Abstract
Diabetic ketoacidosis (DKA) is a life-threatening complication of type 1 diabetes (T1D), which without treatment leads to death. Fulminant type 1 diabetes (FT1D) is a subtype characterised by a markedly rapid and almost complete destruction of pancreatic β-cells, with acute onset leading to severe metabolic derangement and commonly ICU admission. We present a case of an 18-year-old male presenting with FT1D with two rare complications of pneumomediastinum and stress-induced cardiomyopathy (SIC) with significant myocardial necrosis. We also discuss the aetiology of the pneumomediastinum; the latest thoughts on SIC: moving beyond the simple description of ‘Takotsubo cardiomyopathy’; the role of troponins in critical illness; and genetic predisposition for DKA due to FT1D.
Keywords: Fulminant type 1 diabetes, pneumomediastinum, stress induced cardiomyopathy, troponin, Takotsubo cardiomyopathy
Case report
An 18-year-old Filipino male presented to the Emergency Department with a three-day history of lethargy, vomiting, tachypnoea and increasing drowsiness. There was no known past medical history, nor any history of polyuria, polydipsia or weight loss. On initial examination, his vital signs were as follows: heart rate 130 beats/min; blood pressure 105/48 mmHg; respiratory rate 24 breaths/min; oxygen saturation 90% on 6 L oxygen/min; temperature 34.0℃, GCS 8/15 (E1M5V2). He also demonstrated voluntary guarding on abdominal palpation and neck stiffness. He had thin body habitus without central adiposity (BMI 20.6 kg/m2). His arterial blood gas showed pH 7.13; PaO2 7.46 kPa; PaCO2 3.6 kPa; HCO3- 9 mmol/L; BE −20 mmol/L; glucose 50 mmol/L and lactate 5 mmol/L. He was diagnosed with DKA due to new onset T1D and treated with intravenous fluids and a fixed-rate insulin infusion.
Subsequent laboratory analysis of the patient’s blood showed urea 32.6 mmol/L; creatinine 379 µmol/L; glucose 107.3 mmol/L; anion gap 45 mmol/L and ketones 7.1 mmol/L. The serum lipase was not elevated. An initial ECG showed sinus tachycardia with no other acute changes. Due to his altered level of consciousness, he received a CT scan of his brain, which was unremarkable, and a lumber puncture which showed a high glucose but no other abnormalities. His conscious level and blood pressure decreased and he was intubated, ventilated, commenced on a noradrenaline infusion and transferred to the ICU for further management.
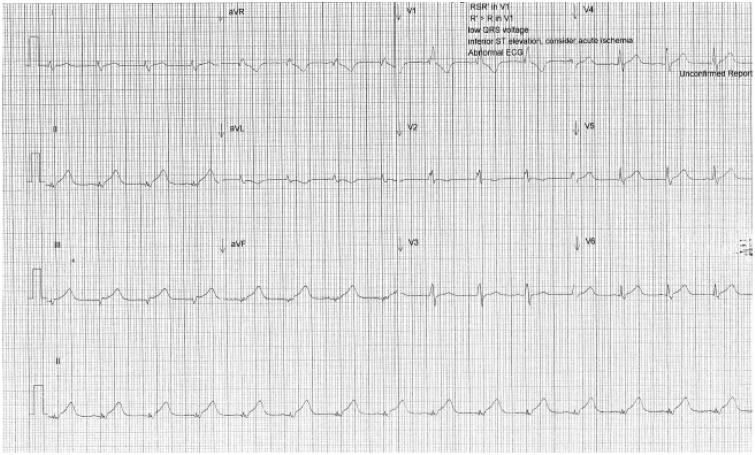
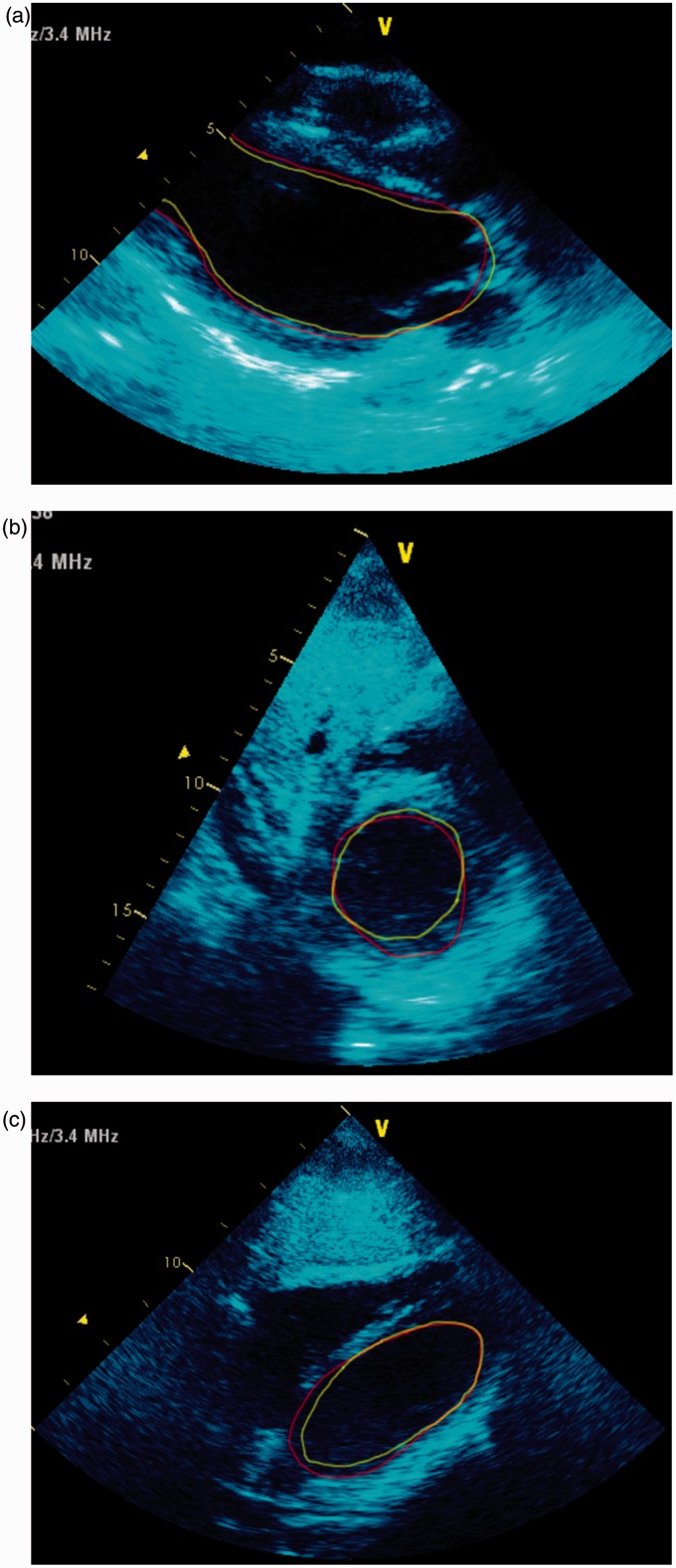
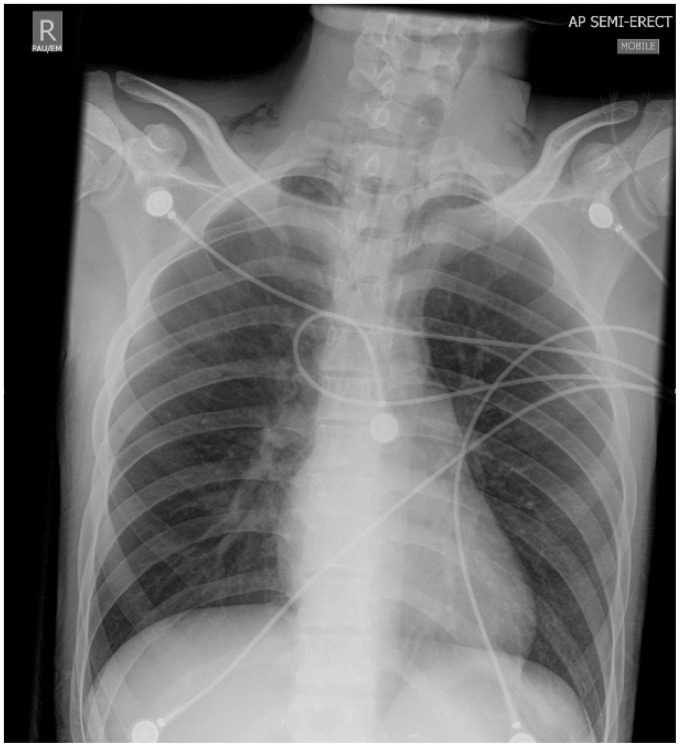
His initial ECG on arrival to the ICU showed ST elevation in leads II, III and aVF with right bundle branch block (Figure 1). Immediate transthoracic echocardiography (TTE) showed global left ventricular (LV) hypokinesis with apical akinesis/dyskinesis (Figures 2(a) to (c) and 3) with the ejection fraction estimated at 15% and severely reduced right ventricular (RV) systolic function. His high-sensitivity troponin, which was retrospectively found to be 61 ng/L on admission, had risen to 1856 ng/L (<15 ng/L) with a correlating creatinine kinase (CK) of 26784 U/L (60–174 U/L). He was also found to have subcutaneous emphysema bilaterally in the supraclavicular fossae on clinical examination, and chest X-ray showed corresponding subcutaneous air and pneumomediastinum (Figure 4). Due to the pneumomediastium, he received a gastrograffin swallow and a CT chest to locate the potential source of air leak. The gastrograffin swallow was normal and CT chest demonstrated significant air in the upper mediastinum and neck (Figures 5a and b). This led to a flexible nasal endoscopy which demonstrated swelling in the posterior pharyngeal wall. It was presumed that he had suffered a rupture of the pharyngeal wall due to severe vomiting which had now spontaneously healed. The pneumomediastinum spontaneously resolved clinically and radiographically.
Figure 1.
ECG on admission showing ST elevation in leads II, III and aVF with RBBB.
Figure 2.
(a) parasternal long axis, (b) subcostal short axis (mitral level) and (c) subcostal long axis. Transthoracic echo on admission. The green line is the inner border of the LV during systole and red line is the inner border during diastole. It can be seen from the figures that there is akinesis of the mid- and apical sections of the LV, and akinesis of most of the basal sections except the inferior sections. This is not classical of Takotsubo, and hence described as SIC.
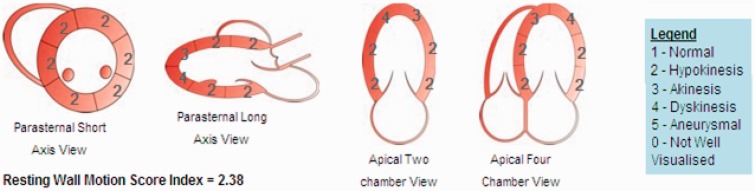
Figure 3.
Echocardiographic pattern of regional wall motion abnormality: basal and mid-ventricle hypokinesis with apical akinesis/dyskinesis. Wall motion score index is a semi-quantitative analysis of regional systolic function = 5-level scoring system for each segment divided by number of segments visualised.
Figure 4.
Initial CXR demonstrating pneumomediastinum with a very distinct border of the cardiac silhouette and associated subcutaneous emphysema bilaterally in the supraclavicular fossae.
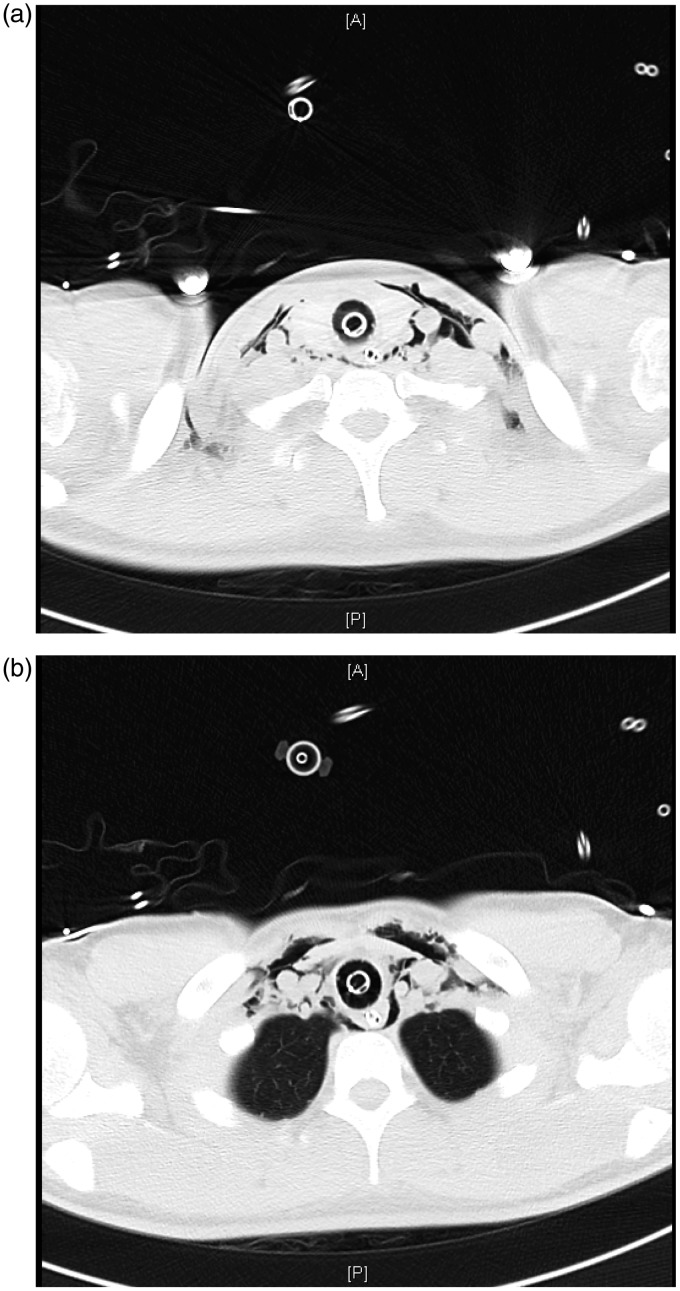
Figure 5.
(a) and (b) computerised tomography (CT) scan of the chest demonstrating pneumomediastinum and subcutaneous emphysema in the supraclavicular fossae and anterior chest wall.
Further investigations showed normal thyroid and cortisol axes; HbA1c of 7.0% (normal range 4.0–6.0; SI Units 53 mmol/mol [normal range 20–42]) and fasting c-peptide <0.1 nmol/L (normal range 0.6–1.4). Antibodies against glutamic acid decarboxylase (GADA), islet cells (ICA) and insulinoma-associated (IA-2) antigens were negative. Over the next 48 hours, his catacholamines and respiratory support were weaned and he was extubated on day 3 with normal cognition. The renal function returned to normal and his LVEF gradually improved, returning to normal at day 5. His troponin peaked at the level of 1856 ng/L and levels returned to normal at day 7, whereas CK peaked at 45651 at day 3 and also returned to normal (Table 1). The ECG changes had resolved by day 3. He was discharged from ICU on day 5 and discharged home on day 11. Follow-up investigations including viral serology (convalescent influenza and eneterovirus), coeliac serology, thyroid antibodies and full autoimmune screen including repeat islet cell antibody testing were negative.
Table 1.
Troponin T (TnT) and creatinine kinase (CK) levels from ICU admission to discharge.
| ICU day | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| TnT ng/L | 1856 | 755 | 498 | 337 | 408 | 387 |
| CK U/L | 26784 | 54948 | 45651 | 16992 | 4590 | 2791 |
Discussion
Fulminant type 1 diabetes
Classic T1D, sometimes referred to as autoimmune diabetes (T1aD), has a variable incidence ranging from lows of 0.1/100,000 per year1 to a high of 40,000 per year (in Finland) and predominately occurs in Caucasian populations. Its aetiology is uncertain, but thought to involve interactions between multiple heterogeneous factors such as genetic factors (e.g. HLA Class 2 genes, plus a few non-HLA loci) interacting with environmental exposures (e.g. viral, dietary or toxic).
In contrast, fulminant type 1 diabetes (FT1D) seems to represent a distinct form or subtype of T1D (sometimes referred to as type 1b). Although reported in Caucasians,2 it predominately occurs in Asians.3 It differs from T1aD in a number of clinical and pathological respects to the point where separate diagnostic criteria for this entity have been published.3
In addition to Asian ethnicity, key features that can help clinicians recognise FT1D as an entity and differentiate it from T1aD include short duration of presenting symptoms (<7 days) with rapid onset of DKA featuring high glucose levels, near-normal glycosylated haemoglobin, low or undetectable c-peptide, and low or undetectable standard autoantibody serology. All of these were present in the case we describe. Classic new-onset antibody-mediated T1aD evolves over weeks to months with gradually progressive metabolic derangement sometimes accelerated towards DKA by an acute precipitant, e.g. superimposed infection. Such cases will usually differ from FT1D by having a longer prodromal period of symptoms (lethargy, polyuria, polydipsia, weight loss, etc.), a high HbA1c (reflecting longer duration of absolute insulin deficiency and hyperglycaemic symptoms) and positive titres or one or more antibodies directed against pancreatic beta cell antigens. C-peptide, a marker of endogenous insulin secretion, is often detectable and even partially recovers once hyperglycaemia and other metabolic derangements have been corrected leading to a temporary potential reduction in insulin requirements during the so-called ‘honeymoon period’ of T1aD. In contrast, in FT1D c-peptide is usually undetectable, reflecting the severity and totality of the underling mechanism of pancreatic injury. This may contribute to additional atypical features such as elevated levels of pancreatic enzymes.3,4
Differing clinical presentation of T1aD and FT1D is reflected in different genetic susceptibilities and the underlying mechanism of pancreatic injury these may induce. Classic T1aD in Caucasian populations usually involves the presence of underlying risk conferring HLA loci such as HLA DR4-DQ8 and DR3-DQ2 and autoantibodies to GAD, IA2, ICA or other cellular targets, leading to progressive humeral B cell-mediated and regulatory T cell-mediated autoimmune endocrine pancreatic damage.1 In contrast, susceptibility to FT1D shows different associations; HLA DR4-DQ4 (encoded by DRB1*04:05-DQB1*04:01) has been reported in up to 41.8% of FT1D in one large Japanese study versus 22.8% for T1aD.3 A large-scale study in the Filipino population confirmed increased frequency of the DRB1*04:05 allele in T1D (but did not separate out FT1D as a subgroup).5 This haplotype is rare in Caucasian populations.3 Studies in other Asian populations have reported other less-common HLA allelic associations with FT1D including HLA DR7.6 It is postulated that these haplotypes may predispose to an accelerated immune response against beta cells causing massive beta cell death and rapid onset of DKA,3 leading to a presentation and natural history distinct from classic T1aD and contributing to features of FT1D such as acute onset, normal HbA1c, undetectable c-peptide and autoantibody negativity.
Pneumomediastinum in DKA
Pneumomediastinum was first described in 1819 by Renee Laennec.7 It can be caused by oesophageal rupture or bowel rupture (air in the abdominal cavity tracts up into the chest), but most commonly results from alveolar rupture followed by centripetal air dissection through the pulmonary interstitium into the mediastinum.8 Clinical signs are usually few but may include subcutaneous emphysema in face, neck, and upper part of the thorax, meaning a high index of suspicion is required, especially in the unconscious patient where history may be limited. The diagnosis can be confirmed on chest X-ray or CT scanning of the thorax. Hamman’s sign9 or Hamman’s crunch, which is a crunching sound, synchronous with the heartbeat and heard over the precordium due to the heart beating against air-filled tissues, is rare. The patient we describe had clinical evidence of subcutaneous emphysema with confirmed pneumomedisatinum on chest X-ray. Hamman’s sign was not present.
Although pneumomediastinum complicating DKA is rare, there are several potential reasons why it may occur. Diabetic keto-acidosis and its associated deep ketotic hyperventilatory pattern may lead to increases in the alveolar pressure of 20–30 mmHg, which may be significant enough to lead to alveolar rupture.10 Additionally, vomiting and retching are common in DKA. Typical patients with diabetes complicated by DKA are young males either undiagnosed or already on insulin. The average age for developing the complication is in the early 20 s. Young men likely have greater musculature and may thus create larger swings in alveolar pressure, leading to an increased risk of alveolar rupture.11 Our patient was not muscular, and indeed had a BMI at the lower end of normal.
One of the major differentials to be considered in patients with vomiting and pneumomediastinum is Boerhaave’s syndrome. The high mortality associated with Boerhaave’s syndrome would lead most clinicians to exclude oesophageal rupture with a contrast oesophagram or computed tomography (CT) scan of the chest with oral contrast.12 However, in a systematic review of spontaneous pneumomediastinum in DKA,13 in all 56 published cases, the condition was self-limiting and oesophageal rupture was not detected in any of the reported cases. The authors suggested that based on this evidence, a restrictive work up should be considered. The patient we describe was unconscious and significantly hypoxic with a PaO2/FiO2 ratio of 80. This is very rare for DKA, hence the further investigation of the cause for the pneumomediastinum.
Stress-induced cardiomyopathy: Beyond Takotsubo
The patient in this report demonstrated globally reduced LV function with localised areas of akinesis, which extended beyond a single epicardial coronary artery distribution. The rapid resolution with supportive management is consistent with a diagnosis of ‘Stress-induced Cardiomyopathy’ (SIC).
SIC is most often used to describe ‘Takotsubo’s cardiomyopathy’ or ‘apical ballooning syndrome’, first reported in the 1990s: a reversible cardiomyopathy precipitated by acute emotion stress in postmenopausal women.14 This form of SIC is characterised by reversible mid-ventricular and apical dyskinesis or ballooning associated with an acute coronary syndrome, in the absence of underlying coronary artery disease. Many variants have been described with varying patterns of isolated ventricular dysfunction which do not match coronary artery blood supply including mid-ventricular variants of Takotsubo’s,15 and reverse Takotsubo’s (basal ventricular akinesis and apical sparing described occasionally associated with phaeochromocytoma).16 SIC has also been described as acute LV dysfunction associated with subarachnoid haemorrhage or stroke, and acute LV dysfunction seen in the critically ill.17 These latter two forms of SIC have a global dysfunction with areas of wall motion abnormality out of keeping with coronary artery distribution as well, but do not have the classical apical ballooning picture.
The pathogenesis of SIC is not known, but the most quoted theory is the sequelae of an autonomic storm. In Takotsubo’s cardiomyopathy, stimulation of the medullary autonomic centre by the limbic system leads to liberation of noradrenaline at the pre- and post-synaptic level, and adrenaline from the adrenal medulla; there is a correlation with supraphysiological levels of plasma catecholamines and stress-related neuropeptides.18
This catecholamine storm constantly stimulates adrenoceptors inducing catecholamine toxicity in the cardiomyocytes.19 This may occur through coronary artery spasm, microvascular impairment, direct myocardial toxicity,20 myocardial inflammation or altered myocardial fatty acid metabolism.21 However, there is a suggestion from the direct recording of muscle sympathetic nerve activity that sympathetic output is reduced in SIC patients compared to controls.22
Other potential mechanisms of LV dysfunction in DKA may include glucose toxicity or high ketone production impairing myocardial glucose utilisation and severe acidosis impairing most steps of excitation–contraction coupling. Only23 few cases of SIC in DKA have been reported17 demonstrating a Takotsubo’s picture. Our patient did not have this classic apical ballooning appearance, and findings were more in keeping with a critical illness cardiomyopathy, where a more global dysfunction is seen. The pathogenesis of this myocardial dysfunction seems unlikely to be majorly different from the other types of SIC.
Troponins in critical illness
Troponin is a complex of three regulatory proteins integral to muscle contraction in skeletal and cardiac muscles. Troponin’s I and T are very specific indicators of myocardial damage and whilst the plasma level is proportional to the extent of acute myocardial necrosis,24 it does not identify the mechanism of injury.25 Cardiac troponins are elevated in up to 50% of critically ill patients in the ICU,19 and whilst a raised troponin in a critically ill patient is an independent predictor of increased mortality, the relationship between myocardial necrosis and troponin level is validated in acute coronary syndrome, but not other forms of critical illness. In the unconscious patient unable to give a history with multi-organ dysfunction syndrome (MODS), the aetiology of raised troponins can be difficult to ascertain.26 For example, renal failure is a common cause of raised troponins in critically ill patients27; however, this does not exclude a concurrent myocardial disease process.
Myocardial infarction (MI) is defined as evidence of myocardial necrosis in a clinical setting consistent with myocardial ischaemia.28 However, this can happen in different clinical settings and the 2007 consensus statement identified these as29; type 1-Spontaneous MI (due to a primary coronary event); type 2-MI due to ischaemic imbalance (e.g. anaemia, shock); type 3-MI resulting in death when biomarker values are unavailable (e.g. sudden cardiac death); type 4a MI related to percutaneous coronary intervention (PCI): type 4b-MI related to stent thrombosis and type 5-MI related to coronary artery bypass grafting (CABG). The criteria were still used in the second revision of the consensus statement in 2012.30 According to this classification, the patient we describe had an MI (type 2 MI) but not in the classical sense of a plaque rupture and vessel occlusion (type 1 MI).
Conclusion
FT1D represents a rare and distinct form of T1D, most commonly occurring in Asian populations. The mechanisms underlying development of FT1D may contribute to rapid and severe metabolic derangement, which probably explain the rare constellation of clinical problems seen in this case. These patients will present to the ICU due to the severity of illness, and rapid recognition of the condition as a distinct form of T1D may allow earlier intervention and optimal clinical outcome.
Due the severity of DKA resulting from FT1D, it can be associated with unusual complications. Pneumomediastinum has not been shown to be associated with oesophageal rupture in DKA, and is more likely to be associated with strenuous breathing and vomiting. SIC is still a concept for which our knowledge is evolving in the context of critical illness, and many previously described conditions such as Takotsubo’s are all part of the spectrum of SIC. High levels of tropinins in critically ill patients should not be discounted, but should be considered within the context of clinical history and examination and interpreted as such when deciding on further treatments.
Consent
This case report is published with the written consent of the patient.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmune Rev 2008; 7: 550–557. [DOI] [PubMed] [Google Scholar]
- 2.Moreau C, Drui D, Arnault-Ouary G, et al. Fulminant type 1 diabetes in Caucasians: a report of three cases. Diab Metab 2008; 34: 529–532. [DOI] [PubMed] [Google Scholar]
- 3.Imagawa A, Hanafusa T, Awata T, et al. Report of the Committee of the Japan Diabetes Society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus. J Diab Invest 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase Y, Imagawa A, Hanafusa T, et al. Fulminant type 1 diabetes as a high risk group for diabetic microangiopathy –a nationwide 5-year-study in Japan. Diabetologia 2007; 50: 531–537. [DOI] [PubMed] [Google Scholar]
- 5.Bugawan TL, Klitz W, Alejandrino M, et al. The association of specific HLA class I and II alleles with type 1 diabetes among Filipinos. Tissue Antigen 2002; 59: 452–469. [DOI] [PubMed] [Google Scholar]
- 6.Feng YF, Yao MF, Li Q, et al. Fulminant type 1 diabetes in China: a case report and review of the literature. Biomed Biotechnol 2010; 11: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur, 1st ed Paris: Brosson & Chaudé, 1819. [Google Scholar]
- 8.Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum. Clinical implications. Arch Intern Med 1939; 64: 913–926. [Google Scholar]
- 9.Hamman L. Spontaneous mediastinal emphysema. Bull John Hopkins Hosp 1939; 64: 1–21. [Google Scholar]
- 10.Bullaboy CA, Jennings RB, Johnson DH, et al. Radiological case of the month: pneumomediastinum and subcutaneous emphysema caused by diabetic hyperpnea. Am J Dis Childhood 1989; 143: 93–94. [PubMed] [Google Scholar]
- 11.Pooyan P, Puruckherr M, Summers JA, et al. Pneumomediastnum, pneumopericardium and epidural pneumatosis in DKA. J Diab Complication 2004; 18: 242–247. [DOI] [PubMed] [Google Scholar]
- 12.Steenkamp D, Patel V, Minkin R. A case of pneumomediastinum: a rare complication of diabetic ketoacidosis. Clin Diab 2011; 29: 76–77. [Google Scholar]
- 13.Pauw RG, Van der Werf TS, Van Dulleman HM, et al. Mediastinal emphysema complicating diabetic ketoacidosis: plea for conservative diagnostic approach. Nether J Med 2007; 65: 10. [PubMed] [Google Scholar]
- 14.Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-myocardial infarction investigations in Japan. J Am College Cardiol 2001; 38: 11–18. [DOI] [PubMed] [Google Scholar]
- 15.Castillo Rivera AM, Ruiz-Bailen M, Rucabado AL. Takotsubo cardiomyopathy – a clinical review. Med Sci Monitor 2011; 17: RA135–RA147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song BG, Chun WJ, Park YH, et al. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol 2011; 34: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chockalingam A, Mehra MA, Dorairajan S, et al. Acute left ventricular dysfunction in the critically ill. Chest 2010; 138: 198–207. [DOI] [PubMed] [Google Scholar]
- 18.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. New Engl J Med 2005; 352: 539–548. [DOI] [PubMed] [Google Scholar]
- 19.Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008; 118: 2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelmoneim SS, Mankad SV, Bernier M, et al. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr 2010; 22: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 21.Kassim TA, Clarke DD, Mai VQ, et al. Catecholamine-induced cardiomyopathy. Endocrinology Practice 2008; 14: 1137. [DOI] [PubMed] [Google Scholar]
- 22.Kurisu S, Inoue I, Kawagoe T, et al. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am College Cardiol 2003; 41: 743–748. [DOI] [PubMed] [Google Scholar]
- 23.Sverrisdóttir YB, Schultz T, Omerovic E, et al. Sympathetic nerve activity in stress-induced cardiomyopathy. Clin Autonomic Res 2012; 22: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohut AR, Jain D. Perfusion and wall motion abnormalities in a patient with diabetic ketoacidosis. J Nucl Cardiol 2009; 16: 316–320. [DOI] [PubMed] [Google Scholar]
- 25.Younger F, Plein S, Barth J, et al. Troponin I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93: 1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim W. Cardiac troponin in the ICU. 2009. Clin Invest Med 32: E405–E410. [DOI] [PubMed]
- 27.Agewall S, Giannitsis E, Jernberg T, et al. Troponin elevation is coronary vs non-coronary disease. Eur Heart J 2011; 32: 404–411. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan V, Jarolim P. Clinician update. how to interpret elevated cardiac troponin levels. Circulation 2011; 124: 2350–2354. [DOI] [PubMed] [Google Scholar]
- 29.Thygessen K, Alpert J, Harvet D, et al. Universal definition of myocardial infarction. Circulation 2007; 116: 2634–2653. [DOI] [PubMed] [Google Scholar]
- 30.Thygessen K, Alpert J, Jaffe A, et al. Third universal definition of myocardial infarction. J Am College Cardiol 2012; 60: 3–4. [DOI] [PubMed] [Google Scholar]