Abstract
Recent studies demonstrate that Th1-type immune responses against a broad spectrum of hepatitis C virus (HCV) gene products are crucial to the resolution of acute HCV infection. We investigated new vaccine approaches to augment the strength of HCV-specific Th1-type immune responses. ELISPOT assay revealed that single or multiple protein immunization using both CpG ODN and Montanide ISA 720 as adjuvants induced much stronger IFN-γ-producing Th1 responses against core, NS3 and NS5b targets than did the formulation without these adjuvants. Protein vaccination using CpG ODN and Montanide ISA 720 as adjuvants also greatly enhanced humoral responses to HCV core, E1/E2 and NS3. When specific IgG isotypes were assayed, protein immunization using CpG ODN and Montanide ISA 720 as adjuvants produced higher titers of IgG2a dominant antibodies than did protein immunization alone, indicating a more Th1-biasedpathway. This increase in IgG2a is consistent with the induction of Th1 cells secreting IFN-γ demonstrated by ELISPOT assay. In conclusion, protein immunization using CpG ODN and Montanide ISA 720 as adjuvants greatly enhanced cellular (Th1 type) as well as humoral immune responses against HCV in Balb/c mice. The use of adjuvants appears critical to the induction of Th1 immune responses during HCV vaccination with recombinant proteins.
Keywords: Hepatitis C virus, Th1-type immune response, Humoral immune response
1. Introduction
Hepatitis C virus (HCV), a positive strand RNA virus and a member of the flavivirus family, is the prime causative agent for post-transfusion hepatitis and is possibly responsible for over 80% of community-acquired hepatitis cases in this country [1–3]. Over 75% of HCV-infected patients develop persistent infection that can ultimately progress to cirrhosis, hepatocellular carcinoma or end-stage liver disease [1–3]. Currently, the optimal treatment for chronic HCV infection is the combination of pegylated IFN-α with ribavirin, but only 50% of treated patients have sustained benefit from antiviral therapy [4,5], emphasizing the need for an effective prophylactic and therapeutic vaccine.
To date, no prophylactic or therapeutic vaccine has proven efficacy. Although antibodies to HCV are detected invariably following infection, humoral immunity, a key aspect of protective immunity in most viral infections, may not play a critical role in viral clearance and recovery from HCV infection [6], perhaps because HCV exists as a quasispecies capable of the rapid emergence of antibody escape mutants [7]. The role of neutralizing antibodies in protective immunity against HCV infection is still unclear. The recent establishment of a transfection system producing HCV pseudoparticles has identified neutralizing antibodies, primarily in patients with chronic infection [8–10]. Though these antibodies can be of high titer and broad reactivity, they have not been associated with viral clearance. Nevertheless, the availability of pseudotype assays and HCV replicating systems provide new tools to develop and assess HCV vaccines [11,12]. Cellular immunity does appear to play an important role in the virological outcome of acute infection and viral clearance correlates with a strong Th1 type immune response [13–17]. A wide variety of vigorous CD4+ T cell responses persist for many years after viral clearance [16]. Analyses of the cytokine profiles of HCV-specific T cells have revealed that persons displaying a Th1 profile (antigen-dependent production of IL-2 and IFN-γ) are more likely to experience viral clearance [15,18,19]. An effective vaccine may require multiple components to induce a strong Th1 immune response.
Our previous studies have shown that immunization with HCV nonstructural protein 3 (NS3) using CpG ODN and liposome as adjuvants greatly enhanced cellular (Th1 type) as well as humoral immune responses compared with DNA or protein immunization alone [20]. In this report we describe a novel protein vaccine regimen using recombinant HCV structural and nonstructural proteins in CpG ODN and Montanide ISA 720 adjuvant, and characterize the immune responses elicited. Montanide ISA 720 is an oil adjuvant composed of a natural metabolizable oil and a highly refined emulsifier from the manide monooleate family [21]. It has been used as the adjuvant combined with HIV and malaria antigens in clinical trials and has shown promise in promoting T cell responses [22,23]. CpG ODN signal through Toll-like receptor 9 (TLR9) [24], inducing both maturation and activation of dendritic cells (DC) and augmenting the production of proinflammatory cytokines [25]. This activation of the innate immune response leads to the induction of specific antibody and Th1 immune responses when CpG ODN are coadministered with protein antigen [20,26–29]. Our data show that this regimen induces potent humoral and cellular (Th1 type) immune responses in the murine model.
2. Materials and methods
2.1. Expression and purification of recombinant HCV core, E1E2, NS3 and NS5b proteins
HCV core cp1-10 (aa 1–164) were expressed, purified and characterized as described previously [30,31]. The endotoxin levels in purified proteins were less than 0.02 EU per µg protein.
HCV envelope proteins E1 and E2 complex (aa 165–746) were produced from a baculovirus-insect cell expression system. Briefly, for the construction of recombinant baculovirus containing HCV core, E1, and E2 genes, a procedure similar to that previously described [32,33], was applied using Bac-To-Bac baculovirus expression systems (Invitrogen Corp., Grand Island, NY). Plasmid pFastBac-HCV-S1 was generated by inserting the HCV core, E1, and E2 genes encoding aa 1–746 (nt342–2579, HCV strain H of genotype 1a) into the blunt-ended BamH 1 restriction enzyme site of pFastBac1 plasmid (Invitrogen Corp., Grand Island, NY). Selection of recombinant bacmid clones from transformed DH10Bac competent cells by pFastBac-HCV-S1, and subsequent identification and purification of recombinant baculovirus from transfected Spodoptera frugiperda Sf9 insect cells were performed according to the manufacture’s protocols. Characterization, expression and purification of HCV E1 and E2 proteins in recombinant baculovirus infected Sf9 insect cells were performed as described previously [32,33]. The endotoxin levels in both E1 and E2 proteins are less than 10 EU per mg of protein.
The full-length HCV NS3 protein (aa 1027–1657) was produced in an E. coli expression system. The construction of HCV NS3 expression vector in E. coli DH5αF’IQ cells, and the expression and purification of recombinant HCV NS3 protein were described previously [20,34,35]. The endotoxin levels in purified proteins were less than 0.02 EU per µg protein.
The full-length HCV NS5b protein (aa 2421–3011) was also produced in an E. coli expression system. Briefly, full-length HCV NS5b gene encoding aa 2421–3011 (nt7602–9374 plus TGA stop codon, HCV strain H of genotype 1a) was inserted into BamH 1 restriction enzyme site of pQE-11 plasmid (Qiagen Inc., Valencia, CA) and expressed in E. coli DH5αF’IQ cells. The expression and purification procedures for full-length HCV NS5b protein were generally the same as for full-length HCV NS3 protein, with minor modification. The endotoxin levels in purified proteins were less than 0.02 EU per µg protein.
2.2. Preparation of CpG ODN emulsified in Montanide ISA 720
Immune-stimulatory CpG ODN (5′-GACGTTGACGTTAGCGT-3′), designated as ‘CpG’ in the text, or non-stimulatory GpC control (5′-GAGCTTGAGCTTAGGCT-3′), designated as ‘GpC control’ in the text, was synthesized with full phosphorothioate backbone by Key-stone Lab (Camarillo, CA, USA) and emulsified with Montanide ISA 720 (SEPPIC, Paris, France) at 7:3 (v/v) oil/aqueous phase ratio. The sequence design of CpG or GpC control was based on the results of Jiao et al. [20].
2.3. Mice immunization
Female BALB/cByJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in approved animal care facilities during the experimental period and were handled following the international guidelines required for experimentation with animals. All animal study protocols were approved by NIH Clinical Center Animal Care and Use Committee. Twelve protein immunization regimes were applied to mice in twelve groups (each group n = 5): 10 µg CpG; 50 µg CpG; 40 µg prot (no CpG); 40 µg prot + 50 µg GpC control; 40 µg prot + 10 µg CpG; 40 µg prot + 50 µg CpG; 8 µg prot + 10 µg CpG; 30 µg prot (no E1E2) + 50 µg CpG; 10 µg core + 10 µg CpG; 10 µg E1E2 + 10 µg CpG; 10 µg NS3 + 10 µg CpG; 10 µg NS5b + 10 µg CpG. On week 0, the mice of all groups were injected intramuscularly with 100 µl of the formulation which contained 0–40 µg of total HCV protein and 0–50 µg CpG and 70% (by volume) Montanide ISA 720 into the tibialis anterior muscle of both legs following 2% isoflurane inhalation anaesthesia. On week 8, mice were bled through the retro-orbital plexus/sinus and serum samples were collected and kept at −70 °C before assay. Then the mice were euthanized and spleens were collected for lymphocyte isolation.
2.4. Assay of anti-HCV antibodies
Eight weeks after protein immunization, mice were bled and sera were kept at −70 °C before assay. Anti-HCV IgG was assayed by ELISA. MaxiSorp Nunc-Immuno plates were coated with recombinant HCV core, NS3 or NS5b protein at 3 µg/ml in coating buffer (20 mM NaHCO3/Na2CO3 pH 9.6, 0.15 M NaCl) and overcoated with 10% milk diluent (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland) in 1 × PBS. For testing anti-envelope antibodies, a modification of a previously described method was used [36]. Briefly, ELISA plates were coated with 4 µg/ml Galanthus nivalis lectin (GNL, vector) at 37 °C for 4 h and then overcoated with 10% milk diluent in 1 × PBS, pH 7.4, for 2 h. HCV envelop proteins were diluted in GNL buffer Q and captured on plates by incubation at room temperature with constant shaking for 2 h. The tested sera were added at 100 µl per well in 5% milk diluent in 1 × PBS containing 0.1% NP-40 with initial dilution of 1:100. Biotinylated goat anti-mouse IgGγ (Kirkegaard & Perry Laboratories) and strepavidin-horseradish per-oxidase (SA-HRP) (Kirkegaard & Perry Laboratories) were added sequentially. One hundred microlitres per well TMB micro-well per-oxidase substrate was used to develop the color and 100 µl per well peroxidase stop solution (Kirkegaard & Perry Laboratories) was added to stop the reaction. Absorbance was read at 450 nm. The IgG titre was determined by end-point dilution. The IgG subtype was determined by the ELISA assay described above except 1:1000 diluted biotinylated rat anti-mouse IgG1 or biotinylated rat anti-mouse IgG2a (both from BD Pharmingen) was used as second antibody. IgG1 and IgG2a concentration (ng/ml)was calibrated against a standard curve using purified mouse IgG1κ or IgG2aκ (2.44–1250 ng, both from BD Pharmingen) as standards, respectively.
2.5. ELISPOT assay for IFN-γ and IL-4
The ELISPOT assay was used to determine IFN-γ and IL-4 secreting cells among the mice spleen cells under the stimulation of recombinant HCV protein. Spleen cells from immunized mice were separated by Ficoll-Paque (Amersham Biosciences, Sweden), adjusted to 4 × 106 cells/ml, and then cultured with recombinant HCV core, E1E2, NS3 and NS5b proteins at 6 µg/ml for 40 h. Ninety six-well filtration plates with 0.45 µm surfactant-free mixed cellulose ester membrane, type MAHAS4510 (Millipore Co., Bedford, MA), were coated with purified anti-cytokine antibody (IFN-γ or IL-4) at the concentration of 10 µg/ml in 20mM borate buffer (pH 8.4) and incubated overnight at room temperature. Then the plates were overcoated with 5% BSA in 1 × PBS for 90min and 4 × 105 spleen cells in 100 µl volume were added to each well and incubated with antigen for 20 h at 37 °C in the presence of 5% CO2, allowing for production and capture of released cytokines. All determinations were run in quadruplicate. After incubation, cells were removed by washing six times with 1 × PBS containing 0.05% NP-40 and two times with distilled water. 1 µg/ml biotinlabeled anti-cytokine monoclonal antibody was used as the capture antibody and 1:4000 diluted streptavidin-horseradish peroxidase was used for antibody detection. Finally, optimal 4CN peroxidase substrate (Bio-Rad, Hercules, CA) was added and incubated for 20–30 min at room temperature to develop the spots. The reaction was stopped by washing with distilled water. The spot-forming units (SFU) representing single cells were counted automatically by ELISPOT reader (AID, Hallbergmoos, Germany) and expressed as SFU per 4 × 105 spleencytes. The number of antigen-specific SFU per well was calculated by subtracting its individual background value (buffer control well without antigen, typically less than 5).
2.6. Statistical analysis
Data were expressed as arithmetic means ± standard error of means (mean ± S.E.M.) or geometric means (GM). Comparison of ELISPOT results between two groups of mice was conducted by using ANOVA. The anti-HCV titers were compared by using the Mann–Whitney U test. All tests were two-tailed, and differences were considered significant for P < 0.05.
3. Results
3.1. Humoral immune responses induced by protein immunization
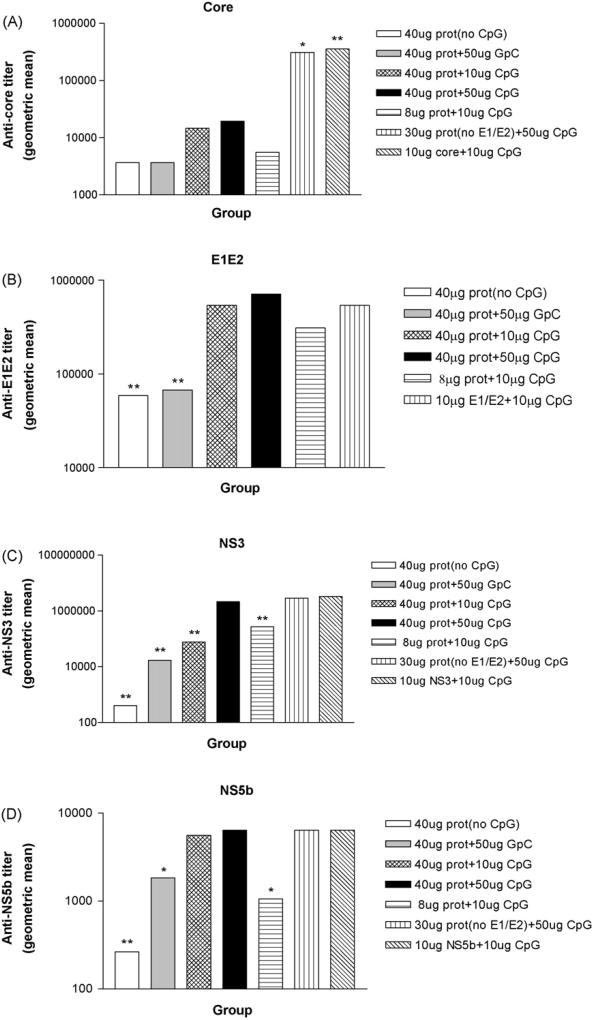
Four weeks after protein boost, the titer of anti-HCV core, E1/E2, NS3 and NS5b IgG was determined by endpoint dilution assay. The results are summarized in Table 1A and Fig. 1A–D.
Table 1.
| A Anti-HCV titers in mice immunized with a combination of HCV recombinant proteins in various formulations (n = 5, each group) | ||||
|---|---|---|---|---|
|
| ||||
| Formulations | Anti-HCV titer (geometric mean)a | |||
|
|
||||
| Core | E1E2 | NS3 | NS5b | |
| 40 µg prot no CpG | 3676 | 58813** | 400** | 264** |
| 40 µg prot + 50 µg GpC control | 3676 | 67559** | 16890** | 1838* |
| 40 µg prot + 10 µg CpG | 14703 | 540470 | 77605** | 5572 |
| 40 µg prot + 50 µg CpG | 19401 | 713155 | 2162000 | 6400 |
| 8 µg prot + 10 µg CpG | 5572 | 310419 | 270235** | 1056* |
| 30 µg prot (no E1E2) + 50 µg CpG | 310419* | – | 2853000 | 6400 |
| 10 µg core + 10 µg CpG | 356578** | – | – | – |
| 10 µg E1E2 + 10 µg CpG | – | 540470 | – | – |
| 10 µg NS3 + 10 µg CpG | – | – | 3277000 | – |
| 10 µg NS5b + 10 µg CpG | – | – | – | 6400 |
| B Isotype analysis anti-HCV core, E1/E2, NS3, NS5b antibodies in mice immunized with a combination of HCV recombinant proteins in various formulationsa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Formulations | n | core | E1E2 | NS3 | NS5b | ||||||||
|
|
|
|
|
||||||||||
| IgG1 | IgG2a | IgG2a/IgG1 | IgG1 | IgG2a | IgG2a/IgG1 | IgG1 | IgG2a | IgG2a/IgG1 | IgG1 | IgG2a | IgG2a/IgG1 | ||
| 40 µg prot no CpG | 5 | 91.45 | 23.96 | 0.26* | 5755.02 | 933.62 | 0.16** | 2.98 | 0.30 | 0.10 | 1.76 | 0.56 | 0.32* |
| 40 µg prot + 50 µg GpC control | 5 | 20.45 | 61.79 | 3.02 | 5205.01 | 2400.07 | 0.46** | 61.06 | 38.83 | 0.64 | 9.25 | 3.19 | 0.35 |
| 40 µg prot + 10 µg CpG | 5 | 35.66 | 227.90 | 6.39 | 9948.31 | 28483.17 | 2.86 | 63.25 | 619.15 | 9.79 | 10.29 | 51.85 | 5.04 |
| 40 µg prot + 50 µg CpG | 5 | 34.78 | 759.91 | 21.85 | 10531.34 | 48365.18 | 4.59 | 3820.61 | 18487.89 | 4.84 | 6.94 | 44.55 | 6.42 |
| 8 µg prot + 10 µg CpG | 5 | 5.90 | 253.92 | 43.04 | 6135.46 | 15078.32 | 2.46 | 532.54 | 1967.48 | 3.69 | 1.37 | 2.80 | 2.04 |
| 30 µg prot (no E1E2) + 50 µg CpG | 5 | 3198.93 | 6939.32 | 2.17* | – | – | – | 9580.35 | 40079.00 | 4.18 | 8.34 | 46.50 | 5.58 |
| 10 µg core + 10 µg CpG | 5 | 12746.02 | 2281.84 | 0.18** | – | – | – | – | – | – | – | – | – |
| 10 µg E1E2 + 10 µg CpG | 5 | – | – | – | 18789.08 | 13426.93 | 0.71* | – | – | – | – | – | – |
| 10 µg NS3 + 10 µg CpG | 5 | – | – | – | – | – | – | 22174.25 | 29164.72 | 1.32 | – | – | – |
| 10 µg NS5b + 10 µg CpG | 5 | – | – | – | – | – | – | – | – | – | 66.40 | 14.13 | 0.21 |
The anti-HCV titers were compared by using the Mann–Whitney U test.
P < 0.05 vs. 40µg prot + 50µg CpG.
P < 0.01 vs. 40µg prot + 50µg CpG.
The titers of anti-HCV core, E1/E2, NS3 and NS5b IgG1 or IgG2a were determined by endpoint dilution assay and expressed as the geometric means. The IgG2a/IgG1 ratios were provided and compared by using the Mann–Whitney U test.
Fig. 1.
Induction of HCV-specific humoral response by protein immunization with CpG adjuvant or GpC control adjuvant. The effect of protein and adjuvant dose is evaluated as well as the inhibitory effect of E1E2 on response to HCV core protein. Twelve protein immunization regimes were applied to mice in twelve groups (n = 5). Protein immunization consisted of 10 µg each of HCV core, E1E2, NS3 and NS5b. Eight weeks after protein immunization, mice were bled and sera were kept at −70 °C before assay. Anti-HCV IgG was assayed by ELISA. The recombinant HCV core (A), E1E2 (B), NS3 (C) or NS5b (D) protein were coated at 3 µg/ml in 96-well plates. The tested sera were serially diluted and applied to the plates. ELISA assay for anti-HCV IgG was performed as described in detail in Section 2. Absorbance was read at 450 nm. The IgG titre was determined by end-point dilution. The results were expressed as geometric means (GM). Comparison of ELISA results between two groups was conducted by using Mann–Whitney U test. Significant P values between groups vaccinated with different regimes are indicated. *P < 0.05 vs. 40 µg prot + 50 µg CpG; **P < 0.01 vs. 40 µg prot + 50 µg CpG. Data not shown for groups immunized with 10 µg CpG and 50 µg CpG, respectively.
The geometric mean antibody titers of protein immunization with CpG (40 µg prot + 50 µg CpG) were 5.3, 12.1, 5405.0 and 24.3 fold higher than protein immunization without CpG for core (P = NS), E1E2 (P < 0.01), NS3 (P < 0.01), and NS5b (P < 0.01), respectively (Table 1A) and 5.3, 10.6, 128.0 and 3.5 fold higher than protein immunization with GpC control for core (P = 0.06), E1E2 (P < 0.01), NS3 (P < 0.01), and NS5b (P < 0.05), respectively (Table 1A). Protein immunization with a smaller CpG dosage (40 µg prot + 10 µg CpG) was 28 fold less effective than CpG dose of 50 µg for NS3 (P < 0.01) but not significantly different for core, E1E2 and NS5b. Immunization with both a smaller protein and CpG dosage (8 µg prot + 10 µg CpG) was 2–8 fold less effective than 40 µg prot + 50 µg CpG for each of the proteins but only significant for NS3 (P < 0.01) and NS5b (P < 0.05) (Table 1A).
When protein immunization with E1E2 (40 µg prot + 50 µg CpG) was compared with protein immunization without E1E2 [30 µg prot (no E1E2) + 50 µg CpG], no significant differences were found in humoral responses against NS3 and NS5b. However, in the absence of E1E2 a significantly stronger humoral response against core (310,419 vs. 19,401, P = 0.03) was observed, suggesting that HCV E1E2, an HCV structural protein, might play an inhibitory role in the induction of HCV core-specific humoral response.
When multi-protein immunization (40 µg prot + 50 µg CpG) was compared with single protein immunization (10 µg core + 10 µg CpG, 10 µg E1E2 + 10 µg CpG, 10 µg NS3 + 10 µg CpG or 10 µg NS5b + 10 µg CpG), no significant differences were found in humoral responses against E1E2, NS3 and NS5b. However, HCV core protein immunization (10 µg core + 10 µg CpG) induced a significantly stronger humoral response against core than multi-protein immunization (40 µg prot + 50 µg CpG) (356,578 vs. 19,401, P < 0.01), which also suggests an inhibitory role of HCV E1E2 in the induction of anti-core humoral immune responses.
3.2. Isotype analysis anti-HCV antibodies induced by protein immunization
Eight weeks after protein boost, the titers of anti-HCV core, E1/E2, NS3 and NS5b IgG1 or IgG2a were determined by endpoint dilution assay. The results are expressed as the geometric means and ratios of IgG2a (Th1 type) to IgG1 (Th2 type) are summarized in Table 1B.
Protein immunization with CpG [40 µg prot + 50 µg or 10 µg CpG, 8 µg prot + 10 µg CpG, 30 µg prot (no E1E2) + 50 µg CpG] induced high levels of both IgG1 and IgG2a for core, E1/E2, and NS3 antigens. The IgG2a/IgG1 ratios involving these active CpG formulations ranged from 2.17 to 43.04 for core, 2.46 to 4.59 for E1/E2, 4.18 to 9.79 for NS3 and 2.04 to 6.42 for NS5b antigens (Table 1B), indicating that the predominant IgG isotype was IgG2a and the immune response was Th1-biased for all HCV antigens administered in active CpG formulations.
Protein immunization either without CpG [40 µg prot (no CpG)] or with GpC control (40 µg prot + 50 µg GpC control) induced lower levels of both IgG1 and IgG2a for core, NS3 and NS5b antigens. The IgG2a/IgG1 ratio ranged from 0.26 to 3.02 for core, 0.16 to 0.46 for E1/E2, 0.10 to 0.64 for NS3 and 0.32 to 0.35 for NS5b antigens (Table 1B), indicating that the predominant IgG isotype for these formulations was IgG1 and that the immune response was Th2-biased for all HCV antigens administered without CpG or with GpC control.
3.3. Induction of strong, broad-spectrum HCV-specific CD4+ Th1-type immune responses by protein immunization
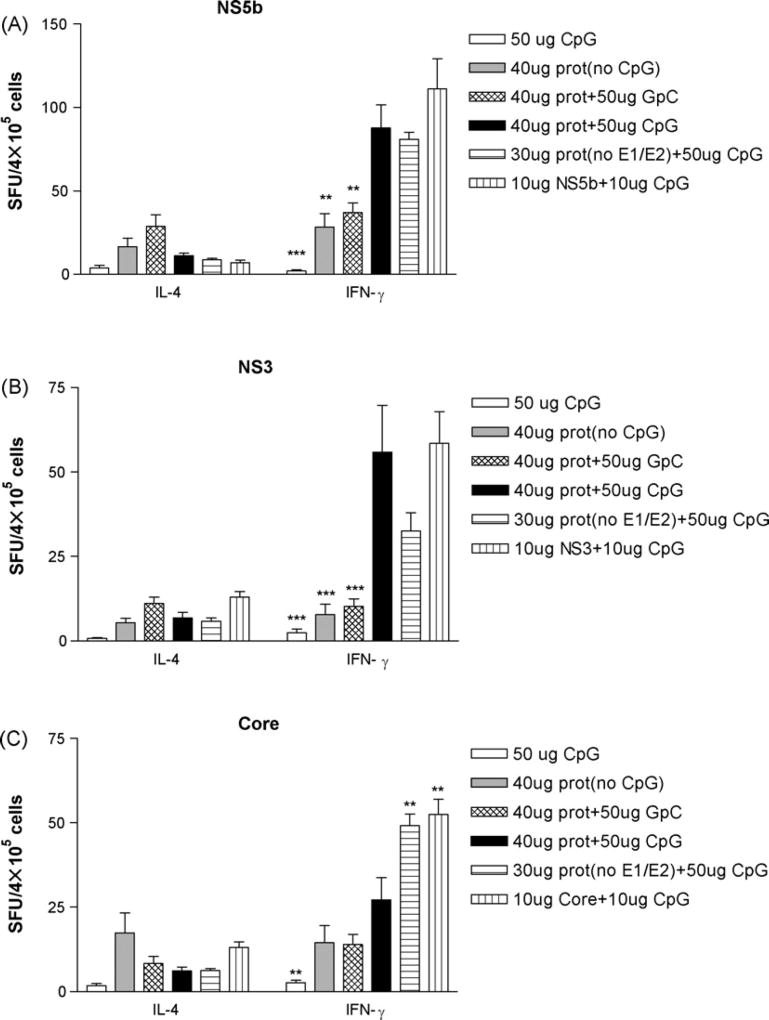
To determine the immune response in immunized mice, spleen cells secreting either Th1 cytokines (IFN-γ), or Th2 type cytokines (IL-4), were detected after stimulation with recombinant HCV structural and nonstructural proteins. The results of the cytokine profile of T helper cells are shown in Fig. 2A–C. The ratios of IFN-γ SFU to IL-4 SFU (IFN-γ/IL-4) are summarized in Tables 2A–2C.
Fig. 2.
Induction of HCV-specific Th1-type immune response by protein immunization. Twelve protein immunization regimes were applied to mice in twelve groups (n = 5, data not shown for 10 µg CpG, 40 µg prot + 10 µg CpG and 8 µg prot + 10 µg CpG). On week 8, mice from all groups were sacrificed and splenocytes were cultured with recombinant HCV NS5b (A), NS3 (B), and core (C) at 6 µg/ml for 40 h. Then 4 × 105 spleen cells in 100 µl volume were added to each well and incubated with antigen for 20 h. ELISPOT assay for IFN-γ and IL-4 were performed as described in detail in Section 2. The number of spot-forming units (SFU) was counted per four hundred thousand cells and the results were expressed as mean ± S.E.M. Comparison of ELISPOT results between two groups of mice was conducted by using ANOVA. Significant P values between groups vaccinated with different regimens are indicated.
Table 2.
| A Induction of HCV NS5b-specific Th1-type immune response as determined by the ratios of IFN-γ SFU to IL-4 SFU in ELISPOT assaya | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Formulation | Number of mice | IFN-γ | IL-4 | IFN-γ/IL-4 | Number of mice with IFN-γ/IL-4 ratio > 1 |
Number of mice with IFN-γ/IL-4 ratio < 1 |
| 40 µg prot no CpG | 5 | 28.25 ± 8.14 | 16.60 ± 5.15 | 2.00 ± 0.48 | 4 | 1 |
| 40 µg prot + 50 µg GpC control | 5 | 37.05 ± 5.84 | 28.80 ± 6.88 | 1.50 ± 0.30 | 3 | 2 |
| 40 µg prot + 50 µg CpG | 5 | 87.85 ± 13.78 | 11.20 ± 1.67 | 8.47 ± 1.77 | 5 | 0 |
| 30 µg prot (no E1E2) + 50 µg CpG | 5 | 80.95 ± 4.19 | 8.85 ± 0.72 | 9.50 ± 1.22 | 5 | 0 |
| 10 µg NS5b + 10 µg CpG | 5 | 111.15 ± 18.22 | 7.10 ± 1.44 | 17.72 ± 3.70 | 5 | 0 |
| B Induction of HCV NS3-specific Th1-type immune response as determined by the ratios of IFN-γ SFU to IL-4 SFU in ELISPOT assaya | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Formulation | Number of mice | IFN-γ | IL-4 | IFN-γ/IL-4 | Number of mice with IFN-γ/IL-4 ratio > 1 |
Number of mice with IFN-γ/IL-4 ratio < 1 |
| 40 µg prot no CpG | 5 | 7.80 ± 3.02 | 5.35 ± 1.29 | 1.26 ± 0.50 | 3 | 2 |
| 40 µg prot + 50 µg GpC control | 5 | 10.25 ± 2.22 | 11.10 ± 1.85 | 0.94 ± 0.13 | 4 | 1 |
| 40 µg prot + 50 µg CpG | 5 | 55.90 ± 13.80 | 6.75 ± 1.63 | 8.56 ± 1.52 | 5 | 0 |
| 30 µg prot (no E1E2) + 50 µg CpG | 5 | 32.55 ± 5.42 | 5.75 ± 1.02 | 5.97 ± 0.99 | 5 | 0 |
| 10 µg NS3 + 10 µg CpG | 5 | 58.50 ± 9.43 | 12.95 ± 1.65 | 4.44 ± 0.23 | 5 | 0 |
| C Induction of HCV core-specific Th1-type immune response as determined by the ratios of IFN-γ SFU to IL-4 SFU in ELISPOT assaya | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Formulation | Number of mice | IFN-γ | IL-4 | IFN-γ/IL-4 | Number of mice with IFN-γ/IL-4 ratio > 1 |
Number of mice with IFN-γ/IL-4 ratio < 1 |
| 40 µg prot no CpG | 5 | 14.45 ± 5.12 | 17.40 ± 5.92 | 1.03 ± 0.35 | 3 | 2 |
| 40 µg prot + 50 µg GpC control | 5 | 13.95 ± 3.03 | 8.35 ± 2.05 | 1.87 ± 0.30 | 5 | 0 |
| 40 µg prot + 50 µg CpG | 5 | 27.20 ± 6.64 | 6.15 ± 1.15 | 5.07 ± 1.82 | 5 | 0 |
| 30 µg prot (no E1E2) + 50 µg CpG | 5 | 49.15 ± 3.47 | 6.20 ± 0.66 | 8.09 ± 0.50 | 5 | 0 |
| 10 µg Core + 10 µg CpG | 5 | 52.45 ± 4.53 | 13.10 ± 1.64 | 4.22 ± 0.48 | 5 | 0 |
The values in the table represent mean±S.E.M. for IFN-γ SFU, IL-4 SFU, and the ratios of IFN-γ SFU to IL-4 SFU (IFN-γ/IL-4) and were calculated using values from all 5 mice.
Protein immunization with CpG [40 µg prot + 50 µg CpG, 30 µg prot (no E1E2) + 50 µg CpG, 10 µg prot (core, NS3 or NS5b) + 10 µg CpG] induced much higher levels of IFN-γ than IL-4 for core, NS3 and NS5b antigens (Fig. 2A–C). The IFN-γ/IL-4 ratio ranged from 4.22 to 8.09 for core, 4.44 to 8.56 for NS3 and 8.47 to 17.72 for NS5b, respectively (Tables 2A–2C), indicating that the immune response was Th1-biased for all the HCV antigens measured.
Protein immunization without CpG [40 µg prot (no CpG)] or with GpC control (40 µg prot + 50 µg GpC control) induced much lower levels of both IFN-γ and IL-4 (Fig. 2A–C). The IFN-γ/IL-4 ratio rangedfrom1.03 to 1.87 for core, 0.94 to 1.26 forNS3and 1.50 to 2.00 for NS5b, respectively (Tables 2A–2C), indicating that the immune responses were only marginally Th1-biased for all the HCV antigens measured.
The number of IFN-γ SFU following protein immunization with CpG (40 µg prot + 50 µg CpG) was significantly higher than that without CpG [40 µg prot (no CpG)] for NS3 (55.90 ± 13.80 vs. 7.80 ± 3.02, P < 0.001) and NS5b (87.85 ± 13.78 vs. 28.25 ± 8.14, P < 0.01), respectively (Fig. 2A–C) and also significantly higher than that of protein immunization with GpC control (Fig. 2A–C), indicating that protein immunization with CpG has the ability to switch the immune response against HCV NS3 and NS5b from the Th2 to the Th1 pathway.
When protein immunization with E1E2 (40 µg prot + 50 µg CpG) was compared with that of without E1E2, no significant differences of the number of IFN-γ SFU were found for NS3 and NS5b. However the number of IFN-γ SFU after protein immunization without E1E2 was significantly higher than that of protein immunization with E1E2 for core (49.15 ± 3.47 vs. 27.20 ± 6.64, P < 0.01), suggesting that HCV E1E2 might play an inhibitory role in the induction of HCV core-specific cellular immune responses (Fig. 2A–C).
When multiple protein immunization (40 µg prot + 50 µg CpG) was compared with single protein immunization (10 µg NS3 + 10 µg CpG or 10 µg NS5b + 10 µg CpG), no significant differences of the number of IFN-γ SFU were found for NS3 and NS5b. However the number of IFN-γ SFU of core protein immunization (10 µg core + 10 µg CpG) was significantly higher than that of multiple protein immunization (40 µg prot + 50 µg CpG) (52.45 ± 4.53 vs. 27.20 ± 6.64, P < 0.01), also suggesting that HCV E1E2 might play an inhibitory role in the induction of HCV core-specific cellular responses (Fig. 2A–C).
4. Discussion
In this report we have demonstrated that vaccination of mice with a single or multiple HCV recombinant protein(s) with CpG ODN + Montanide ISA 720 as adjuvants, elicits robust antigen-specific humoral, and Th1 CD4+ T cell responses, and is superior to the formulation without CpG. Protein vaccination using CpG ODN and Montanide ISA 720 as adjuvants also greatly enhanced humoral responses to HCV core, E1/E2 and NS3. When specific IgG isotypes were assayed, protein immunization produced higher titers of IgG2a dominant antibodies than did protein immunization without CpG, indicating amore Th1-biasedpathway.Moreover, our results also show that this multi-protein immunization with CpG ODN plus Montanide ISA 720 as adjuvants (40 µg prot + 50 µg CpG) induces immune responses to each HCV protein equal to that achieved when these proteins plus adjuvants were given individually.
Several studies in humans have shown that clearance of acute HCV infection is associated with a strong HCV-specific CD4+ T cell response, and that absence or loss of this response was associated with viral persistence or recurrence [13,14]. Analyses of the cytokine profiles of HCV-specific T cells have revealed that persons displaying a Th1 profile (antigen-dependent production of IL-2 and IFN-γ) are more likely to experience viral clearance [13–18]. These results suggest that the intensity of cellular immunity in the early stage of infection is a critical factor in reducing the frequency of chronic infection. Therefore, it is critical that a vaccine strategy induces a strong and broad spectrum of HCV-specific Th1-type immune responses with the robust production of IFN-γ.
The ability to induce strong Th1 immune responses in the current study depends on both the immunomodulatory effects of CpG ODN and the ability of Montanide ISA 720 to co-deliver the antigen and immunomodulator to the same cells. Unlike other commercially available oil adjuvants, Montanide ISA 720 is composed of animal metabolizable oil and thus it is thought to be less reactogenic in humans and when used as the adjuvant in clinical trials of malaria and HIV vaccines has shown promise in promoting T cell responses [22,23]. Montanide ISA 720 exerts adjuvant effects by several mechanisms including the slow release of antigen, recruitment and stimulation of antigen presenting cells, and diffusion of oil droplets to draining lymph nodes [21]. We propose that Montanide ISA 720 can associate with the negatively charged protein antigen through the positively charged polar head of cationic lipids, and subsequently enhance the interaction with the negatively charged cellular membrane. The immunostimulatory activity of CpG ODN requires binding to a cell receptor belonging to the Toll-like receptor family, TLR9 [24], followed by cellular uptake by endocytosis [37]. It has also been reported that CpG can direct tagged antigen specifically to dendritic cells [38]. CpG ODN carries net negative electric charges and has a strong electrostatic attraction to the membrane surface of cationic Montanide ISA 720. This formulation provides a mechanism for rHCV-M720-CpG specifically binding to dendritic cells. CpG ODNs then signal through Toll-like receptor 9 [24], inducing both maturation and activation of dendritic cells (DC), augmenting the production of proinflammatory cytokines [25]. This activation of the innate immune response leads to the induction of specific antibody and Th1 cellular immune responses [20,26–29]. In this study, a strong and optimal HCV-specific humoral and cellular immune responses were elicited by immunization with multiple rHCV protein plus CpG and Montanide ISA 720 as compared to formulations without CpG or with GpC control. These results also indicate a synergistic effect between CpG ODN and M720 in the induction of HCV-specific Th1-biased CD4+ T cell responses.
In this study, protein vaccination using CpG and Montanide as adjuvants also dramatically enhanced humoral responses to recombinant proteins. The ability of CpG ODN to enhance humoral responses has been shown previously in primates [39,40] and humans [41] as well as mice [42], and has been reinforced in this study where vaccination was primarily geared to induce strong T-cell responses. When specific IgG isotypes were assayed, mice immunized with single or multiple HCV recombinant protein(s) with Montanide ISA 720 + CpG produced high titers of IgG2a dominant Ab and a high IgG2a/IgG1 ratio, suggesting a Th1 pathway, while mice immunized with the formulation without CpG produced lowIgG2a and IgG1Aband a lowIgG2a/IgG1 ratio, suggesting a less Th1-biased pathway. Our results also show that multiple protein vaccination with CpG and Montanide as adjuvants leads to IgG2a-dominant humoral immune responses equivalent to that achieved by single protein immunization using the same adjuvants.
Our data also revealed that HCV E1E2, an HCV structural protein, might play an inhibitory role in the induction of HCV core-specific humoral and cellular response. When protein immunization with E1E2 (40 µg prot + 50 µg CpG) was compared with protein immunization without E1E2, no significant differences were found in humoral or cellular immune responses against NS3 and NS5b; however, a significantly stronger humoral and cellular immune response against HCV core was observed in the absence of E1E2 (P < 0.05), suggesting that HCV E1E2, envelope proteins, might play an inhibitory role in the induction of HCV core-specific humoral and cellular immune responses. It has been also reported that E1 envelope protein can be precipitated by anti-core antibody in the presence of core proteins suggesting that the E1 envelope protein physically interacts with the core protein [43].
In summary, our studies have shown that protein vaccination using CpG and Montanide as adjuvants is superior to protein immunization without CpG and that this combination can induce broad and vigorous HCV-specific humoral and cellular (Th1 type)immune responses in BALB/c mice. The ability to switch the immune response pathway depends on both the immunomodulatory effects of CpG oligonuleotides and the ability of Montanide ISA 720 to co-deliver the antigen and immunomodulator to the same cells. This study expands our earlier work to examine the effect of CpG and cationic lipid formulation on the immunogenecity of recombinant HCV antigens. Immune modulation through multi-antigen vaccination combined with protein plus adjuvants could be utilized either for primary immunization in a prophylactic HCV strategy or for secondary immunization in the treatment of chronic HCV infection in combination with anti-viral therapy. A study of the efficacy of this vaccine strategy in primates is now required to test the validity of these encouraging results observed in the murine model.
References
- 1.Cohen J. The scientific challenge of hepatitis C. Science. 1999;285(5424):26–30. doi: 10.1126/science.285.5424.26. [DOI] [PubMed] [Google Scholar]
- 2.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345(1):41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 3.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20(1):17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Rehermann B. Cellular immune response to the hepatitis C virus. J Viral Hepat. 1999;6(Suppl. 1):31–5. doi: 10.1046/j.1365-2893.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiner AJ, Geysen HM, Christopherson C, Hall JE, Mason TJ, Saracco G, et al. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci USA. 1992;89(8):3468–72. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, et al. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci USA. 2003;100(24):14199–204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101(27):10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu MY, Bartosch B, Zhang P, Guo ZP, Renzi PM, Shen LM, et al. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc Natl Acad Sci USA. 2004;101(20):7705–10. doi: 10.1073/pnas.0402458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 12.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102(26):9294–9. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194(10):1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117(4):933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 15.Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, et al. Possible mechanism involving T-lymphocyte response to nonstructural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346(8981):1006–7. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 16.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6(5):578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 17.Racanelli V, Rehermann B. Hepatitis C virus infection: when silence is deception. Trends Immunol. 2003;24(8):456–64. doi: 10.1016/s1471-4906(03)00178-9. [DOI] [PubMed] [Google Scholar]
- 18.Kamal SM, Rasenack JW, Bianchi L, Al Tawil A, El Sayed KK, Peter T, et al. Acute hepatitis C without and with schistosomiasis: correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology. 2001;121(3):646–56. doi: 10.1053/gast.2001.27024. [DOI] [PubMed] [Google Scholar]
- 19.Woitas RP, Lechmann M, Jung G, Kaiser R, Sauerbruch T, Spengler U. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J Immunol. 1997;159(2):1012–8. [PubMed] [Google Scholar]
- 20.Jiao X, Wang RY, Qiu Q, Alter HJ, Shih JW. Enhanced hepatitis C virus NS3 specific Th1 immune responses induced by co-delivery of protein antigen and CpG with cationic liposomes. J Gen Virol. 2004;85(Pt 6):1545–53. doi: 10.1099/vir.0.79896-0. [DOI] [PubMed] [Google Scholar]
- 21.Aucouturier J, Dupuis L, Deville S, Ascarateil S, Ganne V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–8. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 22.Toledo H, Baly A, Castro O, Resik S, Laferte J, Rolo F, et al. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine. 2001;19(30):4328–36. doi: 10.1016/s0264-410x(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 23.Saul A, Lawrence G, Smillie A, Rzepczyk CM, Reed C, Taylor D, et al. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine. 1999;17(23–24):3145–59. doi: 10.1016/s0264-410x(99)00175-9. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Sparwasser T, Vabulas RM, Villmow B, Lipford GB, Wagner H. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur J Immunol. 2000;30(12):3591–7. doi: 10.1002/1521-4141(200012)30:12<3591::AID-IMMU3591>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34(2):417–23. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Babiuk LA, van Drunen Littel-van den Hurk Priming with CpG-enriched plasmid and boosting with protein formulated with CpG oligodeoxynucleotides and Quil A induces strong cellular and humoral immune responses to hepatitis C virus NS3. J Gen Virol. 2004;85(Pt 6):1533–43. doi: 10.1099/vir.0.79821-0. [DOI] [PubMed] [Google Scholar]
- 28.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186(10):1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J Exp Med. 2002;195(12):1565–73. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Berkower I, Ching WM, Wang RY, Alter HJ, Shih JW. Identification of a murine CD4+ T-lymphocyte response site in hepatitis C virus core protein. Mol Immunol. 1996;33(7–8):703–9. doi: 10.1016/0161-5890(96)00010-7. [DOI] [PubMed] [Google Scholar]
- 31.Hu GJ, Wang RY, Han DS, Alter HJ, Shih JW. Characterization of the humoral and cellular immune responses against hepatitis C virus core induced by DNA-based immunization. Vaccine. 1999;17(23–24):3160–70. doi: 10.1016/s0264-410x(99)00130-9. [DOI] [PubMed] [Google Scholar]
- 32.Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67(8):4566–79. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into virus like particles in insect cells. J Virol. 1998;72(5):3827–36. doi: 10.1128/jvi.72.5.3827-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao X, Wang RY, Feng Z, Hu G, Alter HJ, Shih K. DNA immunization encoding the secreted nonstructural protein 3 (NS3) of hepatitis C virus and enhancing the Th1 type immune response. J Viral Hepat. 2004;11(1):18–26. doi: 10.1046/j.1352-0504.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiao X, Wang RY, Feng Z, Alter HJ, Shih JW. Modulation of cellular immune response against hepatitis C virus nonstructural protein 3 by cationic liposome encapsulated DNA immunization. Hepatology. 2003;37(2):452–60. doi: 10.1053/jhep.2003.50051. [DOI] [PubMed] [Google Scholar]
- 36.Patel AH, Wood J, Penin F, Dubuisson J, McKeating JA. Construction and characterization of chimeric hepatitis C virus E2 glycoproteins: analysis of regions critical for glycoprotein aggregation and CD81 binding. J Gen Virol. 2000;81(Pt 12):2873–83. doi: 10.1099/0022-1317-81-12-2873. [DOI] [PubMed] [Google Scholar]
- 37.Krieg AM. Signal transduction induced by immunostimulatory CpG DNA. Springer Semin Immunopathol. 2000;22(1–2):97–105. doi: 10.1007/s002810050019. [DOI] [PubMed] [Google Scholar]
- 38.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167(1):66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164(3):1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 40.Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002;168(4):1659–63. doi: 10.4049/jimmunol.168.4.1659. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG-DNA motif in human primary B cells. J Immunol. 2000;164(2):944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 42.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160(2):870–6. [PubMed] [Google Scholar]
- 43.Lo SY, Selby MJ, Ou JH. Interaction between hepatitis C virus core protein and E1 envelope protein. J Virol. 1996;70(8):5177–82. doi: 10.1128/jvi.70.8.5177-5182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]