Abstract
Purpose
Several options exist for management of clinically localized renal masses suspicious for cancer, including active surveillance, thermal ablation and radical or partial nephrectomy. We summarize evidence on effectiveness and comparative effectiveness of these treatment approaches for patients with a renal mass suspicious for localized renal cell carcinoma.
Materials and Methods
We searched MEDLINE®, Embase® and the Cochrane Central Register of Controlled Trials from January 1, 1997 through May 1, 2015. Paired investigators independently screened articles to identify controlled studies of management options or cohort studies of active surveillance, abstracted data sequentially and assessed risk of bias independently. Strength of evidence was graded by comparisons.
Results
The search identified 107 studies (majority T1, no active surveillance or thermal ablation stratified outcomes of T2 tumors). Cancer specific survival was excellent among all management strategies (median 5-year survival 95%). Local recurrence-free survival was inferior for thermal ablation with 1 treatment but reached equivalence to other modalities after multiple treatments. Overall survival rates were similar among management strategies and varied with age and comorbidity. End-stage renal disease rates were low for all strategies (0.4% to 2.8%). Radical nephrectomy was associated with the largest decrease in estimated glomerular filtration rate and highest incidence of chronic kidney disease. Thermal ablation offered the most favorable perioperative outcomes. Partial nephrectomy showed the highest rates of urological complications but overall rates of minor/major complications were similar among interventions. Strength of evidence was moderate, low and insufficient for 11, 22 and 30 domains, respectively.
Conclusions
Comparative studies demonstrated similar cancer specific survival across management strategies, with some differences in renal functional outcomes, perioperative outcomes and postoperative harms that should be considered when choosing a management strategy.
Keywords: carcinoma, renal cell, comparative effectiveness research, disease management, kidney neoplasms, surgical procedures, operative
Renal masses are a biologically heterogeneous group of tumors ranging from benign neoplasms to cancers that can be indolent or aggressive.1,2 Although the true incidence of renal masses suspicious for malignancy is unknown, approximately 80% of surgically resected tumors are malignant.1,3 All solid renal masses and cystic lesions with solid components are suspicious for renal cell carcinoma, which affects approximately 65,000 new patients yearly and has a 5-year mortality rate of 35%.4
Several options exist for management of clinically localized renal masses suspicious for RCC, including active surveillance, thermal ablation and surgery. Surgery, including PN and RN, is an option for masses of all sizes (clinical stage T1 or T2), although PN is preferred for lesions smaller than 7 cm in diameter (clinical stage T1).5 Given the increased incidence of early, low stage tumors without improvement in cancer related deaths, active surveillance has emerged as an option for patients with small renal masses (4 cm or less, clinical stage T1a), a low likelihood of aggressive malignancy, a procedure limiting comorbidity and/or a limited life expectancy. If thermal ablation is used, which may include cryoablation and radio frequency ablation, the ideal circumstance is a small, clinically localized mass (clinical stage T1) and the procedure can be performed laparoscopically or percutaneously. Each management strategy has relative merits and risks in comparison to the others. As such, professional organizations, including the American Urological Association, European Association of Urology and the National Comprehensive Cancer Network®, refrain from defining strict selection criteria (ie patient or tumor) for particular treatment strategies, and selection criteria vary by organizational guidelines.5–7 Additional controversies exist regarding the ideal management for renal masses of different stages. For example PN has emerged as the recommended treatment for clinical stage T1 renal masses, yet the single RCT comparing RN and PN revealed no difference in overall survival among patients with kidney cancer.8 We performed this systematic review to better compare the effectiveness of the treatment options, taking into consideration oncologic outcomes, renal functional outcomes and complications, as well as competing health risks of patients with a renal mass suspicious for RCC.
METHODS
Data Sources and Searches
We report results from a broader systematic review.9 Full details on methods are available from the evidence report. We searched MEDLINE®, Embase® and the Cochrane Central Register of Controlled Trials from January 1, 1997 (the year the TNM Classification of Malignant Tumours staging system for renal cell carcinoma was modified and the distinctions of T1a/T1b and T2a/T2b were created) through May 1, 2015. Therefore, clinical stage definitions are those of the American Joint Committee on Cancer as follows. T1a is defined as tumor 4 cm or smaller, T1b greater than 4 to 7 cm, T2a greater than 7 to 10 cm and T2b greater than 10 cm, N0 as node negative and M0 as no evidence of distant metastases. We also requested information from device manufacturers and searched ClinicalTrials.gov for relevant studies.
Study Selection, Data Extraction and Quality Assessment
Paired investigators independently screened articles to assess eligibility using predefined criteria to identify controlled studies of the management options or single cohort studies of active surveillance (Appendix 1). Paired investigators abstracted data sequentially and independently assessed risk of bias for individual studies. We used the Cochrane Collaboration tool for assessing risk of bias of RCTs.10 For nonrandomized studies of treatment interventions we used ACEOBAT-NRSI (A Cochrane Eisk of Bias Assessment Tool: for Non-Eandomized Studies of Interventions).11 Differences between reviewers were resolved through consensus.
Data Synthesis and Analysis
All studies were summarized qualitatively. LRFS was defined as the absence of any persistent or recurrent disease in the treated region of the kidney or associated renal fossa after a single, curative intent initial treatment. This definition included persistent enhancement of any treated mass, a visually enlarging neoplasm, new nodularity, failure of regression in size of the treated lesions and new satellite or port site lesions.
We conducted meta-analyses for outcomes using a random effects model with the DerSimonian and Laird method when there were at least 2 sufficiently homogeneous studies. We identified substantial statistical heterogeneity as an I2 statistic with a value greater than 50%. All meta-analyses were conducted using Stata® 12.1.
We graded the strength of evidence using the scheme recommended by the Methods Guide for Effectiveness and Comparative Effectiveness Eeviews.12 Strength of evidence is an assessment that goes beyond evidence hierarchies, which focus solely on study design (RCT, retrospective cohort, etc). Studies evaluating a particular outcome for each pairwise comparison of management strategies are pooled to determine overall level of reporting bias, directness of outcome the studies assess, consistency of findings across included series and precision of effect estimates from the studies. The domains are independently evaluated by 2 graders, followed by discussion to resolve conflicts. We graded the strength of evidence for the outcomes we classified during protocol development as the most important outcomes, including oncologic efficacy, renal functional outcomes, quality of life and OS.
Funding was provided by AHRQ. The funding source had no role in study selection, quality assessment or data synthesis, or in the decision to submit the article for publication.
RESULTS
We identified 20,829 unique citations, of which 13,912 were excluded during the abstract screen. During the full text screening we excluded 1,028 citations. During key question applicability screening we excluded an additional 1,190 articles that did not meet 1 or more inclusion criteria (full report lists excluded articles and reasons for exclusion).9 Overall 147 studies reported in 150 articles were included in the broader review. A total of 40 studies were unrelated to effectiveness (diagnostic questions were evaluated) and are excluded from this article. Therefore, 107 studies reported in 110 articles are included in this review of interventions for management of renal masses suspicious for localized RCC (fig. 1).9 Participant and tumor characteristics are outlined in supplementary tables 1 and 2 (http://jurology.com/). Strength of evidence is given with each comparison, and was at best moderate or low for many outcomes and often insufficient for comparisons involving active surveillance (supplementary tables 3 to 8, http://jurology.com/).
Figure 1.
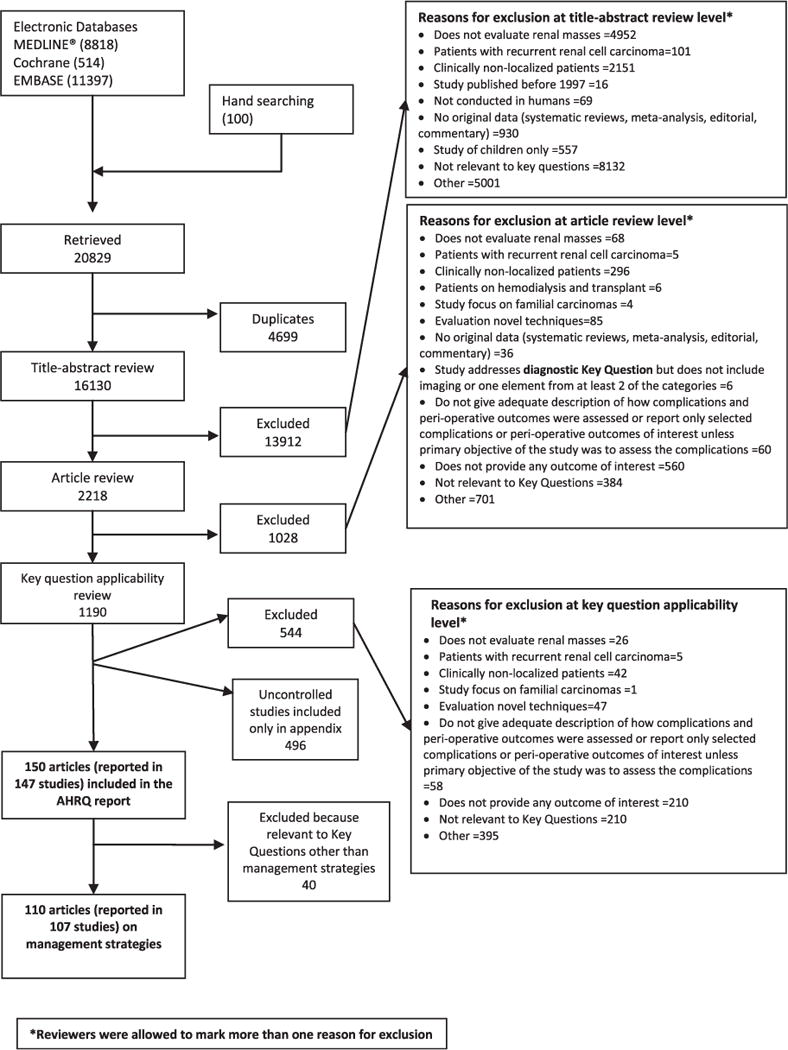
Summary of literature search
Oncologic Outcomes
A total of 60 studies (reported in 61 articles) provided data on at least 1 oncologic efficacy outcome (CSS, metastasis-free survival or LRFS). The series were grouped by category and included RCTs (1 study),8 institutional cohorts (48)13–60 and 11 studies (in 12 articles) of the SEER data set.61–72 An important distinction was made between institutional and SEER studies as the SEER program provides a national perspective but lacks important granular data to account for all potential confounding variables. It is noteworthy that many institutional studies also differ in included variables. In addition, SEER patients cannot be identified as undergoing active surveillance, but only as not having undergone surgery, and, therefore, are labeled as undergoing nonsurgical treatment. Median of median followup was about 60 months for RN and PN cohorts and 48.6 months for thermal ablation cohorts.
Cancer Specific Survival
CSS estimates in the primary analysis among all management strategies were 95% to 100% (median followup 22 to 120 months) and did not differ significantly among treatments (supplementary tables 9 to 11, http://jurology.com/). The strength of evidence was moderate for the finding of equivalent cancer specific survival for RN vs PN and thermal ablation vs RN, low for thermal ablation vs PN, and insufficient for all other comparisons and clinical predictors of CSS (Appendix 2). Figure 2 illustrates the meta-analysis of cancer specific survival among patients undergoing RN and PN. SEER (effect size 1.18 [range 0.94–1.42]) and non-SEER (1.08 [0.87 to 1.33]) studies showed comparable CSS.
Figure 2.
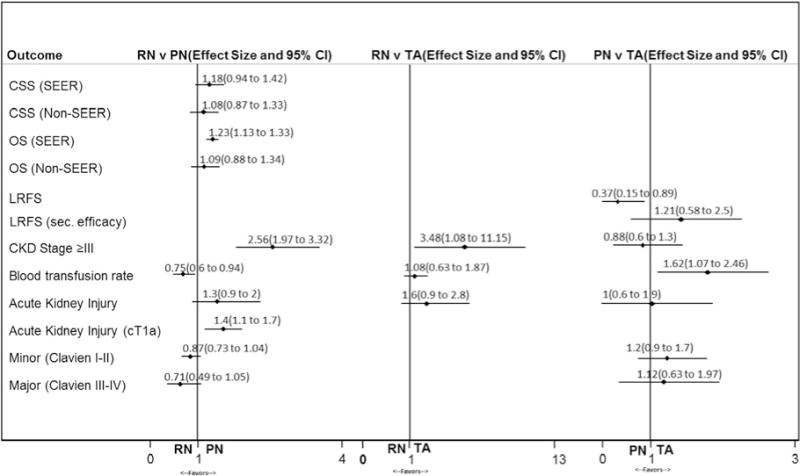
Notable meta-analyses on effectiveness of radical vs partial nephrectomy, radical nephrectomy vs thermal ablation and partial nephrectomy vs thermal ablation. Effect size is given as relative risk for cancer specific survival and overall survival, and odds ratios for other outcomes. CKD, chronic kidney disease. sec., secondary. TA, thermal ablation.
In comparisons of RN and PN subgroup analysis demonstrated cancer specific survival to decrease with increasing tumor size. CSS was 97% vs 98.8%, 91% vs 90% and 82.5% vs 86.7% for clinical stage T1a, T1b and T2 tumors, respectively. One RCT compared cancer specific survival between PN and RN for tumors that were 5 cm or smaller and found no statistically significant differences (hazard ratio 2.06, 95% CI 0.62 to 6.18) after a relatively long median followup of 112 months.8 It is noteworthy that the CI in that series was wide and there were only 12 renal cancer related deaths (4 in the RN group and 8 in the PN group). Comparative analyses of RN and PN indicated that increasing age, larger tumor size and higher tumor grade were the most common predictors of worse cancer specific survival. However, there was no difference in cancer specific survival between RN and PN when stratified by age, tumor size or grade.14,16,27,28,30,35,36,61,62,66,67,71,73,74
Three studies assessed cancer specific survival for nonsurgical management vs RN and PN.67–69 RN and PN resulted in statistically significant improved cancer specific survival compared to nonsurgical treatment in 2 SEER-Medicare studies of patients with clinical stage T1a tumors (hazard ratio for cancer specific mortality 0.58 to 0.62 and 0.42 to 0.45 for RN and PN, respectively, compared to nonsurgical treatment).67,69 Analyses of these SEER data indicated that the cancer specific survival benefit of surgical intervention over nonsurgical treatment may be attenuated in patients 75 years or older or with high cardiovascular risk.68,69 A comparative study that evaluated RN and active surveillance resulted in similar cancer specific survival rates (90.7% vs 94.2%, p = 0.33), albeit with greater tumor size and stage in the patients undergoing surgery.55
Due to a paucity of comparative data, 8 uncontrolled studies of active surveillance were included and consisted of 2 prospective series, 1 population based retrospective study and 5 single institution retrospective series.75–82 CSS rates were excellent, at 98% to 100%, with short followup (12 to 36 months). The prospective DISSRM (Delayed Intervention and Surveillance for Small Renal Masses) Registry compared active surveillance to any immediate surgical intervention and found active surveillance to be noninferior to primary intervention (CSS 100% vs 99%, median followup 2.1 years).76
Metastasis-Free Survival and Local Recurrence-Free Survival
None of the comparative analyses revealed a difference in metastasis-free survival, which ranged from 90.5% to 100% (Appendix 2, supplementary tables 9 to 11, http://jurology.com/), with moderate strength of evidence for no difference between PN and thermal ablation (median of median followup 39.3 and 42.3 months, respectively) and low strength of evidence for no difference in other comparisons. It is noteworthy that metastasis-free survival was similar in the comparative analysis of RN and active surveillance (94.5% vs 94.3%) at a median 53 and 44 months, respectively.55
The RCT comparing PN and RN for tumors 5 cm or smaller did not provide a statistical test but showed a somewhat higher rate of local recurrence for PN (6 patients) compared to RN (1).8 Meta-analysis confirmed no difference in LRFS for RN and PN (median of median followup 51.3 and 46.1 months, respectively) with low strength of evidence (supplementary table 9, http://jurology.com/).
Rates of LRFS were worse for thermal ablation compared to RN (low strength of evidence) and PN (moderate strength of evidence). In the 2 studies specifically comparing RN and thermal ablation LRFS was 97.4% and 100% for RN and 81% and 93% for thermal ablation.13,54 LRFS rates were generally higher in the 14 studies comparing PN and thermal ablation (median 98.9%, IQR 94.6% to 100% vs 93.0%, IQR 89.9% to 96.0%, supplementary table 11, http://jurology.com/). A number of the studies demonstrated secondary efficacy of thermal ablation, which is determined after multiple ablations. Rates of LRFS when considering secondary efficacy ranged from 97% to 100%, and differences between PN and thermal ablation were no longer significant (fig. 2).
Few patients in the uncontrolled active surveillance studies exhibited metastases. Therefore, metastasis-free survival rates reflect cancer specific survival rates (98% to 100% at 12 to 36 months).
Overall Survival
A total of 48 comparative studies (49 articles) addressed OS, of which 1 was a RCT and 9 were studies of the SEER data set reported in 10 articles (Appendix 2, supplementary tables 9 to 11, http://jurology.com/).61,62,64,65,67,68,70,83–85 Median of median followup was 60 months for RN, 30 months for PN and 33.7 months for thermal ablation. Institutional cohorts generally exhibited minimal difference in overall survival between RN and PN (low strength of evidence).18,19,21,22,24,27,28,31,32,36,60,86–88 Authors of studies with statistically significant OS advantage for PN acknowledged differences in baseline characteristics (ie selection bias) that may have contributed to the observed findings.23,25,26,74 In 10 SEER studies comprising the majority of patients in this analysis PN was associated with a statistically significant overall survival benefit compared to RN for clinical stage T1a tumors but not for T1b tumors.61,64,65,67,68,70,83–85 Therefore, meta-analyses were different for SEER (effect size 1.23, range 1.13 to 1.33) and nonSEER studies (1.09, 0.88 to 1.34, supplementary table 9, fig. 2). Finally, the single RCT comparing RN and PN revealed no difference in overall survival among patients with kidney cancer and for those who were clinically and pathologically eligible, although it showed an unexplained benefit of RN compared to PN among the overall cohort.8
In most studies patients undergoing thermal ablation and active surveillance had inferior overall survival outcomes compared to those undergoing RN or PN (low strength of evidence). Many studies acknowledge that patients undergoing thermal ablation and active surveillance are often of advanced age, have multiple comorbidities and are unsuitable for extirpative surgery. SEER studies have demonstrated an overall survival advantage with RN and PN compared to nonsurgical treatment, even in patients with clinical stage T1a tumors, with similar findings of advanced age and comorbidity.67,68 Finally, overall survival ranges from 69% to 94% in uncontrolled studies of active surveillance.75–79,81
Renal Functional Outcomes
In patients undergoing treatment for a renal mass suspicious for clinical stage T1 or T2 RCC kidney function (measured through eGFR) consistently worsens by 1 to 40 ml/minute/1.73 m2 in the immediate postoperative setting (first available postoperative measure within 1 to 7 days) but improves during the next 1 to 6 months and remains relatively stable after that point. This improvement is more pronounced in thermal ablation arms, where final eGFR (primary renal functional outcomes from Kaplan-Meier curves or tabulated data closest to 1 year) is on average 2.83 ml/minute/1.73 m2 better than postoperative eGFR. Improvement is not as pronounced in RN arms, where final eGFR is on average 0.83 ml/minute/1.73 m2 better than postoperative eGFR. In addition, patients with optimal baseline renal function (eGFR greater than 90 ml/minute/1.73 m2) experience less decline in eGFR and no appreciable difference in incidence of chronic kidney disease after undergoing PN compared to those undergoing RN.
RN is associated with worse renal outcomes compared to PN (moderate strength of evidence) and thermal ablation (moderate strength of evidence) with the final eGFR rate decreasing a median of 15 ml/minute/1.73 m2 lower following RN vs PN (−22.4 ml/minute/1.73 m2 vs −7.4 ml/minute/1.73 m2) and 10.3 ml/minute/1.73 m2 lower following RN vs thermal ablation (−13.2 ml/minute/1.73 m2 vs −2.9 ml/minute/1.73 m2, Appendix 2, supplementary tables 9 and 10, http://iurology.com/, and fig. 2). The risk of all stages of chronic kidney disease was highest and estimated to be 32% (average incidence range 2% to 70%) for patients undergoing RN. The risk of stage 3 chronic kidney disease was lower with PN (risk ratio 0.39) compared to RN (moderate strength of evidence) and was 3.48-fold higher with RN compared to thermal ablation (moderate strength of evidence, Appendix 2, supplementary tables 9 and 10, http://jurology.com/, and fig. 2).
Renal functional outcomes were similar between PN and thermal ablation (low strength of evidence). Only 2 comparative studies of renal functional outcomes included active surveillance. These studies revealed greater final eGFR, a smaller change in eGFR and a decreased incidence of stage 3 chronic kidney disease with active surveillance compared to RN (low strength of evidence). The evidence was insufficient to support a conclusion about the reported similarity of final kidney function between active surveillance and the nephron sparing arms because of the sparse data and inconsistencies in reporting of renal functional outcomes.
Quality of Life
Only 4 studies have evaluated comparative health related quality of life outcomes after RN and PN. In general, these studies show that RN may provide better quality of life regarding cancer control, and PN may offer decreased anxiety and depression. The limited number of series and differences in study methods prevented direct comparison of series, leading to an insufficient strength of evidence for all comparisons.
Perioperative Outcomes and Harms
Thermal ablation had the most favorable perioperative outcomes with fewer conversions to open surgery and shorter length of stay compared to RN, and less estimated blood loss, fewer blood transfusions, no conversions to open surgery or RN and shorter length of stay compared to PN. RN had a lower blood transfusion rate than PN (7.3% vs 16.3%, RR 0.75, 95% CI 0.60–0.94) and PN had a higher blood transfusion rate than thermal ablation (4.6% vs 0.4%, RR 1.62, 95% CI 1.07–2.46). The strength of evidence was moderate for comparisons of the perioperative outcomes of RN and PN, and for comparisons of the perioperative outcomes of PN and thermal ablation.
Minor and major complication rates were similar for patients undergoing RN, PN and thermal ablation (low strength of evidence, Appendix 2, supplementary tables 9 to 11, http://iurology.com/ and fig. 2). However, specific complications varied among management strategies. For instance patients undergoing PN had higher rates of urological complications including renal abscess, subsequent intervention, ureteral injury, urine leak and other urological complications compared to those undergoing RN (supplementary table 9, http://jurology.com/). Patients undergoing RN had higher rates of acute kidney injury and nonurological complications but lower rates of bleeding or urine leak compared to those undergoing thermal ablation (supplementary table 10, http://jurology.com/). Patients undergoing PN had higher rates of acute kidney injury, cardiovascular, hematological and respiratory harm but lower rates of infectious disease and wound complications compared to those undergoing thermal ablation (supplementary table 11, http://jurology.com/). The strength of evidence was insufficient for all other comparisons based on inconsistencies in the reporting of harms among studies (Appendix 2). The focus remains on comparative harms given that individual series vary regarding baseline comorbidities, which harms are reported and how these harms are categorized.
DISCUSSION
The findings of this systematic review should be considered in the context of the available evidence, which at times is limited by selection bias and followup as reflected in the strength of evidence ratings. The available literature suggests that overall survival and oncologic outcomes are similar between management strategies. In fact, cancer specific survival was excellent among all modalities and median 5-year survival approached 95% for clinical stage T1a tumors. Overall survival was highly dependent on patient comorbidity and competing risks of mortality. In the retrospective comparative studies, where selection bias exists, patients undergoing PN demonstrated superior overall survival to those undergoing thermal ablation or active surveillance, likely related to their excellent general health. In addition, patients undergoing nonsurgical treatment in SEER studies (ie those who did not or could not undergo surgery) had the worst overall survival compared to those undergoing RN or PN.
In summary, the current evidence does not reveal superiority of a single treatment modality. However, thermal ablation does not achieve local control equivalent to RN or PN without multiple treatments. Therefore, consideration of competing comorbidity is of paramount importance as differences in overall survival are largely driven by patient selection, and certain oncologic measures (cancer specific survival and metastasis-free survival) are generally similar based on the available literature.
As overall and cancer specific survival are driven by patient and tumor characteristics, respectively, consideration of renal functional outcomes, quality of life, perioperative outcomes and harms should be considered for each patient. Therefore, each management strategy offers a profile of outcomes, with relative merits and risks that warrant consideration for each individual. For instance the evidence shows worse renal functional outcomes with RN compared to other management strategies when considering the risk of acute kidney injury, more long-term changes in eGFR and progression of chronic kidney disease. However, RN carries a markedly lower blood transfusion rate and less urological harm than PN. Based on comparative data, thermal ablation has the most favorable perioperative outcomes (less estimated blood loss, shorter length of stay and fewer conversions to open or radical surgery) compared to RN or PN. While the overall rate of postoperative urological and nonurological complications is similar among management strategies, the differential rates of specific postoperative complications vary by strategy. For instance despite similar overall complication rates, PN has the highest rate of postoperative bleeding, while patients undergoing RN have more respiratory harm and acute kidney injury. Since individual risk factors may have an important role in determining a treatment strategy, tailoring treatment to the specific susceptibility of a patient to harms may prove prudent.
We performed in-depth analysis of the existing literature to determine clinical predictors of comparative effectiveness. Data indicated that age, larger tumor size and higher tumor grade were the most common predictors of cancer specific survival, and age and comorbidities were the greatest predictors of overall survival. Limited data exist to explain the role of other clinical factors in predicting oncologic outcomes, overall survival, renal functional outcomes, quality of life, perioperative outcomes and harms among the management strategies. Evidence suggests that larger tumors are more likely to be malignant, and uncontrolled studies indicate that large masses may increase the likelihood of complications during PN (comparative data from this review did not demonstrate any increased risk of complications based on tumor size). Therefore, it may be reasonable to consider RN in patients with larger (clinical stage T1b or T2) tumors.
Studies suggest that baseline renal function is the best predictor of long-term renal functional outcomes regardless of type of surgery, so a patient with a large tumor and chronic kidney disease at baseline (stage 3 or 3b especially) may benefit from a nephron sparing approach. Therefore, the choice of treatment strategy is complex and dependent on patient and tumor characteristics as well as patient and physician preferences regarding risk of recurrence, survival, renal functional outcomes and complications. The current data do not provide strong enough evidence to support a particular surgical approach over another for different types of patient scenarios. The mitigating effects of these characteristics should be considered until stronger evidence is acquired. Future research should be geared toward providing more information to guide the choice of treatment strategy for different types of patients. It is noteworthy that comparative studies are most lacking for active surveillance compared to surgical modalities with insufficient evidence for most strength of evidence categories (Appendix 2).
This systematic review identified a number of shortcomings in the published data, and we offer several recommendations for future research in this area. The major limitation was the high level of selection bias in retrospective series. Whenever possible, prospective studies using objective selection criteria regarding patient and tumor characteristics are recommended. An additional limitation is the imprecise reporting of clinical stage among studies. As nephron sparing approaches are mostly indicated for clinically localized tumors, these series were included regardless of the reporting of clinical stage. However, studies of RN were included only if clinical stage was explicitly stated. We urge all investigators reporting outcomes on renal masses to consistently describe clinical stage since most treatment dilemmas in this patient population are encountered before a pathological diagnosis. Finally, studies of thermal ablation and active surveillance may include patients with benign tumors and may overestimate the efficacy of these treatment strategies. Improved diagnostics and judicious use of renal mass biopsy may improve our understanding of tumor biology in these cohorts. Future research may better objectify the selection of a given management strategy and address issues of cost-effectiveness and long-term sequelae of treatment.
CONCLUSIONS
Comparative studies reveal similar cancer specific survival across management strategies, with some differences in renal functional outcomes, perioperative outcomes and postoperative harms that should be considered when choosing a management strategy. Further research and data are needed to strengthen many aspects of the evidence base.
Acknowledgments
Allen Zhang and Emily Little assisted with data abstraction. Dr. Tim Carey supplied commentary and performed revisions. Dr. Steven Campbell and Dr. Aysegul Gozu provided feedback and insight.
Funded under Contract No. HHSA290201200007l from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Abbreviations and Acronyms
- AHRQ
Agency for Healthcare Research and Quality
- CSS
cancer specific survival
- eGFR
estimated glomerular filtration rate
- LRFS
local recurrence-free survival
- OS
overall survival
- PN
partial nephrectomy
- RCC
renal cell carcinoma
- RCT
randomized controlled trial
- RN
radical nephrectomy
- SEER
Surveillance, Epidemiology and End Results
- SR
systematic review
APPENDIX 1
PICOTS (population, interventions, comparators, outcomes, timing, and setting) for the Key Questions
| Population(s) | Newly diagnosed adults (18 years or older) with solid renal masses (or cystic renal masses with a solid component) suspicious for stage I and II renal cell carcinoma, which corresponds to clinical stage T1 (less than 7 cm and organ confined) or T2 (greater than 7 cm and organ confined) renal masses |
| Interventions |
|
| Comparators | Comparisons include all of the management options listed above |
| Outcomes |
Final health outcomes
Adverse effects of management strategies
Minor versus major Minor (Clavien 1–2)*: conservative management or medications only Major (Clavien 3–4)**: requiring intervention, resulting in permanent disability or death
|
| Type of study | Controlled studies (randomized controlled trials, non-randomized controlled trials, and comparative cohort studies): All comparisons between interventions Uncontrolled studies (single cohort studies): Data from uncontrolled studies that addressed active surveillance are described in the report. Every other uncontrolled study that addressed Key Question 3 is listed in the appendix with the following data: Author, publication year, Study design, Intervention name, Number of patients, Followup, List of outcomes |
| Timing and Setting | Any time point and setting |
Clavien-Dindo system currently used for reporting of complications related to urologic surgical interventions (http://www.surgicalcomplication.info/index-2.html).
APPENDIX 2
Strength of evidence for each domain of comparative effectiveness
| RN v PN | RN v TA | RN v AS | PN v TA | PN v AS | TA v AS | Uncontrolled AS | |
|---|---|---|---|---|---|---|---|
| Strength of Evidence | |||||||
| Cancer-specific survival | Moderate | Moderate | Low | Low | Insufficient | Insufficient | Low |
| Metastasis-free survival | Low | Low | Low | Moderate | Insufficient | Insufficient | Low |
| Local recurrence-free survival | Moderate | Low | Insufficient | Moderate | Insufficient | Insufficient | Insufficient |
| Overall survival | Low | Insufficient | Low | Low | Insufficient | Insufficient | Low |
| Continuous renal functional outcomes (serum creatinine, eGFR) | Moderate | Moderate | Low | Low | Insufficient | Insufficient | Insufficient |
| Categorical renal functional outcomes (CKD, ESRD) | Moderate | Moderate | Low | Low | Insufficient | Insufficient | Insufficient |
| Quality of life | Insufficient | Insufficient | Insufficient | Insufficient | Insufficient | Insufficient | Insufficient |
| Perioperative outcomes | Moderate | Low | Insufficient | Moderate | Insufficient | Insufficient | Insufficient |
| Harms | Low | Low | Insufficient | Low | Insufficient | Insufficient | Insufficient |
AS: active surveillance; CKD: chronic kidney disease; ESRD: end stage renal disease; TA: thermal ablation.
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References 51 through 88 can be obtained at http://jurology.com/.
References
- 1.Kutikov A, Fossett LK, Ramchandani P, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182:41. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:429. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society 2014; Available at http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. Accessed September 24, 2014. [Google Scholar]
- 5.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Available at http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp. Accessed September 24, 2014.
- 8.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Pierorazio PM, Johnson MH, Patel HD, et al. Management of Renal Masses and Localized Renal Cancer. Rockville, Maryland: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 10.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. Oxford: The Cochrane Collaboration; 2011. Available at http://handbook.cochrane.org. Accessed September 24, 2014. [Google Scholar]
- 11.Cochrane. Risk of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACRO-BATNRSI), version 1.0.0. Available at http://www.riskofbias.info. Accessed September 24, 2014.
- 12.Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, Maryland: Agency for Healthcare Research and Quality; 2014. (AHRQ publication No. 10(14)-EHC063-EF). Available at http://www.effectivehealthcare.ahrq.gov. Accessed September 24, 2014. [PubMed] [Google Scholar]
- 13.Lucas SM, Stern JM, Adibi M, et al. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75. doi: 10.1016/j.juro.2007.08.156. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli A, Ficarra V, Bertini R, et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: results of a retrospective, comparative, multi-institutional study. BJU Int. 2012;109:1013. doi: 10.1111/j.1464-410X.2011.10431.x. [DOI] [PubMed] [Google Scholar]
- 15.Barbalias GA, Liatsikos EN, Tsintavis A, et al. Adenocarcinoma of the kidney: nephron-sparing surgical approach vs. radical nephrectomy. J Surg Oncol. 1999;72:156. doi: 10.1002/(sici)1096-9098(199911)72:3<156::aid-jso8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Bedke J, Pritsch M, Buse S, et al. Prognostic stratification of localized renal cell carcinoma by tumor size. J Urol. 2008;180:62. doi: 10.1016/j.juro.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Gratzke C, Seitz M, Bayrle F, et al. Quality of life and perioperative outcomes after retroperitoneoscopic radical nephrectomy (RN), open RN and nephron-sparing surgery in patients with renal cell carcinoma. BJU Int. 2009;104:470. doi: 10.1111/j.1464-410X.2009.08439.x. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RH, Siddiqui S, Lohse CM, et al. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iizuka J, Kondo T, Hashimoto Y, et al. Similar functional outcomes after partial nephrectomy for clinical T1b and T1a renal cell carcinoma. Int J Urol. 2012;19:980. doi: 10.1111/j.1442-2042.2012.03085.x. [DOI] [PubMed] [Google Scholar]
- 20.Indudhara R, Bueschen AJ, Urban DA, et al. Nephron-sparing surgery compared with radical nephrectomy for renal tumors: current indications and results. South Med J. 1997;90:982. doi: 10.1097/00007611-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114:708. doi: 10.1111/bju.12580. [DOI] [PubMed] [Google Scholar]
- 22.Kyung YS, You D, Kwon T, et al. The type of nephrectomy has little effect on overall survival or cardiac events in patients of 70 years and older with localized clinical t1 stage renal masses. Korean J Urol. 2014;55:446. doi: 10.4111/kju.2014.55.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane BR, Fergany AF, Weight CJ, et al. Renal functional outcomes after partial nephrectomy with extended ischemic intervals are better than after radical nephrectomy. J Urol. 2010;184:1286. doi: 10.1016/j.juro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Li JR, Yang CR, Cheng CL, et al. Partial nephrectomy in the treatment of localized renal cell carcinoma: experience of Taichung Veterans General Hospital. J Chin Med Assoc. 2007;70:281. doi: 10.1016/S1726-4901(07)70005-9. [DOI] [PubMed] [Google Scholar]
- 25.Mariusdottir E, Jonsson E, Marteinsson VT, et al. Kidney function following partial or radical nephrectomy for renal cell carcinoma: a population-based study. Scand J Urol. 2013;47:476. doi: 10.3109/21681805.2013.783624. [DOI] [PubMed] [Google Scholar]
- 26.Medina-Polo J, Romero-Otero J, Rodríguez-Antolín A, et al. Can partial nephrectomy preserve renal function and modify survival in comparison with radical nephrectomy? Scand J Urol Nephrol. 2011;45:143. doi: 10.3109/00365599.2010.548082. [DOI] [PubMed] [Google Scholar]
- 27.Milonas D, Skulčius G, Baltrimavičius R, et al. Comparison of long-term results after nephron-sparing surgery and radical nephrectomy in treating 4- to 7-cm renal cell carcinoma. Medicina (Kaunas) 2013;49:223. [PubMed] [Google Scholar]
- 28.Minervini A, Serni S, Tuccio A, et al. Simple enucleation versus radical nephrectomy in the treatment of pT1a and pT1b renal cell carcinoma. Ann Surg Oncol. 2012;19:694. doi: 10.1245/s10434-011-2003-x. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell RE, Gilbert SM, Murphy AM, et al. Partial nephrectomy and radical nephrectomy offer similar cancer outcomes in renal cortical tumors 4 cm or larger. Urology. 2006;67:260. doi: 10.1016/j.urology.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 30.Patard JJ, Shvarts O, Lam JS, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 31.Roos FC, Brenner W, Jäger W, et al. Perioperative morbidity and renal function in young and elderly patients undergoing elective nephron-sparing surgery or radical nephrectomy for renal tumours larger than 4 cm. BJU Int. 2011;107:554. doi: 10.1111/j.1464-410X.2010.09516.x. [DOI] [PubMed] [Google Scholar]
- 32.Roos FC, Brenner W, Thomas C, et al. Functional analysis of elective nephron-sparing surgery vs radical nephrectomy for renal tumors larger than 4 cm. Urology. 2012;79:607. doi: 10.1016/j.urology.2011.10.073. [DOI] [PubMed] [Google Scholar]
- 33.Uchida K, Takahashi A, Masumori N, et al. Partial nephrectomy for small localized renal cell carcinoma. Hinyokika Kiyo. 2004;50:389. [PubMed] [Google Scholar]
- 34.Uzzo RG, Wei JT, Hafez K, et al. Comparison of direct hospital costs and length of stay for radical nephrectomy versus nephron-sparing urgery in the management of localized renal ell carcinoma. Urology. 1999;54:994. doi: 10.1016/s0090-4295(99)00348-9. [DOI] [PubMed] [Google Scholar]
- 35.Weight CJ, Lythgoe C, Unnikrshnan R, et al. Partial nephrectomy does not compromise survival in patients with pathologic upstaging to pT2/pT3 or high-grade renal tumors compared with radical nephrectomy. Urology. 2011;77:1142. doi: 10.1016/j.urology.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 36.Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with inceased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol. 2010;183:1317. doi: 10.1016/j.juro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Dekaj T, Lifshitz DA, Shikanov SA, et al. Laparoscopic radical versus laparosopic partial nephrectomy for clinical T1bN0M0 renal tumors: comparison of perioperative, pathological, and unctional outcomes. J Endourol. 2010;24:1603. doi: 10.1089/end.2009.0312. [DOI] [PubMed] [Google Scholar]
- 38.Kim FJ, Rha KH, Hernandez F, et al. Laparoscopic radical versus partial nephrectomy: assessment of complications. J Urol. 2003;170:408. doi: 10.1097/01.ju.0000076017.26789.6a. [DOI] [PubMed] [Google Scholar]
- 39.Shinohara N, Harabayashi T, Sato S, et al. Impact of nephron-sparing surgery on quality of life in patients with localized renal cell carcinoma. Eur Urol. 2001;39:114. doi: 10.1159/000052422. [DOI] [PubMed] [Google Scholar]
- 40.Janetschek G, Jeschke K, Peschel R, et al. Laparoscopic surgery for stage T1 renal cell carcinoma: radical nephrectomy and wedge resection. Eur Urol. 2000;38:131. doi: 10.1159/000020269. [DOI] [PubMed] [Google Scholar]
- 41.McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 42.Chang X, Zhang F, Liu T, et al. Radiofrequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: long-term clinical and oncologic outcomes. J Urol. 2015;193:430. doi: 10.1016/j.juro.2014.07.112. [DOI] [PubMed] [Google Scholar]
- 43.Klatte T, Mauermann J, Heinz-Peer G, et al. Perioperative, oncologic, and functional outcomes of laparoscopic renal cryoablation and open partial nephrectomy: a matched pair analysis. J Endourol. 2011;25:991. doi: 10.1089/end.2010.0615. [DOI] [PubMed] [Google Scholar]
- 44.Stern JM, Svatek R, Park S, et al. Intermediate comparison of partial nephrectomy and radio-frequency ablation for clinical T1a renal tumours. BJU Int. 2007;100:287. doi: 10.1111/j.1464-410X.2007.06937.x. [DOI] [PubMed] [Google Scholar]
- 45.Tanagho YS, Bhayani S, Kim EH, et al. Renal cryoablation versus robot-assisted partial nephrectomy: Washington University long-term experience. J Endourol. 2013;27:1477. doi: 10.1089/end.2013.0192. [DOI] [PubMed] [Google Scholar]
- 46.Mues AC, Korets R, Graversen JA, et al. Clinical, pathologic, and functional outcomes after nephron-sparing surgery in patients with a solitary kidney: a multicenter experience. J Endourol. 2012;26:1361. doi: 10.1089/end.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67:252. doi: 10.1016/j.eururo.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Youn CS, Park JM, Lee JY, et al. Comparison of laparoscopic radiofrequency ablation and open partial nephrectomy in patients with a small renal mass Korean. J Urol. 2013;54:603. doi: 10.4111/kju.2013.54.9.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillotreau J, Haber GP, Autorino R, et al. Robotic partial nephrectomy versus laparoscopic cryoablation for the small renal mass. Eur Urol. 2012;61:899. doi: 10.1016/j.eururo.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Bensalah K, Zeltser I, Tuncel A, et al. Evaluation of costs and morbidity associated with laparoscopic radiofrequency ablation and laparoscopic partial nephrectomy for treating small renal tumours. BJU Int. 2008;101:467. doi: 10.1111/j.1464-410X.2007.07276.x. [DOI] [PubMed] [Google Scholar]


