Abstract
Treatment and management of kidney disease currently presents an enormous global burden, and the application of nanotechnology principles to renal disease therapy, although still at an early stage, has profound transformative potential. The increasing translation of nanomedicines to the clinic, alongside research efforts in tissue regeneration and organ-on-a-chip investigations, are likely to provide novel solutions to treat kidney diseases. Our understanding of renal anatomy and of how the biological and physicochemical properties of nanomedicines (the combination of a nanocarrier and a drug) influence their interactions with renal tissues has improved dramatically. Tailoring of nanomedicines in terms of kidney retention and binding to key membranes and cell populations associated with renal diseases is now possible and greatly enhances their localization, tolerability, and efficacy. This Review outlines nanomedicine characteristics central to improved targeting of renal cells and highlights the prospects, challenges, and opportunities of nanotechnology-mediated therapies for renal diseases.
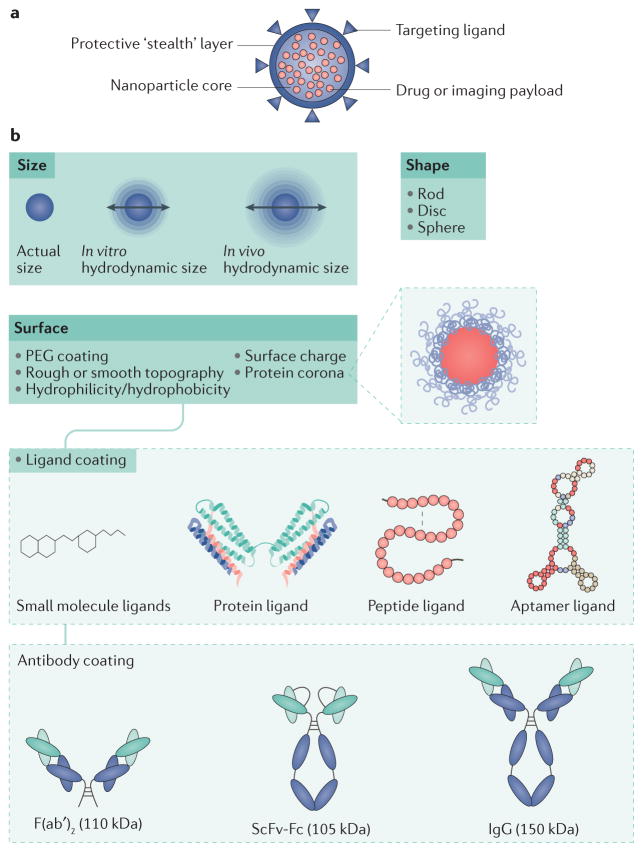
Nanotechnology involves the engineering of atoms and molecules at the submicron scale. The unique optical, electrical, physical, and chemical properties of matter at this scale can yield materials that behave differently from their macroscale counterparts. Inorganic or organic multifunctional nanocarriers, which we refer to as nanoparticles (NPs), can be engineered to deliver drugs or imaging agents with unique characteristics. For example, NP delivery improves pharmacokinetics and biodistribution and enables targeted delivery of drugs to specific tissues, cells, or subcellular compartments. Additionally, achieving spatiotemporal, triggered, and controlled release of a variety of payloads (such as small-molecule drugs, contrast agents, peptides, proteins, deoxy and ribonucleic acids, and their combinations) over extended periods might be possible. Nanomedicines are typically degradable, biodegradable, and bioeliminable structures generally <150 nm in size that can incorporate the aforementioned payloads1–5. Physicochemical properties of nanomedicines such as composition, size, geometry and/or shape, surface charge, surface chemistry (including targeting ligands), hydrophobicity, roughness, rigidity, and elasticity influence their uptake and/or ability to target particular organs and cells6–13 (FIG. 1).
Figure 1. Nanoparticle composition and features.
a | Nanoparticles are composed of a core (payload) encapsulated in a protective layer. The surface can be modified to limit the interactions of the particle with its environment. b | Several parameters such as size, shape and surface modification contribute to the biological and physicochemical properties of nanoparticles. The investigation of such properties is crucial to achieve the translational application of nanomedicines for kidney disease therapy. PEG, polyethylene glycol; ScFv Fc, single chain variable fragment constant fragment.
In the 1970s, colloid-based drug delivery agents designed to improve drug therapeutic efficacy and reduce toxicity were termed NPs14. The term ‘nanoparticle’ is mentioned in over 135,000 publications, and the number of publications on this topic has doubled every other year between 2000 and 2014 (PubMed). Though this rapid growth seems to be slowing, the absolute number of articles published on the topic remains impressive at ~15,000 per year. Monoclonal antibodies took decades to gain significant momentum; similarly, the broad impact of nanotechnology-based therapeutics will become more apparent as these technologies mature15. Exciting developments in NP design, such as the generation of building blocks that release drugs in response to stimuli from the disease environment (such as increased enzymatic activity, the presence of reactive oxygen species or lowered pH) or to externally delivered stimuli (such as heat or ultrasound), are laying the foundations for ‘prompted’ and ‘modular’ drug delivery16–23. Additionally, the ability to co-deliver synergistic therapeutics will further enlarge the arsenal of nanomedicines for the treatment of a variety of diseases and facilitate important combination therapies24,25.
Nanomedicine-based genetic therapies directed to the liver, an organ that filters significant volumes of blood, have now been successfully developed26,27. Such established design principles can also be used to create NPs that can bind to, be taken up by and retained by diseased renal cells. Numerous in vivo NP studies initially designed to target tumours have resulted in serendipitous discoveries of principles underlying kidney targeting and selective accumulation. Such findings raised important ‘structure–activity’ correlations, which can be used to influence NP retention and clearance from the general circulation within kidneys, and these insights could be applied to overcome challenges in the generation of kidney targeted nanomedicines.
In this Review, we discuss the application of nanotechnology to diseases of the kidney. We introduce fundamental concepts of nanomedicine, examine renal anatomy and selective permeability in health and disease, and highlight nanotechnology research aimed at targeting the major renal membranes and cell types involved in disease. Furthermore, we discuss microfluidic- based kidney models and artificial organ-on-a-chip approaches to investigate synthesis, screening and testing of NPs for renal disease therapy. Finally, we examine challenges in the translation of nanomedicines to the clinic.
Nanomedicines
Structure
Depending on the method of preparation or the chemical nature of the building blocks used, NPs can have either a solid or hollow aqueous core (FIG. 1a). Their surface layer can be coated with inert polymers that can shield the NP from blood components. To date, polyethers such as polyethylene glycol (PEG) have been mostly used for this purpose. A variety of payloads can be incorporated within the core or surface layers of the NPs: hydrophilic or hydrophobic small-molecule drugs, proteins, peptides, nucleic acids, or imaging agents. The surface of the NPs can be functionalized with ligands targeted to specific receptors or proteins of interest to achieve ‘active targeting’. Targeting ligands can be antibodies, antibody fragments, antibody mimetics, proteins, peptides, nucleic acids (such as aptamers) and small-molecule ligands (FIG. 1b). Physical characteristics such as NP size can also be used to achieve ‘passive targeting’ and enhanced accumulation in tumours (FIG. 1b). Liposomes were the first nanomedicines to enter the clinic and be marketed, and therefore represent a large portion of clinical- stage nanomedicines. They were followed by polymer–drug conjugates, polymeric micelles, polymeric NPs, protein-based NPs and dendrimers (TABLE 1)15,28.
Table 1.
Clinical use of nanoparticles
| Nanoplatform | Composition | Size | Examples of nanoparticles applied to the clinic | Disease indication | Active pharmaceutical ingredient | Clinical status in 2016 | Refs |
|---|---|---|---|---|---|---|---|
Liposomes
|
Spherical lipid bilayer with an aqueous core | ~100–200nm | Non targeted: Doxil | HIV-related Kaposi sarcoma, ovarian cancer, multiple myeloma | Doxorubicin | Approved | 29–45 |
| Targeted: MM 302 | Her2+ breast cancer | Doxorubicin | Phase II | 63 | |||
| Non targeted, stimuli responsive: ThermoDox | Hepatocellular carcinoma | Doxorubicin | Phase III | 300 | |||
| Non targeted, combination therapy: CPX 351 | Acute myeloid leukemia | Cytarabine and daunorubicin (5:1) | Phase III | 66 | |||
Polymer–drug conjugates
|
Nanosized colloids generated by covalent conjugation of drugs to pendant groups on polymer backbones | ~5–20 nm | Non targeted: Zinostatin | Liver cancer | Styrene maleic anhydride | Approved | 61–62 |
Polymeric micelles
|
Lipids or polymers with a hydrophilic– hydrophobic core– shell structure | ~10–100 nm | Non targeted: Genexol PM | Breast cancer, non-small-cell lung cancer | Paclitaxel | Approved | 52–53 |
Polymeric
|
Solid core, formed by the self-assembly of degradable polymers | ~50–100 nm | Non targeted: Livatag | Liver cancer | Doxorubicin | Phase III | 27 |
| Targeted: BIND 014 | Active targeted delivery to non-small-cell lung cancer, metastatic castration resistant prostate cancer, other metastatic cancers and solid tumours | Docetaxel | Phase II | 64 | |||
Protein-based
|
Albumin-based delivery platform | ~130 nm | Non targeted: Abraxane (Nab paclitaxel) | Breast, lung, and pancreatic cancer | Paclitaxel | Approved | 46–51 |
Dendrimers
|
Symmetrically branched polymeric macromolecules synthesized using polyamides | <10 nm | Non targeted: Vivagel | HIV infection | NA (anionic G4-poly(L-lysine)- type dendrimer displaying 32 napthalene disulfonate surface groups) | Phase III | 67–69 |
Gold-based
|
Spherical, rod or shell based colloids made from the reduction of HAuCl4 | ~27 nm | Non targeted: CYT-6091 | Solid tumours | Tumour necrosis factor | Phase I | 70–72, 80 |
Silica based
|
SiO2 NPs | 20–200 nm | Non targeted: AuroLase (silica core with a gold nanoshell) | Head and neck cancer, lung cancer | NA (laser ablation) | Pilot study | 74,80, 81 |
Iron oxide based
|
Crystalline nanoparticles of Fe3O4/γ-Fe2O3 | 10–20 nm | Non targeted: Nanotherm | Glioblastoma | NA (heat) | Approved | 77 |
Hafnium oxide based
|
HfO2 NPs | ~50 nm | Non targeted: NBTXR3 | Adult soft tissue sarcoma, head and neck cancer | NA (Radiotherapy) | Phase I | 78,79 |
Viral
|
Viral and virus-like nanoparticles | 20–50 nm | Non targeted: Oncolytic poxvirus JX-594 | Stimulation of anti-tumour immune response | Granulocyte colony-stimulating factor | Phase I, Phase II | 92,93, 94 |
Exosomes
|
Naturally secreted cell-derived vesicles | 30–100 nm | Targeted or non targeted: various biologically derived nanosized exosomes | Immunotherapy of melanoma, colon cancer, diabetes, wound-healing, oral mucositis, gastric cancer (biomarker), oropharyngeal cancer (biomarker), thyroid cancer (biomarker) | Various biological payloads | Phase 0, Phase I, Phase II | 95–98, 301 |
Carbon-based
|
Polycyclic aromatic hydrocarbons | >1 μm | Targeted or non targeted: Nanospheres, nanotubes, nanosheets | Breast cancer, lung cancer, other solid tumours | Various poorly soluble therapeutics in high concentrations | Preclinical | 99–103 |
Current therapeutic uses
The majority of nanomedicines generated so far have been developed for cancer therapy, as compromised endothelial barriers in tumours facilitate NP retention (discussed below). Over a dozen NP platforms based on liposomes29–45, albumin46–51, polymeric micelles52–58, and nanosized polymer-drug conjugates59–62 have been approved by the FDA (TABLE 1). A few targeted NPs including Her2 scFv-targeted liposomes (MM-302)63, the first targeted controlled-release polymeric NP BIND-014 (REF. 64), and the first targeted short interfering (si)RNA NP CALAA-01 (REF. 65) are currently being tested in clinical trials for cancer therapy. Although many nanomedicines improve the pharmacokinetics and biodistribution of drugs, so far, only one nanomedicine, CPX351, has increased survival in patients with cancer when directly compared with the conventional parent drug66. These findings underscore the need to rethink strategies for the development of NPs, including potential patient selection to identify those most likely to respond to nanomedicines.
Nanotechnology-enabled diagnostic and therapeutics based on dendrimers67–69, gold70–72, silica73–75, iron oxide71,76,77 and hafnium oxide78,79 are also currently under clinical investigation, with the majority intended for oncological applications (TABLE 1). For example gold NPs decorated with a surface of PEG conjugated to TNFα molecules are in phase I/II trials for solid tumour therapy80. Silica NPs in combination with gold are being tested in thermal ablation therapy of head and neck cancers81. Two MRI contrast agent iron-oxide NPs (Ferumoxtran-10® and Ferumoxytol®) are in clinical trials for cancer imaging82,83. A therapeutic version of iron oxide NPs termed NanoTherm® was approved in Europe in 2010 for the thermal ablation of glioblastomas (magnetic hyperthermia treatment)84. Clinical trials have begun for the investigation of hafnium oxide NPs (phase I), which will be used as radiosensitizers in patients with soft-tissue sarcomas78. Nanomedicines are also undergoing clinical translation for gene therapy85, RNA interference26,65,86–88, and immunotherapy89,90. Viral NPs have found utility in the delivery of a range of therapeutics and have been clinically validated for gene therapy applications91,92,93. For example, the tumour-homing oncolytic pox virus was engineered to generate JX-594, a particle that expresses granulocyte colony-stimulating factor (G-CSF) to potentially increase the immunological antitumour response94. Exosomes are being explored as targeted vectors in drug delivery, where their signalling properties can be exploited to manipulate cellular proteomic functions beyond their current applications in kidney disease biomarker discovery95–98. Other novel nanomaterials including nanodiamonds99–101 and nanographene102,103 are receiving attention in drug- delivery applications: the adsorption of hydrophobic drugs to these surfaces facilitates the delivery of poorly soluble therapeutics in high concentrations.
Developments in nanomedicine have facilitated investigations into the delivery of multiple synergistic active pharmaceutical ingredients25,104,105. For example, the liposomal formulation of cytarabine and dauno-rubicin CPX-351 is under development for high-risk acute myeloid leukemia66. Design advances have also produced nanomedicines that can simultaneously deliver diagnostic and therapeutic agents, termed theranostics106. For example, liposomes containing an MRI contrast agent and an anticancer siRNA payload can be used to dynamically monitor and cause tumour regression107. Nanotheranostic agents that use near infrared absorbing agents to convert optical energy into heat for ablating tumour cells have also been developed and are used for image-guided photothermal therapy with inorganic NPs to treat tumour metastases108–110.
The versatility of NPs (antigens, adjuvants and targeting moieties can be incorporated into single NPs) has also led to their utilization as synthetic vaccines111,112. NPs can accumulate in lymph nodes, target antigen- presenting cells, and co-deliver antigens and/or adjuvants that can trigger impressive cellular and/or humoral responses111–117. The co-delivery of an antigen with a TLR7, TLR8 or TLR9 agonist in polymeric NPs strongly increased humoral and cellular immune responses with minimal systemic production of inflammatory cytokines in mice111,112. This strategy enables the use of TLR agonists as vaccine adjuvants for indications where cellular immunity or a robust humoral response is required.
To date, the vast majority of renal drug delivery studies have mostly investigated the applications of nanosized polymer–drug conjugates, liposomes118–131, or inorganic nanodiagnostic compounds to study renal toxicity132–140. Although organ biodistribution profiles of numerous NPs have been evaluated for other indications than renal diseases (such as tumour therapy), they are quite informative regarding NP structure–activity relationships (concerning NP size and charge) in the kidneys. In glomerular nephropathies, NPs accumulate in the glomeruli, perhaps due to increased vascular permeability and inflammation127,130,141,142. Despite these findings, the kidney has largely been overlooked as a target organ for NP-mediated drug delivery. Few systematic studies of NP glomerular deposition in various kidney diseases and disease stages have been conducted with nanomedicines; however, the ‘tuneable’ properties of NPs and their inherent tendency for glomerular deposition might be exploited for the treatment of kidney diseases.
NPs and systemic circulation
Nanomedicines are currently typically administered via intravenous injection, although research on their oral delivery is under way (with the promise of greater patient compliance)143–147. The biological and physicochemical properties of NPs (FIG. 1b) influence their interactions with biological surroundings and affect their in vivo circulation and biodistribution148,149.
The protein corona
Blood contains ~3,700 distinct proteins, and a few tens of them can adsorb to NP surfaces, forming a ‘protein corona’ (REFS 10,150–156). Adsorption of some plasma proteins (such as immunoglobulins, complement proteins, and fibrinogen) can facilitate clearance by the mononuclear phagocyte system157 and limit NP circulation; conversely, binding of dysopsonin proteins (such as apolipoproteins and albumin) might prolong NP presence in the systemic circulation154–156. Thus, the impetus towards understanding NP interactions with their surroundings in vivo is strong as the protein corona can bestow a new biological identity to NPs158,159.
Surface modifications
To increase NP circulation time, PEGylation of the NP surface to minimize protein binding and render it hydrophilic has been routine practice since the mid-1970s160. Doxil, the first FDA-approved PEGylated ‘stealth’ liposome formulation, has a circulation half-life of up to ~2 days45. Further investigation of the function of the protein corona, however, yielded contradictory results. For example, a 2016 study showed that PEGylated polystyrene NPs are highly prone to selective adsorption of clusterin (a 80 kDa chaperone protein), which reduced their nonspecific macrophage uptake in vitro161. These findings and other results suggest that the stealth effects of PEGylation could be mediated by changes in the nature of the protein corona161,162. Formation of the protein corona can also reduce the efficacy of the targeting ligands coupled to NPs162 or, in some instances, promote organ-specific accumulation, as in the case of apolipoprotein-E-mediated targeting of NPs to hepatocytes in vivo163. Predicting such in vivo responses will require careful analytical techniques and protein fingerprinting to better understand how the NP corona influences the pharmacokinetics, biodistribution, intrarenal biodistribution, and therapeutic efficacy of NPs164–167. In addition, a few animal and human studies have shown potential PEG immunogenicity (presence of anti-PEG antibodies) leading to rapid clearance of PEGylated NPs after repeated administrations168–171. The majority of these studies are based on liposomal NPs and further studies are needed to ascertain the exact immunogenic mechanisms associated with PEGylated NPs.
Pharmacokinetics
Numerous studies in tumour therapy show that NPs can enhance drug delivery, and improve drug Cmax, pharmacokinetics and plasma area under the curve (AUC; a measure of total drug exposure over time), compared to the standard dose and formulation172–175. Hence, NP versus drug pharmacokinetics; encapsulated versus free circulating drugs; drug versus NP Cmax; drug versus NP AUC; and parameters that can further affect plasma versus kidney pharmacokinetics and AUC are important factors to consider for the development of effective nanomedicines for the kidney. The infusion time of drugs that are administered intravenously can vary and drugs usually reach their plasma Cmax during the period of infusion, after which the concentration of the drug diminishes176,177. With NP-encapsulated drugs, the plasma concentration of the released drug is initially low and increases progressively to reach its Cmax at a slower rate than that of the conventional drug64. Of note, the Cmax after drug release from NPs rarely reaches the levels achieved with intravenous injection of the free drug. This kinetic is a major advantage of nanomedicines, as it could reduce drug-associated toxicity, which depends on Cmax64. The free drug and the NP-released drug have similar plasma AUCs, although that of the released drug is generally broad and flat, whereas that of the free drug peaks and has a tail64. These findings imply that NPs might reduce Cmax-associated toxicity, but not AUC-related toxicity, as the total dose of the drug is still released by NPs, albeit at a reduced rate.
Renal anatomy and selective permeability
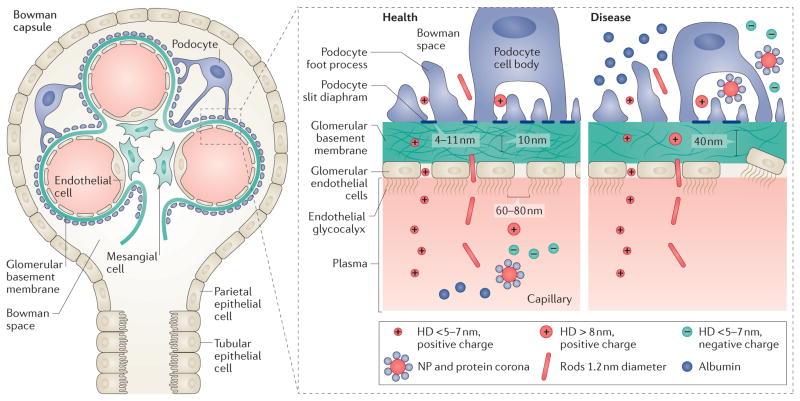
The adult human kidney can contain 1–2.5 million nephrons, which maintain fluid homeostasis, osmoregulation, and waste filtration178. A single nephron consists of two filtration units: the glomerulus and a hairpin-shaped tubule composed of a proximal tubule, the loop of Henle, a distal tubule, and collecting duct179. The glomerulus comprises glomerular endothelial cells (GECs)180, a glomerular basement membrane (GBM)181, podocytes182, mesangial cells183, and parietal epithelial cells184 (FIG. 2).
Figure 2. The kidney glomerulus and the glomerular basement membrane in health and disease.
The glomerular filtration barrier consists of glomerular endothelial cells, the glomerular basement membrane, and podocytes. All solutes and molecules with a molecular weight less than that of albumin (68 kDa) and a hydrodynamic diameter (HD) <5–7 nm can pass this barrier. The glomerular filtration barrier is negatively charged and in healthy states repels negatively charged proteins (such as albumin) and nanoparticles (NPs). In disease, podocyte effacement leads to the breakdown of the barrier and proteinuria. The presence of leaky and abnormal fenestrae can aid the accumulation of large and/or charged nanodrugs in the Bowman space.
Three distinct glomerular filtration barriers separate the vasculature from the urinary spaces and selectively filter blood molecules and ions, yielding a primary urinary filtrate: GECs, the GBM and podocytes179. In the nephrons, the renal tubules and collecting ducts concentrate and balance the urinary filtrate via resorption and secretion of ions, water and certain nutrients through channels and transporters185. Under normal conditions, blood filtration by size and charge by the glomerular barrier ensures that only water and small solutes (urea, glucose, amino acids, and mineral ions) from the plasma cross to the urine179. High molecular-weight plasma components, such as erythrocytes, and negatively charged components, such as albumin, are retained in the blood179. Electrostatic repulsion is thus a predominant filtration parameter, as the glomerular filtration barrier strongly favours the passage of anionic molecules, as shown by the glomerular permeability to larger neutrally charged dextran polymers186. The impairment of this barrier leads to proteinuria, a hallmark of glomerular diseases187.
To escape the general circulation, a NP must cross the glomerular filtration barrier (FIG. 2). Structural features of the glomerular barrier in the setting of renal disease, in particular enlarged endothelial gaps (detailed below), can be exploited for NP delivery to various kidney cells and components.
NP size
NP size has been widely explored to design effective nanomedicines. Most therapeutic NPs are 30–150 nm and are not subject to kidney filtration into the urine, unless they are degraded into particles <10 nm, or the glomerular filtration barrier is damaged by disease (FIG. 2).
In healthy states, colloids and particles with a hydrodynamic diameter up to 5–7 nm fall below the kidney filtration threshold, pass through the glomerulus and are excreted188. NPs below 5.5 nm can be excreted via renal clearance, with an efficiency of >50% of the injected dose 4 h after administration188,189. Of note, some deviations from this threshold have been observed for 12–16 nm inorganic NPs, suggesting that case-by-case investigation of the renal clearance of small-sized NPs is important, as each type of NP has a distinct chemical composition and size-distribution profile133.
Kidney accumulation studies based on size have shown that NP accumulation is restricted to the glomerular mesangium (driven by mesangial cell uptake) and the kidney extracellular matrix, with maximal glomerular deposition for ~80 nm NPs190. One caveat of these studies is that most have been conducted in healthy animals, in which the renal filtration barriers are intact. Considering the influence of NP size on the biodistribution and clearance of nanomedicines, and on size-dependent renal transport, is therefore a crucial parameter for renal nanomedicines.
NP shape
The transport of NPs in the circulation is influenced to a greater degree by the applied convective forces in blood than by Brownian motion. Therefore, shape has an important effect on in vivo performance and biodistribution of NPs, which exist in a wide range of geometries191. For example, a top-down fabrication method termed particle replication in non-wetting templates (PRINT) utilizes lithography techniques to create polymeric NPs of a wide variety of geometries, shapes, and aspect ratios192,193. Cylindrical and discoidal shapes are uniquely subject to blood flow (they have high aspect ratios and minimal regions of curvature), which affects their interaction with macrophages and cell membranes as particles with reduced curvature undergo faster internalization upon incubation with macrophages194,195. Similarly, nanoworm or nanorod (elongated cylindrical) structures show greater tumour accumulation than other shapes196,197. Non-spherical NPs marginate to vessel walls more efficiently than spherical NPs, although they are also more rapidly cleared depending on their aspect ratio and dimension195,198,199. In general, shapes that can accommodate cellular membrane wrapping processes are optimal for cellular internalization200,201.
Interestingly, the kidney can also filter rigid NPs with high aspect ratios but with diameters smaller than the kidney filtration threshold, such as single-walled carbon nanotubes (SWCNTs) with a rod length of 100–1000 nm and diameter of 0.8–1.2 nm199. Even though SWCNTs have higher molecular weights (300–500 kDa) than plasma proteins (30–50 kDa), they are cleared by the kidney, suggesting that aspect ratio influences directional diffusion199. Similar NPs successfully delivered therapeutic siRNA to proximal tubule cells; fibrillar carbon nanotubes delivered two synergistic siRNA payloads targeting Mep1b and Trp53, which reduced the expression of key proteins involved in cisplatin-induced acute kidney injury202. One mechanism proposed to underlie this phenomenon is that hydrostatic forces orient carbon nanotubes perpendicularly to the GBM, enabling the insertion of their <10 nm axis. This shape might be optimal for targeting podocytes, which are difficult to access as they are protected by the fenestrated endothelial cells and the GBM199,203 (FIG. 2).
NP charge
Highly positively charged NPs are rapidly cleared from the circulation by cells of the mononuclear phagocyte system owing to increased protein binding or high affinity interactions with phagocytic cells6,204. Negatively charged NPs can also be more subject to selective cellular uptake (by macrophages, for example) than NPs with neutral surfaces205. NPs with a surface charge of <15 mV had minimal macrophage uptake and long circulation times206. Anionic NPs (surface charge of −10.6 mV) had lower levels of liver and spleen accumulation than near-neutral polymeric micelles (1.3mV)207. Cationically charged NPs undergo high non-specific uptake in a variety of cells (cancer cells and macrophages), and can also achieve endosomal release194. This property is particularly important for the effective delivery of payloads for RNA interference (RNAi), which requires cytosolic delivery for efficacy10,208–210.
Considerations regarding NP charge should also include the charge contribution of targeting ligands that are grafted on NP surfaces for retention in target tissue and/or receptor-mediated endocytosis, leading to increased intracellular drug concentrations1,12,15,211 (FIG. 1). Charge selectivity is also an important criterion for kidney filtration. The suborgan distribution of intravenously administered ultrasmall anionic NPs (3.7 nm quantum dots) at the level of kidney cells was investigated using fluorescence imaging212. The majority of NPs were initially distributed within the peritubular capillaries or the glomerular arterioles, passed through the fenestrated glomerular endothelium, and were gradually taken up by the mesangial cells for up to 30 days212. Interestingly, only trace amounts of quantum dots could be detected in the urine. As the negative charge of the glomerular basement membrane prevents the filtration of anionic quantum dots, cationically charged quantum dots of similar size were found in much higher concentrations in the urine212. These studies provide a framework for understanding charge principles across kidney filtration barriers, which are predominantly negatively charged, in particular as applied to the ultrasmall inorganic NPs often used in imaging applications.
Targeting renal cells
The glomerular endothelial cell barrier
Blood capillaries in the kidneys are lined with GECs, which form the first part of the glomerular filtration barrier. They are characterized by fenestrations and transcellular holes (60–80 nm) filled with an endothelial glycocalyx. The glycocalyx (composed of podocalyxin, sialoglycoprotein, and podoplanin)123 is a polysaccharide-rich >400 nm layer present on 30–40% of GEC surfaces180,213. This layer mechanically limits the access of circulating plasma components to endothelial cell membranes via a filamentous structure and strong negative charge214. The proteoglycan structure effectively makes the fenestrae narrow and restrictive, preventing passage of albumin from plasma, but facilitating water permeability215.
The GECs and endothelial surface layer are important components of the glomerular filtration barrier. Impairment of the endothelial surface layer leads to albuminuria and vascular disease123, and damage to this layer can lead to scarring and glomerulosclerosis123. Preclinical and clinical studies have shown that damage to the endothelial surface layer strongly correlates with diabetes-mellitus-induced proteinuria216,217.
Lipidoid siRNA NPs effectively silenced multiple endothelial genes in the heart, lung, and kidney in vivo with minimal effects on gene expression in hepatocytes or immune cells218. Similar strategies can be used to deliver specific antisense219,220, siRNA26,65,86,87, mRNA88, or DNA inhibitor oligonucleotides85 to damaged GECs for repair and restoration of function. In a mouse model of glomerulonephritis, liposomes coated with an antibody directed against E-selectin, which is specifically expressed on activated endothelial cells during inflammation, were used to successfully deliver dexamethasone to CD31+ GECs, which reduced glomerular endothelial activation and albuminuria after 7 days221. Such studies demonstrate the potential of using disease specific epitopes on the surface of GECs for targeted drug delivery. This approach is particularly beneficial as standard anti-glomerulonephritis therapies consist of glucocorticoids and cytotoxic drugs with systemic adverse effects.
The glomerular basement membrane
The GBM is an anatomic barrier of connective tissue composed of collagen IV, laminin, and proteoglycans (mostly agrin); these proteins are secreted by the GECs and podocytes222. The GBM is an integral component of the glomerular filtration barrier and acts as an intermediary sieving matrix, but can also accumulate pro-angiogenic ligands and secreted factors that mediate cellular communication between podocytes and GECs. Agrin, a major component of the heparin sulfate proteoglycan, has a high negative charge owing to core proteins such as syndecan, glypican, or biglycan with sulfated glycosaminoglycan side chains, which contribute to the net negative charge of the GBM180,181. The charge difference between the GBM and the blood has been proposed to be the main reason for the repulsion of plasma albumin (68 kDa, ~3.6 nm, negative charge).
Scanning electron microscopy studies showed that the GBM is a highly organized non-amorphous mesh-work of interconnected polygonal fibrils 4–10 nm thick223. In healthy states, the core of the GBM is more densely packed (10-nm pores) than the peripheral fibril network; however pores were enlarged up to 40 nm, in a rat proteinuric nephritis model223 (FIG. 2). Subdiffraction resolution stochastic optical reconstruction microscopy (STORM) and STORM-electron microscopy showed that GBM-associated proteins have a highly oriented macromolecular organization of agrin, laminin, and collagen IV224. Mutations in the gene that encodes collagen IV disrupt the self-polymerized collagen network and cause Alport syndrome, a hereditary disease that stems from GBM dysfunction225. Mutation of the LAMB2 gene, which encodes laminin subunit β2, prevents the assembly of the laminin complex LM-521 (a heterodimer composed of the laminin subunits β2, α5 and γ1), which leads to abnormal renal and neuromuscular phenotypes (Pierson syndrome), including splitting of the GBM226. Breakdown of the GBM in Pierson syndrome might be due to reduced levels of laminin trimer polymerization, a process that maintains basement membrane integrity181.
The design of mRNAs targeted to either podocytes or GECs, both of which synthesize the matrix to form and maintain the normal composition of the membrane, might be necessary to repair mutations of the matrix proteins. For example, NPs bearing specific peptide sequences that bind to collagen IV target subendothelial collagen IV in the vasculature227. Interestingly, during renal clearance, cyclodextrin-containing polymer-based cationic NPs that contain siRNAs accumulate and disassemble at the GBM, which might have broader repercussions for NPs that assemble via electrostatic interactions between their matrix and payload228. Whether this property could be exploited to target the GBM is not yet clear. Using targeted NPs to silence GBM protein expression in order to reverse the effects of mutations in the genes encoding laminin and collagen IV would be an interesting avenue to explore in the treatment of hereditary diseases. In addition, NPs targeted to the GBM could help deliver drugs, mRNAs or siRNAs to podocytes and glomerular endothelial cells, because both types of cells sit on the GBM.
Podocytes
Podocytes have unique features such as foot processes and the slit diaphragm, a 40 × 140 Å-wide229 bridge, mainly composed of nephrin, that connects juxtaposed foot processes. The slit diaphragm is porous but prevents the passage of large macromolecules such as proteins230. Since the discovery of nephrin (encoded by NPHS1) and podocin (encoded by NPHS2) as integral components of the slit diaphragm, several other podocyte-related proteins, such as glycogen synthase kinase 3b, have been discovered. Mutations in these proteins are linked to genetic diseases of the glomerulus216.
Foot process effacement is a classical sign of podocyte injury that correlates with the onset of proteinuria, underscoring the importance of these cells as the final size-selective filtration barrier to albumin. As podocytes have limited capacity for repair or regeneration, effective glomerular function is highly sensitive to podocyte number and glomerular filtration surface area216. Podocytes are therefore highly attractive therapeutic targets to treat refractory congenital nephrotic syndromes (such as the Finnish type)231.
In immortalized mouse and human podocytes (AB8/13 cells), activation of TNFα led to increased vascular cell adhesion protein 1 (VCAM-1) expression232. Anti-VCAM-1-coated lipidic NPs were used to deliver the potent immunosuppressant rapamycin to these activated podocytes, which inhibited cell migration232. Similar synthetic lipidic systems, such as synthetic, amphiphilic, interactive transfection reagent (SAINT), have also been used to package therapeutic proteins such as the large GTPase dynamin or antibodies, and were taken up by podocytes and fibroblasts233. The utility of this approach remains to be validated in vivo.
We have shown that polymeric NPs targeted to the neonatal Fc receptor (FcRn) effectively transport insulin across the intestinal barrier143,144. The FcRn signalling pathway is involved in the vectorial transcytosis of IgG and protects IgG and albumin from degradation234. FcRn is also expressed in podocytes, where it prevents IgG from clogging the glomeruli and controls albumin excretion234. NPs that are targeted to FcRn, are taken up via endocytosis, and are capable of releasing their payload from endosomes (for example via pH-sensitive chemistries or other ligands that can cause endosomolysis) might prove an effective means to deliver drugs into podocytes.
The glomerular endothelium and podocytes express several integrin receptors including integrin αvβ3. Activation of integrin αvβ3 induces urokinase plasminogen activator surface receptor signalling in podocytes, which in turn leads to foot process effacement and loss of urinary protein235. In diabetic nephropathy, hyperglycaemia stimulates the secretion of integrin αvβ3 ligands such as vitronectin by vascular cells, leading to the activation of αvβ3 integrins235. Blocking the ligand occupancy of αvβ3 with the F(ab′)2 fragment (obtained by pepsin digestion of an immunoglobulin) of an antibody directed against the extracellular domain of the integrin β3 subunit inhibited the development of diabetic nephropathy in diabetic pigs and rats236,237. Using NPs targeted to integrin αvβ3 that transport siRNA payloads directed against inflammatory pathways in podoctyes could be a promising approach to treat podocyte injury in kidney disease. Inorganic NPs surface modified with a cyclic RGD targeting motif (a sequence originally found in fibronectin) and targeted to integrin αvβ3, underwent receptor-mediated uptake and accumulated in vesicle-like structures in 2D podocyte cultures and whole glomeruli ex vivo238. The identification of podocyte-specific targets, coupled with the identification of specific upregulated pathways that underlie podocyte function in health and disease, will enable the creation of nanomedicines targeted to this important cell type for clinical translation and treatment of glomerulopathies.
Mesangial cells
Mesangial cells function as microvascular pericytes and maintain the structural architecture of the glomerular capillary (FIG. 2). They are primarily responsible for glomerular matrix growth and homeostasis, regulation of filtration surface area, and clearance of apoptotic cells or immune complexes near glomerular capillaries183.
Increased matrix production by mesangial cells and glomerulosclerosis are hallmarks of kidney disease and mesangial cell dysfunction is a key event in diabetic nephropathy. Hyperglycaemia induces excess production of reactive oxygen species, growth factors, and cytokines in mesangial cells183. Targeted NP-mediated drug delivery to mesangial cells would therefore be useful to treat various kidney diseases. NPs have the potential to diffuse and accumulate for prolonged periods in the mesangium if they can successfully cross the glomerular endothelial barrier. In fact, PEGylated gold NPs (with negative surface charge and variable PEG lengths) can be delivered to the kidney mesangium depending on their size167. Intravenously injected 75 nm NPs effectively targeted the kidney mesangium, whereas NPs >130 nm were abundant in the liver and spleen but not detected in the kidney. In this study NPs of ~130 nm in diameter provided an ‘in vivo calibration’ for the size of glomerular endothelial pores in physiologically relevant conditions as values for this threshold are usually derived from direct measurements of transmission and scanning electron microscopy images, for which sample preparation (repeat dehydration) might lead to pore shrinkage167. Cyclodextrin-containing siRNA NPs quickly accumulated in the GBM and also effectively delivered siRNA to mesangial cells to silence enhanced green fluoescent protein (EGFP) expression in the glomeruli of transgenic mice167,239.
Antibody-coated immunoliposomes effectively target mesangial cells. For example, NPs coated with anti-integrin-α8 monoclonal antibodies targeted mesangial cells in lupus glomerulonephritis in mice105. Similarly, NPs coated with OX7, an anti-Thy-1 membrane glycoprotein (Thy-1) monoclonal antibody, targeted the rat mesangium in mesangial proliferative glomerulonephritis127,131. Interestingly, the liposomes investigated in these studies were 130–170 nm in size, suggesting that such NPs can cross the glomerular basement membrane via surface ligand targeting (unlike non-targeted NPs)131. For optimal therapeutic effects, targeted drug delivery systems such as immunoliposomes can be used to deliver high intracellular concentrations of molecules that usually have severe systemic toxicities (glucocorticoids, cyclophosphamide, azathioprine, fatty acids, anticoagulants or antithrombotic agents) to mesangial cells using NPs conjugated to antibodies directed against antigens expressed on mesangial cells.
Tubular epithelial cells
The proximal tubules both secrete waste products and reabsorb useful nutrients into and from the urine122. Two barriers are involved in those processes: the capillary endothelial cells at the basolateral side of the proximal tubular cells and the tubulointerstitium between the capillaries and tubular cells122 (FIG. 2). Renal peritubular capillaries have fenestrations (60–70 nm wide), which are covered by a diaphragm (3–5 nm thick)122. In order to cross the tubulointerstitium, particles need to be smaller than the size of this diaphragm (<5 nm), and positively charged as the fenestrae are negatively charged owing to the presence of heparin sulfate122. Furthermore, the tubulointerstitium also contains fibroblasts and dendritic cells within an extracellular matrix consisting of proteoglycans, glycoproteins, fibrils, and interstitial fluid122. The tubular epithelium is highly susceptible to injury, which leads to tubular cell loss and sublethal proinflammatory and fibrogenic injuries240. Fibrosis is the downstream pathological manifestation of chronic kidney disease and is strongly correlated with disease progression. Hence, NPs designed to deliver drugs that prevent cell death, reduce renal fibrosis and inflammation, and/or promote the regeneration of tubules could be highly valuable241.
An optimal strategy to reduce fibrosis would be to target proximal tubular epithelial cells, as these cells play an important role in the pathogenesis of tubulointerstitial fibrosis. Targeted drug delivery strategies to inhibit pathways that are activated in disease with TGFβ kinase inhibitors, p38 mitogen activated protein kinase (p38 MAPK) inhibitors, Rho-associated kinase (ROCK) inhibitors or PDGF receptor kinase inhibitors, for example, could slow or even prevent progression to renal disease122.
As proximal tubule cells accumulate siRNA, several RNAi-based strategies have been proposed to treat acute kidney injury. NP-mediated delivery of siRNA can increase the accumulation of siRNA molecules within proximal tubule cells and facilitate combination treatments or controlled-release modes of action242,243. As mentioned above, carbon nanotubes with siRNAs against Trp53, Mep1b and Ctr1 reduced renal injury, fibrosis and immune infiltration in a cisplatin-induced model of acute kidney injury202. Although the pharmacokinetic profiles of the carbon nanotubes were comparable in mice and primates, suggesting that this therapy might be amenable to humans, the potential advantages of siRNA delivery using these NPs should also be investigated in the clinic.
A 2015 report showed that 400 nm NPs localize preferentially in the kidney compared to other organs, and selectively accumulate in the renal proximal tubules244. This characteristic suggests that antifibrotic compounds could be delivered directly to proximal tubular cells, which would increase renal specificity and minimize adverse and non-targeted effects; however, further studies are required to confirm these findings.
The effective targeting and optimal transport strategies to deliver xenobiotics and various drug conjugates (with polymers and/or proteins) to tubular cells have been discussed at length elsewhere122. The role of proximal tubular cells transporters, which are involved in the tubular reabsorption and secretion of small molecules, in the efflux of colloidal or nanosized drug-delivery systems remains to be elucidated. Large macromolecular structures are highly unlikely to be transported into tubular cells via this mechanism in healthy states; the transport of NPs and large macromolecules into tubular epithelial cells in disease states needs to be further investigated.
Catechol-derived, low-molecular-weight chitosan NPs were developed and investigated for the treatment of renal fibrosis in a 2014 study120. This self-assembled nanocomplex, designed to selectively release its pay-load inside cellular endosomes for maximal specificity, was specifically absorbed in the proximal tubules in mice. Intravenous delivery of the active anti-fibrosis compound emodin with these NPs attenuated fibrotic progression in a murine model of renal obstruction. Multi-targeted kinase inhibitors, and targeted delivery with nanomedicines, might also achieve antifibrotic effects. For example, sunitinib (a multi-targeted kinase inhibitor) has been evaluated for targeted drug delivery (conjugated to the kidney-specific carrier lysozyme)245, indicating that lysozyme could be utilized as a targeting ligand. A 2015 study investigated the effects of delivering an exogenous microRNA (miR-146a) using polyethylen-imine NPs in a mouse model of renal fibrosis induced by unilateral ureteral obstruction246. These positively charged NPs markedly reduced renal fibrosis by inhibiting inflammation via the suppression of TGFβ1 and Smad mRNA expression, which are responsible for the transcription of profibrotic genes.
A 2015 study showed that 5 nm dextran-based NPs and similar-sized poly(amidoamine) dendrimer NPs were filtered and internalized by renal tubular epithelial cells in a dose and time-dependent manner137. The administration of dextran NPs increased cellular albumin endocytosis and megalin expression but did not affect AQP-1 expression, as measured by dose-dependent urinary output. Similar effects were observed with the dendrimer NPs, which, in addition, led to an increase in clathrin expression. No major renal detrimental effects of dendrimers were reported, although changes in proximal tubule endocytosis and protein cellular expression levels suggest that these parameters should be monitored as researchers develop nanodrugs of various compositions and sizes targeted to the kidney.
Renal cell carcinoma
Renal cell carcinoma is the most lethal urologic cancer and affected patients have limited treatment options247. Over the past decade, cytokine-based therapies that target VEGF and mTORC pathways have improved the treatment of this disease; however, resistance mechanisms exist, which could be mitigated with combination therapies.
Numerous liposomal strategies for drug delivery in renal cell carcinoma have been investigated in preclinical studies, but only a few clinical studies have been carried out248–259. The majority of these studies administrated standard renal disease therapies and used antibody- targeting strategies. PEGylated-liposomal doxorubicin (Doxil) was used to treat patients with refractory renal cell cancer in a phase II clinical trial but had no efficacy and was mildly toxic257. Polymeric NPs have also been investigated in preclinical models for the delivery of the anti-angiogenic compound tetraiodothyroacetic acid to tumour cells in the chick chorioallantoic membrane and in xenografts in mice260.
Targeted therapies and immunotherapies are proving effective for the treatment of metastatic renal cell carcinoma, and several approved targets (such as new immunomodulatory monoclonal antibodies targeting the CTLA-4 or PD-1 pathways) are already available, although the clinical benefits of these new therapies compared to established treatments (tyrosine kinase or mTOR inhibitors) remains to be shown261. Nevertheless, nanomedicine-based approaches are of interest for the targeted delivery of chemotherapies and nanovaccines and their development will require further studies.
Nanotechnology has been widely applied to cancer therapy, and much has been learned regarding the discovery, manufacturing, and preclinical and clinical development of nanomedicines from several types of nonrenal cancer. We can extrapolate and infer from this base of knowledge to improve the design of renal carcinoma nanomedicines. Despite the fact that the safety profiles of most clinically validated NP platforms are superior to those of the parent drug, the nanomedicine does not improve survival when compared head-to-head with the parent drug. This absence of effect might be due, in part, to disease heterogeneity and variations in the degree of the enhanced permeability and retention (EPR) effect, according to which NPs preferentially accumulate in tumour tissues. The EPR effect has been suggested to have a central role in nanomedicine delivery to tumours, underscoring the need to select patients that are most likely to benefit from nanomedicines211,262–266. This effect has been widely investigated in preclinical models but few clinical studies have characterized it in humans or assessed its relevance to therapeutic intervention211,262–264. Furthermore, as understanding of the contribution of the perivascular tumour microenvironment to the penetration depth and uptake of NPs within tumours and into cancer cells has grown, we have progressively recognized that the EPR effect is complex and dynamic. The degree of tumour vascularization, inter-patient genetic variation, and tumour type and aggressiveness are all factors that contribute to this complexity211. Thus, stratification of patients with cancer according to tumour type and characteristics in relation to the likeliness of NP accumulation might be necessary to maximize the benefits of NP-mediated drug delivery through the EPR effect266–268.
In the past two years, preclinical efforts and preliminary clinical studies have begun to explore companion MRI-active nanodiagnostic molecules to dynamically assess response to nanomedicines265,266. Companion diagnostics could also prove valuable to assess NP retention, targeting, and biodistribution in the nephron using existing clinical modalities such as ultrasound, MRI, CT, and radiographs. These methods could be used for the assessment of NP accumulation within renal cell carcinomas269.
In vitro systems to assess NP efficacy
Controlled and optimized in vitro and ex vivo biological assays are required to test renal nanomedicines effectively. Traditional 2D cell culture models do not support complex tissue functions such as epithelial-to-mesenchymal transition or accurately predict the in vivo effects of a drug.
Microfluidics and kidney-on-a-chip
Organs-on-chips are built using microfluidic devices in which multiple cell types are cultured in 2D under continuous perfusion. These systems support tissue patterning in vitro and have been successfully used to study the molecular basis of tissue morphogenesis270. They also enable the screening of nanomedicines under physiological conditions270–272 (FIG. 3). For example, exposure of epithelial monolayers of collecting duct cells and proximal tubule cells to an apical fluid shear stress mimicking the rate found in living kidney tubules in a microchip resulted in enhanced epithelial cell polarization and primary cilia formation and provided an environment resembling that found in vivo, which is amenable to toxicity studies272. In this system, fluid shear stress might have induced actin cytoskeletal rearrangements, resulting in a cellular phenotype akin to that found in human kidneys. A variety of animal and human renal cells cultured in these conditions had a toxicity response similar to that found in vivo. Such systems also effectively recreated epithelial-to-mesenchymal transition, which mediates renal interstitial fibrosis272–277, and facilitated the measurement of P-glycoprotein ATP-binding cassette membrane (PGP, also called multidrug resistance protein 1 transporter activity in response to chemotherapy). Indeed, kidney tubular epithelial cells had more effective PGP efflux activity in baseline conditions under shear flow than without flow, which more closely resembles the in vivo environment than measures with traditional culture systems272.
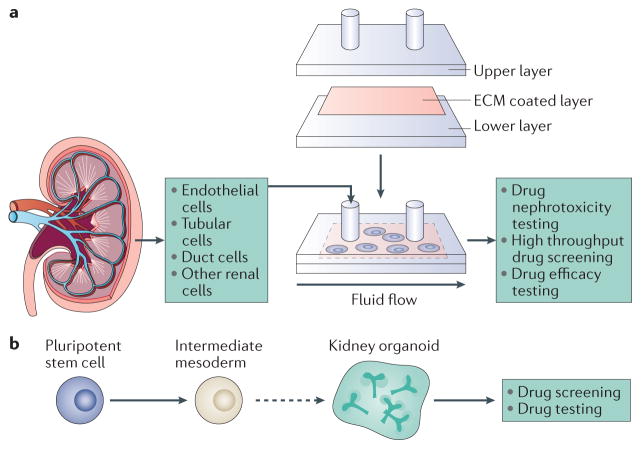
Figure 3. In vitro systems to test nanoparticles.
a | Different types of primary renal cells can be seeded on a porous membrane coated with extracellular matrix (ECM) and cultured in microfluidic devices that mimic physiological conditions with apical fluid shear stress. These devices enable easy fluid sampling and addition of nanodrugs, which facilitates the high throughput testing of nanodrug nephrotoxicity and efficacy. b | Pluripotent stem cells can be induced to differentiatein vitro into mesoderm and renal organoids: 3D structures that contain several renal cell types. Organoids can be used as a platform to screen and test nanodrugs.
Lessons from such studies could prove valuable for screening kidney-targeted drugs and assessing nanodrug nephrotoxicity and efficacy in a more quantitative and dynamic manner than traditional culture systems271,272,278.
3D culture systems
Advances in induced pluripotent stem cell technologies and in the understanding of kidney morphogenesis have enabled the production of mature kidney cells in vitro in 3D structures called organoids279–281 (FIG. 3). Organoids are self-organizing 3D tissues that can be grown from embryonic stem cells, their synthetic induced pluripotent stem cells or organ-restricted adult stem cells282,283.
The kidney includes more than 20 specialized cell types and is an organ with complex structural design, which is not accurately portrayed using traditional 2D culture systems. In contrast, kidney organoids can be grown with more complexity, including more than 500 nephrons and defined glomeruli with Bowman capsule and podocytes, connected to proximal tubules279,284.
One of the next areas of development for kidney organoids is to achieve urine and blood filtration in vitro, which will require a complete kidney structure with a blood system connected to glomerular capillary loops. Establishing patient-derived organoids is also an exciting avenue to develop true personalized medicines, whereby drug response can be predicted according to the patient’s genetic makeup. Furthermore, kidney organoids can facilitate testing of regenerative medicines, combination therapies, gene editing therapies and nanomedicine drug delivery, as they provide an environment akin to that found in the kidney but with the ease of manipulation of an in vivo system285. The toxicity of dendrimer NPs was assessed in a proximal tubule organoid, which expressed toxicity markers previously reported in vivo286. Such functioning proximal tubule cultures provide an environment similar to that found in vivo to assess the nephrotoxicity of nanomedicines and their cargos as they express multiple drug transporters, akin to their in vivo counterparts287.
Challenges to clinical translation
Clinical grade synthesis and screening
NPs are often composed of several components and can have a range of biophysical and chemical properties (size, charge, drug payload, release kinetics and surface targeting ligand) that require screening in a systematic and parallel fashion, while ensuring reproducible synthesis of libraries with such distinct features. Bulk techniques do not always generate uniform NPs even if they can be scaled to yield multi-kilogram batches of particles suitable for clinical development and commercialization288. Microfluidics have improved the synthesis of a variety of NP systems and enabled the controlled synthesis and high-throughput screening of NP libraries288,289. Microfluidic technologies have the potential to become a platform for rapid self-assembly of NPs with a narrow size distribution, tuneable physical and chemical characteristics, and low batch-to-batch variability290–294. Other techniques such as PRINT have also been used to synthesize uniform NPs with precise control of size, shape, chemical composition, drug loading, and surface properties192,193. Analogous to high-throughput drug discovery screening, these technologies could also help to streamline a controlled and high-throughput investigation of kidney NP structure–activity relationships.
Chemistry, manufacturing and controls (CMC) and good manufacturing practice (GMP) requirements must continuously be met as NP technologies transition from preclinical to clinical development, then to commercialization and throughout the market life of the product. Current pharmaceutical manufacturing unit operations that generate first-generation NPs (liposomes and polymeric NPs) meet such demands; however, producing more complex nanomedicines, such as those that incorporate targeting ligands, multidrug combinations, or multi-stage, stimuli-responsive, or theranostic systems, can create additional CMC requisites and require the adaptation of current manufacturing unit operations. The large-scale production of high-quality complex NPs also needs to be addressed. PRINT technology facilitates reproducible fabrication of NPs193, but production at the kilogram scale remains to be demonstrated. Alternative microfluidic technologies such as the coaxial turbulent jet mixer, which provides NP homogeneity, reproducibility, and tuneability, have also been designed and developed for large-scale production of polymeric NPs295. In the future these methodologies can be used to scale-up clinical-stage nanomedicines targeted to the kidney.
In vivo testing
Investigations into in vivo animal models, which can sometimes accurately mimic human diseases, are also important. Some studies have shown pharmacokinetics scaling across species (including humans) for different nanotherapeutics64,86,296,297. For instance, the AUC of polymeric NPs containing camptothecin (CPT) scaled linearly with CPT dose per m2 across species (mice, rats, dogs, and humans), suggesting that NPs behave in a similar way in animals and in humans287. However, interspecies discrepancies exist for albumin binding of CPT (with higher binding affinity found for human albumin), and should be taken into account when considering the pharmacokinetics of the free drug in humans as this property might be important in investigations of NP versus free drug efficacy. Furthermore, NP biodistribution studies in patients are not feasible in most cases. The discrepancies between pre-clinical and clinical models must therefore be considered for NP development and animal models that more closely mimic the heterogeneity and anatomical and histological features of human kidney disease are needed298,299.
Conclusions and Perspectives
As the field of nanomedicine rapidly expands, with several NPs already marketed and many undergoing clinical trials, our accumulated experience and the clinical successes to date form a framework for the creation of the next generation of renal nanomedicines. Refining the generation of nanomaterials with tuneable biophysical properties could yield NPs with highly controllable architectures for kidney binding and retention as well as uptake within important cell types such as podocytes. Fortunately, in the past few years, numerous studies of NP or nano-sized colloids originally intended for tumour targeting have serendipitously yielded important discoveries relevant to kidney targeting and selective renal accumulation. These, in turn, raise important correlations between the structure and the activity of NPs according to their design and biophysical and chemical properties as a function of kidney retention and targeting. We believe that now is an ideal time for collaborative initiatives between nephrologists and nanotechnologists to pioneer and establish promising nanomedicine approaches, with appropriate selection of targeting and therapeutic parameters for the diagnosis and treatment of renal disease.
Key points.
Oncology has benefited greatly from nanotechnology research and investment, yielding important insights that are applicable to kidney nanomedicines
Nanocarriers have the potential to improve the pharmacokinetics, biodistribution, toxicity, and efficacy of drugs
Delivery and retention of nanomedicines in the kidney is challenging and requires a deep understanding of the renal barriers and effective engineering of nanoparticle size, shape, and surface chemistry
To improve drug efficacy and minimize systemic toxicity, nanomedicines can be targeted to distinct kidney cell types and/or to extracellular matrix components such as the glomerular basement membrane
Nanocarriers can deliver drugs, proteins, peptides and nucleic acids, and can facilitate the targeted delivery of genes or RNA interference molecules to treat kidney disorders for which the efficacy of current treatments is limited
Organ-on-a-chip kidneys can streamline the simultaneous screening and testing of nanomedicines in environments that mimic the kidney and accelerate translation of kidney-specific therapeutics
Acknowledgments
O.C.F acknowledges support from the NHLBI HL127464, NCI CA151884, NIBIB EB015419 and the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics. J.C.H is supported by NIH 1R01DK078897, NIH 1R01DK088541, NIH P01-DK-56492, Chinese 973 fund 2012CB517601.
Glossary
- Nanoparticles
Nanoscale particles with modifiable shape and charge capable of carrying a specific payload (drugs, diagnostic molecules etc.).
- Colloid
Substance formed by a non-crystalline material (here nanomaterial) of either natural or synthetic origin dispersed in a solution.
- Organ-on-a-chip
Microfluidic devices used to culture living cells under continuous perfusion in micorometer-sized chambers; they are primarily used to reproduce the physiological functions of tissues and organs as closely as possible.
- PEGylation
Attachment of polyethers to the surface of nanoparticles in order to minimize unwanted interactions with their biological surroundings.
- Cmax
Maximal serum concentration of a drug or nanoparticle achievable after administration.
- Top-down fabrication method
Synthesis of a structure by etching or removal of material from a template or substrate to achieve specific shapes or sizes.
- Particle replication in non-wetting templates
Fabrication technique whereby a pre-particle solution is distributed in a mould with nanosized cavities to generate nanoparticles with precisely defined shape, size, composition and surface properties.
- Aspect ratio
Ratio of the width to the height of a nanoparticle.
- Enhanced permeability and retention (EPR) effect
Describes the accumulation and retention of colloidal nanoparticles within tumour tissue as a result of increased endothelial gap junction distances due to heterogeneous vessel formation and growth.
Footnotes
Author contributions
All authors contributed to researching data for the article, discussion of the article’s content, writing, and review or editing of the manuscript before submission.
Competing interests statement
O.C.F. has financial interests in Selecta Biosciences, Tarveda Therapeutics, and Placon Therapeutics. D.A.A serves on the Board of Directors of Pfizer, Alnylam Pharmaceuticals, Seres Therapeutics, Tarveda Therapeutics and Placon Therapeutics. All other authors declare no conflicts.
References
- 1.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 3.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 4.Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7:1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha K, Bajaj A, Duncan B, Rotello VM. Beauty is skin deep: a surface monolayer perspective on nanoparticle interactions with cells and bio-macromolecules. Small. 2011;7:1903–1918. doi: 10.1002/smll.201100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mu Q, et al. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem Rev. 2014;114:7740–7781. doi: 10.1021/cr400295a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albanese A, et al. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano. 2014;8:5515–5526. doi: 10.1021/nn4061012. [DOI] [PubMed] [Google Scholar]
- 10.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 11.Gratton SE, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anselmo AC, Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.01.007. http://dx.doi.org/10.1016/j.addr.2016.01.007. [DOI] [PubMed]
- 14.Marty JJ, Oppenheim RC, Speiser P. Nanoparticles — a new colloidal drug delivery system. Pharm Acta Helv. 1978;53:17–23. [PubMed] [Google Scholar]
- 15.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116:2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 17.Stuart MA, et al. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 18.Pacardo DB, Ligler FS, Gu Z. Programmable nanomedicine: synergistic and sequential drug delivery systems. Nanoscale. 2015;7:3381–3391. doi: 10.1039/c4nr07677j. [DOI] [PubMed] [Google Scholar]
- 19.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 20.Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1–49. doi: 10.1016/j.mser.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Correa S, Dreaden EC, Gu L, Hammond PT. Engineering nanolayered particles for modular drug delivery. J Control Release. 2016;240:364–386. doi: 10.1016/j.jconrel.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp JA, Shim MS, Heo CY, Kwon YJ. “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18. doi: 10.1016/j.addr.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21:223–232. doi: 10.1016/j.molmed.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabernero J, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 27.Reddy LH, Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J Hepatol. 2011;55:1461–1466. doi: 10.1016/j.jhep.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 28.Wang AZ, et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther. 2008;8:1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyseng-Williamson KA, Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a guide to its use in various malignancies. BioDrugs. 2013;27:533–540. doi: 10.1007/s40259-013-0070-1. [DOI] [PubMed] [Google Scholar]
- 30.Barenholz Y. Doxil® — the first FDA-approved nanodrug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Harrison M, Tomlinson D, Stewart S. Liposomal-entrapped doxorubicin: an active agent in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1995;13:914–920. doi: 10.1200/JCO.1995.13.4.914. [DOI] [PubMed] [Google Scholar]
- 32.Money-Kyrle JF, et al. Liposomal daunorubicin in advanced Kaposi’s sarcoma: a phase II study. Clin Oncol. 1993;5:367–371. doi: 10.1016/s0936-6555(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal E, et al. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. Am J Clin Oncol. 2002;25:57–59. doi: 10.1097/00000421-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Meyerhoff AUS. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28:42–48. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 35.Khemapech N, Oranratanaphan S, Termrungruanglert W, Lertkhachonsuk R, Vasurattana A. Salvage chemotherapy in recurrent platinum-resistant or refractory epithelial ovarian cancer with Carboplatin and distearoylphosphatidylcholine pegylated liposomal Doxorubicin (Lipo-Dox®) Asian Pac J Cancer Prev. 2013;14:2131–2135. doi: 10.7314/apjcp.2013.14.3.2131. [DOI] [PubMed] [Google Scholar]
- 36.Glantz MJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394–3402. [PubMed] [Google Scholar]
- 37.Batist G, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 38.Agosta E, et al. Pharmacogenetics of antiangiogenic and antineovascular therapies of age-related macular degeneration. Pharmacogenomics. 2012;13:1037–1053. doi: 10.2217/pgs.12.77. [DOI] [PubMed] [Google Scholar]
- 39.Gabizon A, et al. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol. 2008;61:695–702. doi: 10.1007/s00280-007-0525-5. [DOI] [PubMed] [Google Scholar]
- 40.Gambling D, Hughes T, Martin G, Horton W, Manvelian G. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg. 2005;100:1065–1074. doi: 10.1213/01.ANE.0000145009.03574.78. [DOI] [PubMed] [Google Scholar]
- 41.Venkatakrishnan K, et al. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br J Clin Pharmacol. 2014;77:998–1010. doi: 10.1111/bcp.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingram I. FDA approves liposomal vincristine (Marqibo) for rare leukemia. Oncology (Williston Park) 2012;26:841. [PubMed] [Google Scholar]
- 43.Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 45.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 46.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–192. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 48.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. doi: 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–1456. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim NK, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Rajeshkumar NV, et al. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 2016;115:442–453. doi: 10.1038/bjc.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SR, et al. A multi-center, late phase II clinical trial of Genexol (paclitaxel) and cisplatin for patients with advanced gastric cancer. Oncol Rep. 2004;12:1059–1064. [PubMed] [Google Scholar]
- 53.Kim TY, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 54.Ediriwickrema A, Zhou J, Deng Y, Saltzman WM. Multi-layered nanoparticles for combination gene and drug delivery to tumors. Biomaterials. 2014;35:9343–9354. doi: 10.1016/j.biomaterials.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talelli M, et al. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;10:93–117. doi: 10.1016/j.nantod.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishiyama N, Matsumura Y, Kataoka K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016;107:867–874. doi: 10.1111/cas.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Oldham EA, Li C, Ke S, Wallace S, Huang P. Comparison of action of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in human breast cancer cells. Int J Oncol. 2000;16:125–132. [PubMed] [Google Scholar]
- 61.Duncan R. Polymer therapeutics: top 10 selling pharmaceuticals — what next? J Control Release. 2014;190:371–380. doi: 10.1016/j.jconrel.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 63.Espelin CW, Leonard SC, Geretti E, Wickham TJ, Hendriks BS. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016;76:1517–1527. doi: 10.1158/0008-5472.CAN-15-1518. [DOI] [PubMed] [Google Scholar]
- 64.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 65.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lancet JE. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol. 2016;34(Suppl):7000. [Google Scholar]
- 67.Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276:579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 68.Roy U, et al. The potential of HIV-1 nanotherapeutics: from in vitro studies to clinical trials. Nanomedicine (Lond ) 2015;10:3597–3609. doi: 10.2217/nnm.15.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev. 2013;65:1316–1330. doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Dreaden EC, Mackey MA, Huang X, Kang B, El-Sayed MA. Beating cancer in multiple ways using nanogold. Chem Soc Rev. 2011;40:3391–3404. doi: 10.1039/c0cs00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anselmo AC, Mitragotri SA. Review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17:1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giljohann DA, et al. Gold nanoparticles for biology and medicine. Angew Chem Int Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paithankar D, et al. Ultrasonic delivery of silica-gold nanoshells for photothermolysis of sebaceous glands in humans: Nanotechnology from the bench to clinic. J Control Release. 2015;206:30–36. doi: 10.1016/j.jconrel.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Yu C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine. 2016;12:317–332. doi: 10.1016/j.nano.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Meng H, et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laurent S, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 77.Maier-Hauff K, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron–oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maggiorella L, et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8:1167–1181. doi: 10.2217/fon.12.96. [DOI] [PubMed] [Google Scholar]
- 79.Field JA, et al. Cytotoxicity and physicochemical properties of hafnium oxide nanoparticles. Chemosphere. 2011;84:1401–1407. doi: 10.1016/j.chemosphere.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 80.Libutti SK, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Gupta S, Li C. Research perspectives: gold nanoparticles in cancer theranostics. Quant Imaging Med Surg. 2013;3:284–291. doi: 10.3978/j.issn.2223-4292.2013.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fortuin AS, Meijer H, Thompson LC, Witjes JA, Barentsz JO. Ferumoxtran-10 ultrasmall superparamagnetic iron oxide-enhanced diffusion-weighted imaging magnetic resonance imaging for detection of metastases in normal-sized lymph nodes in patients with bladder and prostate cancer: do we enter the era after extended pelvic lymph node dissection? Eur Urol. 2013;64:961–963. doi: 10.1016/j.eururo.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 83.Hedgire SS, et al. Enhanced primary tumor delineation in pancreatic adenocarcinoma using ultrasmall super paramagnetic iron oxide nanoparticle-ferumoxytol: an initial experience with histopathologic correlation. Int J Nanomedicine. 2014;9:1891–1896. doi: 10.2147/IJN.S59788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivera Gil P, Huhn D, del Mercato LL, Sasse D, Parak WJ. Nanopharmacy: inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010;62:115–125. doi: 10.1016/j.phrs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Tolcher AW, et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:363–371. doi: 10.1007/s00280-013-2361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schultheis B, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 87.Jensen SA, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Islam MA, et al. Biomaterials for mRNA delivery. Biomater Sci. 2015;3:1519–1533. doi: 10.1039/c5bm00198f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park J, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee IH, et al. Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J Control Release. 2011;155:435–441. doi: 10.1016/j.jconrel.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 91.Park BH, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 92.Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Curr Opin Biotechnol. 2011;22:901–908. doi: 10.1016/j.copbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Czapar AE, et al. Tobacco mosaic virus delivery of phenanthriplatin for cancer therapy. ACS Nano. 2016;10:4119–4126. doi: 10.1021/acsnano.5b07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parato KA, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20:749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morrison EE, Bailey MA, Dear JW. Renal extracellular vesicles: from physiology to clinical application. J Physiol. 2016;594:5735–5748. doi: 10.1113/JP272182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 98.Bitzer M, Ben-Dov IZ, Thum T. Microparticles and microRNAs of endothelial progenitor cells ameliorate acute kidney injury. Kidney Int. 2012;82:375–377. doi: 10.1038/ki.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chow EK, et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med. 2011;3:73ra21. doi: 10.1126/scitranslmed.3001713. [DOI] [PubMed] [Google Scholar]
- 100.Mochalin VN, et al. Adsorption of drugs on nanodiamond: toward development of a drug delivery platform. Mol Pharm. 2013;10:3728–3735. doi: 10.1021/mp400213z. [DOI] [PubMed] [Google Scholar]
- 101.Ho D. Nanodiamond-based chemotherapy and imaging. Cancer Treat Res. 2015;166:85–102. doi: 10.1007/978-3-319-16555-4_4. [DOI] [PubMed] [Google Scholar]
- 102.Jiang T, et al. Furin-mediated sequential delivery of anticancer cytokine and small-molecule drug shuttled by graphene. Adv Mater. 2015;27:1021–1028. doi: 10.1002/adma.201404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valencia PM, et al. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond ) 2013;8:687–698. doi: 10.2217/nnm.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim J, Kim J, Jeong C, Kim WJ. Synergistic nanomedicine by combined gene and photothermal therapy. Adv Drug Deliv Rev. 2016;98:99–112. doi: 10.1016/j.addr.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 106.Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64:1394–1416. doi: 10.1016/j.addr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Kenny GD, et al. Novel multifunctional nanoparticle mediates siRNA tumour delivery, visualisation and therapeutic tumour reduction in vivo. J Control Release. 2011;149:111–116. doi: 10.1016/j.jconrel.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 108.Liang C, Xu L, Song G, Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem Soc Rev. 2016 doi: 10.1039/c6cs00458j. http://dx.doi.org/10.1039/C6CS00458J. [DOI] [PubMed]
- 109.Hessel CM, et al. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011;11:2560–2566. doi: 10.1021/nl201400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferreira MM, Bismuth J, Torresani J. Reversible dissociation of triiodothyronine-nuclear receptor complexes by mercurial and chaotropic reagents. Biochem Biophys Res Commun. 1982;105:244–251. doi: 10.1016/s0006-291x(82)80037-5. [DOI] [PubMed] [Google Scholar]
- 111.Maldonado RA, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112:E156–E165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ilyinskii PO, et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine. 2014;32:2882–2895. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4:148rv149. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 114.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenthal JA, Chen L, Baker JL, Putnam D, DeLisa MP. Pathogen-like particles: biomimetic vaccine carriers engineered at the nanoscale. Curr Opin Biotechnol. 2014;28:51–58. doi: 10.1016/j.copbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Stary G, et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T. cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kodaira H, et al. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials. 2004;25:4309–4315. doi: 10.1016/j.biomaterials.2003.10.097. [DOI] [PubMed] [Google Scholar]
- 119.Borgman MP, et al. Tumor-targeted HPMA copolymer-(RGDfK)–(CHX-A’’-DTPA) conjugates show increased kidney accumulation. J Control Release. 2008;132:193–199. doi: 10.1016/j.jconrel.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiao H, et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials. 2014;35:7157–7171. doi: 10.1016/j.biomaterials.2014.04.106. [DOI] [PubMed] [Google Scholar]
- 121.Yuan ZX, et al. Specific renal uptake of randomly 50% N-acetylated low molecular weight chitosan. Mol Pharm. 2009;6:305–314. doi: 10.1021/mp800078a. [DOI] [PubMed] [Google Scholar]
- 122.Dolman ME, Harmsen S, Storm G, Hennink WE, Kok RJ. Drug targeting to the kidney: advances in the active targeting of therapeutics to proximal tubular cells. Adv Drug Deliv Rev. 2010;62:1344–1357. doi: 10.1016/j.addr.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 123.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62:1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 124.Dolman ME, et al. Dendrimer-based macromolecular conjugate for the kidney-directed delivery of a multitargeted sunitinib analogue. Macromol Biosci. 2012;12:93–103. doi: 10.1002/mabi.201100277. [DOI] [PubMed] [Google Scholar]
- 125.Yuan ZX, et al. Enhanced accumulation of low-molecular-weight chitosan in kidneys: a study on the influence of N-acetylation of chitosan on the renal targeting. J Drug Target. 2011;19:540–551. doi: 10.3109/1061186X.2010.521158. [DOI] [PubMed] [Google Scholar]
- 126.Gao S, et al. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics. 2014;4:1039–1051. doi: 10.7150/thno.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suana AJ, et al. Single application of low-dose mycophenolate mofetil-OX7-immunoliposomes ameliorates experimental mesangial proliferative glomerulonephritis. J Pharmacol Exp Ther. 2011;337:411–422. doi: 10.1124/jpet.110.176222. [DOI] [PubMed] [Google Scholar]
- 128.Tuffin G, Huwyler J, Waelti E, Hammer C, Marti HP. Drug targeting using OX7-immunoliposomes: correlation between Thy1.1 antigen expression and tissue distribution in the rat. J Drug Target. 2008;16:156–166. doi: 10.1080/10611860701848944. [DOI] [PubMed] [Google Scholar]
- 129.Morimoto K, et al. Advances in targeting drug delivery to glomerular mesangial cells by long circulating cationic liposomes for the treatment of glomerulonephritis. Pharm Res. 2007;24:946–954. doi: 10.1007/s11095-006-9213-0. [DOI] [PubMed] [Google Scholar]
- 130.Tuffin G, Waelti E, Huwyler J, Hammer C, Marti HP. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J Am Soc Nephrol. 2005;16:3295–3305. doi: 10.1681/ASN.2005050485. [DOI] [PubMed] [Google Scholar]
- 131.Scindia Y, Deshmukh U, Thimmalapura PR, Bagavant H. Anti-α8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum. 2008;58:3884–3891. doi: 10.1002/art.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Monti DM, et al. Biocompatibility, uptake and endocytosis pathways of polystyrene nanoparticles in primary human renal epithelial cells. J Biotechnol. 2015;193:3–10. doi: 10.1016/j.jbiotec.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Chen H, et al. Gd-encapsulated carbonaceous dots with efficient renal clearance for magnetic resonance imaging. Adv Mater. 2014;26:6761–6766. doi: 10.1002/adma.201402964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang XD, et al. Passing through the renal clearance barrier: toward ultrasmall sizes with stable ligands for potential clinical applications. Int J Nanomedicine. 2014;9:2069–2072. doi: 10.2147/IJN.S64301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond ) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zarschler K, et al. Ultrasmall inorganic nanoparticles: state-of-the-art and perspectives for biomedical applications. Nanomedicine. 2016;12:1663–1701. doi: 10.1016/j.nano.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 137.Nair AV, Keliher EJ, Core AB, Brown D, Weissleder R. Characterizing the interactions of organic nanoparticles with renal epithelial cells in vivo. ACS Nano. 2015;9:3641–3653. doi: 10.1021/acsnano.5b00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen X, et al. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int J Nanomedicine. 2015;10:1–22. doi: 10.2147/IJN.S73538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.L’Azou B, et al. In vitro effects of nanoparticles on renal cells. Part Fibre Toxicol. 2008;5:22. doi: 10.1186/1743-8977-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Z, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Liao J, et al. Effect of steroid-liposome on immunohistopathology of IgA nephropathy in ddY mice. Nephron. 2001;89:194–200. doi: 10.1159/000046067. [DOI] [PubMed] [Google Scholar]
- 142.Ito K, et al. Liposome-mediated transfer of nitric oxide synthase gene improves renal function in ureteral obstruction in rats. Kidney Int. 2004;66:1365–1375. doi: 10.1111/j.1523-1755.2004.00899.x. [DOI] [PubMed] [Google Scholar]
- 143.Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin Drug Deliv. 2015;12:1459–1473. doi: 10.1517/17425247.2015.1018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pridgen EM, et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beloqui A, des Rieux A, Preat V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.04.014. http://dx.doi.org/10.1016/j.addr.2016.04.014. [DOI] [PubMed]
- 146.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu X, et al. Polymeric nanoparticles amenable to simultaneous installation of exterior targeting and interior therapeutic proteins. Angew Chem Int Ed. 2016;55:3309–3312. doi: 10.1002/anie.201509183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi KY, Liu G, Lee S, Chen X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale. 2012;4:330–342. doi: 10.1039/c1nr11277e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cedervall T, et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed. 2007;46:5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 151.Lynch I, Salvati A, Dawson KA. Protein–nanoparticle interactions: what does the cell see? Nat Nanotechnol. 2009;4:546–547. doi: 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- 152.Cedervall T, et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lundqvist M, et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walkey CD, Olsen JB, Guo H, Emili A, Chan WC. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 155.Ritz S, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16:1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 156.Ogawara K, et al. Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanosphere: implications for rational design of nanoparticles. J Control Release. 2004;100:451–455. doi: 10.1016/j.jconrel.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 157.Mahmoudi M, et al. Protein–nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 158.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 159.Chanan-Khan A, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 160.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schottler S, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 162.Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 163.Dong Y, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci USA. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sakulkhu U, et al. Ex situ evaluation of the composition of protein corona of intravenously injected superparamagnetic nanoparticles in rats. Nanoscale. 2014;6:11439–11450. doi: 10.1039/c4nr02793k. [DOI] [PubMed] [Google Scholar]
- 165.Walkey CD, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 166.Bigdeli A, et al. Exploring cellular interactions of liposomes using protein corona fingerprints and physicochemical properties. ACS Nano. 2016;10:3723–3737. doi: 10.1021/acsnano.6b00261. [DOI] [PubMed] [Google Scholar]
- 167.Choi CH, Zuckerman JE, Webster P, Davis ME. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci USA. 2011;108:6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ishida T, et al. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 170.Ichihara M, et al. Anti-PEG IgM response against PEGylated liposomes in mice and rats. Pharmaceutics. 2010;3:1–11. doi: 10.3390/pharmaceutics3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dams ET, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 172.Kalra AV, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74:7003–7013. doi: 10.1158/0008-5472.CAN-14-0572. [DOI] [PubMed] [Google Scholar]
- 173.Koizumi F, et al. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 2006;66:10048–10056. doi: 10.1158/0008-5472.CAN-06-1605. [DOI] [PubMed] [Google Scholar]
- 174.Nakajima TE, et al. Antitumor effect of SN-38-releasing polymeric micelles, NK012, on spontaneous peritoneal metastases from orthotopic gastric cancer in mice compared with irinotecan. Cancer Res. 2008;68:9318–9322. doi: 10.1158/0008-5472.CAN-08-2822. [DOI] [PubMed] [Google Scholar]
- 175.Hamaguchi T, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nagai N, et al. Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol. 1996;39:131–137. doi: 10.1007/s002800050548. [DOI] [PubMed] [Google Scholar]
- 177.Barpe DR, Rosa DD, Froehlich PE. Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur J Pharm Sci. 2010;41:458–463. doi: 10.1016/j.ejps.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 178.Puelles VG, et al. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 179.Eaton DC, Pooler J. Vander’s Renal Physiology. 8. McGraw-Hill Medical Publishing; 2013. [Google Scholar]
- 180.Satchell S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol. 2013;9:717–725. doi: 10.1038/nrneph.2013.197. [DOI] [PubMed] [Google Scholar]
- 181.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318:973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Armelloni S, et al. Podocytes: recent biomolecular developments. Biomol Concepts. 2014;5:319–330. doi: 10.1515/bmc-2014-0020. [DOI] [PubMed] [Google Scholar]
- 183.Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–985. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 184.Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol. 2014;10:158–173. doi: 10.1038/nrneph.2014.1. [DOI] [PubMed] [Google Scholar]
- 185.Brown D, Wagner CA. Molecular mechanisms of acid–base sensing by the kidney. J Am Soc Nephrol. 2012;23:774–780. doi: 10.1681/ASN.2012010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang RL, Deen WM, Robertson CR, Brenner BM. Permselectivity of the glomerular capillary wall: III. Restricted transport of polyanions. Kidney Int. 1975;8:212–218. doi: 10.1038/ki.1975.104. [DOI] [PubMed] [Google Scholar]
- 187.Mallipattu SK, He JC. A new mechanism for albuminuria-induced podocyte injury. J Am Soc Nephrol. 2013;24:1709–1711. doi: 10.1681/ASN.2013070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Choi HS, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Choi HS, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zuckerman JE, Davis ME. Targeting therapeutics to the glomerulus with nanoparticles. Adv Chronic Kidney Dis. 2013;20:500–507. doi: 10.1053/j.ackd.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 191.Toy R, Peiris PM, Ghaghada KB, Karathanasis E. Shaping cancer nanomedicine: the effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine (Lond ) 2014;9:121–134. doi: 10.2217/nnm.13.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rolland JP, et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 193.Xu J, et al. Future of the particle replication in nonwetting templates (PRINT) technology. Angew Chem Int Ed. 2013;52:6580–6589. doi: 10.1002/anie.201209145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Park JH, et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv Mater. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chauhan VP, et al. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gentile F, et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 199.Ruggiero A, et al. Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci USA. 2010;107:12369–12374. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 202.Alidori S, et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci Transl Med. 2016;8:331ra339. doi: 10.1126/scitranslmed.aac9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lacerda L, et al. Carbon-nanotube shape and individualization critical for renal excretion. Small. 2008;4:1130–1132. doi: 10.1002/smll.200800323. [DOI] [PubMed] [Google Scholar]
- 204.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10:487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 206.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 207.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)–poly(D,L-lactide) block copolymer micelles with modulated surface charge. J Control Release. 2001;77:27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 208.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 210.Zhu X, et al. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc Natl Acad Sci USA. 2015;112:7779–7784. doi: 10.1073/pnas.1505629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liang X, et al. Short- and long-term tracking of anionic ultrasmall nanoparticles in kidney. ACS Nano. 2016;10:387–395. doi: 10.1021/acsnano.5b05066. [DOI] [PubMed] [Google Scholar]
- 213.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40:828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 216.Lal MA, Young KW, Andag U. Targeting the podocyte to treat glomerular kidney disease. Drug Discov Today. 2015;20:1228–1234. doi: 10.1016/j.drudis.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 217.Wu HY, et al. Diagnostic performance of random urine samples using albumin concentration versus ratio of albumin to creatinine for microalbuminuria v screening in patients with diabetes mellitus: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:1108–1115. doi: 10.1001/jamainternmed.2014.1363. [DOI] [PubMed] [Google Scholar]
- 218.Dahlman JE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dritschilo A, et al. Phase I study of liposome-encapsulated c-raf antisense oligodeoxyribonucleotide infusion in combination with radiation therapy in patients with advanced malignancies. Clin Cancer Res. 2006;12:1251–1259. doi: 10.1158/1078-0432.CCR-05-1260. [DOI] [PubMed] [Google Scholar]
- 220.Elazar V, et al. Sustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasis. Int J Cancer. 2010;126:1749–1760. doi: 10.1002/ijc.24890. [DOI] [PubMed] [Google Scholar]
- 221.Asgeirsdottir SA, et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294:F554–F561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 222.Groffen AJ, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem. 1998;46:19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 223.Hironaka K, Makino H, Yamasaki Y, Ota Z. Pores in the glomerular basement membrane revealed by ultrahigh-resolution scanning electron microscopy. Nephron. 1993;64:647–649. doi: 10.1159/000187418. [DOI] [PubMed] [Google Scholar]
- 224.Suleiman H, et al. Nanoscale protein architecture of the kidney glomerular basement membrane. eLife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kashtan CE. Alport syndrome & thin basement membrane nephropathy. GeneReviews. https://www.ncbi.nlm.nih.gov/books/NBK1207/ (updated 25 nov 2015)
- 226.Mohney BG, et al. A novel mutation of LAMB2 in a multigenerational mennonite family reveals a new phenotypic variant of Pierson syndrome. Ophthalmology. 2011;118:1137–1144. doi: 10.1016/j.ophtha.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kamaly N, et al. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci USA. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zuckerman JE, Choi CH, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci USA. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol. 1999;10:2440–2445. doi: 10.1681/ASN.V10112440. [DOI] [PubMed] [Google Scholar]
- 230.Tassin MT, Beziau A, Gubler MC, Boyer B. Spatiotemporal expression of molecules associated with junctional complexes during the in vivo maturation of renal podocytes. Int J Dev Biol. 1994;38:45–54. [PubMed] [Google Scholar]
- 231.Mallipattu SK, He JC. The podocyte as a direct target for treatment of glomerular disease? Am J Physiol Renal Physiol. 2016;311:F46–F51. doi: 10.1152/ajprenal.00184.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Visweswaran GR, et al. Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-harnessed SAINT-based lipid carrier system. PLoS ONE. 2015;10:e0138870. doi: 10.1371/journal.pone.0138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chiang WC, et al. Establishment of protein delivery systems targeting podocytes. PLoS ONE. 2010;5:e11837. doi: 10.1371/journal.pone.0011837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chaudhury C, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wei C, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 236.Maile LA, et al. Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology. 2014;155:4665–4675. doi: 10.1210/en.2014-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Maile LA, et al. Blocking αVβ3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats. J Diabetes Res. 2014;2014:421827. doi: 10.1155/2014/421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pollinger K, et al. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small. 2012;8:3368–3375. doi: 10.1002/smll.201200733. [DOI] [PubMed] [Google Scholar]
- 239.Zuckerman JE, Gale A, Wu P, Ma R, Davis ME. siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther. 2015;25:53–64. doi: 10.1089/nat.2014.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ramos AM, et al. Designing drugs that combat kidney damage. Expert Opin Drug Discov. 2015;10:541–556. doi: 10.1517/17460441.2015.1033394. [DOI] [PubMed] [Google Scholar]
- 241.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol. 2015;11:233–244. doi: 10.1038/nrneph.2014.246. [DOI] [PubMed] [Google Scholar]
- 242.Morishita Y, et al. siRNAs targeted to Smad4 prevent renal fibrosis in vivo. Sci Rep. 2014;4:6424. doi: 10.1038/srep06424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zheng X, et al. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. 2006;82:1781–1786. doi: 10.1097/01.tp.0000250769.86623.a3. [DOI] [PubMed] [Google Scholar]
- 244.Williams RM, et al. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015;15:2358–2364. doi: 10.1021/nl504610d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dolman ME, et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int J Nanomedicine. 2012;7:417–433. doi: 10.2147/IJN.S26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Morishita Y, et al. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine. 2015;10:3475–3488. doi: 10.2147/IJN.S82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pascual D, Borque A. Epidemiology of kidney cancer. Adv Urol. 2008;2008:782381. doi: 10.1155/2008/782381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Koshkina NV, et al. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7:3258–3262. [PubMed] [Google Scholar]
- 249.Boorjian SA, et al. Phase 1/2 clinical trial of interferon α2b and weekly liposome-encapsulated all-trans retinoic acid in patients with advanced renal cell carcinoma. J Immunother. 2007;30:655–662. doi: 10.1097/CJI.0b013e31805449a8. [DOI] [PubMed] [Google Scholar]
- 250.Kulkarni AA, Vijaykumar VE, Natarajan SK, Sengupta S, Sabbisetti VS. Sustained inhibition of cMET–VEGFR2 signaling using liposome-mediated delivery increases efficacy and reduces toxicity in kidney cancer. Nanomedicine. 2016;12:1853–1861. doi: 10.1016/j.nano.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 251.Liu J, et al. Comparison of sorafenib-loaded poly (lactic/glycolic) acid and DPPC liposome nanoparticles in the in vitro treatment of renal cell carcinoma. J Pharm Sci. 2015;104:1187–1196. doi: 10.1002/jps.24318. [DOI] [PubMed] [Google Scholar]
- 252.Takaha N, et al. Significant induction of apoptosis in renal cell carcinoma cells transfected with cationic multilamellar liposomes containing the human interferon-β gene through activation of the intracellular type 1 interferon signal pathway. Int J Oncol. 2012;40:1441–1446. doi: 10.3892/ijo.2012.1377. [DOI] [PubMed] [Google Scholar]
- 253.Umebayashi M, Makizono T, Ichihara H, Matsumoto Y, Ueoka R. Inhibitory effects of cationic hybrid liposomes on the growth of human renal cell carcinoma. Anticancer Res. 2010;30:327–337. [PubMed] [Google Scholar]
- 254.Umebayashi M, Matsumoto Y, Ueoka R. Inhibitory effects of three-component hybrid liposomes containing cationic lipids without drugs on the growth of human renal tumor cells in vitro. Biol Pharm Bull. 2008;31:1816–1817. doi: 10.1248/bpb.31.1816. [DOI] [PubMed] [Google Scholar]
- 255.Yamamoto K, et al. Significant antitumor activity of cationic multilamellar liposomes containing human interferon-β gene in combination with 5-fluorouracil against human renal cell carcinoma. Int J Oncol. 2008;33:565–571. [PubMed] [Google Scholar]
- 256.Nakanishi H, et al. Significant antitumoral activity of cationic multilamellar liposomes containing human IFN-β gene against human renal cell carcinoma. Clin Cancer Res. 2003;9:1129–1135. [PubMed] [Google Scholar]
- 257.Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in renal cell cancer. Invest New Drugs. 2002;20:101–104. doi: 10.1023/a:1014428720551. [DOI] [PubMed] [Google Scholar]
- 258.Tian JQ, et al. In vitro enhanced cytotoxicity of tumor-infiltrating lymphocytes transfected with tumor necrosis factor-related apoptosis-inducing ligand and/or interleukin-2 gene in human renal cell carcinoma. Urology. 2006;67:1093–1098. doi: 10.1016/j.urology.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 259.Akita H, et al. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J Control Release. 2015;200:97–105. doi: 10.1016/j.jconrel.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 260.Yalcin M, et al. Tetraidothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res. 2009;29:3825–3831. [PubMed] [Google Scholar]
- 261.Kasenda B, Larkin J, Gore M. Immunotherapies in early and advanced renal cell cancer. Prog Tumor Res. 2015;42:1–10. doi: 10.1159/000436988. [DOI] [PubMed] [Google Scholar]
- 262.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 263.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 264.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 265.Miller MA, et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med. 2015;7:314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ramanathan RK, et al. Pilot study in patients with advanced solid tumors to evaluate feasibility of ferumoxytol (FMX) as tumor imaging agent prior to MM398, a nanoliposomal irinotecan (nalIRI) [abstract] Cancer Res. 2014;74(19 Suppl):CT224. [Google Scholar]
- 267.Koukourakis MI, et al. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol. 1999;17:3512–3521. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 268.Arrieta O, et al. High liposomal doxorubicin tumour tissue distribution, as determined by radiopharmaceutical labelling with 99mTc-LD, is associated with the response and survival of patients with unresectable pleural mesothelioma treated with a combination of liposomal doxorubicin and cisplatin. Cancer Chemother Pharmacol. 2014;74:211–215. doi: 10.1007/s00280-014-2477-x. [DOI] [PubMed] [Google Scholar]
- 269.Grenier N, Merville P, Combe C. Radiologic imaging of the renal parenchyma structure and function. Nat Rev Nephrol. 2016;12:348–359. doi: 10.1038/nrneph.2016.44. [DOI] [PubMed] [Google Scholar]
- 270.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 271.Wilmer MJ, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016;34:156–170. doi: 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 272.Jang KJ, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb ) 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 273.Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 274.Snouber LC, et al. Analysis of transcriptomic and proteomic profiles demonstrates improved Madin–Darby canine kidney cell function in a renal microfluidic biochip. Biotechnol Prog. 2012;28:474–484. doi: 10.1002/btpr.743. [DOI] [PubMed] [Google Scholar]
- 275.Zhou M, Ma H, Lin H, Qin J. Induction of epithelial-to-mesenchymal transition in proximal tubular epithelial cells on microfluidic devices. Biomaterials. 2014;35:1390–1401. doi: 10.1016/j.biomaterials.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 276.Kim S, Takayama S. Organ-on-a-chip and the kidney. Kidney Res Clin Pract. 2015;34:165–169. doi: 10.1016/j.krcp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Adler M, et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol. 2016;27:1015–1028. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kim S, et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8:015021. doi: 10.1088/1758-5090/8/1/015021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mae S, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Schmidt-Ott KM. How to grow a kidney: patient-specific kidney organoids come of age. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw256. http://dx.doi.org/10.1093/ndt/gfw256. [DOI] [PubMed]
- 281.Li Z, et al. 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell. 2016;19:516–529. doi: 10.1016/j.stem.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 283.Takasato M, Little MH. A strategy for generating kidney organoids: recapitulating the development in human pluripotent stem cells. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.08.024. http://dx.doi.org/10.1016/j.ydbio.2016.08.024. [DOI] [PMC free article] [PubMed]
- 284.Takasato M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 285.Reininger-Mack A, Thielecke H, Robitzki AA. 3D-biohybrid systems: applications in drug screening. Trends Biotechnol. 2002;20:56–61. doi: 10.1016/s0167-7799(01)01880-7. [DOI] [PubMed] [Google Scholar]
- 286.Astashkina AI, et al. Nanoparticle toxicity assessment using an in vitro 3D kidney organoid culture model. Biomaterials. 2014;35:6323–6331. doi: 10.1016/j.biomaterials.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 287.Astashkina AI, Mann BK, Prestwich GD, Grainger DWA. 3D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials. 2012;33:4700–4711. doi: 10.1016/j.biomaterials.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 288.Valencia PM, et al. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano. 2013;7:10671–10680. doi: 10.1021/nn403370e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kamaly N, et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Karnik R, et al. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- 291.Valencia PM, et al. Single-step assembly of homogenous lipid-polymeric and lipid-quantum dot nanoparticles enabled by microfluidic rapid mixing. ACS Nano. 2010;4:1671–1679. doi: 10.1021/nn901433u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rhee M, et al. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv Mater. 2011;23:H79–H83. doi: 10.1002/adma.201004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chen D, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 294.Kim Y, et al. Mass production and size control of lipid–polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012;12:3587–3591. doi: 10.1021/nl301253v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lim JM, et al. Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer. ACS Nano. 2014;8:6056–6065. doi: 10.1021/nn501371n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zuckerman JE, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Eliasof S, et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Natl Acad Sci USA. 2013;110:15127–15132. doi: 10.1073/pnas.1309566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kohan DE. Kidney cell-specific knockdown: anything but simple. Am J Physiol Renal Physiol. 2015;309:F1007–F1008. doi: 10.1152/ajprenal.00434.2015. [DOI] [PubMed] [Google Scholar]
- 299.de Caestecker M, et al. Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol. 2015;26:2905–2916. doi: 10.1681/ASN.2015070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.National Library US of Medicine. ClinicalTrialsgov. 2014 https://clinicaltrials.gov/ct2/show/NCT00617981?term.
- 301.Escudier B, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]