Abstract
Recent research suggests that prosocial outcomes in sharing games arise from prefrontal control of self-maximizing impulses. We used continuous Theta Burst Stimulation (cTBS) to disrupt the functioning of two prefrontal areas, the right dorsolateral prefrontal cortex (DLPFC) and dorsomedial prefrontal cortex (DMPFC). We used cTBS in the right MT/V5, as a control area. We then tested subjects’ prosocial inclinations with an unsupervised Dictator Game in which they allocated real money anonymously between themselves and low and high socioeconomic status (SES) players. cTBS over the two prefrontal sites made subjects more generous compared to MT/V5. More specifically, cTBS over DLPFC increased offers to high SES players, while cTBS over DMPFC caused increased offers to low SES players. These data, the first to demonstrate an effect of disruptive neuromodulation on costly sharing, suggest that DLPFC and MPFC exert inhibitory control over prosocial inclinations during costly sharing, though they may do so in different ways. DLPFC may implement contextual control, while DMPFC may implement a tonic form of control. This study demonstrates that humans’ prepotent inclination is toward prosocial outcomes when cognitive control is reduced, even when prosocial decisions carry no strategic benefit and concerns for reputation are minimized.
Keywords: Empathy, Prosocial Behavior, Cognitive Control, Transcranial Magnetic Stimulation, Neuroeconomics
Introduction
Are humans primarily selfish or selfless? Correspondingly, does socialization constrain our selfishness or warp our altruism? This question is perhaps overly simplistic, yet it has the advantage of reducing complex social behavior to something tractable in the laboratory. The question has its philosophical roots in Rousseau and Hobbes’ debate over the “state of nature”, i.e. humanity’s “natural”, precivilized state. Hobbes considered human nature to be an inherently nasty, brutish thing that is saved and improved by civilization. Rousseau, in contrast, thought that humankind began as “noble savages”, which have become warped into beasts by civilization. Whether we apply this debate to individual socialization or to our species’ evolution, the central question remains the same: is our prepotent impulse in social transactions to self-maximize and compete, or to share and cooperate?
While it is likely that self-maximizing and prosocial impulses coexist dynamically beginning early in life, the notion that we are primarily self-maximizing has dominated theory in politics, economics, and social psychology for a long time. Two recent studies examining the effect of Transcranial Magnetic Stimulation (TMS) on economic behavior are indeed interpreted along these lines. The studies found that subjects playing as responders in the Ultimatum Game (in which subjects must accept or reject divisions of money) accepted more unfair offers following disruption of the right DLPFC by low-frequency TMS (Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006, van’t Wout, Kahn, Sanfey, & Aleman, 2005). This finding has been interpreted as suggesting that disruptive TMS over prefrontal cortex produced disinhibition of a prepotent self-maximizing impulse. However, those results could also be interpreted as the manifestation of disinhibition of prosocial impulses. After all, accepting an unfair offer also results in a larger payoff for the opposing player. Indeed, we propose that our prepotent impulse in costly sharing paradigms is towards prosocial decisions, fostered by reflexive forms of empathy that are tonically and contextually inhibited by prefrontal systems involved in top-down control (Christov-Moore & Iacoboni, 2015).
Lower animals as well as primates exhibit apparently altruistic behavior, often risking their lives to protect others within the group (Preston & De Waal, 2002). Children and toddlers also demonstrate spontaneous sympathetic and prosocial behavior in response to the distress of others (Eisenberg & Fabes, 1990). Economic games such as the Prisoner’s Dilemma or the Dictator Game show outcomes inconsistent with a self-maximizing drive (Camerer & Thaler, 1995; Engel, 2001; Liebe & Tutic, 2010), leading some to conclude that prosocial outcomes may have a subjective reward value or utility during decision-making (Fehr & Camerer, 2007; Fehr & Fischbacher, 2003). Indeed, a 2006 study on neural correlates of charitable donations found that the mesolimbic reward system is engaged by donations in the same way as when monetary rewards are obtained (Moll, Krueger, Zahn, Pardini, de Oliveira-Souza, & Grafman, J., 2006). This suggests that humans possess a reflexive prosocial inclination.
In addition to the dorsolateral prefrontal cortex (DLPFC), the dorsomedial prefrontal cortex (DMPFC) may also be instrumental in modulating the distinction between self and other during social interactions and decision-making (Camerer & Thaler, 1995; Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006; van’t Wout, Kahn, Sanfey, & Aleman, 2005; Miller & Cohen, 2001; Spengler, von Cramon, & Brass, 2010; Taylor, Borckardt, & George, 2012). The DMPFC is important for manipulating self and other perspectives (Amodio & Frith, 2006), as well as the tonic control of spontaneous imitation (mimicry) (Cross, Torrisi, Reynolds-Losin, & Iacoboni, 2013; Lhermitte, 1983; Spengler, von Cramon & Brass, 2010). Furthermore, disruptive Theta-Burst stimulation (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005) to DMPFC was recently found to reduce bias towards out-group members (Holbrook, Izuma, Deblieck, Fessler, & Iacoboni, 2015).
To further test the contrasting hypotheses that humans are instinctively self-maximizers or prosocial, we examined sharing behavior towards players of high and low socioeconomic status (SES) in the Dictator Game (DG), which is a more direct measure of subjects’ prosocial or self-maximizing inclinations, as opposing players cannot reject the subject’s offers. We transiently disrupted the activity of two cortical sites in DLPFC and DMPFC that were previously implicated in the inhibitory control of prosocial inclinations during the DG (Christov-Moore & Iacoboni, in press). If self-maximization is the primary drive being inhibited by prefrontal control (as suggested by Knoch et al., 2005 and van’t Wout et al., 2005), then disruption of prefrontal control should result in lower offers to opposing players. If prosociality is, on the contrary, the primary drive inhibited by prefrontal control, then disruption of prefrontal control should result in higher offers to opposing players.
Methods
Subjects
A total of 58 subjects participated in this experiment (30 women, 28 men, mean age = 21.31 years, age range: 18–35 years). Subjects were all right-handed, with no history of drug and alcohol abuse and no prior or concurrent diagnosis of any neurological, psychiatric or developmental disorders. Participants were compensated $25 per hour and received additional compensation ($0–30) depending on their performance on the DG. Subjects were randomly assigned to stimulation in either the right DLPFC (9 females and 10 males), DMPFC (10 females and 9 males) or MT/V5 areas (11 females, 9 males). Subjects underwent continuous theta burst stimulation (cTBS) to either right DLPFC, DMPFC or MT/V5 before playing the DG. All subjects underwent two experimental sessions. All aspects of this experiment were previously approved by the UCLA Institutional Review Board.
Magnetic Resonance Imaging
The first experimental session, identical for all subjects, consisted of a high-resolution T1-weighted structural scan (TR=1900 ms, TE=2.26 ms, TI=100 ms, flip angle=9°, matrix size=192×192, FOV=250 mm, 176 slices, 1.0 × 1.0 × 1.0 mm voxels). These imaging data were collected to guide the location of the stimulation site. Subjects also performed two tasks of pain perception and facial emotion observation and imitation. Since these two tasks and their results were not used in this non-invasive neuromodulation study, we will not describe them in detail here.
Transcranial Magnetic Stimulation
Subjects’ cTBS session occurred approximately a week after their MRI session. The right DLPFC and DMPFC regions-of-interest (ROI) were constructed in standard space (MNI 152 template) using 10mm diameter spheres centered on voxels in right DLPFC (x = 40mm, y = 24mm, z = 38mm) and right DMPFC (x = 8mm, y = 24mm, z = 54mm). These ROI’s were centered on coordinates where activation during affective and somatosensory neural resonance tasks showed the highest correlation with subjects’ modulation of DG offers in response to players’ SES in a prior experiment (Christov-Moore & Iacoboni, 2015). That is, the activity of these areas during neural resonance tasks was correlated with the tendency to share less money with high SES players, compared to low SES players, in the DG. The rationale behind this experimental design choice is that these coordinates should represent areas of maximal control during the DG that we aim to disrupt with cTBS. The control ROI was constructed in an identical fashion around an area of no a priori interest in MT/V5 (x = 48mm, y = −74mm, z = 0mm), centered on a peak of activation from the same experiment (obtained while subjects viewed a video clip displaying an object in motion) (figure 1). ROI’s were registered to each subject’s native space using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), and used to accurately target the TMS coil using frameless stereotaxy as implemented in Brainsight (Rogue Research).
Figure 1.
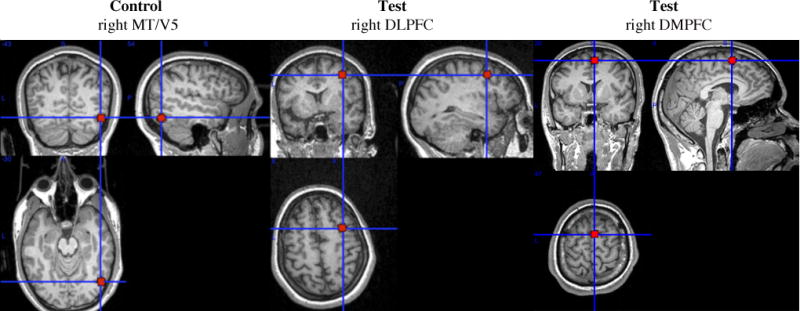
Stimulation sites. Control site: area MT/V5 (x = 48mm, y = −74mm, z = 0mm). Test sites: DLPFC (x = 40mm, y = 24mm, z = 38mm) and DMPFC (x = 8mm, y = 24mm, z = 54mm). ROI’s are displayed on a single subject’s high-resolution T1-weighted image in neurological orientation.
In accordance with previous studies (Klucharev, Moniek, Munneke, Smidts & Fernandez, 2011; Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006), active motor threshold (AMT) was measured in the left first dorsal interosseous for DLPFC and MT/V5, and in the anterior tibialis for DMPFC. AMT was defined as the minimum intensity that produced MEPs of ≥200 uV in 5 of out 10 consecutive stimulations, while subjects concurrently flexed the recorded muscle. Then, cTBS was delivered to the right DLPFC or DMPFC in the test groups and to the right MT/V5 in the control group. cTBS consists of triplets of TMS pulses at 50 Hz, delivered at 5 Hz for a total of 600 pulses over 40 seconds, with intensity set at 80% AMT, using a second-generation Magstim Rapid stimulator. Stimulation was delivered to MT/V5 and right DLPFC using a flat figure-eight coil, while stimulation to right DMPFC was delivered using an angled coil (commonly used to reach deeper stimulation sites, such as DMPFC) (after Klucharev, Moniek, Munneke, Smidts & Fernandez, 2011). Subjects were unaware of their group affiliation (test or control). After cTBS, subjects waited for 5–7 minutes before commencing the DG, consistent with the previously reported stabilization of post-TBS effects (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005), during which time they were briefed on the DG. Before and during briefing, they were comprehensively informed that the study protocol involved no deception.
Dictator Game
For the DG, subjects were asked to divide a sum of money ($10) with another player as they saw fit. The DG consisted of 24 trials with 24 different digital profiles of various ages and race. Each profile contained a name, a headshot from the NimStim facial stimulus set (Tottenham, Tanaka, Leon, McCarry, Nurse, Hare, Marcus, Westerlund, Casey, & Nelson, 2009) and a yearly income (SES), which was either low ($18,000–$30,000/yr) or high ($70,000–200,000/yr). This allowed us to test how people’s apparently altruistic generosity (a proxy for the relative utility of the player’s welfare) varied as a function of the opposing player’s SES. SES (a proxy for perceived need) is a contextual factor which previous studies have shown to inhibit generosity – people give less money to players with lower perceived need (Engel, 2011; Liebe & Tutic, 2010). In each trial, subjects were shown a profile displayed on a computer screen. They had 5 seconds to observe the profile, after which an onscreen prompt (“How much $ would you like to offer?”) appeared in the lower right corner of the profile. Subjects then had 5 seconds to make an offer by pressing a number key (0–10) on a standard keyboard. Subjects interacted with each player only once. This design allowed us to isolate the relative utility of subjects’ and players’ welfare separate from strategic concerns, as the subjects’ compensation was dependent only on their allocation of funds. This is distinct from the Ultimatum game, in which opposing players can reject subjects’ offers, which adds a personal strategic value to prosocial decisions.
The DG was designed such that half of the profiles (n=12) represented real people in Los Angeles (contacted prior to the study) who would actually receive the money subjects offered in the DG. In order to maintain the profiles’ anonymity, we created false names and used NimStim headshots matched for age, gender, and race. Subjects and virtual players were compensated for three randomly selected trials (list randomizer, www.random.org) so that, in any given trial, a subject’s decision might affect the welfare of a real person and themselves. This was done to encourage engagement of naturalistic social cognitive processes; previous studies have shown that different behavior and neural activation ensues if subjects believe they are playing the game with a computer (van 't Wout, Kahn, Sanfey, & Aleman, 2006). In order to reduce the possible effect of concerns for reputation and experimenter observation, subjects played the DG alone in a closed room, unobserved and unrecorded. To ensure their anonymity, a research assistant with whom subjects did not interact in the experiment was assigned to process their deidentified data. Subjects were comprehensively informed about all of these controls and questioned to assure that they understood the task parameters and controls.
Four subjects who voluntarily expressed disbelief in the controls following the Dictator Game were excluded from the study (and do not figure in the 58 subjects reported above). The requirement for anonymity also obviously prevented us from performing brain imaging during the DG.
Self Reported Empathy
Following the DG, subjects filled out the Interpersonal Reactivity Index (IRI). The IRI (Davis, 1983) is a widely used (in relation to self-other resonance)(Avenanti, Minio-Paluello, Bufalari, & Aglioti, 2009; Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008) and previously validated (Litvack-Miller, McDougall, & Romney, 1997) questionnaire designed to measure both cognitive and affective components of empathy. It consists of 24 statements that the subject rates on a 5-point scale ranging from 0 (Does not describe me very well) to 5 (Describes me very well). The statements are calculated to test four theorized subdimensions of empathy: Fantasizing Scale (FS) measures the tendency to take the perspective of fictional characters; Empathic Concern (EC) measures sympathetic reactions to the distress of others; Perspective Taking (PT) measures the tendency to take others’ perspective and Personal Distress (PD), which measures aversive reactions to the distress of others. Scores were summed for each sub-dimension (measured by 6 items) to make 4 scores per subject. We included the PT, PD, FS and EC subscales as covariates in our analyses of DG behavior.
Results
We first examined the normality of offers using a Shapiro-Wilk test and found that offers to low SES players were approximately normally distributed (MT/V5 p=.354, DLPFC p=.522), except for the DMPFC group (p=.005). Offers to high SES players violated the assumption of normality, likely due to a floor effect (MT/V5 p=.048, DLPFC p=.04, DMPFC p=.029). In addition, a Levene test of homogeneity of error variance within each SES condition between groups found that offers to high SES players additionally violated the assumption of homogeneity F (2,55) = 8.89, p < .0001, while offers to low SES players did not F (2,55) = .236, p = .791.
To accommodate non-normality and heteroscedasticity between groups, we employed a repeated-measures linear mixed model with robust standard errors, including gender and the PT, PD, FS and EC subscales of the Interpersonal Reactivity Index as covariates (to control for possible effects of gender and individual differences in trait empathy). We found a trending effect of group on offers F (2,107) = 2.589, p = .08, a highly significant effect of player SES (high or low) on offers F (2,107) = 153.175, p < .0001, and a significant group × player SES interaction F (2,107) = 3.976, p = .022. We did not find a significant effect of trait empathy or gender on offers. Accordingly, a follow-up analysis performed without these covariates’ inclusion did not change the main effects of player SES, group or the interaction term.
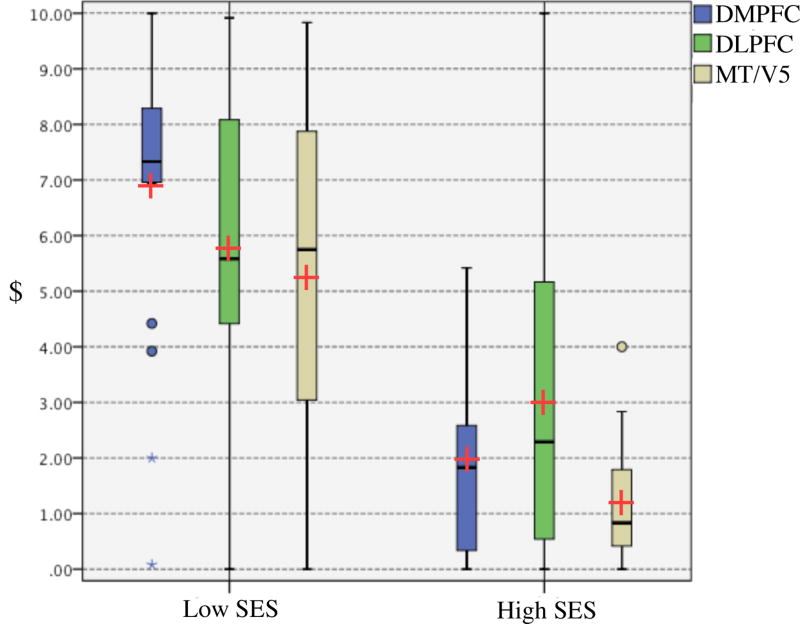
Post-hoc tests of simple effects (t-tests with robust standard error) were conducted to test our hypothesis that offers were higher in the DLPFC and DMPFC groups relative to controls. Accordingly, alpha level was set at .05, one tailed. We found a highly significant increase in mean offers to high SES players between the DLPFC and MT/V5 group, t (107) = 2.777, p = .003 (Table 1). There was also a significant increase in mean offers to low SES players between the DMPFC and MT/V5 group, t (107) = 1.818, p = .036 (Table 1). We additionally calculated effect sizes using Cohen’s d for these two post-hoc analyses, and found an effect size of .95 for the DLPFC/High SES comparison, and an effect size of .59 for the DMPFC/Low SES comparison. All analyses were carried out using SPSS (V.21, SPSS Inc., Chicago, Illinois, USA).
Table 1.
Sample sizes and means with 95% Confidence Interval (CI) and robust estimated standard errors of dictator game offers (in $) by group and condition.
| Group | n | Low SES offers x̄ (95%CI) |
σ | High SES offers x̄ (95%CI) |
σ |
|---|---|---|---|---|---|
|
| |||||
| MT/V5 | 20 | 5.29 (4.00, 6.58) | 0.649 | 1.22 (0.73, 1.70) | 0.244 |
| MPFC | 19 | 6.88 (5.76, 8.00) | 0.566 | 1.98 (1.09, 2.87) | 0.449 |
| DLPFC | 19 | 5.85 (4.78, 6.92) | 0.54 | 3.00 (1.80, 4.20) | 0.605 |
Discussion
These results demonstrate that disruptive stimulation to right DLPFC and DMPFC increased subjects’ offers, suggesting that these prefrontal areas exert an inhibitory influence on a prepotent prosocial inclination. However, these prefrontal systems seem to control prosocial decision making differently. Player SES was found to have a strong inhibitory effect on prosocial decisions, suggesting that the high SES condition evokes top-down contextual control to a greater extent than the low SES condition. Accordingly, transient disruption of the DLPFC seems to have reduced the inhibitory effect of contextual cues (offers to high SES players), while transient disruption of DMPFC seemed to reduce context-independent control (offers to low SES players).
These data suggest that DLPFC may implement a form of context-sensitive inhibition, consistent with its proposed role in integrating cognition and emotional responses during decision-making (Fehr & Camerer, 2007; Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006). DLPFC stimulation had the greatest effect on offers to high SES players, where contextual inhibition is most likely to play a role relative to tonic inhibition. DMPFC may implement tonic control (consistent with its possible role in the tonic control of automatic imitation, Lhermitte, 1983; Spengler, von Cramon, & Brass, 2010), as its effect is most pronounced on offers to low SES players, a condition in which contextual inhibition plays a smaller role.
Recent studies suggest that our prosocial inclinations may originate from our tendency to share in the internal states and behavior of others, a contagion-like process we will refer to as “self-other resonance”. A likely neural mechanism for self-other resonance is neural resonance, the tendency to recruit similar neural systems for the pain, emotions and behavior of the self and other people (Zaki & Ochsner, 2012). Neural resonance has been correlated with prosocial behavior (Hein, Silani, Preuschoff, Batson & Singer, 2010; Hein, Lamm, Brodbeck, & Singer, 2011; Ma, Wang & Han, 2011), suggesting that our ability to share in the experiences of others may compel us to include the welfare of others in our decisions (Preston & De Waal, 2002; Smith, 2006). However, neural resonance and prosocial behavior covary with contextual factors like affiliation, race, gender, perceived need and social distance (Camerer & Thaler, 1995; Cheng, Chen, Lin, Chou, & Decety, 2010; Hogeveen, Inzlicht, & Obhi, 2014; Gu & Han, 2007; Guo, Zheng, Zhang, Zhu, Jianqi, Wang, Dienes, & Yang, 2012; Lamm, Nusbaum, Meltzoff, & Decety, 2007; Liebe & Tutic, 2010; Loggia, Mogil, & Bushnell, 2008; Reynolds Losin, Iacoboni, Martin, Cross, & Dapretto, 2012; Reynolds Losin, Iacoboni, & Dapretto, 2014; Reynolds Losin, Woo, Krishnan, Wager, Iacoboni, & Dapretto, 2015; Singer, Seymour, O'Doherty, Stephan, Dolan, & Frith, 2006; Zaki & Ochsner, 2012). This context-sensitivity may result from mechanisms of inhibitory cognitive control via prefrontal systems, influencing the relative utility of others’ outcomes in our decisions.
We theorize that when we make costly decisions about others’ welfare in the Dictator Game, we implicitly assess the relative utility of their welfare versus our own. Neuroeconomics research suggests that in order to do this, we automatically form models of the people we are dealing with (Krueger, Grafman, & McCabe, 2008), which may evoke the sensory, affective and motor imagery we associate with other people. This imagery, much like real-time biological stimuli, may drive self-other resonance (and its inhibitory control), thus increasing the respective utility of the other person’s welfare. Indeed, there is evidence that affective/motivational structures implicated in neural resonance and empathy (like the amygdala and anterior insula) interact with systems involved in cognitive control (like the DLPFC and ventromedial prefrontal cortex) during social decision-making (Bechara, Damasio, Damasio, & Lee, 1999; Camerer, 2003; Hare, Camerer, Knoepfle, & Rangel, 2010; Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003).
It is important to note that prosocial decision-making often occurs in circumstances in which the biological stimuli typically associated with neural resonance (Zaki & Ochsner, 2012) are relatively sparse. In the Dictator Game employed here, the only biological stimuli associated with players are headshots with neutral facial expressions. However, neural resonance is correlated with prosocial behavior in circumstances involving similarly sparse biological stimuli (Christov-Moore & Iacoboni, 2015; Hein, Silani, Preuschoff, Batson, & Singer, 2012; Hein, Lamm, Brodbeck, & Singer, 2011; Ma, Wang, & Han, 2011). The relative presence of biological stimuli likely has an effect on our prosocial inclinations, since biological stimuli as sparse as a photograph have been found to increase charitable donations (Genevsky, Daniel, Paul, & Knutson, 2013). In addition, the prefrontal systems affected in this study are likely involved in a wide range of regulatory functions that may have also been disrupted by TBS. Further outcome measures are necessary to elucidate these effects. Furthermore, though the electrophysiology of TMS stimulation is not well understood, there is evidence that the propagation of TMS effects along white-matter tracts likely depends on the connectivity of the stimulated ROI, implying that the effects of TMS are at circuit level (Nummenmaa, McNab, Savadiej, Okada, Hämäläinen, Wang, Wald, Pascual-Leone, Wedeen, & Raj, 2014). Future studies should incorporate Diffusion Tensor Imaging parameters into their statistical models in order to improve the estimation of TMS effects.
Manipulations designed to diminish the role of top-down control in decision-making (increased cognitive load) have been shown to increase fair offers in the Dictator Game (Schulz, Fischbacher, Thoni & Utikal, 2014). However, to our knowledge, this is the first evidence for a successful effect of neuromodulation on costly sharing. Previous studies have suggested that prefrontal control areas (specifically the right DLPFC) exert an inhibitory influence on self-maximization (Knoch, Pascual-Leone, Meyer, Treyer, & Fehr, 2006; van’t Wout, Kahn, Sanfey, & Aleman, 2005). Our study suggests an opposite interpretation. In agreement with our hypothesis, these data provide causal evidence that right DLPFC and DMPFC are important for exerting an inhibitory influence on prereflective prosocial inclinations, since the areas we targeted here had previously demonstrated activity inversely correlated with offers in the DG. This finding represents a step towards a proximate explanation for humans’ tendency to deviate from rational self-interest. This suggests that our primary drive in non-strategic social transactions may in fact be to behave prosocially, perhaps due to reflexive forms of empathy that blur the boundaries between individuals.
Figure 2.
Box-and-whisker plots of average offers to high and low income/SES players by stimulation site. Edges of box indicate 1st and 3rd quantiles. Dots and stars indicate significant outliers. Means are indicated by red crosses (+), while medians are indicated by black horizontal lines (−). All outliers were included in the analysis.
Acknowledgments
This work was supported by the National Institute of Mental Health under grant R21 MH097178 to M.I., and by the National Science Foundation under a Graduate Fellowship Grant DGE-1144087 to L.C.M. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund.
References
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neuro. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio-Paluello I, Bufalari I, Aglioti SM. The pain of a model in the personality of an onlooker: Influence of state-reactivity and personality traits on embodied empathy for pain. NeuroImage. 2009;44(1):275–83. doi: 10.1016/j.neuroimage.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial pre-frontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer CF. Strategizing in the brain. Science. 2003;300:1673–1675. doi: 10.1126/science.1086215. [DOI] [PubMed] [Google Scholar]
- Camerer C, Thaler RH. Anomalies: Ultimatums, dictators and manners. J Econ Persp. 1995;9(2):209–219. [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, Decety J. Love hurts: An fmri study. NeuroImage. 2010;51(2):923–9. doi: 10.1016/j.neuroimage.2010.02.047. [DOI] [PubMed] [Google Scholar]
- Christov-Moore L, Iacoboni M. Emotions In Interaction:Towards a Supraindividual Study of Empathy. In: Martinovski B, editor. Emotion in Group Decision and Negotiation. Springer; 2015. pp. 4–36. [Google Scholar]
- Cross KA, Torrisi S, Reynolds Losin EA, Iacoboni M. Controlling Automatic Imitative Tendencies: Interactions Between Mirror Neuron and Cognitive Control Systems. NeuroImage. 2013;83:493–504. doi: 10.1016/j.neuroimage.2013.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- Eisenberg N, Fabes RA. Empathy: Conceptualization, Measurement, and Relation to Prosocial Behavior. Motiv Emotion. 1990;14(2):131–149. [Google Scholar]
- Engel C. Dictator games: A meta study. Exp Econ. 2011;14(4):583–610. [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: The neural circuitry of social preferences. Trends Cogn Sci. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425(6960):785–91. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Genevsky A, Daniel V, Paul S, Knutson B. Neural Underpinnings of the Identifiable Victim Effect: Affect Shifts Preferences for Giving. J Neurosci. 2013;33(43) doi: 10.1523/JNEUROSCI.2348-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. NeuroImage. 2007;36(1):256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Guo X, Zheng L, Zhang W, Zhu L, Jianqi L, Wang Q, Dienes Z, Yang Z. Empathic neural responses to others' pain depend on monetary reward. Soc Cog Affect Neuro. 2012;7(5):535–41. doi: 10.1093/scan/nsr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30(2):583–90. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hein G, Lamm C, Brodbeck C, Singer T. Skin conductance response to the pain of others predicts later costly helping. PloS One. 2011;6(8):e22759. doi: 10.1371/journal.pone.0022759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J, Inzlicht M, Obhi SS. Power changes how the brain responds to others. J Exp Psychol Gen. 2014;143(2):755–62. doi: 10.1037/a0033477. [DOI] [PubMed] [Google Scholar]
- Holbrook C, Izuma K, Deblieck C, Fessler DMT, Iacoboni M. Neuromodulation of Group Prejudice and Religious Belief. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Klucharev Vasily, Moniek A, Munneke M, Smidts Ale, Fernández Guillén. Downregulation of the Posterior Medial Frontal Cortex Prevents Social Conformity. J Neurosci. 2011;31:33. doi: 10.1523/JNEUROSCI.1869-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Krueger F, Grafman J, McCabe K. Neural correlates of economic game playing. Philos T Roy Soc B. 2008;363(1511):3859–74. doi: 10.1098/rstb.2008.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PloS One. 2007;2(12):e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte F. "Utilization behaviour" and its relation to lesions of the frontal lobes. Brain. 1983;106:237–55. doi: 10.1093/brain/106.2.237. [DOI] [PubMed] [Google Scholar]
- Liebe U, Tutic A. Status groups and altruistic behavior in dictator games. Ration. Soc. 2010;22(3):353–380. [Google Scholar]
- Litvack-Miller W, McDougall D, Romney DM. The structure of empathy during middle childhood and its relationship to prosocial behavior. Genet Soc Gen Psych. 1997;123:303–324. [PubMed] [Google Scholar]
- Loggia ML, Mogil JS, Bushnell MC. Empathy hurts: Compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136(1–2):168–76. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang C, Han S. Neural responses to perceived pain in others predict real-life monetary donations in different socioeconomic contexts. NeuroImage. 2011;57(3):1273–80. doi: 10.1016/j.neuroimage.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa A, McNab JA, Savadiej P, Okada Y, Hämäläinen MS, Wang R, Wald LL, Pascual-Leone A, Wedeen VJ, Raj T. Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stim. 2014;7(1):80–84. doi: 10.1016/j.brs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. NeuroImage. 2008;39(4):2076–85. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, De Waal F. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25(01):1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Reynolds Losin EA, Iacoboni M, Martin A, Cross K, Dapretto M. Race modulates neural activity during imitation. NeuroImage. 2012;59(4):3594–603. doi: 10.1016/j.neuroimage.2011.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds Losin EA, Iacoboni M, Dapretto M. Neural processing of race during imitation: self-similarity versus social status. Hum Brain Mapp. 2014;35(4):1723–39. doi: 10.1002/hbm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds Losin EA, Woo C, Krishnan A, Wager TD, Iacoboni M, Dapretto M. Brain and psychological mediators of imitation: Sociocultural versus physical traits. Cult Brain. doi: 10.1007/s40167-015-0029-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science (New York, N.Y.) 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schulz JF, Fischbacher U, Thöni C, Utikal V. Affect and fairness: Dictator games under cognitive load. J Econ Psych. 2014;41:77–87. [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Cognitive empathy and emotional empathy in human behavior and evolution. Psych Rec. 2006;56(1):3. [Google Scholar]
- Spengler S, von Cramon DY, Brass M. Resisting motor mimicry: Control of imitation involves processes central to social cognition in patients with frontal and temporo-parietal lesions. Soc Neuro. 2010;5(4):401–16. doi: 10.1080/17470911003687905. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rtms-induced analgesia. Pain. 2012;153(6):1219–25. doi: 10.1016/j.pain.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiat Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Wout M, Kahn RS, Sanfey AG, Aleman A. Affective state and decision-making in the ultimatum game. Exp Brain Res. 2006;169(4):564–8. doi: 10.1007/s00221-006-0346-5. [DOI] [PubMed] [Google Scholar]
- van't Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16(16):1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Ochsner K. The neuroscience of empathy: Progress, pitfalls and promise. Nat Neuro. 2012;15(5):675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]