Abstract
Introduction
Early rehabilitation in critically ill patients has been demonstrated to be safe and is associated with many positive outcomes. Despite this, there are inconsistencies in the early active rehabilitation that patients receive on intensive care units. The aims of this study were to quantify the amount of active rehabilitation provided for patients in a District General Hospital intensive care unit and to identify specific barriers encountered.
Methods
Data were collected over a six-week period during March and April 2013. All patients admitted to the intensive care unit at St Peter’s Hospital for more than 48 h were included. For every treatment session, the treating physiotherapist recorded what type of treatment took place. Treatments were classified as either non-active or active rehabilitation. Non-active rehabilitation included chest physiotherapy, passive range of movement exercises and hoisting to a chair. Active rehabilitation was defined as any treatment including active/active-assisted exercises, sitting on the edge of the bed, sitting to standing, standing transfers, marching on the spot or ambulation. Classification of rehabilitation was based upon internationally agreed intensive care unit activity codes and definitions. All barriers to active rehabilitation were also recorded.
Results
The study included 35 patients with a total of 194 physiotherapy treatment sessions. Active rehabilitation was included in 51% of all treatment sessions. The median time to commencing active rehabilitation from intensive care unit admission was 3 days (range 3–42 [IQR 3–7]). The most frequent barriers to active rehabilitation were sedation and endotracheal tubes, which together accounted for 50% of the total barriers.
Conclusion
The study provides useful benchmarking of current rehabilitation activity in a District General Hospital intensive care unit and highlights the most common barriers encountered to active rehabilitation. Longer duration studies incorporating larger sample sizes are warranted. Future studies should utilise the internationally agreed intensive care unit activity codes to improve comparability.
Keywords: Critical care, rehabilitation, intensive care, benchmarking, physiotherapy
Introduction and aims
With decreasing mortality following critical illness, there has been a shift in emphasis towards the long-term outcomes of patients surviving the intensive care unit (ICU). Guidelines published by the National Institute for Health and Care Excellence (NICE) for the rehabilitation of the critically ill patient highlight the severe physical morbidity suffered by patients confined to bed in critical care units for prolonged periods of time.1 Physical morbidity often persists for many months after the patients have recovered from their initial illness. The NICE guidelines call for early identification of rehabilitation needs and for rehabilitation to commence as soon as possible.1 In order to implement the guidelines, we need to understand current practice and usual care.2 Recent studies investigating rehabilitation practices in ICU have called for the benchmarking of current practice in a standardised format.2
Early rehabilitation in critically ill patients has been demonstrated to be safe3 and is associated with reductions in delirium, duration of mechanical ventilation, improved physical function at hospital discharge4 and reduced lengths of both ICU and hospital stay.5 Despite these outcomes, many patients do not receive early active rehabilitation. Thomas et al.6 report that only 55% of all physiotherapy episodes carried out in their district general ICU over a three-month period included active rehabilitation.
Barriers to rehabilitation cited in the literature include over sedation and poor sedation management, lack of appropriate staff, inappropriate vascular access positions, conflict with other planned procedures and agitation.7,8 In 2012, a stakeholder conference called for identification of barriers to early rehabilitation in order to identify strategies to increase physical activity of patients in critical care.9
The aims of this current study were to quantify the amount of active rehabilitation provided for patients in the ICU at St Peter’s Hospital and to identify specific barriers encountered.
Method
Data were collected prospectively over a six-week period during March and April 2013. All patients admitted to the nine-bedded general ICU at St Peter’s Hospital for more than 48 h were included in the study. Demographic data including age, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, diagnosis and duration of mechanical ventilation were recorded for each patient along with the presence of any co-morbidities affecting mobility. For the duration of each patient’s ICU stay, the indication for physiotherapy treatment was documented daily. For every treatment session, the treating physiotherapist recorded what type of treatment took place. Treatments were classed as either non-active or active rehabilitation. Non-active treatment included chest physiotherapy, passive range of movement exercises and hoisting to a chair. Active rehabilitation was defined as any treatment including active/active-assisted exercise (including use of the MOTOmed Letto bike in active-assisted mode), sitting on the edge of the bed, sitting to standing (±standing hoist), standing transfers, marching on the spot or ambulation. Classification of rehabilitation was based upon the internationally agreed ICU activity codes and definitions which were developed at the Fifth Annual International ICU Physical Medicine and Rehabilitation meeting in San Francisco.10
All barriers to active rehabilitation were recorded. Barriers to rehabilitation identified by previous studies along with those identified by the researchers from prior experience of working on the ICU were included as tick boxes. The tick boxes reflected the presence of an endotracheal tube (ETT), sedation, femoral vas-cath, ‘unstable’, staffing and lack of equipment. A free text space stating ‘Other (please specify)’ was also included to record any other barriers to rehabilitation that might occur. No limit to the number of barriers that could be recorded was made. Data collection was completed by all physiotherapists providing a service to ICU including weekend staff.
Data were analysed using Microsoft Excel 2010 and SPSS version 22. Descriptive statistics were employed to establish what percentage of physiotherapy sessions included active rehabilitation, the median time from ICU admission to active rehabilitation commencing and the most common barriers to active rehabilitation. Means have been reported where data were normally distributed and medians where it was not. Normality was tested in SPSS version 22 with the use of Skewness values, Kutosis and histograms.
The study was registered with and approved by Ashford and St Peter’s Hospitals NHS Foundation Trust audit department.
Results
The data collected during the study period were complete with the exception of one day at a weekend where no data were recorded by the physiotherapists on duty.
The study included 35 patients with a total of 194 physiotherapy treatment sessions. Demographics are displayed in Table 1. Pre-existing co-morbidities affecting mobility were recorded in 37% (n = 13) patients. Mobility was considered to be affected if patients required assistance or a walking aid to mobilise, lacked the exercise tolerance to mobilise for more than 60 m without rest or required ambulatory oxygen. No patient included in the study was entirely wheelchair or bed bound.
Table 1.
Demographics of the study population.
| Age, years | 52 (18–90 [51–65]) |
| Length of ICU stay, days | 5 (2–69 [3–8.25]) |
| Length of hospital stay, days | 19 (3–87 [8.25–29]) |
| Duration of MV, days | 2 (0–67 [2–4]) |
| APACHE II score | 23 (4.8) |
| Gender | |
| Male | 19 (54%) |
| Female | 16 (46%) |
| Primary diagnosis | |
| Gastrointestinal-post surgery | 14 (40%) |
| Genitourinary | 5 (14.5%) |
| Post AAA repair | 4 (11.5%) |
| Cardiovascular | 4 (11.5%) |
| Respiratory | 4 (11.5%) |
| Gastrointestinal-other | 2 (5.5%) |
| Neurological | 2 (5.5%) |
Data are reported as median (range [IQR]), mean (SD) or number (percentage), as appropriate. MV: mechanical ventilation; APACHE II: Acute Physiology and Chronic Health Evaluation; AAA: abdominal aortic aneurysm.
Active rehabilitation was included in 51% (n = 99) of all physiotherapy treatment sessions. The median time to commencing active rehabilitation from ICU admission was 3 days (range 3–43 [IQR 3–7]).
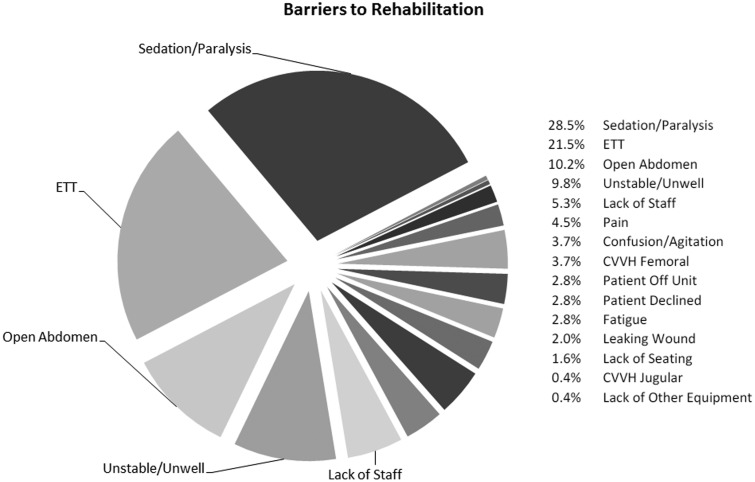
The most frequent barriers to active rehabilitation were sedation and presence of an ETT, which together accounted for 50% (n = 123) of total barriers to rehabilitation. Figure 1 demonstrates common barriers to rehabilitation encountered.
Figure 1.
All barriers to rehabilitation recorded and their percentage proportion. ETT: endotracheal tube; femoral CVVH: continuous veno-venous haemofiltration via femoral vein; jugular CVVH: continuous veno-venous haemofiltration via jugular vein.
Discussion
There are limited published studies on the incidence of active rehabilitation within general ICUs in the United Kingdom and even less data available on common barriers to rehabilitation.3,5,6 Differences in ICU setting along with variation in physiotherapy practice, staffing levels and expertise in different countries often make comparison of studies in ICU rehabilitation difficult. Differences in methodology and definitions of active rehabilitation compound difficulties in comparing results of studies. Thomas et al.6 provide data in a similar population to the current study and investigated rehabilitation activity within an eight-bedded general ICU.
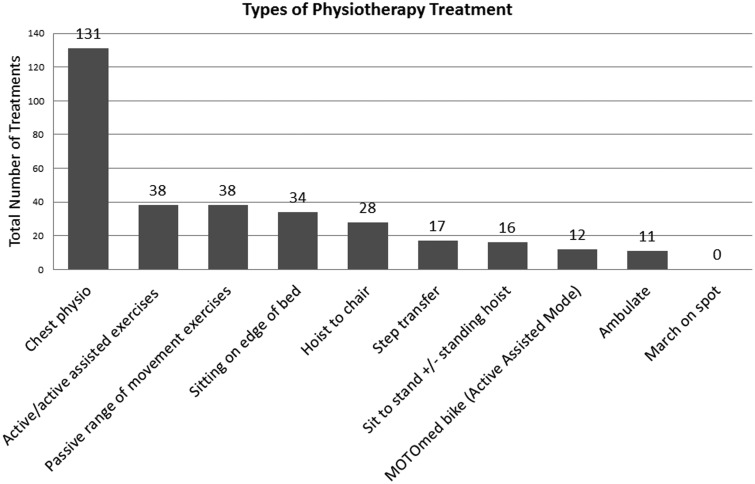
The percentage of treatments involving some form of active rehabilitation in this study was similar to that of Thomas et al.6 They reported active rehabilitation in 55% of all treatment sessions whilst in the current study 51% of treatment sessions included active rehabilitation. There were also similarities between types of active rehabilitation carried out. Activities tended to follow a functional hierarchy with lower level activities being carried out more frequently than higher level activities. In both studies, the most common form of active rehabilitation was active/active-assisted exercises followed by sitting on the edge of the bed. The least common activity was ambulation. Figure 2 shows the frequency with which different physiotherapy interventions, both active and non-active, were carried out in the current study.
Figure 2.
Frequency of types of physiotherapy treatment undertaken.
Thomas et al.6 do not provide information on the number of days from ICU admission to the first active rehabilitation episode, so comparisons with the current study cannot be made. Bailey et al.3 reported a mean time from initial ICU admission to commencing active rehabilitation of 6.6 days. The current study reports a median time to active rehabilitation of 3 days. Data distribution and therefore statistical tests employed make comparisons between the two studies difficult. In addition, the lowest level of active rehabilitation recorded by Bailey et al.3 was sitting on the edge of the bed; hence, active or active-assisted exercises would not have been classed as active rehabilitation. In the current study, the median time to sitting on the edge of the bed was 10 days. Bailey et al.’s3 study was conducted in a specialist tertiary respiratory ICU; hence, these results should be compared with caution.
Table 2 indicates the length of time patients took to achieve key functional milestones. We would expect the activity milestones to follow a functional hierarchy with patients achieving the lower level functional milestones sooner than more advanced milestones. These data do not reflect this pattern. One reason for this may have been under reporting of lower level milestones in more able patients, for example, when patients achieved multiple milestones in the same physiotherapy treatment session or when they achieved them with the nursing staff rather than during physiotherapy sessions. Bailey et al.3 report data on length of time to activity milestones. In their study, milestones did follow a functional hierarchy, but direct comparisons with the current study are difficult as they report mean values rather than median values.
Table 2.
The length of time in days that patients took to achieve key functional milestones.
| Milestone | Days, median (range [IQR]) |
|---|---|
| SOEOB (n = 7) | 10 (2–46 [4–29]) |
| STS ± hoist (n = 5) | 17 (4–69 [9–56]) |
| Step transfer (n = 12) | 2.5 (2–15 [2–4.5]) |
| March on spot (n = 0) | N/A |
| Ambulate (n = 7) | 10 (4–19 [5–15]) |
SOEOB: sit on edge of bed; STS: sit to stand.
Table 3 displays the percentage of patients who achieved functional milestones whilst on ICU during the data collection period. The table indicates that higher level milestones were achieved less often than lower level milestones. Patients achieving the ambulation milestone had longer ICU stays than that of those who did not, suggesting that those recorded as non-achievers may have been discharged from ICU prior to reaching the more advanced milestones. The achievement of functional milestones does not appear to be related to illness severity measured by APACHE II score, which was similar between achievers and non-achievers.
Table 3.
Percentage of patients achieving functional milestone.
| Functional milestone | Functional milestone achieved | Functional milestone not achieved |
|---|---|---|
| SOEOB | ||
| n (%) | 20 (57%) | 15 (43%) |
| APACHE II, mean (SD) | 23 (4.47) | 24.5 (4.03) |
| ICU LOS, days (median [IQR]) | 4.5 (3–69 [3–12.25]) | 5 (3–46 [4–6.5]) |
| STS ± hoist | ||
| n (%) | 18 (51%) | 17 (49%) |
| APACHE II, mean (SD) | 25.3 (4.6) | 24 (3.91) |
| ICU LOS, days (median [IQR]) | 4.5 (3–69 [3–16.75]) | 5 (3–46 [4–5]) |
| Step transfer | ||
| n (%) | 14 (40%) | 21 (60%) |
| APACHE II, mean (SD) | 21 (3.69) | 22.5 (4.46) |
| ICU LOS, days (median [IQR]) | 4 (3–23 [3–9]) | 5 (3–69 [4–8]) |
| March on spot | ||
| n (%) | 0 (0%) | 0 (0%) |
| APACHE II, mean (SD) | n/a | n/a |
| ICU LOS, days (median [IQR]) | n/a | n/a |
| Ambulate | ||
| n (%) | 7 (20%) | 28 (80%) |
| APACHE II, mean (SD) | 22 (4.49) | 24 (4.06) |
| ICU LOS, days (median [IQR]) | 10 (4–23 [5.5–19.5]) | 4 (3–69 [3–5.75]) |
ICU LOS: intensive care unit length of stay; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOEOB: sit on edge of bed; STS: sit to stand.
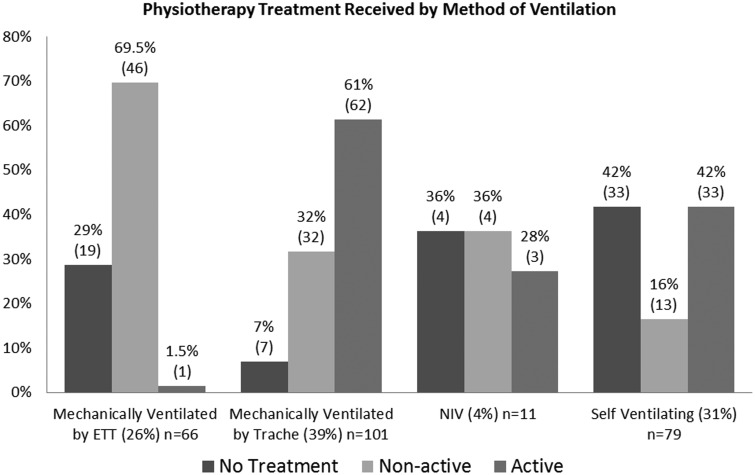
One of the biggest barriers to active rehabilitation was the presence of an ETT. Figure 3 demonstrates patients mechanically ventilated via an ETT received minimal active rehabilitation. One treatment of active-assisted bed exercises was the only rehabilitation carried out in the ETT patient group. In contrast, for patients mechanically ventilated via a tracheostomy, 61% of treatment sessions involved some component of active rehabilitation. A similar level of active rehabilitation was seen in patients who were self-ventilating to those mechanically ventilated via a tracheostomy. It should be noted that the number of treatment episodes with patients ventilated via non-invasive ventilation was small (n = 7), and therefore, data relating to this patient group should be interpreted with caution due to the risk of systemic measurement error.
Figure 3.
Physiotherapy treatment received by method of ventilation. ETT: endotracheal tube; Trache: tracheostomy tube; NIV: non-invasive ventilation (included patients on continuous positive airway pressure).
There may be a number of reasons why patients with an ETT received minimal active rehabilitation. These patients may have been more acutely unwell than those in other groups. It is not possible to state this definitively, as no daily measurement of illness acuity was recorded during the study period. The patient being too unstable to participate in rehabilitation was only recorded as a barrier in 2% of treatment episodes with patients ventilated via an ETT. No specific criteria were set as to when a patient should be considered too unstable for physiotherapy and this was left to individual physiotherapists’ judgement. Reasons recorded by physiotherapists included the patient being cardiovascularly unstable, pyrexial or having high oxygen requirements. It is possible that being ‘too unstable’ may have been under reported in cases where other obvious barriers to rehabilitation co-existed. Where sedation was recorded as a barrier to rehabilitation, there was a 5.5% incidence of patients being considered too unstable for treatment. Where both sedation and ETT were recorded as a barrier to rehabilitation, there was a 6% incidence of patients being too unstable for treatment. Future studies may wish to record a daily illness acuity score to establish more objectively the degree to which illness acuity limits rehabilitation.
Bailey et al.3 demonstrated that it is safe to mobilise patients with an ETT. However, in the current study, sedation levels meant patients with an ETT tube could not mobilise and were limited in their ability to actively participate in rehabilitation. Sedation was recorded as a barrier to rehabilitation in 100% of treatment episodes for patients with an ETT. Whilst there may have been occasions when patients were too unstable to participate in rehabilitation, it does appear the main barrier to rehabilitation whilst ventilated via an ETT was the sedation levels required for the patient to tolerate the ETT.
Carrying out earlier tracheostomies may help to reduce levels of sedation and thus facilitate earlier participation in rehabilitation. The TrachMan study found a moderate but significant reduction in sedation levels in patients who underwent early tracheostomy (<4 days) compared to those receiving late tracheostomy (>10 days).11 It should be noted, however, that the majority of patients continued to receive sedatives after their tracheostomy was performed.
It is clear that careful consideration of when to carry out tracheostomy is required. The results of the TrachMan study indicated performing tracheostomies earlier than day 4 may result in patients receiving unnecessary tracheostomies.11 The authors recommend delaying tracheostomy until at least day 10 of a patient’s ICU stay.11 The mean number of days from ICU admission to tracheostomy in the current study was 15 days (SD 9). One patient in the study was ventilated for 25 days via an ETT before receiving a tracheostomy. During this time, no active rehabilitation was undertaken. Given that almost all patients in the current study received a late tracheostomy (as per TrachMan criteria), it is possible reducing time to tracheostomy may have allowed these patients to begin active rehabilitation earlier without increasing the risk of unnecessary tracheostomy. Whilst there are many different factors requiring consideration in deciding when to perform a tracheostomy, we must be aware that delaying the procedure may have an impact on patients’ ability to participate in active rehabilitation.
The placement of some lines and attachments was identified as a barrier to rehabilitation. In particular, femoral intravascular catheters for CVVH (continuous veno-venous haemofiltration) limit the degree of hip flexion a patient can perform, thus restricting the rehabilitation in which they can participate. Consideration to ease of mobility should be given when deciding where to place lines and attachments. Placing intravascular catheters in alternative sites may enable patients to exercise and transfer out of bed more easily.
Often active rehabilitation was not carried out due to clinical reasons, such as the patients being unstable, unwell or too fatigued. Clinicians need to make judgements to balance rehabilitation with other critical care demands on the patient such as ventilator weaning. According to the internationally agreed ICU activity codes, activities such as being hoisted to a chair are not considered active rehabilitation. However, along with many other interventions in ICU, hoisting to a chair can increase oxygen consumption and energy expenditure.12 Physiotherapists must use their skill and experience to judge the clinical appropriateness of active rehabilitation for each individual patient.
Lack of available staff was reported as a barrier to rehabilitation. Rehabilitation of ICU patients requires highly skilled staff. The ICU patient group is often dependent and requires several physiotherapists and nurses to facilitate active rehabilitation. Therefore, if any professional group has a lack of staff, patients will receive less rehabilitation. Lack of staffing was recorded as a barrier to rehabilitation regardless of which part of the multi-disciplinary team was lacking resources. The promotion of good inter-disciplinary communication and teamwork can optimise rehabilitation opportunities during periods of staff shortage.
Lack of equipment presented as an occasional barrier to rehabilitation with the most common problem being a lack of appropriate seating. As the literature investigating barriers to rehabilitation is so sparse, it is difficult to ascertain whether this is a local or more global issue.
Lee and Fan13 discuss future developments which may facilitate early rehabilitation in critically ill patients. Included in their discussion is use of new sedative agents and techniques such as daily sedative interruption to allow early rehabilitation.7 Also discussed is the increasing use of alternatives to mechanical ventilation such as extracorpeal membrane oxygenation which minimises the need for sedation and analgesia.7
Study limitations and future investigations
The main limitations of this study were the small sample size and data collection period plus the single centre design. Longer duration studies incorporating larger sample sizes are warranted. Additionally, it would be useful to compare variations in rehabilitation practice across different ICU settings and populations. To improve comparability of studies, future investigations should utilise the internationally agreed ICU activity codes.10
Future studies may wish to collect daily measurements of illness acuity. This would allow more objective analysis of the degree to which illness acuity contributes to patients receiving longer periods of ventilation via ETT, higher levels of sedation and being unable to participate in active rehabilitation.
Data were collected by the physiotherapists providing the critical care service. All physiotherapists were aware of the purpose of the study, which means that the results could have been influenced by the Hawthorne effect.14 Physiotherapists may have modified their treatment as a result of participating in the study and therefore a true picture of ‘usual care’ may not have been captured.
It was beyond the scope of this study to consider the relationship between quantity of rehabilitation and functional outcome. Further investigations are necessary to consider the impact of quantity, quality and intensity of rehabilitation on functional outcomes in ICU survivors.
Conclusion
The results of this study provide us with a better understanding of current rehabilitation activity in critical care, and barriers most commonly encountered. As a result, we can begin to address these barriers, thereby meeting rehabilitation needs and combating the high levels of physical morbidity currently endured by critical care survivors. Ultimately, overcoming these barriers will require the creation of an ICU culture and multidisciplinary team that prioritises early rehabilitation and where facilitating early activity is a key consideration in clinical decision-making.
Acknowledgements
The authors would like to thank the physiotherapy staff at Ashford and St Peter’s Hospitals NHS Foundation Trust for their assistance in data collection. They especially thank Zoe Richards, Laura Brockway and Freya Bloor. They also thank Tina Thomas for her assistance with data entry and Amanda Thomas for her input and guidance in preparing this work for publication.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.National Institute of Health and Clinical Excellence (NICE). Rehabilitation after critical illness, London: NICE, 2009. [PubMed] [Google Scholar]
- 2.Parker A, Tehranchi KM, Needham DM. Critical care rehabilitation trials: the importance of ‘usual care’. Crit Care 2013; 17: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey P, Thomsen GE, Spuhler VJ. Early activity is feasible and safe in respiratory failure patients. Crit Care Med 2007; 35: 139–145. [DOI] [PubMed] [Google Scholar]
- 4.Schweickert WD, Pohlman MC, Pohlman AS. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet (London, England) 2009; 373: 1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris PE, Goad A, Thompson C. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008; 36: 2238–2243. [DOI] [PubMed] [Google Scholar]
- 6.Thomas A, Wright K, Mill L. The incidence of physiotherapy practice and rehabilitation activities within a general intensive care unit. J Assoc Chart Physiother Resp Care 2009; 49: 3–8. [Google Scholar]
- 7.Pohlman MC, Schweikert WD, Pohlman AS. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med 2010; 38: 2089–2094. [DOI] [PubMed] [Google Scholar]
- 8.Zanni JM, Korupolu R, Fan E. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care 2010; 25: 254–262. [DOI] [PubMed] [Google Scholar]
- 9.Needham DM, Davidson J, Cohen H. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40: 502–509. [DOI] [PubMed] [Google Scholar]
- 10.Hodgson C. ICU Activity Codes and Definitions, http://www.mobilization-network.org/Network/Documents.html (2012, accessed 18 June 2014).
- 11.Young D, Harrison DA, Cuthbertson BH. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA 2013; 309: 2121–2129. [DOI] [PubMed] [Google Scholar]
- 12.Zafiropoulos B, Alison J, McCarren B. Physiological responses to the early mobilisation of the intubated, ventilated abdominal surgery patient. Aust J Physiother 2004; 50: 95–100. [DOI] [PubMed] [Google Scholar]
- 13.Lee CM, Fan E. ICU-acquired weakness: what is preventing its rehabilitation in critically ill patients? BMC Med 2012; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke RH, Kaul JD. The Hawthorne experiments: first statistical interpretation. Am Sociolog Rev 1978; 43: 623–643. [Google Scholar]