Abstract
According to the U.S. Department of Energy and the European Union, tellurium is a critical element needed for energy and defense technology. Thus methods are needed to recover tellurium from waste streams. The objectives of this study was to determine the feasibility of utilizing upflow anaerobic sludge bed (UASB) reactors to convert toxic tellurite (TeIV) oxyanions to non-toxic insoluble elemental tellurium (Te0) nanoparticles (NP) that are amendable to separation from aqueous effluents. The reactors were supplied with ethanol as the electron donating substrate to promote the biological reduction of TeIV. One reactor was additionally amended with the redox mediating flavonoid compound, riboflavin (RF), with the goal of enhancing the bioreduction of TeIV. Its performance was compared to a control reactor lacking RF. The continuous formation of Te0 NPs using the UASB reactors was found to be feasible and remarkably improved by the addition of RF. The presence of this flavonoid was previously shown to enhance the conversion rate of TeIV by approximately 11-fold. In this study, we demonstrated that this was associated with the added benefit of reducing the toxic impact of TeIV towards the methanogenic consortium in the UASB and thus enabled a 4.7-fold higher conversion rate of the chemical oxygen demand. Taken as a whole, this work demonstrates the potential of a methanogenic granular sludge to be applied as a bioreactor technology producing recoverable Te0 NPs in a continuous fashion.
Keywords: tellurite, tellurium nanoparticles, continuous bioreactors, redox mediators, riboflavin
Graphical Abstract
1. INTRODUCTION
Tellurium (Te) is a scarce and valuable element which is part of the chalcogen group. The concentration of this metalloid in the earth’s crust ranges between 0.005 to 0.027 ppm (Wedepohl, 1995; Ba et al., 2010). Te is found in copper ores, as well as, associated with gold and silver in minerals, such as, calaverite (AuTe2) and silvanite (AgAuTe4) (Taylor, 1999; Chasteen et al., 2009). The most stable forms of Te in the environment are tellurate TeO42− ((+6), TeVI), tellurite TeO32− ((+4), TeIV), elemental Te0 (0) and telluride (−2) (Zannoni et al., 2008). Te is commercially obtained through different processes which include the leaching of the metalloid from the anode slimes produced during the electrolytic recovery of copper (Morrison, 1977; Taylor, 1999). Tellurite is the predominant soluble species of Te in leachates from copper and gold mining (Kyle et al., 2011; Fan et al., 2013).
The soluble oxyanions of Te, especially TeIV, are highly toxic to most microorganisms. The observed toxic effects towards different microbial communities were found to be related to the cells growth conditions (either in planktonic form or as biofilms) and related to the oxidation state of the Te oxyanions. Escherichia coli and Staphylococcus aureus were more affected by TeIV than by TeVI (Turner et al., 2012). For both of these bacteria planktonic cells were more sensitive than biofilms. Te oxyanions inhibited distinct trophic groups in an anaerobic methanogenic mixed culture differently. TeIV was very toxic to acetate- and hydrogen- consuming trophic groups; while, TeVI was moderately toxic to the acetate consuming- and non-inhibitory to the hydrogen consuming- trophic groups of the same mixed culture (Ramos-Ruiz et al., 2016b). Thus the presence of these soluble oxyanions in the environment can potentially negatively affect anaerobic treatment processes such as the methanogenic phase in a landfill.
Due to the increasing number of reported applications of Te, this element has been regarded as a critical element by the US Department of Energy and by the European Union because a shortage of its supply might compromise the development of energy and defense related advanced technologies (de Boer and Lammertsma, 2013). In recent years, Te has been largely used as an additive in the rubber vulcanization process, as well as, an alloy to improve the thermo-electrical and opto-electronic properties of metal and glass. This metalloid plays a major role in the transition to clean energy technology. Te is currently used to produce CdTe thin-film photovoltaic cells, one of the most common types of solar panels commercially available (U.S. Department of Energy’s National Renewable Energy Laboratory, 2013), as well as, to manufacture Bi2Te3 thermoelectric modules (Amatya and Ram, 2011). Te has also been used to develop novel materials such as Te-based fluorescent quantum dots which have the potential to be used as probes in biosensors (Deng et al., 2007). To insure the proper supply of Te it is imperative to develop efficient processes to recover Te from mining residues and from leachates of decommissioned Te-containing products, such as CdTe solar panels in the future.
Biotechnology can provide an environmentally-friendly and cost-effective alternative to recover elemental Te0 from soluble Te in aqueous diluted streams since different microorganisms have been found to be capable of reducing a wide range of oxidized elements to their insoluble zerovalent forms (Korbekandi et al., 2009; Narayanan and Sakthivel, 2010). Several microorgansims are able to catalyze TeVI/TeIV reduction to Te0, depositing nanoparticles inside and outside the cells. The bacteria Bacillus selenitireducens and Sulfurospirillum barnesii were reported to grow using TeIV and TeVI as electron acceptors, respectively (Baesman et al., 2007; Baesman et al., 2009). In a previous work, we studied the ability of an unadapted methanogenic consortium in granular sludge to reduce both Te oxyanions to Te0 in batch experiments. The methanogenic consortium was found to be capable of reducing both Te species utilizing the endogenous substrates in the sludge biomass as the electron donor, and the presence of an external source of electrons, such as H2, had modest effects on increasing the reduction rates(Ramos-Ruiz et al., 2016a). TeIV was reduced considerably faster than TeVI (Ramos-Ruiz et al., 2016a). The Te oxyanion reduction rates were greatly enhanced by the addition of redox mediating compounds. Redox mediators are quinones or flavins that shuttle electrons from the cells to oxidized compounds (Van der Zee and Cervantes, 2009). 2-Hydroxy-1,4-naphthoquinone (lawsone) increased the rate of TeIV reduction, as well as, the quantity of Te0 NPs formed outside the cells in batch tests where the facultative photosynthetic bacterium, Rhodobacter capsulatus (Borghese et al., 2014), was supplied with malate as the electron-donating substrate. Riboflavin (RF) accelerated the rate of TeIV reduction by anaerobic methanogenic granular sludge up to 10.8- fold (Ramos-Ruiz et al., 2016a).
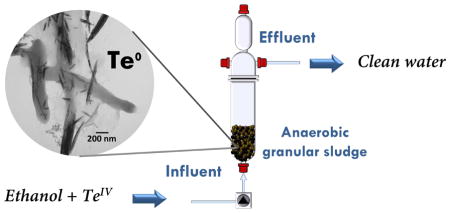
Among biological technologies, upflow anaerobic sludge blanket (UASB) reactors use anaerobic mixed cultures to treat wastewater in a continuous fashion. UASB reactors have proven to be effective to remove heavy metals, such as, cadmium (Cd) and zinc (Zn) from waste waters under sulfidogenic conditions (Goncalves et al., 2007). Furthermore, UASB reactors have been successfully used to study the recovery of the precious platinum group metal, palladium (Pd) (Pat-Espadas et al., 2016b). The present study was aimed at evaluating the potential to use UASB technology to reduce TeIV to Te0 NP using a methanogenic microbial consortium in granular sludge provided with ethanol as an exogenous source of electron-donating substrate. The impact of RF to act as a redox mediator on facilitating the reduction process was tested by amending it to one of two UASB columns.
2. MATERIALS AND METHODS
2.1. Chemicals
TeIV as Na2TeO3 and riboflavin (RF) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and the Te standard (1000 ppm in 3% HNO3) was purchased from RICCA Chemical Company (Arlington, TX, USA).
2.2. Biomass source
An anaerobic granular biofilm obtained from a full scale up-flow anaerobic sludge blanket (UASB) reactor at Mahou’s (beer brewery in Guadalajara, Spain) wastewater treatment plant, was used as the source of inoculum. This biomass contained 0.0657 g volatile suspended solids (VSS) g−1 wet wt. The maximum methanogenic activities of the sludge were 565.8±63.8 mg methane expressed as chemical oxygen demand (COD-CH4) gVSS−1 day−1 and 570.9±25.9 mg COD-CH4 gVSS−1 day−1 for the assays utilizing acetate and hydrogen as substrate, respectively. The sludge was stored at 4 °C.
2.3. Set-up of the continuous reactors
Two laboratory-scale upflow anaerobic granular sludge bed (UASB) reactors (270 mL) were run in parallel (see Fig S1 in supplementary information (SI)) in four different periods of time, based on the changes in TeIV concentration in the influent as shown in Table S1 in SI, and which are briefly discussed below. Each reactor was inoculated to a final concentration of 10 g VSS L−1 of anaerobic granular biofilm. Both reactors were continuously operated for 146 days at room temperature (23 ± 2 °C) with hydraulic retention times (HRT) of ~0.6 days. In order to study the effect of the redox mediator riboflavin (RF) on the reduction of TeIV, one reactor (R1) was operated as a control without the addition of RF, while the mineral medium (M1, please refer to SI) used in the second reactor (R2) was spiked with different concentrations of the redox mediator during their continuous operation. The conditions of operation for both reactors during the four time periods are summarized in Table S1 in SI. Briefly, the first period of time, which comprises the initial 20 days of operation, was used as an acclimation stage and for this, the influents of both reactors were supplemented with basal media (M1, please see SI) lacking TeIV and containig ~50 mg L−1 (104.0 mg COD L−1) of ethanol as substrate. In period II (day 20 to 50), both reactors were supplemented with mineral medium (M1 please see SI) containing 10 mg L−1 of TeIV, ~50 mg L−1 of ethanol, and one reactor was amended with RF at a molar ration Te:RF 1:0.5. During period III (day 50 to 100) the concentration of TeIV in the basal medium (M1 please see SI) was increased to 20 mg L−1 and two different levels of ethanol were used, ~ 50 mg L−1 (days 50 to 75) and ~ 140 mg L−1 ( 291.3 mg COD L−1; days 75 to 100); two different Te:RF molar ratios were also supplied to R2 (1:0.25 for days 50 to 75, and 1:0.5 for days 75 to 100). In period IV (days 100 to 146), the concentration of TeIV was increased to 40 mg L−1, the amount of ethanol was also raised to ~ 280 mg L−1 (582.7 mg COD L−1) and the Te:RF molar ratio in R2 was 1:0.25.
Liquid samples obtained periodically from the influent and effluent streams of the reactors were centrifuged in centrifuge tubes at 12000 rpm for 10 min, and the supernatants were diluted into a 2% v/v HNO3 solution. The acidified samples were analyzed for total soluble Te as described in SI section. In order to account for potential losses of Te0 NPs in the effluents of the reactors, selected liquid samples were filtered through 25 nm membrane filters (EMD Millipore, Billerica, MA, USA); the filtrates were acidified to a final concentration of 2% v/v HNO3 and analyzed for dissolved Te using an ICP-OES as described in SI section. The presence of Te0 NPs was then estimated as the difference between the Te concentration from the centrifugation and filtration steps.
Samples of the influent and effluent ports were taken for the immediate measurement of pH according to standard methods, and of ethanol and acetate as described in SI section. The amount of ethanol and acetate (as a product of ethanol degradation), soluble Te, and the pH of the influents and effluents of the bioreactors were used as indicators of the performance of the reactors.
2.4. Activity kinetics batch bioassays
Two independent batch experiments were conducted to investigate the impact of the concentration of TeIV and the effect of the biomass concentration on the rate of TeIV reduction. These experiments were performed in 590 mL serum bottles (Wheaton, Millville, NJ, USA) amended with different amounts of granular sludge (from 0.19 to 3 g VSS L−1), 370 mL of a liquid mixture containing different amounts of two (0.5 or 5 g TeIV L−1) stock solutions to attain concentrations between 1.2 ± 0.2 – 74.6 ± 14.3 mg TeIV L−1 according of the purpose of the assay, mineral basal medium (M2, please see SI), and different stoichiometric excesses of hydrogen (H2) as the electron donor was supplied to the 220 mL of headspace proving 10.4 mmol H2 gas L−1liq (excesses ranging from 6.6- to 664-fold based on e− equivalents). The experimental conditions utilized in these experiments are summarized in Table S2 in SI. Prior to the addition of H2 to the bottles, a gas mixture of N2/CO2 (80:20 v/v) was bubbled through the liquid phase of the opened flasks for 3 min, and then after closing the bottles with a butyl rubber septum and an aluminum seal, the N2/CO2 mixture was passed through the headspace of the bottles for an additional 4 min. using an inlet and outlet needle inserted at the top of the stoppers, to eliminate the remaining O2 and ensure anaerobic conditions in the experiments. After O2 was eliminated from the flasks, H2 was provided to the bottles as a gas mixture of H2:CO2 (80:20 v/v) with an overpressure of 8 psi (0.54 atm) by inverting them and injecting the gas directly to the liquid phase, in order to attain the desired concentration (10.4 mmol H2 gas L−1 liq).
All the experiments were carried out as duplicate replicates and incubated in the dark at 30°C on a 105 rpm orbital shaker. Samples of the liquid phase were periodically withdrawn with a syringe to study the reduction of TeIV. Afterwards, the samples were transferred to centrifugal filters (Amicon® ultra-4 3K, EMD Millipore, Billerica, MA, USA) and immediately centrifuged (Centrifuge 5804, Eppendorf, Enfield, CT, USA) at 4,500 rpm for 25 min. The filtrate was transferred to a 2% v/v HNO3 solution to be preserved before analyzing for soluble Te as described in SI section.
2.5. Quantification of Te0 NPs associated to the anaerobic sludge
On the last day of operation (day 146), the liquid media, the anaerobic granular sludge, and the glass beads at the bottom of the columns (used to distribute evenly the influent and to prevent any loss of granular sludge to the influent line), were separated. The granular sludge was then homogenized by manually stirring using a lab spatula, and three samples of 1.505 ± 0.028 g of each reactor were digested using a mixture of 9 mL of concentrated HNO3 (70% wt.) and 3 mL of concentrated HCl (37% wt.) according to EPA standard procedures (Environmental Protection Agency, 2007) to quantify the total amount of Te associated to the biomass. Digested samples were diluted in DI water to reach a HNO3 concentration of 2% v/v and were analyzed for Te using an ICP-OES as described in SI section, and the Te content of the sample was extrapolated to obtain the Te associated to the full amount of biomass used in the reactors. To determine the amount of Te0 NPs formed outside the cells, 16.29 ± 1.12 g of the homogenized anaerobic sludge of each reactor were transferred to 590 mL serum bottles (Wheaton, Millville, NJ, USA), along with 100 mL of the mineral medium (M1) used in the influent of both reactors. The bottles were then closed and shaken to separate the external colloidal material from the cells; the bottles were allowed to settle for 1.5 min to separate the coarser material as described in a previous work (Ramos-Ruiz et al., 2016a), and 4 samples of the liquid suspension containing colloidal material and soluble Te species were withdrawn. Two samples were digested and diluted as mentioned above, while the rest of the samples were transferred to centrifugal filters (Amicon® ultra-4 3KDa, EMD Millipore, Billerica, MA, USA) and immediately centrifuged (Centrifuge 5804, Eppendorf, Enfield, CT, USA) at 4,500 rpm for 25 min. After this step, the filtrates containing only dissolved Te species were transferred to a 2% v/v HNO3 solution. All acidified samples were analyzed for total Te using an inductively coupled plasma-optical emission spectroscopy instrument (ICP-OES Optima 2100 DV, Perkin-Elmer TM, Shelton, CT) as described in SI. The amount of Te0 NPs formed outside the cells was then calculated as the difference between the total amount of Te (colloidal and dissolved) obtained in the digestion and the dissolved Te estimated in the centrifugal filtration steps. The fraction of extracellular Te0 NPs was then estimated after dividing the amount of Te0 NPs formed outside the cells by the total amount of Te associated to the biomass.
3. RESULTS
3.1. Kinetic Bioassays using H2 as electron donor
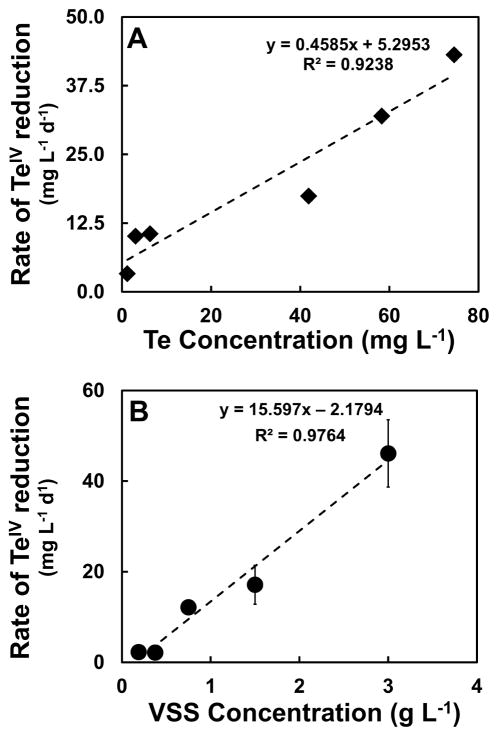
Two batch experiments were set up to investigate the effect of the initial concentration of TeIV and of the initial biomass concentration on the reduction of TeIV. For the experiment designed to study the effect of the initial concentration of Te, a fixed amount of biomass (1.5 g VSS L−1) and six different concentrations of TeIV were provided. Figure S2A in SI shows the time course of Te reduction as a function of the initial concentration of TeIV supplied to the bottles. In the figure it can be observed that even at the highest concentration used in the experiment (74.6 ± 14.3 mg TeIV L−1) the sludge was able to carry out TeIV reduction; however, a lag phase of ~21 hours was observed in that treatment. On the other hand, a rapid loss of TeIV was observed starting at day 0 for the treatments with an initial TeIV concentration of 41.9 and 58.3 mg TeIV L−1. The three lowest initial TeIV concentrations tested had classic time course patterns expected for a first order reaction (rates progressively decreasing as the TeIV was consumed). The higher concentration treatments had first order patterns at incubation times of 21 h and beyond. Figure 1A shows the initial rates of TeIV reduction as a function of the initial concentration of the oxyanion (the rate was calculated after the lag phase for the highest concentration treatment). These rates increased proportionally with the initial concentration of the oxyanion. A strong dependence of the reduction rates to the initial Te concentration is evident (R2=0.9238), confirming the first order nature of the TeIV reduction kinetics.
Figure 1.
Rate of TeIV reduction as a function of the initial TeIV concentration (Panel A) and of the initial concentration of biomass in the bioassays (Panel B).
The enzyme content and other reduced cofactors of the anaerobic biofilm are expected to be in direct relationship with the biomass concentration; therefore, it is expected that the Te reducing activity might be related to the anaerobic granular sludge concentration within the systems. To test the effect of the biomass concentration on TeIV reduction, 30 mg L−1 of TeIV were supplied to the bottles along with five different amounts of biomass. Figure S2B in SI shows the time course of Te reduction as a function of the sludge concentration. The rates of reduction increased with the corresponding increase of the biomass, the bottles containing ≥ 0.75 g VSS L−1 were able to reduce the full amount of Te in less than five days. The treatments with less biomass < 0.75 g VSS L−1 showed slower reduction rates, and the treatment with the lowest amount of biomass (0.19 g VSS L−1) had a lag phase of 3 days, after which the reduction occurred. Figure 1B summarizes the reduction rates as a function of the biomass concentration. These rates increase proportionally with the biomass concentration providing strong evidence a dependence of the rate on the biomass concentration (R2=0.9764).
3.2. Continuous reduction of TeIV
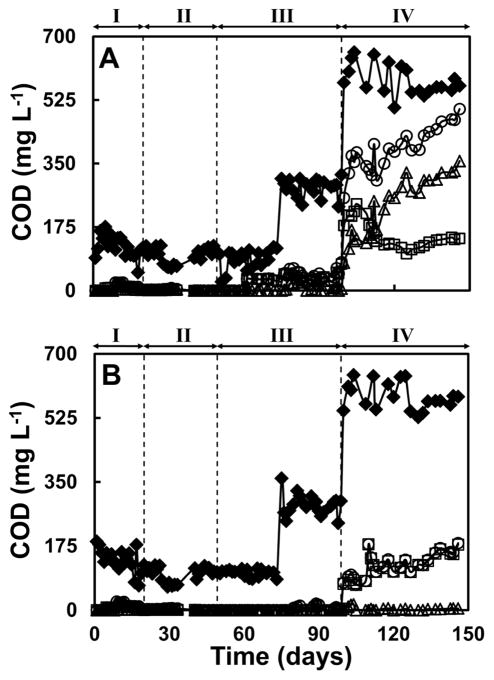
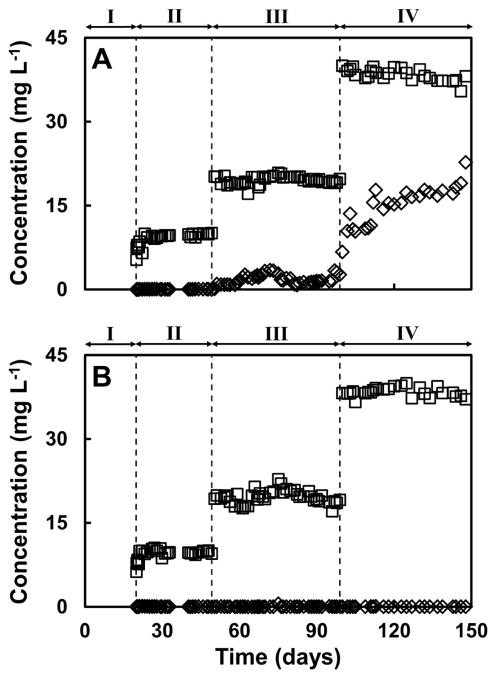
Figures 2 and 3 present the time course of the concentrations of COD, expressed as mg COD L−1, (including ethanol and acetate) and of soluble Te in the influent and effluent of the UASB reactors used in this study, respectively. The first period of time (days 0 to 20) was used as an acclimation stage for the sludge supplied only with ethanol in the influent. Steady state removal of ethanol was achieved in both reactors from the beginning of the period as can be seen in Figure 2. During the second period of operation (days 20 to 50), the influents of the reactors were spiked with 10 mg TeIV L−1. Ethanol was mostly removed throughout this period of operation in both reactors; however, a decrease in COD removal of ~10% (Figure S3) was observed in the reactor lacking RF after approximately 30 days of operation, but the decreased removal was recovered by day 40. As can be observed in Figure 3, the biomass was able to fully reduce TeIV to Te0 in both reactors, since no soluble TeIV was measured in the effluents. Important differences in the performances of the bioreactors became evident during the third period of operation (days 50 to 100) at which time the concentrations of TeIV in the influents were increased to 20 mg TeIV L−1. In the case of the reactor lacking riboflavin (R1), the COD removal decreased to ~40% (Figure S3) around day 60. The decrease in the COD removal efficiency was reflected by an accumulation of acetate in this period as can be observed in Figure 2A. The reduction of TeIV was also affected as evidenced by a higher effluent concentration of the oxyanion. Around day 70, only 80% of the TeIV in the influent was reduced during this stage as can be observed in Figure 3A. However, when the concentration of ethanol in the influent was increased from 98 to 285 mg COD L−1 (day 75 to 100), the reduction of TeIV fully recovered. The removal of COD also increased to 80–90% but, the degradation of ethanol was affected since a small accumulation of ethanol and acetate started to become evident just prior to day 100 (Figure 2A). The most noteworthy response of this reactor was observed during the fourth period of operation (days 100 to 150) when the concentration of TeIV in the influent was augmented to 40 mg TeIV L−1 and the concentration of ethanol was increased further. A remarkable loss of TeIV reduction capacity was observed in R1 when only 59.6 ± 11.2% of the Te of the influent was reduced, and a progressive trend in TeIV reduction deterioration was observed (only 33% was reduced during the last day of operation, Figure 3A). The inhibition of the biomass in R1 was also evident due to the presence of a considerable amount of ethanol and acetate in the effluent. During this period the removal of COD was only 29.4±12.0% and a progressive trend in COD removal deterioration was also observed (the removal of COD decreased dramatically to 14.3% at the end of the operation time).
Figure 2.
Performance of the reactors in terms of the time course of the COD concentration in the influents and effluents of both reactors. Panel A, reactor lacking RF (R1). Panel B, reactor amended with RF (R2). Legends: (◆), total COD in the influent; (○), total COD in the effluent; (□), acetate-COD in the effluent; (Δ), ethanol-COD in the effluent. The vertical lines indicate the time when the concentrations of TeIV in the influents were increased.
Figure 3.
Time course of TeIV concentration in the influent (□) and effluent (◇) of the continuous reactors during the time of operation. Panel A, reactor lacking riboflavin (R1). Panel B, reactor supplied with riboflavin (R2). The vertical lines indicate the time when the concentrations of Te in the influents were increased.
The reactor amended with riboflavin (R2) was able to sustain TeIV reduction during the whole period of operation without further complications. The biomass was able to degrade ethanol and completely remove COD as can be observed in Figures 2B and 3B. During the fourth period of operation (days 100 to 150), R2 continued to completely reduce TeIV (Figure 3B) presumably due to enhanced reductive capacity afforded by RF. Although inhibition of the biomass in this reactor was observed, it was remarkably lower compared to R1, the COD removal was sustained at 77.8 ± 6.1 %.(Figure S3 in SI).
3.3. Mass balances at the end of the operation period
At the end of the experiment (day 146), samples of the biomass obtained from the bioreactors were digested and analyzed to corroborate the amount of TeIV retained as elemental Te NPs in the columns both, outside and inside of the cells. The distribution of Te between the phases of both reactors is summarized in Table 1.
Table 1.
Recovery of Te in different fractions at the end of the experiment.
| R1 | R2 | |
|---|---|---|
|
| ||
| ΣQTe inf (g) | 1.63 | 1.69 |
| ΣQTe eff (g) | 0.461 | 0.002 |
| ΣQTe eff/ΣQTe inf (%) | 28.3 | 0.1 |
| ΣQTe inf − ΣQTe eff (g) | 1.17 | 1.69 |
| (ΣQTe inf − ΣQTe eff)/ΣQTe inf (%) | 71.7 | 99.9 |
| XTe (g) | 1.165±0.0002 | 0.673±0.024 |
| Te in glass beads (g) | - | 0.391±0.007 |
| ΣQTe inf − ΣQTe eff- XTe-Tein glass beads (g) | - | 0.619 |
| XTe/(ΣQTe inf − ΣQTe eff) (%) | 99.6 | 39.8 |
| (XTe + Tein glass beads)/(ΣQTe inf - ΣQTe eff) (%) | 99.6 | 63.0 |
| CollTe (g) | 0.105±0.001 | 0.028±5.85×10−5 |
| UFTe (g) | 4.175×10−3±4.38×10−5 | 9.44×10−5 ±2.30×10−6 |
| CollTe-UFTe (g) | 0.101±0.001 | 0.028±5.62×10−6 |
| Te0 NP/(XTe) (%) | 8.68±0.10 | 39.3* |
This percentage was estimated assuming that the Te associated to the beads at bottom of R2 was formed externally to the cells.
Cumulative Te fed to the column (ΣQTe inf)
Cumulative Te discharge with effluent (ΣQTe eff)
Fraction of cumulative Te discharge with effluent compared to total Te fed to the column (ΣQTe eff/ΣQTe inf)
Estimated cumulative Te retained in the column (ΣQTe inf − ΣQTe eff)
Fraction of cumulative Te retained in the column compared to total Te fed to the column (ΣQTe inf − ΣQTe eff)/ΣQTe inf
Te found retained by the sludge (XTe)
Total Te found associated to glass beads (Tein glass beads)
Total Te lost to the glass beads of the reactors (ΣQTe inf − ΣQTe eff− XTe-Tein glass beads)
Total Te recovered by sludge digestion (XTe/(ΣQTe inf − ΣQTe eff))
Total Te recovered by digestion and attached to glass beads (XTe + Te in glass beads)/(ΣQTe inf − ΣQTe eff) (%)
Total Te associated to the sludge (see section 2.6, soluble and in suspension) after 1.5 min settling (CollTe)
Total soluble Te associated to the sludge in suspension after 1.5 min settling (see section 2.6, Te passing through 3KDa filter (UFTe))
Te0 NPs formed extracellularly associated to the sludge (CollTe-UFTe)
Fraction of Te0 found as extracellular material (Te0 NP/(XTe))
In the reactor lacking RF (R1), the Te recovered by the digestion of the sludge along with the Te measured in the effluent of R1 accounted for 99.7% of the cumulative amount of TeIV fed during the full time of operation. The amount of Te0 NPs lost to the effluent of the reactor was determined as described in section 2.5. The results showed that a non-significant amount of colloidal Te was present in the effluent of R1. The fraction of Te leaving the column in particulate form (colloidal fraction) varied from 0.6 to 11.5% of the total Te in the effluent, for the selected samples collected at different moments of the operation period; while, the remaining Te left the column completely dissolved. The small amount of colloidal material found in the effluent, as well as, in the digestion of the well homogenized sludge of R1 at the end of operation period, suggest a potential formation of intracellular material over extracellular precipitation.
In the case of R2, the Te recovered from the digestion of the sludge, the Te measured in the effluent of the reactor, and the amount found attached to the glass beads used at the base of reactor (intended to uniformly distribute the influent), accounted for 63.1% of the cumulative TeIV fed to the reactor during the 146 days of operation. The incomplete recovery of Te by the digestion is potentially due to a focussed accumulation of Te at the bottom of the column where incomplete homogenization of the biomass may have occurred during sampling for the aforementioned digestion. To account for a potential release of Te0 NPs to the effluent of the reactor, the same procedure used in R1 was followed. The results showed that a more important amount of colloidal Te was present in the effluent of reactor R2 compared to that of R1. According to the results obtained from the filtrations of the effluent, it was calculated that between 13.8 to 84.5% of the total Te was present in particulate form in this stream while the rest was dissolved. The latter suggests an important formation of extracellular material compared to intracellular precipitation.
3.4. Te0 NPs characterization
Samples of the biomass obtained from both bioreactors were analyzed by TEM and XRD to investigate the nature and shape of the particles of Te0 formed inside the columns. Figures S4 and S5 show the TEM images of the material produced internally and externally to the cells in the bioreactors.
In general, the Te0 NPs were closely associated to the biomass in the bioreactor R1 as shown in Figures S4C and S6 (in SI), while in the case of the reactor containing RF (R2), the NPs were found mostly dispersed and away from the cells (data not shown). With respect to the size of the NPs in both reactors, a highly heterogeneous material was found. In both reactors small individual needle shards with lengths ranging between 30 to 300 nm with diameters of ~ 10 nm were observed randomly oriented. Rossettes, as those shown in Figures S4D and S6 (in SI) were only found in the reactor lacking RF. Bundles formed with orderly oriented rod shaped NPs were also discovered in both bioreactors; however, those produced in R1 (lengths ranging from 90 to 315 nm) were larger than those precipitated in R2 (lengths ranging from 140 to 190 nm) as shown in Figure S4 in SI (Panels A and B).
Internal accumulation of Te0 NPs was also corroborated in the cytoplasm and in the periplasmic membranes of the microorganisms in the biofilms coming from both reactors. More cellular damage and internal accumulation was evident in the biomass of R1 as is evident in Figure S5 in SI. Important membrane damage can be observed in the bottom right of Figure S5C as well as larger Te0 NPs inside the cells compared to the NPs of R2 (Figures S5A and B).
The XRD analyses showed that the solid product collected from the sludge of both bioreactors at the end of the operation time (day 146) corresponded to elemental Te0. Figure S7 shows the XRD patterns for the aggregates found in both reactors. The marks indicate the positions of the expected peaks in the Te diffraction pattern which correspond to the characteristic diffraction peaks for crystalline Te0. According to the XRD patterns, the samples coming from the reactor lacking riboflavin contained a larger amount of Te0 (either internalized or outside the cells) than those coming from the RF amended reactor (R2). These results support the idea that the material in the reactor with RF was mainly deposited in the lower part of the column.
4. DISCUSSION
4.1. Ethanol as the exogenous source of electrons
The addition of an external electron donor was intended to provide electrons needed to carry out TeIV reduction in the continuous columns. In a previous work, we found that the sludge itself contains significant endogenous substrate to carry out the reduction of 20 mg TeIV L−1 in batch experiment; however, the addition of an external electron donor, such as, H2 stimulated the reduction reaction of TeIV (Ramos-Ruiz et al., 2016a). Therefore, the presence of an external electron donor ensures the adequate supply of electron equivalents required to reduce TeIV in a continuous process, and at the same it might represent several benefits, such as, the increase of the TeIV reduction rate. Based on the production of methane estimated from the decay of the biomass in the anaerobic granules, the amount of endogenous substrate in the sludge corresponds to 60 to 166 mg chemical oxygen demand (COD) g−1 VSS (Field et al., 2004; Tapia-Rodriguez et al., 2010). Considering the amount of sludge used to inoculate the bioreactors (10 g VSS Lreactor −1 in a volume of 0.27 L), the endogenous capacity of the reactors was estimated to be between 0.162 to 0.4482 g COD. The total amount of TeIV supplied to the reactors would require approximately of 0.23 g COD-TeIV. For this reason, addition of an excess external electron donor was needed, especially when considering that there is competition with other processes for electrons such as methanogenesis.
Ethanol was used as the external electron donor to support the reduction of TeIV throughout this work. Ethanol was selected since it represents a safer and more economical option compared to other electron donors which have proven to be effective in stimulating the reduction of Te oxyanion, such as H2 (Ramos-Ruiz et al., 2016a). H2 is one of the products of the anaerobic fermentation of ethanol by the acetogenic- bacteria, which are present in methanogenic anaerobic sludge, as can be noted in eq 1.
| (1) |
The effect of ethanol might be comparable to H2 since it is in fact the biogenic H2 that is the source of effective electrons. In batch experiments, where the reduction of 20 mg TeIV L−1 was studied using different external electron donors, H2 increased the rate of TeIV reduction 1.30-times with respect to the systems lacking external electron donor. The other product of ethanol fermentation, acetate, was found to be a poor electron donating substrate for TeIV (Ramos-Ruiz et al., 2016a). Likewise compared to ethanol and H2, acetate was also a poor electron-donor for the bioreduction of other oxidized compounds such as AsV, UVI, and PdII (Field et al., 2004; Tapia-Rodriguez et al., 2010; Pat-Espadas et al., 2016a) in anaerobic sludge.
To overcome the competition with other processes, ethanol was added in different excesses with respect to the amount stoichiometrically required to reduce the TeIV in the influent of the columns during the four stages of operation (7-, 14-, and 20- times). The effectiveness of ethanol as electron donor, as well as, the importance of adding it in excess was evident during the third period of operation when the lowest ethanol excess was used (7- times). The electron-donor supply capacity of the anaerobic reactor was apparently depleted as a small accumulation of TeIV in the effluent of the reactors was measured during this stage. TeIV accumulation in the effluent disappeared when the ethanol supplied to the influent was increased at day 75 to achieve a 20-fold excess.
4.2. Continuous reduction of TeIV oxyanion to recoverable Te0 NPs
During periods II and III of operation, the non-adapted anaerobic methanogenic granular sludge used in this study was able to perform TeIV reduction, up to a concentration of 20 mg TeIV L−1 in the influent to elemental Te0 in a continuous fashion with reduction efficiencies ranging from 83% to 96% in the case of R1 (lacking RF), and of ≥ 99.5% in R2 (with RF). In the third period of operation, when the supply of electron equivalents was restricted due to the low excess of ethanol supplied to the reactors, an accumulation of TeIV caused mild inhibition of the fermenting bacteria which did not recover after the reduction of TeIV was restored around day 70 in R1. No toxic effect towards the fermenting bacteria was evident in R2 throughout periods II and III of operation. In the fourth period of operation, when the concentration of TeIV was increased to 40 mg TeIV L−1, the granular sludge in R1 lost its TeIV-reducing capacity. The remarkable toxic effects of TeIV towards the fermenting bacteria was evident from an important accumulation of ethanol and acetate in the effluent of R1 around day 100. On the other hand, the presence of a low concentration of RF in R2 as a redox mediating agent allowed for the TeIV reducing capacity of the sludge to remain intact during the full time of operation. As a consequence, only moderately toxic effects towards the ethanol fermenting bacteria were observed in R2.
To date, only few studies have assessed the recovery of Te0 NPs in a continuous mode (Basnayake et al., 2001; Rajwade and Paknikar, 2003). In one of the studies, a continuous stirred tank reactor (CSTR) was set up to assess the production of Te0 by means of TeIV reduction using Pseudomonas mendocina MCM B-180 and sucrose as a substrate. The CSTR was able to sustain the complete reduction of 100 mg TeIV L−1 in the influent (translated to a TeIV load of 1.40 mg TeIV h−1 Lreactor) (Rajwade and Paknikar, 2003). These results correlate well with our findings with the reactor lacking RF in which 20 mg TeIV L−1 (representing a TeIV load of 1.53 mg TeIV h−1 Lreactor) were effectively removed from the influent; however, the TeIV reduction capacity of our continuous system was remarkably improved with the use of low concentrations of RF in a way that the reactor was able to carry out the reduction of a higher volumetric load of TeIV (3.06 mg TeIV h−1 Lreactor).
This type of technology has been also successfully used to investigate the recovery of some precious metals like palladium (Pd) using ethanol as substrate (Pat-Espadas et al., 2016b) for a maximum concentration of 15 mg PdII L−1 in the influent of the reactor, achieving removal efficiencies of 98.9 ± 0.7%. The removal of the chalcogen selenate (SeVI) was also investigated in a UASB reactor using a methanogenic consortium and lactate as the electron donating substrate. Complete removal of SeVI at 1.4 mg SeVI L−1 in the influent was achieved in the reactor (Lenz et al., 2008). Our results suggest that UASB technology represents a viable option to recover Te0 NPs from solubilized Te obtained from decommissioned materials, such as, CdTe solar panels, and from mining aqueous waste streams. The reactor’s performance might be importantly enhanced by the addition of a redox mediator, such as, RF.
4.3. Effect of riboflavin on TeIV reduction
Several advantages of the use of RF were observed during the operation of the continuous columns in the present study. This redox mediator (RM) lowered the toxic effects of TeIV by forming poorly bioavailable Te0 NPs, and most importantly, the RF amended reactor was able to reduce twice the amount of Te compared to the one lacking RF. An increase in the rate of TeIV reduction caused by the presence of the catalyst RF might explain these operational advantages.
The toxic effects of TeIV towards the microorganisms in the granular sludge were remarkably lower in R2 compared to R1. The acetogenic- and acetoclastic- microorganisms in the methanogenic sludge were highly inhibited at the highest concentration of TeIV tested of 40 mg Te L−1 in R1; while, in R2, the RF amendment greatly lowered the toxic effects, which were only moderately impacting the acetoclastic- microorganisms (based on observing some residual acetate). The high toxicity of TeIV towards methanogens in anaerobic granular sludge was recently reported (Ramos-Ruiz et al., 2016b). The acetoclastic methanogens were more sensitive towards TeIV than the hydrogen consuming methanogens. The estimated 50% inhibiting concentration (IC50) of the methanogenic acetoclastic activity was 8.6 mg TeIV L−1 (Ramos-Ruiz et al., 2016b). TeIV has also been reported to be very toxic for most bacteria at very low concentrations as of 1 mg L−1 (Taylor, 1999). The minimal concentration required to eliminate a biofilm of Escherichia coli was 1.8 mg TeIV L−1 (Turner et al., 2012).
To the best of our knowledge, this is the first report of applying a redox mediator to enhance the continuous bioreduction of TeVI oxyanions. Redox mediators, such as, RF, quinones, and humus, are compounds known to stimulate biological reactions by facilitating the transfer of electrons from cells to oxidized inorganic compounds (e.g. selenate and palladiumII). This enables the direct reduction of inorganic compounds by the reduced form of the redox mediators(Van der Zee and Cervantes, 2009). In the previous work, we tested the effectiveness of four different RM compounds in the reduction of TeIV (lawsone, AQDS, riboflavin and hydroxocobalamin) at two different Te/RM molar ratios (Ramos-Ruiz et al., 2016a). RF had the greatest impact on the reduction of the TeIV increasing rates by 3.6- and 10.8- fold when the Te/RF molar ratios were held at 10:1 and 1:1, respectively. The effect of other RM compounds (lawsone, AQDS, menadione) on the reduction of TeIV has also been reported before (Wang et al., 2011). Lawsone increased the rate of TeIV reduction by 10-fold in a system using the bacterium E. coli with glucose supplied as electron donor (Wang et al., 2011). Lawsone almost doubled the TeIV reduction rate for the photosynthetic bacterium Rhodobacter capsulatus when pyruvate was used as substrate. The stimulation however was found to be independent of the lawsone concentration (Borghese et al., 2014).
The catalytic properties of RF observed in this work might be explained by the fact that the standard redox potential (E0′ for pH 7) of the RM is between those of the electron donor and electron acceptor reactions (H2 oxidation and Te oxyanions reduction). The standard redox potentials for the chemical species involved in this reduction are 2H+/H2 E0′=−0.414 V; RFox/RFred E0′= −0.208 V (Bird et al., 2011); and TeIV (HTeO3−/Te0) E0′ = 0.196 V (calculated from reported E0 values (Bouroushian, 2010)). The effectiveness of an electron shuttle is dependent of the energy of activation of their reduction and oxidation (Van der Zee et al., 2001). For these reasons, RF was suspected to decrease the energy of activation of the redox reactions, and as a consequence the TeIV reduction reaction occurred faster.
CONCLUSION
The feasibility of continuously converting TeIV oxyanion to recoverable Te0 NPs with methanogenic granular sludge in a UASB fed ethanol as the electron donating substrate was demonstrated for the first time. The sludge used in this work was able to sustain the reduction of high loads of the highly toxic oxyanion TeIV. Due to its redox mediating properties, RF greatly enhanced the performance of the UASB by increasing the rate of TeIV reduction. The faster formation of non-toxic Te0 NPs due to the increase of the Te reduction rate resulted in a better detoxification; thus, indirectly RF improved TeIV reduction. All these encouraging findings, might be used as the basis for the development of a safe, environmentally friendly and cost- effective large-scale process to recover Te0 from aqueous streams containing TeIV
Supplementary Material
Highlights.
A continuous anaerobic bioreactor converted TeIV to elemental Te0 nanoparticles
TeIV reduction rates depended on biomass and TeIV concentrations
Ethanol used as an electron donating substrate promoted TeIV reduction
Riboflavin used as redox mediator greatly improved TeIV reduction
Riboflavin markedly lowered TeIV toxicity to chemical oxygen demand removal
Acknowledgments
This work was funded in part by a grant of the National Institute of Environment and Health Sciences-supported Superfund Research Program (NIH ES-04940). ARR was partly funded by CONACyT and PROMEP. We are also very grateful to Dr. T. Day, Dr. S. Roberts for their valuable assistance with the TEM, and XRD analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amatya R, Ram RJ. Trend for thermoelectric materials and their earth abundance. J Electron Mater. 2011;41:1011–1019. [Google Scholar]
- Ba LA, Doring M, Jamier V, Jacob C. Tellurium: an element with great biological potency and potential. Org Biomol Chem. 2010;8:4203–4216. doi: 10.1039/c0Ob00086h. [DOI] [PubMed] [Google Scholar]
- Baesman SA, Bullen TD, Dewald J, Zhang D, Curran S, Islam FS, Beveridge TJ, Oremland RS. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl Environ Microb. 2007;73:2135–2143. doi: 10.1128/AEM.02558-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baesman SM, Stolz JF, Kulp TR, Oremland RS. Enrichment and isolation of Bacillus beveridgei sp nov. a facultative anaerobic haloalkaliphile from Mono Lake, California, that respires oxyanions of tellurium, selenium, and arsenic. Extremophiles. 2009;13:695–705. doi: 10.1007/s00792-009-0257-z. [DOI] [PubMed] [Google Scholar]
- Basnayake RST, Bius JH, Akpolat OM, Chasteen TG. Production of dimethyl telluride and elemental tellurium by bacteria amended with tellurite or tellurate. Appl Organomet Chem. 2001;15:499–510. [Google Scholar]
- Bird LJ, Bonnefoy V, Newman DK. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011;19:330–340. doi: 10.1016/j.tim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Borghese R, Baccolini C, Francia F, Sabatino P, Turner RJ, Zannoni D. Reduction of chalcogen oxyanions and generation of nanoprecipitates by the photosynthetic bacterium Rhodobacter capsulatus. J Hazard Mater. 2014;269:24–30. doi: 10.1016/j.jhazmat.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Bouroushian M. Electrochemistry of Metal Chalcogenides. Springer-Verlag; Berlin: 2010. [Google Scholar]
- Chasteen TG, Fuentes DE, Tantalean JC, Vasquez CC. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. Fems Microbiol Rev. 2009;33:820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- de Boer MA, Lammertsma K. Scarcity of Rare Earth Elements. Chemsuschem. 2013;6:2045–2055. doi: 10.1002/cssc.201200794. [DOI] [PubMed] [Google Scholar]
- Deng ZT, Zhang Y, Yue JC, Tang FQ, Wei Q. Green and orange CdTe quantum dots as effective pH-sensitive fluorescent probes for dual simultaneous and independent detection of viruses. J Phys Chem B. 2007;111:12024–12031. doi: 10.1021/jp074609z. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. Method 3051a. Microwave assisted acid digestion of sediments, sludge, soils and oils 2007 [Google Scholar]
- Fan YQ, Yang YX, Xiao YP, Zhao Z, Lei Y. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: Thermodynamic evaluation and experimental study. Hydrometallurgy. 2013;139:95–99. [Google Scholar]
- Field JA, Sierra-Alvarez R, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. Facile reduction of arsenate in methanogenic sludge. Biodegradation. 2004;15:185–196. doi: 10.1023/b:biod.0000026697.10029.b2. [DOI] [PubMed] [Google Scholar]
- Goncalves MMM, Da Costa ACA, Leite SGF, Sant’Anna GL., Jr Heavy metal removal from synthetic wastewaters in an anaerobic bioreactor using stillage from ethanol distilleries as a carbon source. Chemosphere. 2007;69:1815–1820. doi: 10.1016/j.chemosphere.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotechnol. 2009;29:279–306. doi: 10.3109/07388550903062462. [DOI] [PubMed] [Google Scholar]
- Kyle JH, Breuer PL, Bunney KG, Pleysier R, May PM. Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing. Part 1: Mineralogy, aqueous chemistry and toxicity. Hydrometallurgy. 2011;107:91–100. [Google Scholar]
- Lenz M, Van Hullebusch ED, Hommes G, Corvini PFX, Lens PNL. Selenate removal in methanogenic and sulfate-reducing upflow anaerobic sludge bed reactors. Water Res. 2008;42:2184–2194. doi: 10.1016/j.watres.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Morrison BH. 4047939. United States Patent Office; Slimes treatment process. 1977
- Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interfac. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Pat-Espadas AM, Field JA, Otero-Gonzalez L, Razo-Flores E, Cervantes FJ, Sierra-Alvarez R. Recovery of palladium(II) by methanogenic granular sludge. Chemosphere. 2016a;144:745–753. doi: 10.1016/j.chemosphere.2015.09.035. [DOI] [PubMed] [Google Scholar]
- Pat-Espadas AM, Field JA, Razo-Flores E, Cervantes FJ, Sierra-Alvarez R. Continuous removal and recovery of palladium in an upflow anaerobic granular sludge bed (UASB) reactor. J Chem Technol Biot. 2016b;91:1183–1189. [Google Scholar]
- Rajwade JM, Paknikar KM. Bioreduction of tellurite to elemental tellurium by Pseudomonas mendocina MCM B-180 and its practical application. Hydrometallurgy. 2003;71:243–248. [Google Scholar]
- Ramos-Ruiz A, Field JA, Wilkening JA, Sierra-Alvarez R. Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environ Sci Technol. 2016a;50:1492–1500. doi: 10.1021/acs.est.5b04074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ruiz A, Zeng C, Sierra-Alvarez R, Teixeira LH, Field JA. Microbial toxicity of ionic species leached from II-VI semiconductor materials, cadmium telluride (CdTe) and cadmium selenide (CdSe) Chemosphere. 2016b;162:131–138. doi: 10.1016/j.chemosphere.2016.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rodriguez A, Luna-Velasco A, Field JA, Sierra-Alvarez R. Anaerobic bioremediation of hexavalent uranium in groundwater by reductive precipitation with methanogenic granular sludge. Water Res. 2010;44:2153–2162. doi: 10.1016/j.watres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Taylor DE. Bacterial tellurite resistance. Trends Microbiol. 1999;7:111–115. doi: 10.1016/s0966-842x(99)01454-7. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Borghese R, Zannoni D. Microbial processing of tellurium as a tool in biotechnology. Biotechnol Adv. 2012;30:954–963. doi: 10.1016/j.biotechadv.2011.08.018. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Energy’s National Renewable Energy Laboratory. Department of Energy; Washington: 2013. [last accessed: 09/13/2016]. http://www.nrel.gov/docs/fy15osti/62580.pdf. [Google Scholar]
- Van der Zee FP, Bouwman RHM, Strik D, Lettinga G, Field JA. Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnol Bioeng. 2001;75:691–701. doi: 10.1002/bit.10073. [DOI] [PubMed] [Google Scholar]
- Van der Zee FR, Cervantes FJ. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol Adv. 2009;27:256–277. doi: 10.1016/j.biotechadv.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Liu GF, Zhou JT, Wang J, Jin RF, Lv H. Quinone-mediated reduction of selenite and tellurite by Escherichia coli. Bioresour Technol. 2011;102:3268–3271. doi: 10.1016/j.biortech.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Wedepohl KH. The composition of the continental-crust. Geochim Cosmochim Ac. 1995;59:1217–1232. [Google Scholar]
- Zannoni D, Borsetti F, Harrison JJ, Turner RJ. The bacterial response to the chalcogen metalloids Se and Te. In: Poole RK, editor. Adv Microb Physiol. Vol. 53. 2008. pp. 1–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.