Abstract
Bronchial thermoplasty is an endoscopic therapy for severe asthma. The previously reported, randomised sham-controlled AIR2 (Asthma Intervention Research 2) trial showed a significant reduction in severe asthma exacerbations, emergency department visits and hospitalisations after bronchial thermoplasty. More “real-world” clinical outcome data is needed.
This article compares outcomes in bronchial thermoplasty subjects with 3 years of follow-up from the ongoing, post-market PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study with those from the AIR2 trial.
279 subjects were treated with bronchial thermoplasty in the PAS2 study. We compared the first 190 PAS2 subjects with the 190 bronchial thermoplasty-treated subjects in the AIR2 trial at 3 years of follow-up. The PAS2 subjects were older (mean age 45.9 versus 40.7 years) and more obese (mean body mass index 32.5 versus 29.3 kg·m−2) and took higher doses of inhaled corticosteroids (mean dose 2301 versus 1961 μg·day−1). More PAS2 subjects had experienced severe exacerbations (74% versus 52%) and hospitalisations (15.3% versus 4.2%) in the 12 months prior to bronchial thermoplasty. At year 3 after bronchial thermoplasty, the percentage of PAS2 subjects with severe exacerbations, emergency department visits and hospitalisations significantly decreased by 45%, 55% and 40%, respectively, echoing the AIR2 results.
The PAS2 study demonstrates similar improvements in asthma control after bronchial thermoplasty compared with the AIR2 trial despite enrolling subjects who may have had poorer asthma control.
Short abstract
A comparison of the results obtained thus far in PAS2 with AIR2 evaluating bronchial thermoplasty in severe asthma http://ow.ly/sB0X30csDuE
Introduction
The Alair™ Bronchial Thermoplasty System (Boston Scientific, Marlborough, MA, USA) was developed as a novel system designed to deliver radiofrequency energy to the airways of asthmatic subjects. Bronchial thermoplasty is a nonpharmacological, endoscopic treatment for subjects aged ≥18 years with severe persistent asthma that is not well controlled with inhaled corticosteroids (ICSs) and long-acting β-agonists (LABAs). During the bronchial thermoplasty procedure, radiofrequency energy is used to heat the airway walls in a controlled manner. The mechanism of action is, in part, a lasting reduction in airway smooth muscle (ASM) mass due to the heat produced during the procedure [1–5]. This effect was demonstrated in dogs in the 1980s [6] and in humans more recently in four clinical reports that demonstrated this reduction in ASM in biopsy samples taken from small numbers of subjects with asthma who underwent bronchial thermoplasty [1, 2, 4, 5]. One of these studies reported that the reduction in ASM was still apparent in all nine subjects who consented to a second bronchoscopy and biopsy at least 24 months after bronchial thermoplasty [4]. The reduction in ASM is associated with clinical improvements seen in subjects undergoing bronchial thermoplasty [2, 3]. Histopathological changes other than ASM reduction have also been demonstrated (e.g. a decrease in the thickness of the reticular basement membrane due to reduced collagen type I deposition has also been shown in these clinical studies). Additional mechanisms of action may also contribute to symptom reduction, including structural effects on neuroendocrine epithelial cells and bronchial nerve endings [3]. Future studies may help to further define these mechanisms [3, 7].
Several randomised controlled clinical trials of bronchial thermoplasty, such as the AIR2 (Asthma Intervention Research 2) trial and others, have shown significantly improved clinical outcomes and relative safety in subjects who underwent bronchial thermoplasty [8–11]. In addition to these randomised controlled trials, open-label studies including the PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study are ongoing to confirm the safety and efficacy of bronchial thermoplasty when performed in clinical practice [12, 13].
The pivotal AIR2 trial was a randomised sham-controlled trial of the Alair system in subjects with severe persistent asthma. This trial demonstrated that over a 12-month follow-up period after bronchial thermoplasty treatment, bronchial thermoplasty-treated subjects experienced clinically relevant improvements. Specifically, subjects in this study reported improved Asthma Quality of Life Questionnaire (AQLQ) scores and reductions in the number of severe asthma exacerbations they experienced after bronchial thermoplasty. These improvements persisted through 5 years of follow-up. The detailed methods and results from the AIR2 trial are described by Wechsler et al. [11] and Castro et al. [14] .
The Alair system received pre-market approval from the US Food and Drug Administration (FDA) in April 2010. Subsequently, the PAS2 study was initiated in fulfilment of FDA requirements for pre-market approval. Here, we describe an interim analysis of accumulating data for the first 190 subjects enrolled in the PAS2 study and compare the 3-year follow-up data with the results obtained in subjects from the pivotal AIR2 trial at the same time-point.
Methods
Study design
The PAS2 study was designed to demonstrate the short- and long-term treatment effectiveness and the safety profile of the Alair system in clinical practice. It is a prospective, open-label, observational, multicentre clinical study (ClinicalTrials.gov: NCT01350336) that began enrolment in 2011 at 27 centres in the USA (n=23) and Canada (n=4), and was approved by the ethics committee at each site. All participants in the study provided written informed consent. PAS2 is currently an active study with the last subject expected to exit in January 2020 after completing 5 years of follow-up.
Study subjects
PAS2 subjects were enrolled if their asthma was inadequately controlled despite optimised treatment with high ICS and LABA doses. Subjects in the PAS2 study were allowed to take medications in addition to ICSs and LABAs/short-acting β-agonists (SABAs), including low doses of oral corticosteroids (OCSs). A comparison of the inclusion and exclusion criteria for the PAS2 study and the AIR2 trial is shown in table 1. A total of 284 study subjects were enrolled in the PAS2 study between 2011 and 2014, and 279 of these subjects were treated with at least one bronchial thermoplasty procedure. At the time of the current analysis, 252 subjects remain actively enrolled in long-term follow-up or have completed the final follow-up at 5 years.
TABLE 1.
Differences in inclusion and exclusion criteria between the PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study and AIR2 (Asthma Intervention Research 2) trial
| PAS2 | AIR2 | |
| Inclusion | Age 18–65 years Able to provide written informed consent Willing and able to comply with study protocol ICS >1000 μg·day−1 (beclomethasone equivalent), LABA ≥80 μg salmeterol or equivalent May also be taking leukotriene modifiers and/or anti-IgE OCS ≤10 mg·day−1 Pre-bronchodilator FEV1 % pred ≥60% Nonsmoker for ≥1 year (if former smoker, <10 pack-years total smoking history) Able to undergo outpatient bronchoscopy procedures Has had at least 2 days of asthma symptoms in the last 4 weeks AQLQ score ≤6.25 during baseline period |
Age 18–65 years Able to provide written informed consent Willing and able to comply with study protocol including requirements for taking and abstaining from medications ICS >1000 μg·day−1 (beclomethasone equivalent), LABA ≥100 μg salmeterol or equivalent May also be taking leukotriene modifiers and/or anti-IgE OCS ≤10 mg·day−1 Pre-bronchodilator FEV1 % pred ≥60% Nonsmoker for ≥1 year (if former smoker, <10 pack-years total smoking history) Able to undergo outpatient bronchoscopy procedures Has had at least 2 days of asthma symptoms in the last 4 weeks AQLQ score ≤6.25 during baseline period PC20 <8 mg·mL−1 per methacholine inhalation test using standardised methods |
| Exclusion | Participation in another trial within 6 weeks of baseline period involving respiratory intervention Over the last 7 days of a 4-week medication stable period, rescue medication usage exceeds an average of 8 puffs·day−1 SABA, 4 puffs·day−1 rescue bronchodilator or two nebuliser treatments·day−1 Post-bronchodilator FEV1 % pred <65% History of life-threatening asthma (previous intubation or ICU admission in prior 2 years) ≥4 lower respiratory tract infections in previous 12 months ≥3 hospitalisations for asthma in the previous 12 months ≥4 pulses of systemic corticosteroids in the past 12 months Known sensitivity to medications required to perform bronchoscopy Other respiratory diseases including emphysema, cystic fibrosis, vocal cord dysfunction, mechanical upper airway obstruction, Churg–Strauss syndrome, allergic aspergillosis Segmental atelectasis, lobar consolidation, significant or unstable pulmonary infiltrate, or pneumothorax confirmed by chest radiography Cardiovascular disease including myocardial infarction, angina, cardiac dysfunction, cardiac dysrhythmia, conduction defect, cardiomyopathy or stroke Known aortic aneurysm Significant comorbid illness including cancer, renal failure, liver disease or cerebral vascular disease Uncontrolled hypertension Implanted electrical stimulation device Known coagulopathy Any other medical condition that could interfere with study participation in the opinion of the investigator |
Participation in another trial within 6 weeks of baseline period involving respiratory intervention Over the last 7 days of a 4-week medication stable period, rescue medication usage exceeds an average of 8 puffs·day−1 SABA, 4 puffs·day−1 rescue bronchodilator or two nebuliser treatments·day−1 Post-bronchodilator FEV1 % pred <65% History of life-threatening asthma (previous intubation or ICU admission in prior 2 years) ≥4 lower respiratory tract infections in previous 12 months ≥3 hospitalisations for asthma in the previous 12 months ≥4 pulses of systemic corticosteroids in the past 12 months Known sensitivity to medications required to perform bronchoscopy Known systemic hypersensitivity or contraindication to methacholine chloride or other parasympathomimetic agents Undergoing immunosuppressant therapy (i.e. methotrexate) Use of β-adrenergic blocking agents Use of anticoagulant medication Insulin-dependent diabetes Pregnant/nursing mother or plans to become pregnant within the next year Other respiratory diseases including emphysema, cystic fibrosis, vocal cord dysfunction, mechanical upper airway obstruction, obstructive sleep apnoea, Churg–Strauss syndrome, allergic aspergillosis Segmental atelectasis, lobar consolidation, significant or unstable pulmonary infiltrate, or pneumothorax confirmed by chest radiography Interstitial lung disease Chronic sinus disease Uncontrolled gastro-oesophageal reflux disease History of epilepsy Cardiovascular disease including myocardial infarction, angina, cardiac dysfunction, cardiac dysrhythmia, conduction defect, cardiomyopathy or stroke Known aortic aneurysm Significant comorbid illness including cancer, renal failure, liver disease or cerebral vascular disease Uncontrolled hypertension Implanted electrical stimulation device Known coagulopathy Psychiatric or other disorder that could interfere with study participation in the opinion of the investigator |
Differences are indicated in italics. ICS: inhaled corticosteroid; LABA: long-acting β-agonist; OCS: oral corticosteroid; FEV1: forced expiratory volume in 1 s; AQLQ: Asthma Quality of Life Questionnaire; PC20: provocative concentration reducing FEV1 by 20% from baseline; SABA: short-acting β-agonist; ICU: intensive care unit.
Treatment
All PAS2 subjects were scheduled to undergo three bronchoscopy procedures performed ∼3 weeks apart. Bronchial thermoplasty treatments were administered per FDA labelling by the investigators. The right lower lobe of the lung was treated during the first session, the left lower lobe during the second session and both upper lobes during the third session. Bronchial thermoplasty was performed using the Alair system as previously described [10, 11].
Follow-up
PAS2 subjects were evaluated at each bronchoscopy visit and at 6 weeks after the last procedure (the end of the treatment period). Subsequently, subjects were scheduled to be seen at annual office visits up to 5 years after the bronchial thermoplasty treatments. Subjects were also contacted by phone every 3 months between annual office visits. During each phone call and at each office visit, information from each subject about adverse events experienced, maintenance asthma medications, severe exacerbations, emergency department visits for asthma symptoms and hospitalisations for asthma symptoms was solicited using a standardised, structured interview questionnaire. At each annual office visit, a physical examination was also performed, and forced expiratory volume in 1 s (FEV1) was measured both pre- and post-bronchodilator.
Outcome measures
The primary end-point of the PAS2 study is the proportion of subjects experiencing severe exacerbations during the subsequent 12-month period (for years 2, 3, 4 and 5) compared with the first 12-month period after bronchial thermoplasty. A severe exacerbation is defined for the PAS2 study as a worsening of asthma symptoms requiring the use of systemic corticosteroids (tablets, suspension or injection). For subjects already taking OCSs on a daily or alternate-day basis, a severe exacerbation is defined as a worsening of symptoms requiring an increase in the daily dose of systemic corticosteroids. This definition is consistent with the National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma [15]. Unlike the AIR2 trial, the PAS2 study did not include a doubling of ICS dose as part of the definition of a severe exacerbation. Other end-points related to the safety of the Alair system include respiratory adverse events, serious adverse events, and measurements for pre- and post-bronchodilator FEV1.
Adverse event monitoring
Adverse events were actively solicited and recorded at each follow-up visit in the post-treatment period. Periprocedural adverse events inside the treatment period were reported separately. Investigators reported the severity of each event during the study.
Statistical analyses
Baseline demographics, characteristics and outcomes of the first 190 PAS2 subjects were compared with those seen in the 190 AIR2 subjects who received the bronchial thermoplasty treatment. The proportion of PAS2 and AIR2 subjects experiencing severe exacerbations, emergency department visits for respiratory symptoms and hospitalisations for respiratory symptoms during the 12-month period preceding bronchial thermoplasty treatment was compared with the proportion of subjects experiencing these events during the 3 years after treatment completion. Continuous variables were summarised with sample size, mean, standard deviation, and minimum and maximum; binary variables were summarised with proportions (numerator over denominator). Comparisons between the PAS2 study and AIR2 trial were done using a t-test for continuous variables and Fisher's exact test for binary variables. Repeated measures analysis in a logistic model was used to look for differences in proportions across time.
Results
The characteristics from the first 190 subjects who were enrolled in the PAS2 study were included in this analysis and compared with those reported for the 190 bronchial thermoplasty subjects included in the AIR2 trial. 168 out of these 190 PAS2 subjects completed 3 years of follow-up and the outcomes at this time-point were compared with those seen in the 165 AIR2 subjects who reached their 3-year follow-up visit.
Baseline demographics and clinical characteristics
The demographics, baseline characteristics and baseline medications for the PAS2 subjects are summarised in table 2. Compared with the results from the previously reported AIR2 trial, the PAS2 subjects were on average 5.2 years older (45.9 versus 40.7 years; p<0.0001) and had been diagnosed with asthma for longer before study enrolment (25.6 versus 22.9 years; p=0.059). PAS2 subjects also had a higher BMI (32.5 versus 29.3 kg·m−2; p<0.0001) than AIR2 subjects. They had a mean baseline pre-bronchodilator FEV1 % pred of 79.6% and a mean baseline post-bronchodilator FEV1 % pred of 84.8% (bronchodilator reversibility 5.2%). Their mean AQLQ score was 4.17. Subjects enrolled in the PAS2 study were more likely to have experienced exacerbations (74.2% versus 51.6%; p<0.0001) and hospitalisations (15.3% versus 4.2%; p=0.0003) in the 12 months prior to bronchial thermoplasty than the AIR2 subjects. PAS2 and AIR2 subjects had similar rates of emergency department visits (27.4% versus 28.9%; p=0.7322).
TABLE 2.
Baseline demographics and characteristics for subjects enrolled in the PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study and AIR2 (Asthma Intervention Research 2) trial
| PAS2 | AIR2, bronchial thermoplasty | p-value | |
| Subjects | 190 | 190 | |
| Demographics | |||
| Age years | 45.87±11.39 (190) (18.00–66.00) |
40.69±11.89 (190) (18.00–63.00) |
<0.0001 |
| Female | 61.6 (117/190) | 57.4 (109/190) | 0.4032 |
| BMI kg·m−2 | 32.50±7.72 (190) (18.48–61.27) |
29.29±6.16 (190) (17.63–52.77) |
<0.0001 |
| Baseline medication usage | |||
| ICS dose μg·day−1 | 2301.04±807.46 (189) (750.00–6480.00) |
1960.74±745.19 (190) (880.00–6000.00) |
<0.0001 |
| LABA dose μg·day−1 | 106.87±39.36 (189) (75.00–416.67) |
116.8±34.39 (189) | 0.0031 |
| SABA puffs·day−1 | 2.38±1.48 (182) (1.00–18.00) |
2.24±1.29 (168) (1.00–8.00) |
0.3451 |
| Other asthma medications | |||
| OCS | 18.9 (36/190) | 4.2 (8/190) | <0.0001 |
| Dose mg·day−1 | 9.13±2.66 (35) (5.00–17.00) |
11.88±15.51 (8) (5.00–50.00) |
0.3125 |
| Methylxanthines | 4.2 (8/190) | 2.6 (5/190) | 0.3972 |
| Leukotriene modifier | 44.2 (84/190) | 0.0 (0/190) | <0.0001 |
| Omalizumab | 15.8 (30/190) | 1.1 (2/190) | <0.0001 |
| Dose mg·day−1 | 266.83±88.67 (30) (150.00–405.00) |
350.00±35.36 (2) (325.00–375.00) |
0.2026 |
| Other | 40.5 (77/190) | 31.1 (59/190) | 0.0541 |
| Any of the above maintenance medications | 70.5 (134/190) | 33.7 (64/190) | <0.0001 |
| Quality of life measurement: AQLQ | 4.17±1.33 (190) (1.56–6.59) |
4.30±1.17 (190) (1.63–6.28) |
0.2936 |
| ERS/ATS guidelines: modified definition of severe asthma# | 94.7 (180/190) | 82.1 (156/190) | 0.0001 |
| Spirometry | |||
| Pre-bronchodilator FEV1 % pred | 79.63±13.10 (190) (59.52–125.93) |
77.83±15.65 (190) (50.15–120.27) |
0.2255 |
| Post-bronchodilator FEV1 % pred | 84.82±12.90 (190) (65.09–130.69) |
86.06±15.76 (190) (56.09–130.29) |
0.4009 |
| Pre-bronchodilator FEV1 L | 2.54±0.65 (190) (1.28–4.93) |
2.59±0.73 (190) (1.31–5.68) |
0.4517 |
| Post-bronchodilator FEV1 L | 2.70±0.66 (190) (1.40–5.18) |
2.87±0.79 (190) (1.34–5.83) |
0.0225 |
| Medical history: how long the subject has been diagnosed with asthma years | 25.62±14.46 (190) (1.00–60.00) |
22.91±13.37 (190) (1.00–59.00) |
0.0591 |
| Prior 12 months | |||
| Subjects | |||
| Severe exacerbations | 74.2 (141/190) | 51.6 (98/190) | <0.0001 |
| Hospitalisations for asthma | 15.3 (29/190) | 4.2 (8/190) | 0.0003 |
| Emergency department visits for asthma | 27.4 (52/190) | 28.9 (55/190) | 0.7322 |
| Events | |||
| Severe exacerbations | 1.57±1.15 (190) (0.00–3.00) |
0.88±1.03 (190) (0.00–3.00) |
<0.0001 |
| Hospitalisations for asthma | 0.21±0.53 (190) (0.00–2.00) |
0.05±0.27 (190) (0.00–2.00) |
0.0003 |
| Emergency department visits for asthma | 0.52±1.16 (190) (0.00–10.00) |
0.74±1.71 (190) (0.00–10.00) |
0.1409 |
Data are presented as N, mean±sd (n or N) (range) or % (n/N), unless otherwise stated. BMI: body mass index; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; SABA: short-acting β-agonist; AQLQ: Asthma Quality of Life Questionnaire; ERS: European Respiratory Society; ATS: American Thoracic Society; FEV1: forced expiratory volume in 1 s. #: the definition used for severe asthma was modified from the ERS/ATS guideline definition as Asthma Control Questionnaire and the Asthma Control Test scores were not collected for both studies; a subject was considered to have severe asthma if one of the following was true: baseline ICS ≥2000 μg·day−1 (beclomethasone equivalent) and LABA/leukotriene modifier usage; or two or more severe exacerbations in the 12 months prior to first bronchial thermoplasty; or one or more hospitalisations in the 12 months prior to first bronchial thermoplasty; or post-bronchodilator FEV1 % pred <80% and FEV1/forced vital capacity <0.7.
There were also significant differences in baseline medication usage between the PAS2 study and AIR2 trial. In the PAS2 study, the mean ICS dosage at baseline was 2301 μg·day−1 (beclomethasone equivalent), while in the AIR2 trial, the mean daily ICS dose at baseline was significantly lower (p<0.0001) at 1961 μg·day−1. In addition, at baseline, 18.9% of PAS2 subjects were taking OCSs at a mean dose of 9 mg·day−1, 44.2% were taking leukotriene modifiers and 15.8% were taking omalizumab. In contrast, only 4.2% of the bronchial thermoplasty subjects in AIR2 were taking OCSs at baseline (at a mean dose of 11.9 mg·day−1), no subjects were taking leukotriene modifiers and only 1.1% were taking omalizumab (p<0.0001 for each of these medications).
Based on a modified version of the European Respiratory Society/American Thoracic Society guideline definition for severe asthma [16], there were 94.7% severe asthmatic subjects in the PAS2 study and 82.1% severe asthmatic subjects in the AIR2 trial (p=0.0001). As Asthma Control Questionnaire and the Asthma Control Test scores were not collected for both studies, we applied the components of the definition we had available (table 2).
Medication reduction at 3 years after bronchial thermoplasty
By their 3-year follow-up visits, the PAS2 subjects were able to significantly reduce their mean ICS daily dose to 2070 μg·day−1 (p=0.003) (table 3). In the AIR2 trial, the mean ICS daily dose was also significantly reduced to 1841 μg·day−1 (p=0.006). In addition, the percentage of PAS2 study subjects who were taking daily OCSs to improve asthma control was reduced from 18.9% at baseline to 10.2% in the third year after bronchial thermoplasty (p=0.0004) (table 3). This decrease was not apparent in the AIR2 trial (p=0.52), where a lower percentage (4%) of the bronchial thermoplasty subjects were on maintenance OCS medication at baseline.
TABLE 3.
Medication usage at baseline and at each yearly follow-up visit for subjects in the PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study and AIR2 (Asthma Intervention Research 2) trial treated with at least one bronchial thermoplasty procedure
| Baseline | Year 1 | Year 2 | Year 3 | |
| PAS2 subjects# | ||||
| ICS dose μg·day−1 | 2301.0±807.5 (189) (750.0–6480.0) |
2108.5±938.3 (175) (440.0–7500.0) |
1981.7±1051.1 (164) (176.0–8100.0) |
2069.7±1158.2 (157) (176.0–8100.0) |
| LABA dose μg·day−1 | 106.9±39.4 (189) (75.0–416.7) |
106.2±50.7 (172) (20.0–416.7) |
104.2±49.8 (156) (20.0–416.7) |
104.9±53.5 (149) (20.0–416.7) |
| SABA puffs·day−1 | 2.4±1.5 (182) (1.0–18.0) |
2.4±1.5 (173) (1.0–18.0) |
2.4±1.5 (166) (1.0–18.0) |
2.4±1.5 (162) (1.0–18.0) |
| OCS | 18.9 (36/190) | 8.9 (16/180) | 11.8 (20/170) | 10.2 (17/166) |
| Dose mg·day−1 | 9.1±2.7 (35) (5.0–17.0) |
8.5±3.2 (14) (5.0–15.0) |
15.8±7.9 (19) (5.0–30.0) |
14.6±6.9 (15) (5.0–30.0) |
| Leukotriene modifier | 44.2 (84/190) | 45.0 (81/180) | 42.4 (72/170) | 43.4 (72/166) |
| Omalizumab | 15.8 (30/190) | 14.4 (26/180) | 14.1 (24/170) | 14.5 (24/166) |
| AIR2 subjects# | ||||
| ICS dose μg·day−1 | 1960.7±745.2 (190) (880.0–6000.0) |
1970.9±765.0 (177) (55.0–6000.0) |
1830.4±869.8 (158) (110.0–6000.0) |
1840.9±901.8 (151) (200.0–6000.0) |
| LABA dose μg·day−1 | 122.2±50.6 (190) (24.0–500.0) |
115.8±35.3 (171) (24.0–200.0) |
114.9±40.5 (148) (24.0–200.0) |
116.7±42.7 (143) (24.0–250.0) |
| SABA puffs·day−1 | 2.2±1.3 (168) (1.0–8.0) |
2.0±1.0 (153) (1.0–8.0) |
2.0±0.9 (135) (1.0–8.0) |
2.0±0.9 (134) (1.0–8.0) |
| OCS | 4.2 (8/190) | 3.9 (7/181) | 4.8 (8/165) | 3.7 (6/162) |
| Dose mg·day−1 | 11.9±15.5 (8) (5.0–50.0) |
7.1±2.2 (7) (5.0–10.0) |
8.1±3.5 (8) (5.0–15.0) |
7.3±2.5 (6) (4.0–10.0) |
| Leukotriene modifier | 0.0 (0/190) | 0.0 (0/181) | 0.0 (0/165) | 0.0 (0/162) |
| Omalizumab | 1.1 (2/190) | 1.1 (2/181) | 1.8 (3/165) | 1.9 (3/162) |
Data are presented as mean±sd (n or N) (range) or % (n/N). ICS: inhaled corticosteroid; LABA: long-acting β-agonist; SABA: short-acting β-agonist; OCS: oral corticosteroid. #: N=190, unless otherwise indicated.
Severe exacerbations
During the third year of follow-up after the last bronchial thermoplasty procedure, 39.9% of the PAS2 study subjects experienced at least one severe asthma exacerbation (figure 1a and b, and supplementary table S1). This represents a 44.6% relative decrease in severe exacerbations after bronchial thermoplasty when compared with the 12 months prior to treatment (74.2% versus 39.9%; p<0.0001). This was similar to the 36.8% relative decrease in severe exacerbations reported for the AIR2 trial during year 3 after bronchial thermoplasty. There was a similar reduction in the number of severe exacerbations from baseline to 3 years (1.57 versus 0.64 events·subject−1; p<0.0001) (supplementary table S2).
FIGURE 1.
a) Proportion of subjects with severe exacerbations and b) severe exacerbation rates. c) Proportion of subjects with emergency department visits for respiratory symptoms and d) emergency department visit rates. e) Proportion of subjects with hospitalisations and f) hospitalisation rates. Error bars represent 95% CI.
Other end-points
In PAS2 subjects, we observed a 55% relative decrease in subjects with emergency department visits for respiratory symptoms in year 3 following bronchial thermoplasty compared with the 12 months prior to the treatment (27.4% versus 10.7%; p=0.0005) (figure 1c and d, and supplementary table S3). The AIR2 trial data showed an even larger 72.3% reduction in emergency department visits after bronchial thermoplasty. There was also a significant reduction in emergency department visits from baseline to 3 years (0.52 versus 0.18 events·subject−1; p=0.003) for PAS2 subjects (supplementary table S4).
The number of hospitalisations for PAS2 subjects was also recorded for the 12 months prior to bronchial thermoplasty and for each year following the treatment (figure 1e and f, and supplementary table S5). In the year prior to bronchial thermoplasty, 15.3% of the PAS2 study subjects were hospitalised at least once for asthma symptoms. Only 7.1% of the PAS2 subjects were hospitalised during year 3 after bronchial thermoplasty (p=0.055; relative decrease 40%). The AIR2 trial reported relative decreases of up to 25% in the 3 years post-bronchial thermoplasty. There was also a reduction in hospitalisations from baseline to 3 years (0.21 versus 0.10 events·subject−1; p=0.25) for PAS2 subjects; however, this reduction was not significant (supplementary table S6).
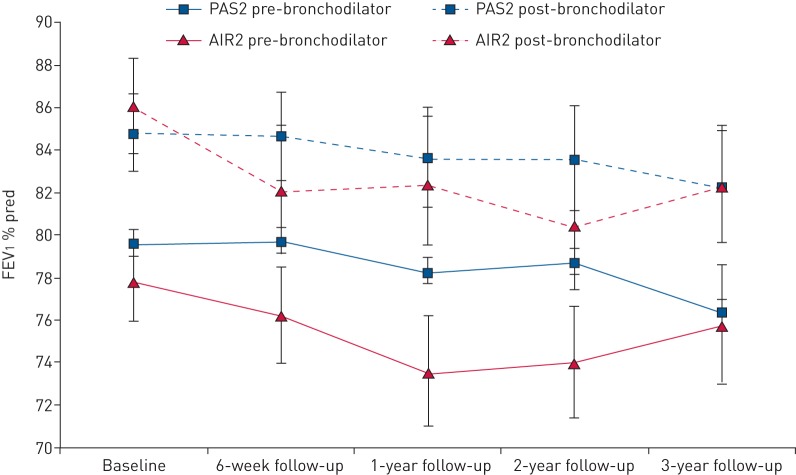
Finally, the PAS2 results confirm those from previous studies [3, 10, 17, 18] indicating that there is no effect on spirometric parameters of lung function following bronchial thermoplasty. Pre-bronchodilator FEV1 remained unchanged from baseline throughout the 3-year follow-up period in both the PAS2 study and AIR2 trial (figure 2 and supplementary table S8). In both the PAS2 study and AIR2 trial, post-bronchodilator FEV1 remained higher than pre-bronchodilator values at all times, indicating reversibility of asthma in the subjects. At baseline, PAS2 subjects had a pre-bronchodilator FEV1 % pred of 79.6% and a post-bronchodilator FEV1 % pred of 84.8% (percentage bronchodilator reversibility 5.2%). At the year 3 follow-up visit, PAS2 subjects had a pre-bronchodilator FEV1 % pred of 76.3% and a post-bronchodilator FEV1 % pred of 82.3% (percentage bronchodilator reversibility 6.1%). These values were similar to those in the AIR2 trial. The bronchodilator reversibility was significantly higher for AIR2 subjects at baseline compared with PAS2 subjects (p<0.0001), but this measure was no longer significantly different at 3 years (p=0.48).
FIGURE 2.
Forced expiratory volume in 1 s (FEV1) % pred over time in the AIR2 (Asthma Intervention Research 2) trial and PAS2 (Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma) study. Error bars represent 95% CI.
Adverse events
It is known that bronchoscopy can worsen asthma-related symptoms in the short term as well as induce other procedure-related complications that are associated with interventional pulmonary procedures in general, particularly in severe asthmatic subjects [19–21]. Overall, the respiratory adverse events observed in the PAS2 study and AIR2 trial during the treatment and post-treatment phases appear similar (supplementary table S9). Our data reveal that the percentages of subjects with periprocedural adverse events requiring hospitalisation or prolongation of hospitalisation during the treatment phase were comparable between the PAS2 study (13.2%) and AIR2 trial (8.4%) (p=0.19). We did, however, observe statistically significant differences between the PAS2 study and AIR2 trial during the treatment period in terms of severe exacerbations (55.8% versus 40.5%; p=0.004) and emergency department visits (15.8% versus 5.3%; p=0.0012), suggesting the risk of these events is higher in the PAS2 subjects during bronchial thermoplasty treatment.
There were no statistically significant differences in respiratory-related serious adverse events between the PAS2 study and AIR2 trial during the follow-up period outside the treatment phase.
Discussion
Subjects with difficult-to-control asthma suffer significant morbidity even while prescribed multiple medications, including high doses of ICSs, LABAs and systemic corticosteroids. Alternative management strategies for these subjects are required in order to improve their quality of life. Bronchial thermoplasty represents a nonpharmacological strategy that may assist in the management of these subjects, but while the data from several large clinical trials of bronchial thermoplasty (including the AIR, AIR2 and RISA (Research in Severe Asthma) trials [10, 17, 22]) indicate that the procedure is safe and effective, subjects enrolled in clinical trials are not representative of the most severe asthma cases seen in “real-world” clinical practice due to strict study eligibility criteria. Thus, available information on the safety and efficacy of bronchial thermoplasty in these most severe asthmatic subjects is limited. A few recent publications have reported on a limited number of severe asthmatic subjects and have indicated clinical improvement after bronchial thermoplasty in these subjects as well as acceptable rates of adverse events [1, 3–5]. In addition, Burn et al. [23] reported on subjects undergoing bronchial thermoplasty between 2011 and 2015 in the UK that were included in the Difficult Asthma Registry and the Hospital Episode Statistics database in order to obtain data on true “real-world” bronchial thermoplasty cases. They concluded that these subjects were on average older with worse baseline lung function and quality of life, and that after bronchial thermoplasty, more of these subjects experienced adverse events compared with previous clinical trials but that more data was required.
The AIR2 trial and PAS2 study described here are two of the largest studies to date of the Alair system for bronchial thermoplasty. Both studies were designed to evaluate the effectiveness of bronchial thermoplasty as well as durability of treatment and procedural safety in subjects with poorly controlled asthma who remained symptomatic despite treatment with the current standard of care. The eligibility criteria for the PAS2 study were largely the same as those used for the AIR2 trial as reported by Castro et al. [10], with a few important exceptions. Subjects in the AIR2 trial were required to be using LABAs at higher dosages than PAS2 subjects. Moreover, in the AIR2 trial, subjects were required to have a provocative concentration reducing FEV1 by 20% from baseline <8 mg·mL−1 per methacholine inhalation test, whereas no methacholine inhalation test was required for PAS2 subjects. AIR2 subjects could not be enrolled in the study if they had known systemic hypersensitivity or contraindication to methacholine chloride or other parasympathomimetic agents. Additionally, subjects with more comorbidities were allowed to enter the PAS2 study. These comorbidities included insulin-dependent diabetes, obstructive sleep apnoea, cardiac dysfunction, interstitial lung disease, chronic sinus disease, uncontrolled gastro-oesophageal reflux, epilepsy and known coagulopathy. These study entry criteria make the PAS2 study particularly important, because it is the first large-scale, prospective study performed in a post-market setting to validate previous clinical trial results in a population of subjects with severe asthma that are being managed in clinical practice. We acknowledge that the study entry criteria for PAS2 have some restrictions and thus the most severe asthma subjects seen in clinical practice were not included in the PAS2 study. However, recent studies have reported on subjects that were even more severe than those enrolled in PAS2, suggesting that bronchial thermoplasty improves asthma control across the spectrum of severe uncontrolled asthmatic subjects [10, 11].
The PAS2 study data presented a unique opportunity to gain insight into the safety and effectiveness of bronchial thermoplasty in subjects that may have had poorer asthma control at baseline than those included in previous clinical trials, such as AIR2. Therefore, we performed an analysis on the first 190 enrolled PAS2 subjects when a comparable number of them (n=168) reached the 3-year follow-up visit, so that a comparison between these PAS2 subjects and the subjects from the AIR2 randomised controlled trial, in which 190 subjects were enrolled and 165 reached a 3-year follow-up visit, could be made. Indeed, the PAS2 results presented here confirm the findings from the AIR2 trial [10, 11]. Specifically, the PAS2 study validates the AIR2 trial results showing that bronchial thermoplasty is a safe procedure, and that it significantly reduces steroid exacerbations, emergency department visits and hospitalisations in severe asthmatic subjects compared with the 12 months before bronchial thermoplasty treatment. For example, 76.5% of the PAS2 study subjects who had at least one severe exacerbation during the 12 months preceding their bronchial thermoplasty treatment experienced a decrease of at least one severe exacerbation per year by year 3 after bronchial thermoplasty. This was similar to the reduction in severe exacerbation rates reported in AIR2.
The PAS2 study data also contain some important differences from the AIR2 trial. Differences in baseline demographics and clinical correlates in the 12 months prior to bronchial thermoplasty indicate that the PAS2 subjects may have more severe asthma than the bronchial thermoplasty subjects enrolled in the AIR2 trial. They also have more comorbidities associated with higher BMI and older age, particularly cardiovascular disease. The PAS2 subjects also have a higher incidence of chronic sinus disease. However, more AIR2 subjects than PAS2 subjects had dermatological comorbidities (supplementary table S10). Despite this, the PAS2 subjects may indeed resemble “real-world” bronchial thermoplasty patients more closely. The PAS2 study data contribute to the body of literature that supports the safety and efficacy of bronchial thermoplasty in “real-world” populations. In terms of adverse events, the PAS2 study did show increases in both severe exacerbations and emergency department visits during the treatment period compared with the AIR2 trial. This could be due to a higher risk for more severe events in this population or because these subjects with more comorbidities have a lower threshold for healthcare utilisation.
Importantly, the PAS2 data suggest that the treatment effect of bronchial thermoplasty is both consistent and durable even in this population of subjects with poorly controlled asthma who may have associated comorbidities and more severe asthma. There was a continual improvement in asthma control during the first 3 years after bronchial thermoplasty treatment. Despite the improvements in these clinical correlates, there appears to be no difference in pre- and post-bronchodilator FEV1 over the 3 years of follow-up in the PAS2 study. This observation is consistent with that seen in the AIR2 trial, as well as with other reports on the use of bronchial thermoplasty in severe asthmatic subjects [10–12, 17, 24]. This is a reassuring observation and adds to the data indicating that over the long-term, bronchial thermoplasty does not adversely affect lung function.
Even though the PAS2 study may have included subjects that likely had more severe asthma compared with those included in AIR2, study subjects receiving bronchial thermoplasty have reduced their medication usage in the 3 years following treatment. There was a durable reduction in ICS usage at the 3-year follow-up. Moreover, even in PAS2 subjects with poorer asthma control, the percentage of subjects on daily maintenance OCSs significantly decreased after bronchial thermoplasty. OCS dosage for those subjects who remained on maintenance OCSs did not decrease, however, possibly due to the low initial dosage (OCS ≤10 mg·day−1) mandated at inclusion. This apparent reduction in the percentage of subjects taking daily maintenance OCSs is encouraging, because daily OCS use is associated with significant side-effects, including the development of obesity, diabetes and osteoporosis. Thus, identification of a treatment such as bronchial thermoplasty that allows a decrease in steroid exposure in severe asthmatic subjects will result in improved patient experiences and outcomes.
This work does have several important limitations. First, geographical differences in the locations of the investigational sites varied between the PAS2 study, which included subjects from North America only, and the AIR2 trial, which was more global and included patients from North America, Europe, Brazil and Australia. These geographic differences might have had an impact on the subject characteristics and comorbidities seen in each study. Second, as the PAS2 subjects are being followed for 5 years after bronchial thermoplasty, this article contains data on only a subgroup of subjects included in the study who have completed 3 years of follow-up. Additional analysis of the entire cohort at 3 and 5 years may further validate our observations, thus far consistent with the results of the AIR2 randomised controlled trial. The analysis is further limited by differences between the PAS2 study and AIR2 trial. Due to its follow-up schedule, the PAS2 study collected AQLQ data only at baseline and not at the post-procedure follow-up visits due to concerns over the ability of this tool to reliably capture asthma-related quality of life when only collected once annually. Therefore, we were unable to include this measure in our analysis. Also, the PAS2 study and AIR2 trial used slightly different definitions of severe asthma exacerbations, although a post hoc evaluation for these definition differences showed a difference of only one severe exacerbation. Moreover, the indirect comparison between the randomised AIR2 trial and the single-arm PAS2 study may be limited by cross-trial differences we failed to identify in our analysis. Finally, further subgroup analysis is needed help identify which asthma subjects are most likely to benefit from the bronchial thermoplasty procedure in the “real-world”. This would benefit asthma subjects currently not well managed with optimised pharmacological therapeutic treatment options.
Conclusions
The PAS2 study subjects described in this article appear to be slightly sicker than the subjects in the AIR2 trial based on baseline characteristics. The PAS2 study shows, similar to the AIR2 trial, that bronchial thermoplasty is safe and that subjects have markedly lower rates of steroid exacerbations, hospitalisations and emergency department visits at 3 years after bronchial thermoplasty compared with the 12 months prior to bronchial thermoplasty treatment, and that the treatment effect is consistent and durable. The study subjects also sustainably reduced their asthma medication, including complete discontinuation of maintenance OCSs for a significant number of subjects. Notably, the PAS2 study is a prospective nonrandomised clinical study, and comparing its results to a randomised controlled trial is challenging due to potential for exogenous bias and confounding factors outside those baseline demographics and clinical characteristics measured. Notwithstanding these caveats, this study offers useful results to clinicians convinced of the efficacy of bronchial thermoplasty within the confines of a randomised controlled trial, but who questioned its “real-world” clinical benefits. Although the prospectively enrolled PAS2 study population in this article is described as “real-world”, the study eligibility criteria mean that the most severe subjects often seen in clinical practice were not included. Nevertheless, the 3-year results from this subset of subjects in the PAS2 study inspire confidence, because they suggest that the “real-world” results obtained after bronchial thermoplasty in the PAS2 study echo those observed in the AIR2 randomised controlled trial.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00017-2017_Supplement (387.8KB, pdf)
Disclosures
S. Bansal ERJ-00017-2017_Bansal (1.2MB, pdf)
G. Chupp ERJ-00017-2017_Chupp (1.2MB, pdf)
L. Cohn ERJ-00017-2017_Cohn (1.2MB, pdf)
G.M. Grubb ERJ-00017-2017_Grubb (1.2MB, pdf)
S. Khatri ERJ-00017-2017_Khatri (1.2MB, pdf)
J.N. Kline ERJ-00017-2017_Kline (1.2MB, pdf)
C. McEvoy ERJ-00017-2017_McEvoy (1.2MB, pdf)
E. McMullen ERJ-00017-2017_McMullen (1.2MB, pdf)
A. Shifren ERJ-00017-2017_Shifren (1.2MB, pdf)
R. Strauven ERJ-00017-2017_Strauven (1.2MB, pdf)
Acknowledgements
Other members of the PAS2 Study Group (in addition to the authors) were: Gerard Silvestri (Medical University of South Carolina, Charleston, SC, USA), Mark Dransfield (University of Alabama Lung Health Center, Birmingham, AL, USA), Andrew Haas (University of Pennsylvania Presbyterian Medical Center, Philadelphia, PA, USA), Momen Wahidi (Duke University Medical Center, Durham, NC, USA), Michael Simoff (Henry Ford Hospital, Detroit, MI, USA), Jamie Hey (Pulmonary Associates of Richmond, Richmond, VA, USA), Stephen Ryan (MultiCare Pulmonary Specialists, Tacoma, WA, USA), Richard Kahlstrom (St Joseph Medical Center, Tacoma, WA, USA), Scott Ferguson (University of Wisconsin-Madison, Madison, WI, USA), Carla Lamb (Lahey Hospital and Medical Center, Burlington, MA, USA), Samaan Rafeq (St Elizabeth's Medical Center, Brighton, MA, USA), Marc McClelland (Spectrum Health Hospitals, Grand Rapids, MI, USA), Kyle Hogarth (University of Chicago, Chicago, IL, USA), Kyle Enfield (University of Virginia Health System, Charlottesville, VA, USA), Ron Olivenstein (Montreal Chest Institute, Montreal, QC, Canada), Jennifer Toth (Penn State University/Hershey Medical Center, Hershey, PA, USA), Ted Lawson (Surrey Memorial Hospital, Surrey, BC, Canada), Adnan Majid (Beth Israel Deaconess Medical Center, Boston, MA, USA) and Mark Fitzgerald (University of British Columbia, Vancouver, BC, Canada).
The authors thank Jennifer L. Olson, a medical writer employed by Boston Scientific (Marlborough, MA, USA), for editorial assistance with this manuscript.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Support statement: This study was funded by Boston Scientific Corporation. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
Contributor Information
Collaborators: Other members of the PAS2 Study Group, Gerard Silvestri, Mark Dransfield, Andrew Haas, Momen Wahidi, Michael Simoff, Jamie Hey, Stephen Ryan, Richard Kahlstrom, Scott Ferguson, Carla Lamb, Samaan Rafeq, Marc McClelland, Kyle Hogarth, Kyle Enfield, Ron Olivenstein, Jennifer Toth, Ted Lawson, Adnan Majid, and Mark Fitzgerald
References
- 1.Chakir J, Haj-Salem I, Gras D, et al. . Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc 2015; 12: 1612–1618. [DOI] [PubMed] [Google Scholar]
- 2.Pretolani M, Dombret MC, Thabut G, et al. . Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med 2014; 190: 1452–1454. [DOI] [PubMed] [Google Scholar]
- 3.Pretolani M, Bergqvist A, Thabut G, et al. . Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathological correlations. J Allergy Clin Immunol 2016; 139: 1176–1185. [DOI] [PubMed] [Google Scholar]
- 4.Salem IH, Boulet LP, Biardel S, et al. . Long-term effects of bronchial thermoplasty on airway smooth muscle and reticular basement membrane thickness in severe asthma. Ann Am Thorac Soc 2016; 13: 1426–1428. [DOI] [PubMed] [Google Scholar]
- 5.Denner DR, Doeing DC, Hogarth DK, et al. . Airway inflammation after bronchial thermoplasty for severe asthma. Ann Am Thorac Soc 2015; 12: 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danek CJ, Lombard CM, Dungworth DL, et al. . Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 7.Dombret MC, Alagha K, Boulet LP, et al. . Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev 2014; 23: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro M, Cox G. Asthma outcomes from bronchial thermoplasty in the AIR2 trial. Am J Respir Crit Care Med 2011; 184: 743–744. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Rubin A, Laviolette M, et al. . Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011; 107: 65–70. [DOI] [PubMed] [Google Scholar]
- 10.Castro M, Rubin AS, Laviolette M, et al. . Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wechsler ME, Laviolette M, Rubin AS, et al. . Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013; 132: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bicknell S, Chaudhuri R, Lee N, et al. . Effectiveness of bronchial thermoplasty in severe asthma in ‘real life’ patients compared with those recruited to clinical trials in the same centre. Ther Adv Respir Dis 2015; 9: 267–271. [DOI] [PubMed] [Google Scholar]
- 13.Doeing DC, Mahajan AK, White SR, et al. . Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma 2013; 50: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro M, Musani AI, Mayse ML, et al. . Bronchial thermoplasty: a novel technique in the treatment of severe asthma. Ther Adv Respir Dis 2010; 4: 101–116. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. NAEPP Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication 97-4051 Bethesda, National Institutes of Health, 2007. [Google Scholar]
- 16.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 17.Cox G, Thomson NC, Rubin AS, et al. . Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356: 1327–1337. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm CP, Chipps BE. Bronchial thermoplasty: a review of the evidence. Ann Allergy Asthma Immunol 2016; 116: 92–98. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowski J, Pratt S. Anesthesia for bronchoscopy In: Ernst A, ed. Introduction to Bronchoscopy. New York, Cambridge University Press, 2009; pp. 46–61. [Google Scholar]
- 20.Waxman A. Flexible bronchoscopy: indications, contraindications, and consent In: Ernst A, ed. Introduction to Bronchoscopy. New York, Cambridge University Press, 2009; pp. 78–84. [Google Scholar]
- 21.de Blic J, Marchac V, Scheinmann P. Complications of flexible bronchoscopy in children: prospective study of 1,328 procedures. Eur Respir J 2002; 20: 1271–1276. [DOI] [PubMed] [Google Scholar]
- 22.Pavord ID, Cox G, Thomson NC, et al. . Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 23.Burn J, Sims AJ, Keltie K, et al. . Procedural and short-term safety of bronchial thermoplasty in clinical practice: evidence from a national registry and Hospital Episode Statistics. J Asthma 2016; in press [http://dx.doi.org/10.1080/02770903.2016.1263652]. [DOI] [PubMed] [Google Scholar]
- 24.Cox PG, Miller J, Mitzner W, et al. . Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24: 659–663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00017-2017_Supplement (387.8KB, pdf)
S. Bansal ERJ-00017-2017_Bansal (1.2MB, pdf)
G. Chupp ERJ-00017-2017_Chupp (1.2MB, pdf)
L. Cohn ERJ-00017-2017_Cohn (1.2MB, pdf)
G.M. Grubb ERJ-00017-2017_Grubb (1.2MB, pdf)
S. Khatri ERJ-00017-2017_Khatri (1.2MB, pdf)
J.N. Kline ERJ-00017-2017_Kline (1.2MB, pdf)
C. McEvoy ERJ-00017-2017_McEvoy (1.2MB, pdf)
E. McMullen ERJ-00017-2017_McMullen (1.2MB, pdf)
A. Shifren ERJ-00017-2017_Shifren (1.2MB, pdf)
R. Strauven ERJ-00017-2017_Strauven (1.2MB, pdf)