronic venous disease patient-derived endothelial cells. Mediators Inflamm 2013; 2013: 423407–423407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallegos AM, Bevan MJ. Driven to autoimmunity: the nod mouse. Cell 2004; 117: 149–151. [DOI] [PubMed] [Google Scholar]
- 16.Vlahopoulos S, Boldogh I, Casola A, et al. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 1999; 94: 1878–1889. [PubMed] [Google Scholar]
- 17.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta 2010; 411: 1570–1579. [DOI] [PubMed] [Google Scholar]
- 18.Steyers CM, Miller FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014; 15: 11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page P, DeJong J, Bandstra A, et al. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. Int J Cell Biol 2014; 2014: 601063–601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol 2014; 810: 1–16. [PubMed] [Google Scholar]
- 21.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008; 4: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983; 221: 1181–1183. [DOI] [PubMed] [Google Scholar]
- 23.Bjørnevik K, Riise T, Casetta I, et al. Sun exposure and multiple sclerosis risk in Norway and Italy: The EnvIMS study. Multiple Scler J 2014; 20: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 24.Garg M, Lubel JS, Sparrow MP, et al. Vitamin D and inflammatory bowel disease–established concepts and future directions. Aliment Pharmacol Ther 2012; 36: 324–344. [DOI] [PubMed] [Google Scholar]
- 25.Kassi E, Adamopoulos C, Basdra EK, et al. Role of vitamin D in atherosclerosis. Circulation 2013; 128: 2517–2531. [DOI] [PubMed] [Google Scholar]
- 26.Carrelli AL, Walker MD, Lowe H, et al. Vitamin D deficiency is associated with subclinical carotid atherosclerosis: the Northern Manhattan study. Stroke 2011; 42: 2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong W, Gu B, Gu Y, et al. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol 2014; 140: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uberti F, Lattuada D, Morsanuto V, et al. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab 2014; 99: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 29.Klode J, Stoffels I, Körber A, et al. Relationship between the seasonal onset of chronic venous leg ulcers and climatic factors. J Eur Acad Dermatol Venereol 2011; 25: 1415–1419. [DOI] [PubMed] [Google Scholar]
- 30.Dentali F, Ageno W, Rancan E, et al. Seasonal and monthly variability in the incidence of venous thromboembolism: A systematic review and a meta-analysis of the literature. Thromb Haemost 2011; 106: 439–447. [DOI] [PubMed] [Google Scholar]