Abstract
Chronic pain after traumatic brain injury (TBI) is very common, but the mechanisms linking TBI to pain and the pain-related interactions of TBI with peripheral injuries are poorly understood. Chemokine receptors play an important role in both pain and brain injury. In the current work, we pursued the hypothesis that the epigenetically regulated CXC chemokine receptor 2 (CXCR2) is a crucial modulator of nociceptive sensitization induced by TBI. For these studies, we used the rat lateral fluid percussion model of TBI. Histone actyltransferase activity was blocked using anacardic acid beginning immediately following injury, or delayed for seven days prior to administration. The selective CXCR2 antagonist SCH527123 administered systemically or intrathecally was used to probe the role of chemokine signaling on mechanical hindpaw sensitization after TBI. The expression of the CXCR2 receptor was accomplished using real-time PCR, immunohistochemistry, and Western blotting, while epigenetic regulation was assessed using chromatin immunoprecipitation assay. The spinal levels of several pain-related mediators including CXCL1, an endogenous ligand for CXCR2, as well as brain-derived neurotrophic factor and prodynorphin were measured by enzyme-linked immunosorbent assay. We observed that anacardic acid potently blocked and reversed mechanical hindpaw sensitization after TBI. The same drug was able to prevent the upregulation of CXCR2 after TBI, but did not affect the spinal expression of other pain mediators. On the other hand, both systemically and intrathecally administered SCH527123 reversed hindpaw allodynia after TBI. Most of the spinal CXCR2 appeared to be expressed by spinal cord neurons. Chromatin immunoprecipitation experiments demonstrated TBI-enhanced association of the CXCR2 promoter with acetylated-H3K9 histone protein that was also reversible using anacardic acid. Taken together, our findings suggested that TBI causes the upregulation of spinal CXCR2 through an epigenetic mechanism ultimately supporting nociceptive sensitization. The use of CXCR2 antagonists may, therefore, be useful in pain resulting from TBI.
Keywords: Traumatic brain injury, CXCR2, histone acetyltransferase, anarcardic acid, chronic pain
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, affecting all ages and demographics.1 In the United States alone, approximately 1.7 million new cases are reported annually.2,3 Severe TBI results in death in roughly 5% of individuals and long-term disability in greater than 40% of those affected.4,5 The vast majority of these injuries are, however, mild, though even mild injuries have been associated with a number of adverse consequences. TBI results in wide-ranging physical and psychological sequelae including motor deficits, spasticity, memory impairment, anxiety, depression, and other changes with variable patterns of onset and persistence.6,7 One of the most common complaints of patients after TBI is chronic pain; it has been estimated that more than 50% of TBI patients develop chronic pain at some point after their injuries.8 TBI was observed to confer an odds ratio of 5.0 for chronic pain in a recently described military cohort.9 Unlike some of the more severe motor and cognitive consequences, chronic pain after TBI appears to be at least as likely to be experienced by those with mild injuries as more severe ones.8
C-X-C Motif Chemokine Receptor 2 (CXCR2) is a chemokine receptor expressed on the cellular surface of leukocytes including neutrophils, mast cells and monocytes, endothelial cells, and a range of other cells throughout the human body, which was cloned for the first time in 1991 from the human cell line HL-60.10 Several high-affinity endogenous ligands for CXCR2 have been discovered including the C-X-C chemokine ligand CXCL1.11,12 Accumulating evidence suggests that CXCR2 and its corresponding chemokine ligands regulate pain, including pain related to cancer, nerve damage, inflammation, surgery, and chronic opioid administration.13–18 Unclear at this point is how pain after TBI is supported and whether CXCR2 participates although several reports have suggested that CXCR2 is upregulated in the brain after TBI.19–21
The molecular mechanisms underlying the upregulation of CXCR2 in inflammatory and pain-related states remain ill defined. Recently published studies have demonstrated that the expression of CXCR2 involves epigenetic mechanisms. For example, Kiguchi et al.22 reported that the administration of the histone acetyltransferase inhibitor anacardic acid (AA) suppressed the upregulation of CXCR2 in the injured sciatic nerve site in the partial sciatic nerve ligation neuropathic pain model. Our lab demonstrated a role for CXCR2 in postsurgical pain using a mouse incisional model, and showed that CXCR2 was upregulated through histone acetylation in spinal cord tissue.15,16 Moreover, acetylation of histone proteins in spinal cord neurons has been reported in a rat TBI model.23 Here, we hypothesized that TBI causes nociceptive sensitization at least in part by causing the upregulation of spinal CXCR2 expression.
Methods
Animals
Male Sprague–Dawley rats (300 ± 20 g) from Harlan (Indianapolis, IN, USA) were used in these experiments. Each cage housed two rats in 30 × 30 × 19-cm isolator cages with solid floors covered with 3 cm of wood chip bedding, and food and water were available ad libitum. Animals were kept under standard conditions with a 12-h light–dark cycle (6 am to 6 pm). All experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the animal subject guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All in vivo experiments were performed between 10 am and 4 pm in the Veterinary Medical Unit of the Veterans Affairs Palo Alto Health Care System.
TBI surgery
TBI was performed with a modification of the lateral fluid percussion (LFP) rat model described previously.24–26 Briefly, rats were anesthetized using isoflurane inhalation and secured prone in a stereotactic frame. A midline incision in the head was made in the scalp, and underlying periosteum removed. To deliver LFP, a craniotomy of 5 mm in the rat skull was performed between the Bregma and lambda sutures, and centered approx. 2 mm to the right of the midline. Using a method commonly employed in human craniotomies, the bone flap was moved under the adjacent scalp providing better preservation. A female luer-lock hub was implanted at the craniotomy site and fixed using cyanoacrylate glue. Dental acrylic was then applied to the exposed rat skull to secure the lure-lock hub. Following slight recovery, the luer-lock hub was connected to the LFP apparatus (Amscien Instruments, USA), and a pressure wave of 1.5 atm, (±0.1 atm) or no pressure wave (sham) was applied to rat dura. Thereafter, the luer-lock hub and dental acrylic were removed, the bone flap replaced and the overlying wound closed using 4-0 silk suture. Both TBI and sham rats were allowed to recover in their home cages until awake and responsive.
Drug administration
Anarcardic acid (Cayman Biochemical, Ann Arbor, Michigan) and SCH527123 (MCE, Medical Express, NJ) were freshly dissolved in DMSO and diluted in 0.9% saline (final DMSO concentration 10%). For AA experiments, AA (5.0 mg/kg, 100 µl) or vehicle were administered daily via intra-peritoneal (i.p.) injection for seven days beginning immediately after surgery, and in other experiments the initiation of daily injections was delayed for one week after TBI.
For experiments involving systemic SCH527123 administration, animals received SCH527123 (5.0 mg/kg) or vehicle daily via intra-peritoneal (i.p.) injection for seven days beginning immediately after TBI surgery. For Intrathecal (i.t.) injection, rats were slightly anesthetized using isoflurane inhalation, and SCH527123 (4 µg, 2 µl) was injected intrathecally (i.t.) between the L4 and L5 vertebra on day 7 post-TBI.
Behavioral testing
Mechanical allodynia—Mechanical withdrawal thresholds were measured using a modification of the up-down method and von Frey filaments as described previously.27 Animals were placed on wire mesh platforms in clear cylindrical plastic enclosures of 20 cm diameter and 30 cm in height. After 30 min of acclimation, fibers of sequentially increasing stiffness with initial bending force of 2.0 g were applied to the plantar surface of the hind paw, and left in place 5 s with enough force to slightly bend the fiber. Withdrawal of the hind paw from the fiber was scored as a response. When no response was obtained, the next stiffer fiber in the series was applied in the same manner. If a response was observed, the next less stiff fiber was applied. Testing proceeded in this manner until four fibers had been applied after the first one causing a withdrawal response, allowing the estimation of the mechanical withdrawal threshold using a curve fitting algorithm.28
Immunohistochemistry
The techniques employed for immunohistochemical analysis of spinal cord tissue were based on those described previously by our group.16 Briefly, animals were euthanized with CO2 and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, via the ascending aorta. The spinal cords were extruded, and the lumbar section was collected at time points before and at day 2 after TBI surgery. The collected issue was incubated in 0.5 M sucrose in PBS overnight, mounted in Tissue-Tek OCT embedding compound (Sakura Finetek), frozen and cut into 10 µm sections.
Blocking of the sections took place at 4℃ for 1 h in PBS containing 10% normal donkey serum, followed by exposure to the primary antibodies including rabbit anti-CXCR2 antibody (1:50, Santa Cruz, Dallas, TX) and mouse anti-NeuN (1:300, Millipore) overnight at 4℃. Sections were then rinsed and incubated with fluorescein-conjugated secondary antibodies against the primary antibodies (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. Double-labeling immunofluorescence was performed with donkey anti-mouse IgG conjugated with cyanine dye 3 or donkey anti-rabbit IgG conjugated with fluorescein isothiocyanate secondary antibodies. Confocal laser scanning microscopy was carried out using a Zeiss LSM/510 META microscope (Thornwood, NY). Sections from control and experimental animals were processed in parallel. Control experiments omitting either primary or secondary antibody revealed no significant staining.
Enzyme immunoassay for CXCL1, BDNF, and dynorphin levels in spinal cord
Rat spinal cord (L4, 5 lumbar enlargement) was collected after behavioral testing and frozen immediately on dry ice. The spinal cord tissue was cut into fine pieces in ice-cold PBS, pH 7.4, containing a cocktail of protease inhibitors (Roche Applied Science) followed by homogenization using a Dounce tissue grinder. Homogenates were centrifuged for 15 min at 12,000 g, 4℃. The supernatants were aliquoted and stored at −80℃ until required for enzyme immunoassay (EIA) performance. Total protein contents in all tissue extracts were measured using the DC protein assay kit (Bio-Rad). Brain-derived neurotrophic factor (BDNF), prodynorphin (PDYN), and CXCL1 levels were determined in duplicate using respective EIA kits, according to the manufacturer’s instructions. BDNF EIA kit was from LifeSpan BioSciences. PDYN concentration was measured using the PDYN EIA kit (antibodies-online). CXCL1 level was determined with an EIA kit from R&D Systems. These assay systems detect rat BDNF, PDYN, and CXCL1 with sensitivity of 12.29 pg/ml, 3.9 pg/ml, and 1.3 pg/ml, respectively. The concentrations of BNDF, PDYN, and CXCL1 proteins were calculated from the standard curve at each assay. Each protein concentration was expressed as pg/mg total protein.
Western blot
To test whether TBI can induce the expression of C-X-C motif chemokine receptor 2 protein (CXCR2) in the spinal cord posttrauma, rat spinal cord (L4, 5 lumbar enlargement) was collected after treatment and frozen immediately on dry ice. Spinal cord samples were later homogenized in ice-cold Tris buffer with 0.7% (v/v) β-mercaptoethanol and 10% glycerol. Homogenates were centrifuged at 12,000 g for 15 min at 4℃. Total protein concentration of the supernatant was measured with BCA protein assay kit (Thermo Scientific). Then equal amounts of protein (50 µg) were subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a polyvinylidene difluorided membrane. The blots were blocked with 5% non-fat dry milk in tris-buffered saline and incubated with primary CXCR2 antibody on a rocking platform overnight at 4℃. After three washes in Tris-buffered saline with 0.5% Tween-20 (TBST), the membrane was further incubated with HRP-conjugated secondary antibody for 1 hr at room temperature. The membrane was then washed again with TBST, and proteins were detected using ECL chemiluminescence reagent (Thermo Scientific). Bands were quantified by densitometry of digitalized images. Each blot was then stripped and re-probed with β-actin antibody, allowing normalization of expression between samples. All antibodies were from Santa Cruz Biotechnology. The band intensity was analyzed using National Institutes of Health ImageJ. CXCR2/ACTIN band intensity ratio was calculated to demonstrate the change of the specific protein after treatments. The results of all assays were confirmed by repeating the experiment three times.
Chromatin immune precipitation assay
The assay was performed following the protocol adopted and refined previously by our lab.29 Rats were sacrificed using carbon dioxide asphyxiation and the spinal cord lumbar dorsal horn segments were quickly dissected on a pre-chilled surface at day 7 after TBI. Tissue was flash frozen in liquid nitrogen and stored at −80℃ until use. Later, small pieces of minced tissue were cross-linked using 1% formaldehyde and sonicated on ice. The sonicated chromatin was clarified by centrifugation, aliquoted, and snap-frozen in liquid nitrogen. To perform chromatin immune precipitation (ChIP), chromatin (150 μl) was diluted 10-fold and purified with specific antibody against H3K9 acetylated histone protein (Millipore, Waltham, MA) or IgG antibody as control, and protein G-agarose (60 μl). The DNA that was released from the bound chromatin after cross-linking reversal and proteinase K treatment was precipitated and diluted in low-TE buffer (1 mM Tris, 0.1 mM EDTA). Quantitative PCR of target gene promoter enrichment in ChIP samples was done using an ABI prism 7900HT system and SYBR green. Five microliters of input ChIP or IgG sample were used in each reaction in duplicate for four biological samples (N = 4) in each condition. Percentage input was calculated for both the ChIP and IgG samples and then fold enrichment was calculated as a ratio of the ChIP to IgG. An in-plate standard curve determined amplification efficiency30, and the 100 fold dilution factor for the input was included.
Gene expression
For CXCR2 and CXCL1 gene expression, the spinal lumbar dorsal horn segments were quickly dissected on a pre-chilled surface on day 2 and day 7 after TBI, and then total RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA purity and concentration were determined spectrophotometrically as described previously for spinal cord.29 cDNA was synthesized from total RNA using random hexamer primer and a First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Briefly, 1 µg of total RNA was mixed with 4 μl of 10 × RT buffer, 8 μl of 25 mM MgCl2, 4 μl 0.1 M DTT, 1 μl RNasin, 2 μl SSII (50 u/μl), 5 μl hexomers, and RNase-free water to 40 μl. Incubation was then carried out at 42℃ for 60 min followed by heat inactivation at 70℃. Finally, 1 μl RNase H was added to each reaction and incubated at 37℃ for 20 min to degrade the RNA. For real-time quantitative PCR, reactions were conducted in a volume of 10 μl using the SYBR green I master kit (Life Technologies, Foster City, CA). Briefly, 5 μl of a mixture of 2x SYBR green and primers was loaded with 5 μl diluted cDNA template in each well. Following this, 8 μl mineral oil was loaded in each well to prevent loss of solution. Using an ABI prism 7900HT system, PCR was carried out using the parameters 52℃, 5 min → 95, 10 min then [95℃, 30 s→ 60℃, 60 s] for 40 cycles. The primer sequences: CXCR2: atctttgctgtggtcctcgt (forward) and ggggttaagacagctgtgga (reverse); CXCL1: ggattcacttcaagaacatccag (forward) and agcatcttttggacaatcttctg (reverse). Samples were analyzed in triplicate. β-actin was used as an internal control. Quantification was performed with ΔΔCt method.
Rat epigenetic gene expression measurements were performed using the Rat Epigenetic Chromatin Modification Enzymes RT2 Profiler™ PCR Array (Qiagen, Valencia, CA) according to the handbook. It profiles the expression of 84 key genes encoding enzymes known or predicted to modify genomic DNA and histones to control gene expression, which are listed in Supplementary Tables in Supplementary Data section. cDNA synthesis from 1 µg of total RNA was made in 20 μl with RT2 first strand kit (Qiagen, Valencia, CA). After reverse-transcription reaction, cDNA sample was suitably diluted with RNase-free water for PCR analysis. PCR components were prepared with 2 x RT2 SYBR green mastermix, diluted cDNA sample, and RNase-free water. 10 μl of the PCR components of each sample was added to corresponding well of the Profiler PCR Array. Using an ABI prism 7900HT system, PCR was carried out using the parameters 95℃, 10 min then [95℃, 15 s→ 60℃, 60 s] for 40 cycles. PCR data analysis was performed using PCR Array Data Analysis Software (http://www.sabiosciences.com/rt_pcr_product/HTML/PARN-085 Z.html#accessory). The expression of candidate genes showing at least 1.5-fold changes in expression on the array were validated using a similar PCR method with custom primers and 2x SYBR green mastermix. The specific gene primers were ordered from Qiagen. The gene information was listed in Table 1 (Valencia, CA).
Table 1.
Gene expression alteration induced by TBI on day 2 and day 7.
| Gene symbol | Fold regulation | Time (Day) |
|---|---|---|
| Ciita | 1.579 | 2 |
| Crebbp | −1.8312 | 2 |
| Aurkb | 1.7803 | 7 |
| Ciita | 1.8557 | 7 |
| Dnmt3b | −1.7449 | 7 |
| Rnf20 | −1.5055 | 7 |
Statistical analysis
For multiple group comparisons, two-way analysis of variance followed by Newman–Keuls post hoc testing was employed. Prism 6 (GraphPad Software) was used for statistical calculations. Significance was set as p < 0.05. Data are presented as mean values ± standard error of the mean (SEM).
Results
Effects of AA on TBI-induced allodynia
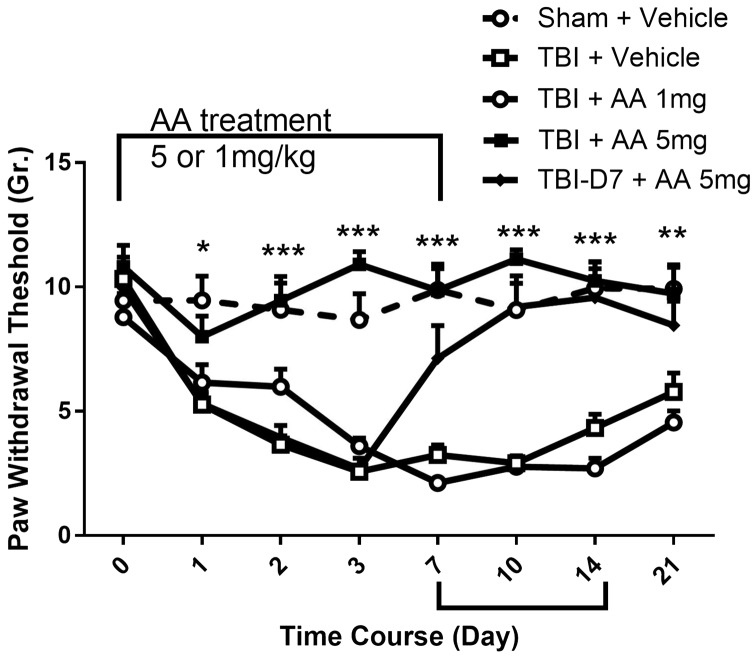
Histone acetylation is a major mechanism governing neuroplasticity. Previous laboratory studies have shown that the activation of histone acetyltransferase activity is required for maximal sensitization and duration of allodynia after incision and other injuries.16 Using AA, a well-characterized non-subtype selective histone acetylation transferase (HAT) inhibitor, we determined the likely contributions of HAT to nociceptive sensitization after TBI. We used this compound at a dose of 1 and 5.0 mg/kg, with the latter dose shown previously to have large effects on sensitization after hindpaw incision.16 In Figure 1 it is shown that the administration of 5 mg/kg AA blocked and reversed nociceptive sensitization after TBI. When AA was administered beginning immediately after injury and continued for seven days, rats failed to develop the mechanical allodynia observed in the vehicle-treated rats. Sensitization after cessation of 5 mg/kg AA administration was similar to control group. To further refine our understanding of AA’s effects, we treated TBI animals with AA beginning seven days after TBI and continuing for another seven days. When administered in this way, the AA-treated animals showed a decrease in hindpaw allodynia resulting in the full normalization of mechanical thresholds.
Figure 1.
Effect of anacardic acid (AA) on TBI-induced nociceptive sensitization induced in rats. AA 1 or 5 mg/kg (i.p.) was injected daily from day 0 to day 7 beginning immediately after injuries. In addition, one group of TBI animals received AA 5 mg/kg beginning on day 7 postinjury and continuing for seven days. Nociceptive sensitivity in hindpaws contralateral to TBI was assessed using von Frey fibers at the indicated time points prior to daily injections. Threshold values are expressed as the mean ± SEM, N = 6. *p < 0.05; **p < 0.01; ***p < 0.001 comparison of contralateral paws in vehicle-treated rats to the paws of AA-treated animals.
Effects of CXCR2 antagonist on nociceptive sensitization after TBI
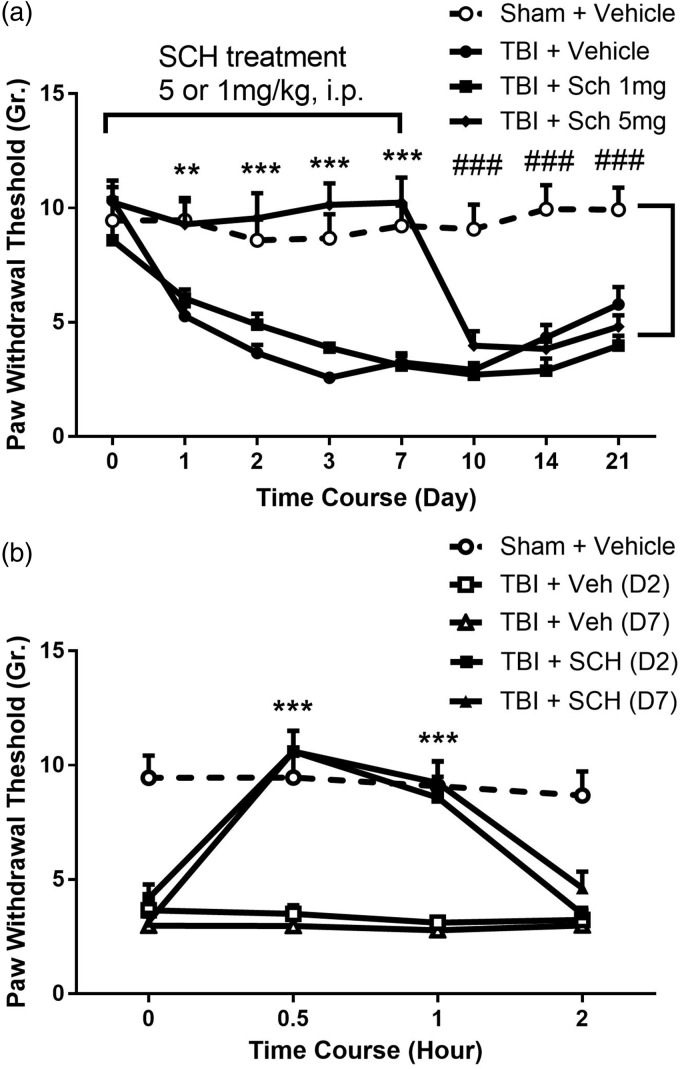
TBI can cause chronic pain in patients and nociceptive sensitization in rodent TBI models.24 Recent work suggests that TBI activates epigenetic changes in spinal dorsal horn neurons through as-yet unidentified mechanisms. Although the expression of many genes is potentially altered though epigenetic changes, the spinal CXCL1/CXCR2 chemokine signaling pathway has been shown to be epigenetically upregulated and participates in mechanical sensitization after limb injury.15,16 Therefore, we hypothesized that the highly selective CXCR2 antagonist SCH52712331,32 would reduce nociceptive sensitization after TBI. As demonstrated in Figure 2(a), TBI induced a persistent mechanical allodynia from day 1 to day 21. SCH527123 administered 5 mg/kg caused a profound reversal of allodynia 1 h after injection, and the effect was consistent over the course of seven days of administration although a lower dose had no discernable effect. However, the nociceptive sensitivity returned to the level of TBI-vehicle-treated group after SCH527123 administration was terminated suggesting that the drug did not alter the underlying mechanism of sensitization.
Figure 2.
Effects of CXCR2 blockade on TBI-induced nociceptive sensitization. (a) systemic adminsitration of SCH527123 (SCH, 1 or 5 mg/kg, i.p.) was performed daily from day 0 to day 7 beginning immediately after TBI. Nociceptive sensitivity was assessed using von Frey fibers 1 h after daily SCH injections during the treatment period. (b) Lumbar intrathecal injection of SCH, 2 µg in 2 µL, on the effects of TBI-induced nociceptive sensitization in day 2 and day 7 injury animals was tested from 0 to 2 h after injection. Threshold values are expressed as mean ± SEM, N = 6. **p < 0.01; ***p < 0.001 comparison of contralateral paws in vehicle-treated rats to the paws of SCH-treated animals; ###p < 0.001 comparison of SCH-treated groups to sham + vehicle groups. TBI: traumatic brain injury; SCH: SCH527123.
Since we hypothesized it was spinal CXCL1/CXCR2 signaling that is critical for post-TBI sensitization, we administered SCH527123 into the lumbar intrathecal space. Consistent with our hypothesis, TBI rats given intrathecal SCH527123 showed a rapid reversal of nociceptive sensitization when the drug was given either two or seven days after TBI (Figure 2(b)).
Effects of AA on CXCR2 and CXCL1 gene expression in spinal cord tissue
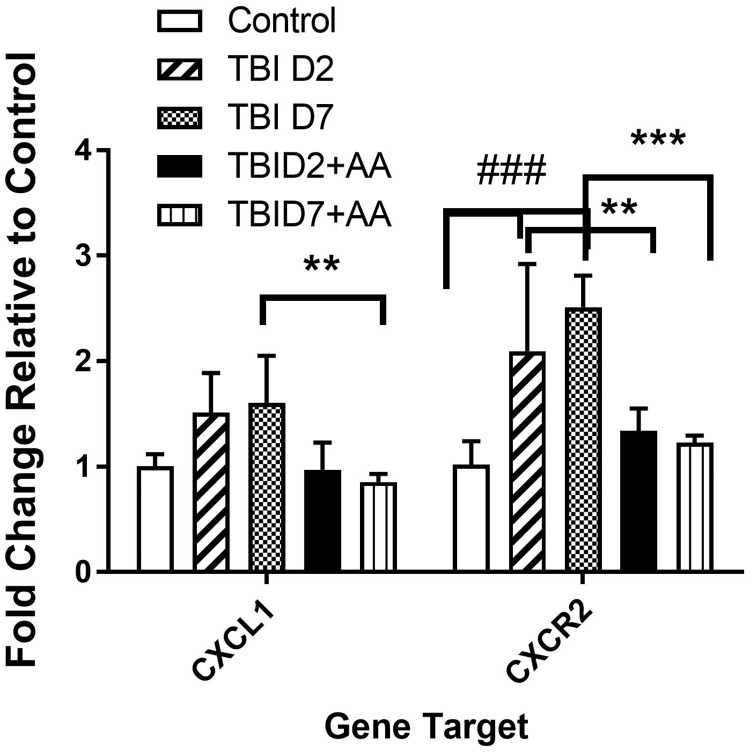
We used real-time qPCR to detect the changes in CXCL1 and CXCR2 gene expression in spinal cord tissue after TBI and AA treatment. In Figure 3, the data presented suggest that TBI induced a significant increase in CXCR2 after TBI. On the other hand, while there were trends toward increased expression of CXCL1, these did not reach statistical significance two or seven days after TBI. The HAT inhibitor AA when administered beginning immediately after injury prevented the increases in spinal CXCR2 mRNA.
Figure 3.
Effect of the HAT inhibitor AA on the expression of spinal CXCL1 and CXCR2 induced by TBI on day 2 and 7 poster injury. CXCL1 and CXCR2 gene expression in spinal cord lumbar area was detected by real-time PCR. Samples from 5 animals per group were included (N = 5). **p < 0.01; ***p < 0.001 comparing TBI to TBI + AA; ### in comparison to TBI to control group. The values are expressed as mean ± SEM. TBI: traumatic brain injury; AA: anacardic acid; HAT: histone acetylation transferase.
Effects of AA on CXCR2 protein expression in spinal cord
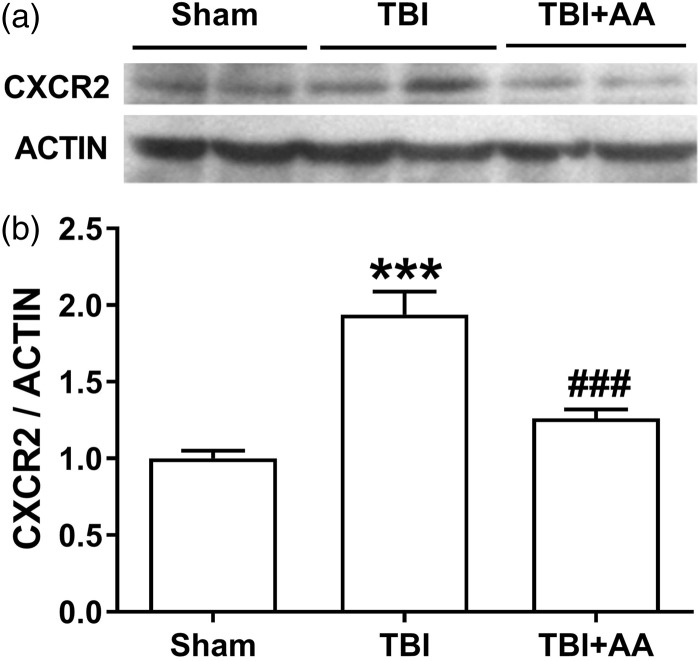
To complement the CXCR2 mRNA experiments, we measured CXCR2 protein expression in lumbar spinal cord after TBI and AA treatment. The data presented in Figure 4 demonstrate that TBI caused an increase in CXCR2 protein expression seven days after injury. However, AA treatment significantly suppressed this increase.
Figure 4.
Effect of the HAT inhibitor AA on spinal CXCR2 protein levels on day 7 post-TBI injury. Protein expression was detected by Western blot. The upper panel was a representative image of CXCR2 protein. The bottom panel displays a bar chart of CXCR2 expression. The protein expression was normalized to the housekeeping gene actin. The values are displayed as mean ± SEM, N = 6. ***p < 0.001 in comparison to the sham group; ###P < 0.001comparison of TBI to TBI treated with AA group. TBI: traumatic brain injury; AA: anacardic acid; HAT: histone acetylation transferase.
Effects of AA on pain-related mediators in spinal cord tissue
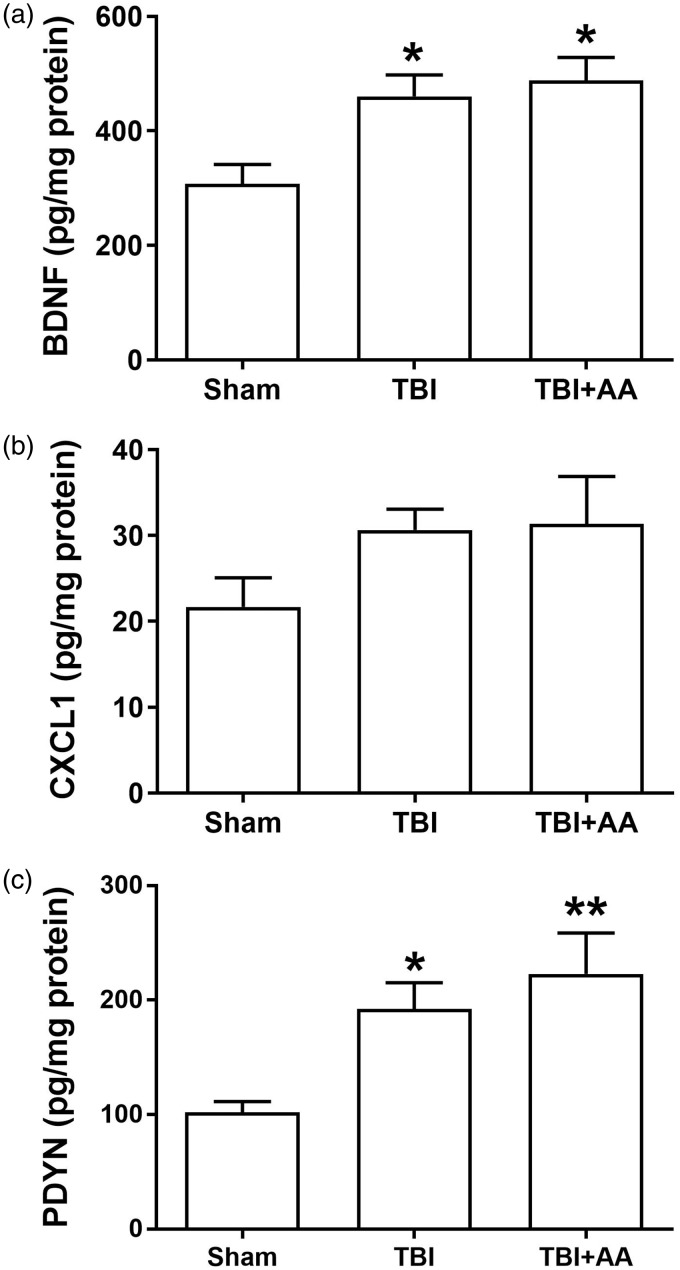
To detect possible effects of AA on protein levels of CXCL1 and additional epigenetically regulated pain-related mediators including BDNF and PDYN, we conducted ELISA studies. Consistent with the mRNA studies, the data in Figure 5 show that TBI did not elevate CXCL1 protein levels although there was a trend toward greater abundance. Moreover, levels of BDNF and PDYN were significantly increased on day 7 post-TBI, but AA had no significant effects on these increases suggesting that the analgesic effects of AA were not through the reduction of abundance of spinal BDNF and PDYN.
Figure 5.
Alteration of BDNF, CXCL1, and prodynorphin in contralateral lumbar spinal cord after TBI. Protein levels of (a) brain-derived neurotrophic factor (BDNF), (b) C-X-C Motif Chemokine Ligand 1(CXCL1), and (c) prodynorphin seven days after TBI injuries. Animals were treated with vehicle or AA as described in Materials and Methods. The values are displayed as mean ± SEM with pg/mg protein), N = 6. *p < 0.05, **p < 0.01 comparison of TBI-sham groups with TBI+ vehicle, TBI + AA group. TBI: traumatic brain injury; AA: anacardic acid; BDNF: brain-derived neurotrophic factor.
Location of CXCR2 expression in spinal cord
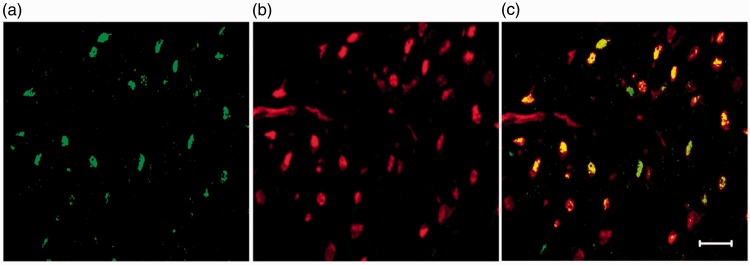
To identify the type of cell expressing CXCR2 in lumbar spinal tissue, we undertook immunohistochemichal experiments. Figure 6 presents data showing that CXCR2 protein was primarily co-localized with the neuronal marker NeuN in the sensory-related dorsal horn region of the spinal cord.
Figure 6.
Spinal CXCR2 immunohistochemistry. Double staining for CXCR2 (a) and NeuN markers (b) demonstrates strong association with CXCR2 in neuronal cells (c) in the contralateral side of spinal cord lumbar dorsal horn at day 2 after TBI. Arrows showed double positive cells. Scale bar = 20 µm. TBI: traumatic brain injury.
CXCR2 expression is regulated by histone acetylation
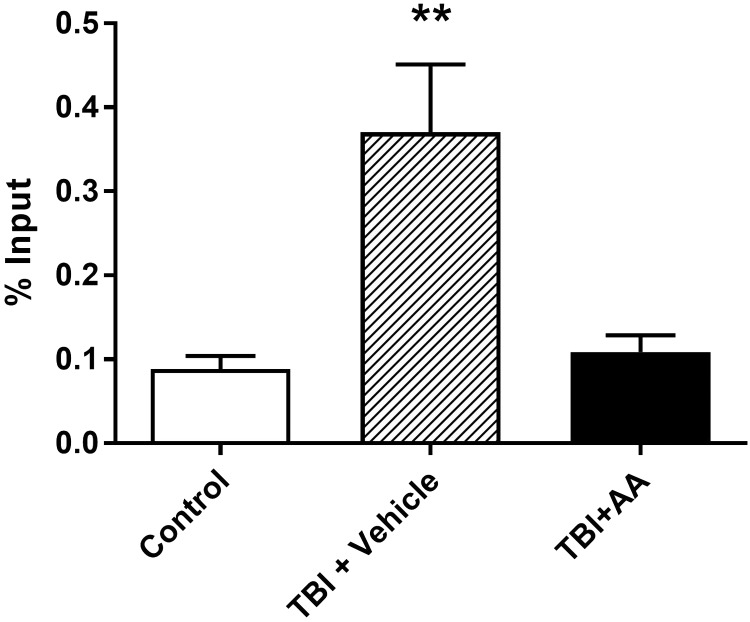
We next examined the association of acetylated histone protein with the CXCR2 gene promoter in light of the effects of AA on CXCR2 expression and previous evidence demonstrating this mechanism of epigenetic control of CXCR2 expression in the setting of neuropathic and postsurgical pain.14,16 To accomplish this, we employed ChIP analysis of the CXCR2 gene promoter association with acetylated histone H3 (Ac-H3K9) in spinal cord tissue. The ChIP assay revealed a significantly enhanced association of Ac-H3K9 with the promoter region of CXCR2 seven days after TBI (Figure 7). However, the treatment of animals with AA to block HAT activity significantly suppressed this enhancement consistent with AA reducing TBI-enhanced CXCR2 by blocking spinal histone acetylation.
Figure 7.
Changes of CXCR2 in promoter region histone acetylation in contralateral spinal cord tissue after TBI and TBI with AA treatment detected using a ChIP assay and real-time PCR. Data are presented as the means ± SEM. **p < 0.01; N = 4. TBI: traumatic brain injury; AA: anacardic acid.
Epigenetic gene expression in spinal cord tissue
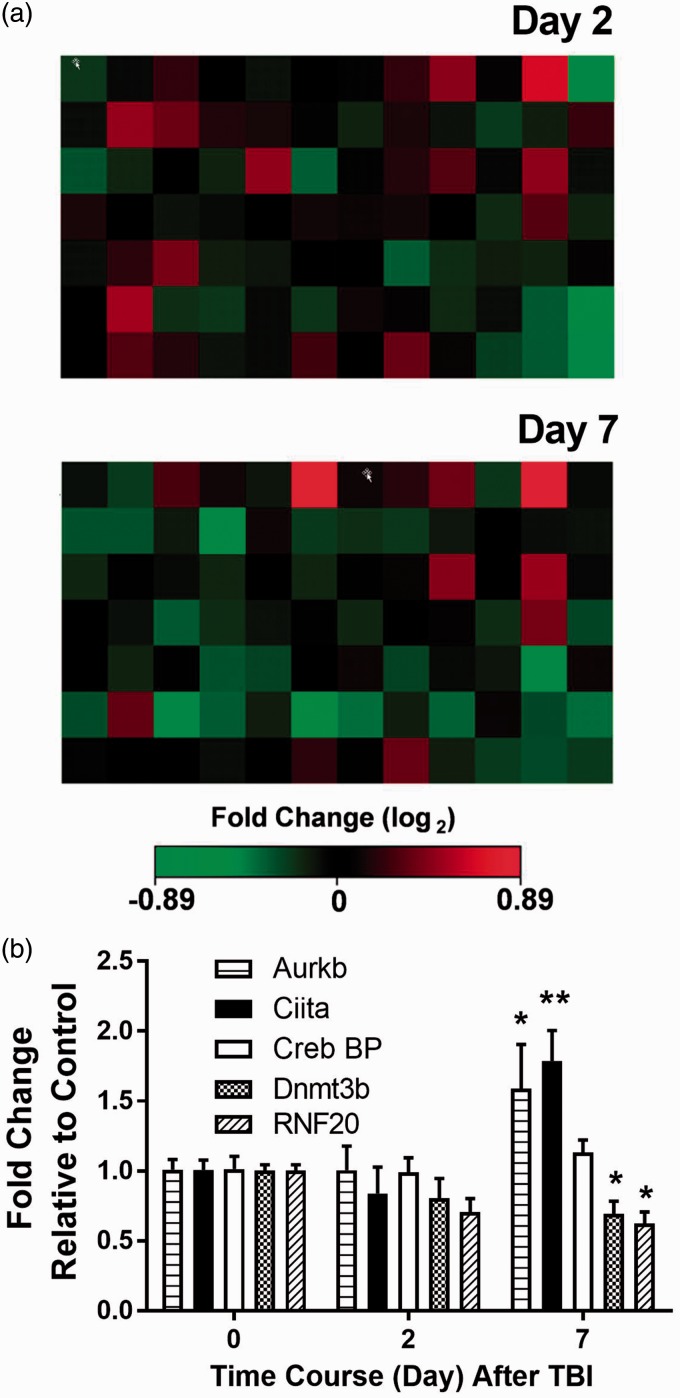
We used an epigenetic chromatin modification enzymes array to determine if there were alterations in the expression of genes coding for epigenetic enzymes in spinal cord tissue. This PCR array profiles the expression of 84 key genes encoding enzymes known or predicted to modify genomic DNA and histone conformation and therefore gene expression. They include DNA methyltransferases, histone cetyltransferases, histone methyltransferases SET domain proteins, histone phosphorylation, histone ubiquitination, histoned, DNA, and histone demethylases. We used 1.5-fold alteration as an initial threshold to identify two genes involved in histone acetylaton (Ciita and Crebbp), one involved in histone phosphorylation (Aurkb), one involved in histone ubiquitination (Rnf20), and one DNA methyltransferase (Dnmt3b) as displayed in Table 1, and the heat map of these genes in Figure 8(a) and (b). Confirmatory PCR studies demonstrated the increased expression of Ciita and Aurkb and decreased expression of DNMT3b and Rnf20 at seven days post-TBI.
Figure 8.
The expressions of genes involved in epigenetic processes were screened in spinal cord lumbar tissue using a PCR array. (a) Heat maps of gene expression in spinal cord two and seven days after TBI; (b) Candidate genes with expression changed at least 1.5-fold in the PCR array experiment were validated by real-time qPCR. Data are presented as the means ± SEM. * < 0.05; **p < 0.01; N = 4. TBI: traumatic brain injury.
Discussion
TBI affects individuals of all ages and constitutes a major injury-related healthcare issue. Approximately two million people in the United States suffer TBI each year with the majority being mild in character and not requiring hospitalization or extensive rehabilitative therapy.3,33 The recent recognition of the high prevalence of TBI in athletes and military service members has focused attention on the long-term consequences of TBI. Cognitive and psychological changes are commonly associated with TBI, but chronic pain in the forms of headaches, back pain, limb pain is gaining recognition as well.34 This TBI-related pain contributes to long-term disability and suffering. The very high prevalence of pain in those with polytrauma involving TBI and additional injuries also suggests that TBI might alter pain signaling mechanisms.34,35 Even those with mild injuries are not immune from pain-related sequalae, and, regrettably, there are no specific treatments for TBI-related pain.8 A key problem in advancing our ability to treat TBI-related pain is our lack of understanding of TBI-related pain mechanisms. In these studies, we used a rat fluid percussion model of TBI to explore the enhancement of nociceptive signaling transmission at a central nervous system site distant from but in neural continuity with the brain, namely the spinal cord. Our data suggest that epigenetically upregulated spinal chemokine signaling supports nociceptive sensitization in rats after TBI. Conceivably, similar mechanisms may increase the prevalence or severity of pain after TBI in humans.
Many mechanisms may contribute to persistent pain after TBI in humans and prolonged nociceptive sensitization in our rat model. These mechanisms include damage to ascending sensory pathways including the spinothalamic tract and thalamus, neuroplastic changes in pain-related cortical areas including the sensory and motor cortices, and the dysregulation or interruption of descending pain modulatory mechanisms. The latter descending mechanisms seem especially likely to be linked to our observations as they functionally connect the TBI-vulnerable midbrain and brainstem regions to the spinal cord sensory processing circuits where we observed enhanced expression of CXCR2 receptors and epigenetic changes in gene expression. The periaqueductal gray matter (PAG), locus coeruleus,36 and rostral ventromedial medulla are three such centers involved in regulating spinal nociceptive signal processing through descending fibers.37 Recently provided reports show that deficient descending inhibitory tone can support TBI-related and other forms of headache.38,39 Patients with headaches post-TBI had diminished responses in conditioned pain modulation testing, an assay of descending control efficiency.39 Additional experiments using a closed-head model of TBI provided data suggesting that descending control from the locus coeruleus and possibly other brain structures was diminished in these animals.40 Finally, MRI scans of TBI patients provide evidence of dysregulation of the PAG, and these changes were observed to be proportional to the pain experienced by the individual patients.41,42
While we did not directly study descending modulatory tracts, we did examine the consequences of TBI on spinal dorsal horn epigenetic gene regulation. Epigenetic mechanisms were of particular interest as the nociception-related changes in the rat TBI model we employed persisted for weeks, and the pain syndromes experienced by TBI patients can be very chronic. Epigenetic mechanisms are particularly strongly associated with persistent changes in cell functions. They include DNA methylation and covalent changes in histone proteins. Chromatin acetylation plays pivotal roles in chronic pain. Here, we chose to begin by studying the effects of a HAT inhibitor as recent data from our laboratory demonstrated that the levels of acetylated H3K9 histone protein were elevated after TBI in dorsal horn neurons.23 Furthermore, we showed in the same group of experiments that drugs that blocked the deacetylation of histones greatly prolonged nociceptive sensitization in this rat model. In the present study, the HAT inhibitor AA very efficaciously blocked sensitization if administered systemically beginning soon after TBI, and could even reverse established sensitization if given a week after TBI although the latter effects took a few days to reach maximal levels. Importantly, our nociceptive measurements were made approximately 23 h from the time of the previous AA injections suggesting that long-lived epigenetic rather than short-term non-specific pharmacological factors were operative. However, our systemic administration experiments do not exclude non-spinal sites as targets for AA or HAT inhibition. In addition, the AA treatments affected the CXCR2 mRNA levels and reduced the association of acetylated H3K9 histone protein with the promoter of the CXCR2 gene as hypothesized. TBI-enhanced levels of BDNF and PDYN were not altered by AA suggesting that their expression is regulated by other mechanisms after TBI. Thus, TBI-induced effects on spinal cord functions may involve a number of mechanisms.
We did attempt to determine whether changes in the expression of genes involved in epigenetic regulation were present or changed in spinal tissue after TBI. Our array studies targeted 84 such genes, and in general, only very small differences were observed. The two genes achieving statistically significant expression increases were Aurkb (aurora B kinase) and Ciita (class II major histocompatibility complex transactivator). While neither gene product appears to have direct histone acetylase or deacetylase activities, Ciita has been shown to interact with CBP/P300 to regulate histone acetylation levels and, consequently, gene expression.43 Somewhat arguing against a role for Ciita in spinal tissue after TBI is the increase in expression only at day 7 after injury, while CXCR2 expression is upregulated as early as day 2. In addition, DNA Methyltransferase 3 Beta (DNMT3b) was significantly reduced in the spinal cord after TBI. DNMT3b is an important element in epigenetic methylation changes. DNA methylation is an epigenetic modification associated with transcriptional repression of promoters. Therefore, deduction of DNMT3b expression may enhance the gene expression in spinal tissue after TBI, although we did not seek to identify target genes.
Our studies focused on CXCR2 receptor signaling for a number of reasons. First, spinal populations of this receptor have been shown to mediate nociceptive sensitization in models of neuropathic pain,44 inflammation,45 incisional pain,16 and opioid-induced hyperalgesia.46 The pain-related upregulation of the CXCR2 receptor has been shown to rely on changes in histone acetylation.16 In addition, CXCR2 has been noted to be upregulated in the brains of animals after experimental TBI,19–21 although our report appears to be the first to show upregulation at a distant site. The present work demonstrated not only that the systemic injection of a highly selective CXCR2 antagonist provided profound analgesia sustained over days of treatment, but that the selective lumbar spinal intrathecal administration of this drug was analgesic while vehicle had no effect. These observations suggest that spinal CXCR2 receptors are involved in nociceptive sensitization after TBI. Our immunohistochemical studies demonstrated that the majority of spinal CXCR2 receptors are located on dorsal horn neurons again consistent with previous reports16,17 though the regulation of CXCR2 expression and actions of the CXCR2 antagonist may also occur in non-neuronal cell populations expressing CXCR2, which appear to be limited in number. Additional studies did not, however, identify elevated levels of CXCL1in homogenates of spinal cord tissue as has been shown after peripheral injury.16 While it is perhaps not necessary for there to be a profound change in ligand expression to achieve nociceptive sensitization, it might also be the case that only small numbers of cells, for example, astrocytes, have an enhanced expression of CXCL1,17,45,47 or that other CXCR2 ligands, for example, CXCL2, CXLC3, and CXCL4 are service to stimulate the CXCR2 receptor.48
In summary, pain is often experienced in TBI patients including in areas remote from the site of injury. We have little information suggesting why this occurs, or how we might rationally approach the treatment of these individuals. Our animal model demonstrates that epigenetically modulated neurochemical changes occur in spinal cord tissue after TBI perhaps due to damage to descending neural systems. CXCR2 contributes to nociceptive sensitization after TBI, which is associated with epigenetic regulation of chromatin DNA acetylation. The observation that the selective CXCR2 antagonist SCH527123 potently reverses sensitization after TBI suggests that we may be able to use this or similar compounds to control pain after TBI.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the DOD CDMRP award MR130295, and VA Merit Review Grant 1I01RX001776 to JDC.
References
- 1.Hyder AA, Wunderlich CA, Puvanachandra P, et al. The impact of traumatic brain injuries: a global perspective. Neuro Rehabil 2007; 22: 341–353. [PubMed] [Google Scholar]
- 2.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ 2011; 60: 1–32. [PubMed] [Google Scholar]
- 3.Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol 2015; 127: 3–13. [DOI] [PubMed] [Google Scholar]
- 4.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006; 21: 375–378. [DOI] [PubMed] [Google Scholar]
- 5.Rutland-Brown W, Langlois JA, Thomas KE, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil 2006; 21: 544–548. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MP, Carmine H. Long-term health implications of individuals with TBI: a rehabilitation perspective. Neuro Rehabil 2012; 31: 85–94. [DOI] [PubMed] [Google Scholar]
- 7.Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol 2015; 35: 64–82. [DOI] [PubMed] [Google Scholar]
- 8.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA 2008; 300: 711–719. [DOI] [PubMed] [Google Scholar]
- 9.Higgins DM, Kerns RD, Brandt CA, et al. Persistent pain and comorbidity among Operation Enduring Freedom/Operation Iraqi Freedom/operation New Dawn veterans. Pain Med 2014; 15: 782–790. [DOI] [PubMed] [Google Scholar]
- 10.Murphy PM and Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 1991; 253: 1280–1283. [PubMed]
- 11.Olson TS and Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol 2002; 283: R7–28. [DOI] [PubMed]
- 12.Van Damme J, Decock B, Conings R, et al. The chemotactic activity for granulocytes produced by virally infected fibroblasts is identical to monocyte-derived interleukin 8. Eur J Immunol 1989; 19: 1189–119410. [DOI] [PubMed]
- 13.Cao DL, Qian B, Zhang ZJ, et al. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res Bull 2016; 127: 219–225. [DOI] [PubMed] [Google Scholar]
- 14.Kiguchi N, Kobayashi Y, Maeda T, et al. Epigenetic augmentation of the macrophage inflammatory protein 2/C-X-C chemokine receptor type 2 axis through histone H3 acetylation in injured peripheral nerves elicits neuropathic pain. J Pharmacol Exp Ther 2012; 340: 577–587. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Sahbaie P, Liang D, et al. Opioids enhance CXCL1 expression and function after incision in mice. J Pain 2014; 15: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Sahbaie P, Liang DY, et al. Epigenetic regulation of spinal CXCR2 signaling in incisional hypersensitivity in mice. Anesthesiology 2013; 119: 1198–1208. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZJ, Cao DL, Zhang X, et al. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013; 154: 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou YQ, Gao HY, Guan XH, et al. Chemokines and their receptors: potential therapeutic targets for bone cancer pain. Curr Pharm Des 2015; 21: 5029–5033. [DOI] [PubMed] [Google Scholar]
- 19.Otto VI, Stahel PF, Rancan M, et al. Regulation of chemokines and chemokine receptors after experimental closed head injury. Neuroreport 2001; 12: 2059–2064. [DOI] [PubMed] [Google Scholar]
- 20.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 2010; 30: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valles A, Grijpink-Ongering L, de Bree FM, et al. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis 2006; 22: 312–322. [DOI] [PubMed] [Google Scholar]
- 22.Kiguchi N, Kobayashi Y, Saika F, et al. Epigenetic upregulation of CCL2 and CCL3 via histone modifications in infiltrating macrophages after peripheral nerve injury. Cytokine 2013; 64: 666–672. [DOI] [PubMed] [Google Scholar]
- 23.Liang D-Y, Sahbaie P, Sun Y, et al. TBI-induced nociceptive sensitization is regulated by histone acetylation. IBRO Reports 2017; 2: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feliciano DP, Sahbaie P, Shi X, et al. Nociceptive sensitization and BDNF up-regulation in a rat model of traumatic brain injury. Neurosci Lett 2014; 583: 55–59. [DOI] [PubMed] [Google Scholar]
- 25.Ling GS, Lee EY and Kalehua AN. Traumatic brain injury in the rat using the fluid-percussion model. Curr Protoc Neurosci 2004; Chapter 9: Unit 9 2. [DOI] [PubMed]
- 26.McIntosh TK, Vink R, Noble L, et al. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 1989; 28: 233–244. [DOI] [PubMed] [Google Scholar]
- 27.Guo TZ, Offley SC, Boyd EA, et al. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain 2004; 108: 95–107. [DOI] [PubMed] [Google Scholar]
- 28.Poree LR, Guo TZ, Kingery WS, et al. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg 1998; 87: 941–948. [DOI] [PubMed] [Google Scholar]
- 29.Liang DY, Li X, Clark JD. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J Pain 2013; 14: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Neumann M, Hansen K, et al. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma 2011; 28: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holz O, Khalilieh S, Ludwig-Sengpiel A, et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J 2010; 35: 564–570. [DOI] [PubMed] [Google Scholar]
- 32.Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther 2012; 11: 1353–1364. [DOI] [PubMed] [Google Scholar]
- 33.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995-2009. J Safety Res 2012; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- 34.Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev 2013; 50: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 35.Sayer NA, Cifu DX, McNamee S, et al. Rehabilitation needs of combat-injured service members admitted to the VA Polytrauma Rehabilitation Centers: the role of PM&R in the care of wounded warriors. PMR 2009; 1: 23–28. [DOI] [PubMed] [Google Scholar]
- 36.Ucal M, Kraitsy K, Weidinger A, et al. Comprehensive Profiling of modulation of nitric oxide levels and mitochondrial activity in the injured brain: an experimental study based on the fluid percussion injury model in rats. J Neurotrauma 2017; 34: 475–486. [DOI] [PubMed] [Google Scholar]
- 37.De Felice M, Ossipov MH. Cortical and subcortical modulation of pain. Pain Manag 2016; 6: 111–120. [DOI] [PubMed] [Google Scholar]
- 38.Boyer N, Dallel R, Artola A, et al. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 2014; 155: 1196–1205. [DOI] [PubMed] [Google Scholar]
- 39.Defrin R, Riabinin M, Feingold Y, et al. Deficient pain modulatory systems in patients with mild traumatic brain and chronic post-traumatic headache: implications for its mechanism. J Neurotrauma 2015; 32: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bose P, Hou J, Nelson R, et al. Effects of acute intrathecal baclofen in an animal model of TBI-induced spasticity, cognitive, and balance disabilities. J Neurotrauma 2013; 30: 1177–1191. [DOI] [PubMed] [Google Scholar]
- 41.Jang SH, Park SM, Kwon HG. Relation between injury of the periaqueductal gray and central pain in patients with mild traumatic brain injury: observational study. Medicine 2016; 95: e4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strigo IA, Spadoni AD, Lohr J, et al. Too hard to control: compromised pain anticipation and modulation in mild traumatic brain injury. Transl Psychiat 2014; 4: e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Jiang Y, Lu L, et al. MHC class II transactivator represses human IL-4 gene transcription by interruption of promoter binding with CBP/p300, STAT6 and NFAT1 via histone hypoacetylation. Immunology 2007; 122: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G, Park CK, Xie RG, et al. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 2014; 137: 2193–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao DL, Zhang ZJ, Xie RG, et al. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol 2014; 261: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang LH, Xu GM, Wang Y. Up-regulation of CXCL1 and CXCR2 contributes to remifentanil-induced hypernociception via modulating spinal NMDA receptor expression and phosphorylation in rats. Neurosci Lett 2016; 626: 135–141. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Zhu MD, Zhang X, et al. NFkappaB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation 2014; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Y, Fischer FR, Hancock WW, et al. Macrophage inflammatory protein-2 and KC induce chemokine production by mouse astrocytes. J Immunol 2000; 165: 4015–4023. [DOI] [PubMed] [Google Scholar]