Abstract
Candida spp. cause various infections involving the skin, mucosa, deep tissues, and even life-threatening candidemia. They are regarded as an important pathogen of nosocomial bloodstream infection, with a high mortality rate. As a result of prolonged exposure to azoles, the therapeutic failure associated with azoles resistance has become a serious challenge in clinical situations. Therefore, novel, alternative antifungals are required urgently. In the present study, the CLSI M-27A broth microdilution method and the 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay were used to evaluate the antifungal effects of magnolol against various standard Candida strains in planktonic mode and biofilm formation, respectively. The antifungal activity of magnolol was demonstrated in planktonic C. albicans and non-albicans Candida species, especially fluconazole-resistant Candida krusei, with the minimum inhibitory concentrations ranging from 10 to 40 μg/mL. The BMIC90 (minimum concentration with 90% Candida biofilm inhibited) values of magnolol ranged from 20 to 160 μg/mL, whereas the BMIC90 values of fluconazole were more than 128 μg/mL. As an alternative and broad-spectrum antifungal agent, magnolol might be of benefit to the treatment of refractory Candida infection.
Keywords: magnolol, inhibition, Candida spp., biofilm
Introduction
The genus Candida, an opportunistic pathogen, is prone to attack immunocompromised hosts or those with debilities, causing the infection of the skin, mucosa, deep-tissues, or even the life-threatening candidemia.1 With the use of potent antibiotics; immunosuppressive and cytotoxic agents; and implanted devices, as well as prolonged intensive care unit stays, the risk of Candida-associated nosocomial infections is increasing remarkably. According to a survey from the US National Nosocomial Infections Surveillance System, Candida species are the fourth most common cause of nosocomial bloodstream infection, with a mortality rate of 35%.2
Azoles, such as fluconazole and itraconazole, are the most frequently prescribed antifungals in candidiasis therapy, which destroy the cellular structures of fungi by inhibiting the biosynthesis of membranous ergosterol.3 However, long-term or repeat exposure to azoles in refractory infection can induce the emergence of resistant strains.4 Among C. albicans isolates from candidemia patients and human immunodeficiency virus (HIV)-positive patients with oropharyngeal candidiasis, 0%–4.3% and 9.5% were reported to be fluconazole resistant, respectively.5–8 In recent years, the incidence of infections caused by non-albicans Candida species (NACS), including C. glabrata, C. dubliniensis, and C. krusei, increased.9 Approximately 26% of Candida bloodstream infections investigated in the USA were attributed to C. glabrata,10 and 1.5%–32% of HIV-positive populations were infected with C. dubliniensis.11,12 Azoles-induced Candida species screening is responsible for the increased infection by NACS. Under the stress of azoles, the species susceptible to azoles are inhibited, leaving the resistant species to grow richer.13 C. krusei is intrinsically azoles-resistant, while the resistance of C. glabrata may be acquired. Their ability to take up exogenous sterols allows C. glabrata to grow in the presence of azoles.14 Despite C. dubliniensis being mostly sensitive to azoles, it can develop azole resistance during antifungal treatment.15 The incidence was reported to be 23% in HIV-positive individuals.16 Therefore, a novel, alternative agent is needed against a broad range of fungi.
Magnolol, a lignin compound, was extracted initially in the 1930s from the dried bark of the stem, root, or branch of the traditional Chinese medicinal plant Magnolia officinalis. Previous studies demonstrated that magnolol could inhibit the growth of Helicobacter pylori remarkably,17 as well as other pathogens localized in the oral cavity, including Streptococcus mutans,18 Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia.19 The inhibitory activities of magnolol against Cryptococcus neoformans, Aspergillus niger, and C. albicans were also demonstrated.18,20 Nevertheless, NACS-associated infections have increased notably, and the effect of magnolol on NACS, especially the resistant species, remains unclear. Therefore, in this study, the activities of magnolol against various Candida spp. were evaluated in planktonic mode and in biofilm formation.
Materials and methods
Organisms and culture condition
Five different standard strains of C. albicans (ATCC90028), C. krusei (ATCC6258), C. dubliniensis (MYA646), C. glabrata (ATCC90030), and C. parapsilosis (ATCC22019), obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA), were used in the study. C. parapsilosis (ATCC22019) was used as a quality control isolate.
All yeasts were cultured aerobically on Sabouraud dextrose agar (SDA) plates (BioMérieux Industry Co. Ltd., Shanghai, China) for 48 h at 37°C, and stored at 4°C ready for use.
Drug preparation
Commercial powders of magnolol and fluconazole (Figure 1) were obtained from the National Institutes for Food and Drug Control (Beijing, China). The purity was measured by high-performance liquid chromatography and determined to be about 98.8% for magnolol, 99.8% for fluconazole. The drugs were dissolved in dimethyl sulfoxide (Sigma-Aldrich Co., St Louis, MI, USA), and stored at a concentration of 1.28×105 μg/mL for magnolol, and 1.28×104 μg/mL for fluconazole, at −80°C.
Figure 1.
Structures of magnolol and fluconazole.
Antifungal activity of magnolol against planktonic Candida cells
Susceptibility testing of planktonic yeast cells to magnolol was performed following the CLSI M-27A broth micro-dilution method.21 The frozen magnolol solution was thawed and diluted in Roswell Park Memorial Institute (RPMI) 1640 medium (containing L-glutamine) (Life Technologies Co., Madison, WI, USA), which was buffered to pH =7.0 using 0.165 M 3-morpholinopropane-1-sulfonic acid (Sigma-Aldrich Co.). The magnolol solution (100 μL of a 2-fold dilution) was pipetted into each well of a 96-well microtiter plate. The final concentration of magnolol ranged from 2.5 to 1,280 μg/mL.
Fresh yeast cells were harvested and washed twice with PBS (pH =7.2). Yeast suspensions at 1×104 cells/mL were prepared using RPMI 1640 medium. Aliquots of 100 μL of the yeast suspension was inoculated into each well containing the magnolol solution, and incubated for 48 h at 37°C. The minimum inhibitory concentration (MIC) was determined on visual inspection. The MIC was defined as the lowest concentration, at which no yeast could be seen to grow. The experiment was performed in triplicate.
As a positive control, the MICs of fluconazole against the planktonic yeasts were determined in parallel, and the final concentration of fluconazole ranged from 0.25 to 128 μg/mL.
Preparation of standard yeast suspensions for biofilm studies
Yeast cells were grown on an SDA plate for 18 h at 37°C. A loopful of the yeast was then inoculated into yeast nitrogen base medium (YNB, Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) supplemented with 50 mM glucose in an orbital shaker at 80 rpm. After overnight incubation, the yeast cells were harvested. After washing twice in PBS, yeast suspensions at 1×107 cells/mL were prepared in YNB (pH =7.0) medium containing 100 mM glucose.
Biofilm formation
A previously described method was used for Candida bio-film formation.22 Briefly, aliquots of 100 μL of the standard yeast suspensions were pipetted into each well of polystyrene microtiter plates and incubated for 90 min at 37°C in a shaker at 80 rpm, which allowed the yeast cells to attach to the well surface. Thereafter, the yeast suspensions were aspirated, and each well was washed gently with 100 μL of sterilized PBS. Following the pipetting of 200 μL of YNB medium supplemented with 100 mM glucose into each well, 4 μL of 2-fold dilutions of magnolol solutions were added to each well, the final concentrations of magnolol ranged from 5 to 2,560 μg/mL. The microtiter plates were subsequently incubated at 37°C in a shaker at 80 rpm. After 6, 12, 24, or 48 h of incubation, the yeast suspensions were aspirated. The 4 time-points were set up based on the developmental phases during the period of Candida biofilm formation. Each well was washed twice with sterilized PBS to remove unattached cells.
The influence of fluconazole on Candida biofilm production was also studied, and the final concentration of fluconazole ranged from 0.25 to 128 μg/mL.
XTT reduction assay
This assay was used to determine the biofilm activity by measuring the reduction of 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT).23 XTT (Sigma-Aldrich Co.) was dissolved in PBS at 1 mg/mL. After sterilization through a 0.22-μm filter, the XTT solution was stored at −80°C until use. Menadione (0.4 mM; Sigma-Aldrich Co.) was prepared in acetone immediately before the assay. Before each assay, the thawed XTT solution was mixed with the menadione solution at a ratio of 5 to 1 by volume. Following the prewash, 200 μL of XTT-menadione-PBS reagent was added to each well containing adherent yeast cells, and incubated in the dark. Three hours later, 100 μL of the supernatant in each well was transferred to new wells. The color of the supernatants in each well was measured using a microplate reader (model: EL ×808) (BioTek Instruments, Inc., Waltham, MA, USA) at 490 nm. The absorbance value of each solution was read as the optical density (OD) value. The experiment was performed in triplicate and the average result was used. The yeast suspension without drug was regarded as the drug-free control.
The BMIC90 was defined as the minimum concentration with 90% Candida biofilm inhibited, of which produced 90% reduction of OD value compared with the drug-free control.
Result
Antifungal activity of magnolol against planktonic Candida cells by broth microdilution
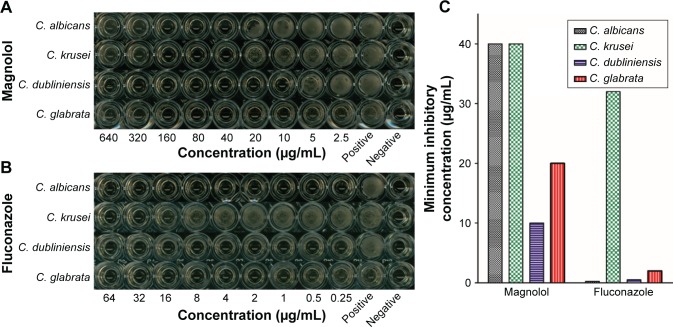
Magnolol demonstrated in vitro inhibitory activities against planktonic C. albicans, as well as non-albicans Candida, in terms of their MICs (Figure 2). Of the tested strains, C. dubliniensis was most susceptible to magnolol, with a MIC of 10 μg/mL, followed by C. glabrata with a MIC of 20 μg/mL, and C. albicans with a MIC of 40 μg/mL, which was equal to that of C. krusei (Table 1).
Figure 2.
The inhibitory effects of magnolol on planktonic-mode Candida cells using the CLSI M-27A broth microdilution method.
Notes: The quantity of Candida cells was reduced as the drug concentrations of the wells increased. At the MICs or over, no yeast cells were observed to grow. (A) Results for magnolol; (B) results for fluconazole; (C) the MICs of magnolol and fluconazole for C. albicans, C. krusei, C. dubliniensis, and C. glabrata.
Abbreviation: MICs, minimum inhibitory concentrations.
Table 1.
Activities of magnolol against planktonic-mode and 48-h biofilm production of various Candida spp.
| Planktonic-mode (MIC, μg/mL)
|
Biofilm (BMIC90, μg/mL)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans | C. krusei | C. dubliniensis | C. glabrata | C. albicans | C. krusei | C. dubliniensis | C. glabrata | |
| Magnolol | 40 | 40 | 10 | 20 | 160 | 80 | 20 | 40 |
| Fluconazole | 0.25 | 32 | 0.5 | 2 | >128 | >128 | >128 | >128 |
Notes: MIC is the minimum concentration of the drugs that inhibited 100% of the yeast growth on visual inspection. BMIC90 is the minimum concentration of the drugs that produced 90% reduction of optical density value compared with the drug-free control.
Abbreviations: MIC, minimum inhibitory concentration; BMIC90, the minimum concentration with 90% Candida biofilm inhibited.
Corresponding to the standard recommended by National Committee for Clinical Laboratory Standards (NCCLS) [21], fluconazole showed antifungal activities against C. albicans, C. dubliniensis, and C. glabrata with MICs of 0.25, 0.5, and 2 μg/mL, respectively, while C. krusei was resistant to fluconazole, with a MIC of 32 μg/mL.
Inhibitory effects of magnolol on Candida biofilm formation by the XTT reduction assay
Magnolol was remarkably effective at inhibiting Candida spp. biofilm formation compared with fluconazole. At the mature-stage of biofilm (48 h), the BMIC90 of magnolol against C. albicans was 160 μg/mL. C. dubliniensis and C. glabrata were more susceptible to magnolol, with the BMIC90 values of 20 and 40 μg/mL, respectively. Even C. krusei, the fluconazole-resistant strain, demonstrated sensitivity to magnolol, with a BMIC90 of 80 μg/mL. In comparison, fluconazole showed higher BMIC90 values for all strains, at over 128 μg/mL. No definitive values were identified because of the restricted concentrations of fluconazole used in the study (0.25–128 μg/mL) (Table 1).
Compared with the planktonic-mode, the BMIC90 values of fluconazole increased by 4–500 times against 48 h yeast-biofilms, changing from 0.25 to 32 μg/mL to over 128 μg/mL. By contrast, the BMIC90 values of magnolol ranged from 20 to 160 μg/mL for the 4 Candida spp., which were only 2–4 times higher than the MICs in planktonic form (ranging from 0.25 to 32 μg/mL; Table 1).
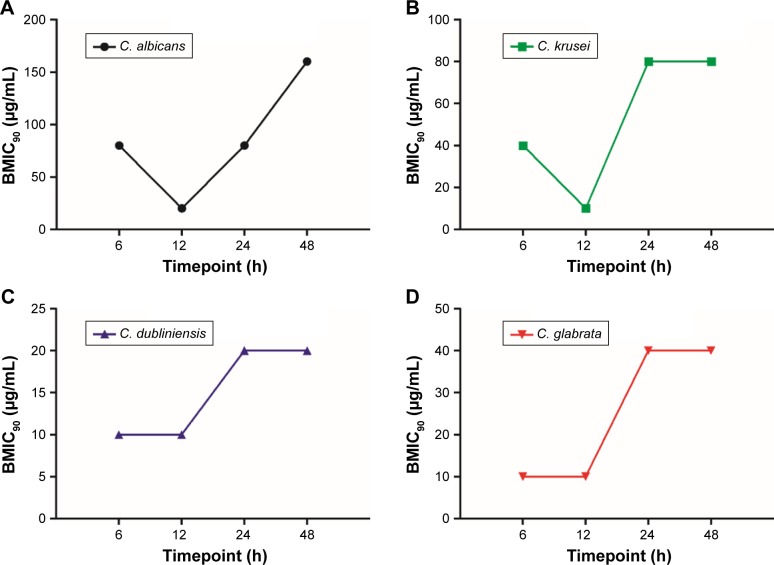
Based on the developmental phases during the period of Candida biofilm formation, 4 different time-points were chosen to evaluate the effect of magnolol on Candida biofilm formation. All yeast strains were most vulnerable to magnolol at the maturation age of 12 h. The BMIC90 of C. albicans was 20 μg/mL, C. krusei, C. dubliniensis and C. glabrata were all 10 μg/mL. With the maturation of the biofilms, the BMIC90 values showed a tendency to increase (Figure 3), changing from 10 to 20 μg/mL to 20–80 μg/mL (from 12 to 24 h). With the exception of C. albicans, the BMIC90 values of the other strains reached a high level, which was maintained until 48 h. From 12 to 48 h of biofilm development, the BMIC90 of C. albicans continued to increase. This result suggested that the inhibitory effect of magnolol on yeast biofilm production reached a peak at around 12 h, which coincided with the start of maturation, after which, the yeast biofilm became more tolerant to magnolol (Figure 3).
Figure 3.
Inhibitory activities of magnolol against Candida biofilm formation at different time-points using the XTT reduction assay.
Notes: (A) C. albicans; (B) C. krusei; (C) C. dubliniensis; (D) C. glabrata.
Abbreviations: BMIC90, the minimum concentration with 90% Candida biofilm inhibited; XTT, 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide.
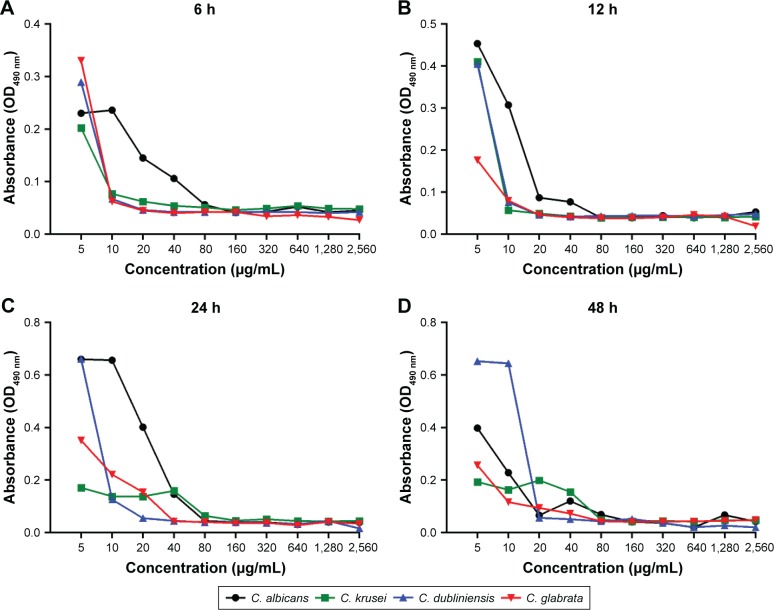
Using the XTT reduction assay, the biofilm metabolic activities of the Candida strains were identified by the microplate reader in terms of absorbance (OD490 nm) (Figure 4). At the 4 time-points observed, the decreases in absorbance correlated with the increasing magnolol concentrations. After 6 h of culture, the metabolic activities of the strains except for C. albicans, decreased rapidly. At 12 h, greatly reduced metabolic activity was observed in all strains. Thereafter, the rate of decline slowed in all strains. This result implied that the fungicidal efficacy of magnolol was relatively high up to 12 h.
Figure 4.
Influence of different concentrations of magnolol on Candida biofilm activity during biofilm maturation, as assessed by the XTT reduction assay.
Note: Assessed at 6 (A), 12 (B), 24 (C), and 48 (D) h.
Abbreviation: OD, optical density.
Discussion
Magnolol, the major chemical compound purified from the traditional Chinese medicinal plant M. officinalis, has been demonstrated to have various pharmacological functions in the treatment of illness, including antianxiety,24 analgesic,25 smooth muscle relaxing,26 anti-tumorigenic,27 and antimicrobial activities. Magnolol is active against Gram-positive and acid-fast bacteria,17–19 H. pylori, and Propionibacterium acne.17 By reducing the secretion of IL-8 and TNF-α induced by P. acne, magnolol may exhibit anti-inflammatory effects.28 The inhibitory effects of magnolol against clinical isolates of C. albicans are remarkable, with the MIC values ranging from 16 to 32 μg/mL.18,20 It was confirmed in the present study using the planktonic standard strain of C. albicans (ATCC90028), in which the MIC of magnolol was 40 μg/mL. Besides C. albicans, the potent antifungal effects of magnolol for NACS, including C. krusei, C. dubliniensis, and C. glabrata in the planktonic form, were determined with MICs ranging from 10 to 40 μg/mL. C. krusei is intrinsically fluconazole-resistant, and C. dubliniensis,15 C. glabrata,14 and C. albicans29 may develop fluconazole-resistance in clinical antifungal therapy. Fluconazole targets mainly membranous ergosterol. Therefore, mutations of the ergosterol biosynthesis gene, Erg11, and the overexpression of drug efflux pumps Mdr1p and Cdr1p/Cdr2p, are responsible for fluconazole resistance in C. albicans.30 In C. glabrata, the resistance to fluconazole is attributed to its ability to take up exogenous sterols instead of the altered cell membrane sterols. On the other hand, mutations in the Pdr1 gene are also involved in fluconazole resistance of C. glabrata.31 Although the precise mechanism is unclear, C. krusei is considered to resist fluconazole via the efflux pump activity,32 as well as the reduced azole affinity for Erg11p.33 In this regard, our data suggested that magnolol had a broad antifungal spectrum mechanism that differs from that of fluconazole. Membranous ergosterol may be not the target of magnolol, or at least, not the only one.
In recent years, although C. albicans is still the predominant cause of candidiasis, NACS-associated infections have increased notably. C. glabrata is isolated frequently from patients with vulvovaginal or urinary candidiasis because of its affinity for epithelial cells of the vagina33 and urethra.34 Its rapid dissemination throughout the body contributes to C. glabrata’s increasing prevalence in candidemia cases.35 In the USA, C. krusei was responsible for 2.7% of NACS-associated infections.36 It is considered an important risk factor causing Candida infection among patients with hematological malignancies and bone marrow transplants.37 C. dubliniensis was either the second or the third most commonly identified pathogenic fungi in patients with HIV/AIDS,38,39 and was associated with 2%–7% of candidemia cases.40,41 In oral infections, C. glabrata or C. krusei was responsible for 42% of Sjogren’s syndrome cases combined with oral candidiasis.42 For this reason, as an alternative antifungal agent, magnolol may potentially benefit the treatment of NACS-associated infections, particularly the infection caused by the azoles-resistant species.
In nature, most microorganisms prefer growth in the form of biofilm, in which one or more other species are embedded within an extracellular matrix (ECM), comprising a complex community.43 Approximately 65% of all clinical infections are biofilm-associated.44 A biofilm is significantly less susceptible to antimicrobial agents, being 10–1,000 times more resistant to antimicrobial agents than the planktonic form.45 Notably, the concentrations of magnolol required in the present study to reduce 90% of metabolic activity were just 2 to 4 times higher for biofilms than for planktonic cells, whereas the concentrations of fluconazole required increased by 4 to 500 times. Moreover, magnolol was more active than fluconazole in inhibiting biofilm formation of Candida spp. for the BMIC90 values for fluconazole were all >128 μg/mL for C. albicans, C. krusei, C. dubliniensis, and C. glabrata, whereas the BMIC90 values for magnolol ranged from 20 to 160 μg/mL for them. Despite the BMIC90 of magnolol against C. albicans being 160 μg/mL, the other isolates were more susceptible, with BMIC90 values in the range of 20–80 μg/mL, whereas the BMIC90 of fluconazole against all strains were over 128 μg/mL.
Multiple mechanisms are responsible for biofilm-associated resistance, including drug efflux pumps, delayed penetration of the antimicrobial agent through the biofilm matrix, decrease of growth rate or cell metabolism.46,47 Fluconazole-associated resistance attributes to the overexpression of drug efflux pumps genes Mdr1 and Cdr1/Cdr2, as well as the alteration of ergosterol biosynthesis pathway.48 However, a different mechanism of magnolol from fluconazole could be indicated because of a broad antifungal range of magnolol against Candida spp., including intrinsic fluconazole-resistant isolate. Few studies focus on the antifungal mechanisms of magnolol. In the report of Sun et al magnolol was considered to inhibit C. albicans biofilm formation through decreasing the yeasts’ adhesive and morphological transitional abilities, and its fungicidal capabilities.20 In addition, cell wall component β-1,3-glucan may also be a target of magnolol as echinocandin family antifungals.49 Levels of β-1,3-glucan on Candida cell walls as well as in ECM were significantly elevated in biofilm-form than in planktonic-mode, which may benefit the biofilm fluconazole-resistance.50 Moreover, since magnolol was demonstrated to take effect around the cellular logarithmic phase in the present study, the inhibitory effects of cell growth may also be a possible mechanism of magnolol against biofilm formation. However, further studies are needed.
Candida biofilm formation involves several specific stages: 1) The early phase (60–90 min). In this stage, round yeast cells adhere to the substrate; 2) The developmental phase: attached cells proliferate to form a basal layer, and biofilm formation begins; 3) The biofilm maturation stage: complex layers of polymorphic cells develop and become encased in an ECM (24 h); 4) The dispersal stage: some round yeast cells disperse from the biofilm to seed new sites.51 In the different developmental stages, the biofilm has different biological behaviors. With the maturation of the biofilm, Candida spp. cells exhibited increased tolerance to magnolol. To obtain 90% biofilm reduction, increased amounts of magnolol were required. In the present study, the lowest dosage of magnolol required was at around 12 h of culture, after which the BMIC90 values started to increase remarkably. It is reasonable to assume that magnolol attacks yeast cells in the logarithmic phase (16–18 h) when the cells are sensitive to environmental changes.52 Using the XTT reduction assay, the biofilm metabolic activity of Candida spp. was assessed. Our data showed that with the increasing magnolol concentrations, the metabolic activities of the biofilms decreased. The reduction proceeded through the course of biofilm formation, which suggested that the effects of magnolol on Candida spp. biofilm formation were concentration-dependent.
In summary, in contrast to fluconazole, the antifungal spectrum of magnolol was broad. Various Candida spp., including C. albicans, C. krusei, C. dubliniensis, and C. glabrata were susceptible to magnolol, both in plank-tonic mode and biofilm form. Magnolol was more active than fluconazole at inhibiting biofilm formation of Candida spp. The effect was concentration-dependent, and might act during the logarithmic phase of yeast growth. As an alternative antifungal agent, magnolol might be beneficial to treat NACS-associated infections, particularly those caused by azoles-resistant species. Nevertheless, the safety and the antifungal effect in vivo require further evaluation.
Acknowledgments
This study was supported by the Natural Science Foundation of China (grant number 81670991).
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20(Suppl 6):5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 2.Calderone RA. Introduction and historical perspectives. In: Calderone RA, editor. Candida and Candidiasis. Washington, DC: ASM Press; 2002. pp. 3–13. [Google Scholar]
- 3.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003;11(6):272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 4.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11(2):382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enwuru CA, Ogunledun A, Idika N, et al. Fluconazole resistant opportunistic oro-pharyngeal Candida and non-Candida yeast-like isolates from HIV infected patients attending ARV clinics in Lagos, Nigeria. Afr Health Sci. 2008;8(3):142–148. [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn Microbiol Infect Dis. 2012;73(1):45–48. doi: 10.1016/j.diagmicrobio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. Isavuconazole, micafungin, and 8 comparator antifungal agents’ susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis. 2015;82(4):303–313. doi: 10.1016/j.diagmicrobio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Ying Y, Zhang J, Huang SB, et al. Fluconazole susceptibility of 3,056 clinical isolates of Candida species from 2005 to 2009 in a tertiary-care hospital. Indian J Med Microbiol. 2015;33(3):413–415. doi: 10.4103/0255-0857.158569. [DOI] [PubMed] [Google Scholar]
- 9.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3(11):685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 10.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 11.Faggi E, Pini G, Campisi E, Martinelli C, Difonzo E. Detection of Candida dubliniensis in oropharyngeal samples from human immunodeficiency virus infected and non-infected patients and in a yeast culture collection. Mycoses. 2005;48(3):211–215. doi: 10.1111/j.1439-0507.2005.01129.x. [DOI] [PubMed] [Google Scholar]
- 12.Pontón J, Rüchel R, Clemons KV, et al. Emerging pathogens. Med Mycol. 2000;38(Suppl 1):225–236. doi: 10.1080/mmy.38.s1.225.236. [DOI] [PubMed] [Google Scholar]
- 13.González Gravina H, González de Morán E, Zambrano O, et al. Oral Candidiasis in children and adolescents with cancer. Identification of Candida spp. Med Oral Patol Oral Cir Bucal. 2007;12(6):E419–E423. [PubMed] [Google Scholar]
- 14.Nakayama H, Izuta M, Nakayama N, Arisawa M, Aoki Y. Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice. Antimicrob Agents Chemother. 2000;44(9):2411–2418. doi: 10.1128/aac.44.9.2411-2418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea S, López-Ribot JL, Wickes BL, et al. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 2002;46(6):1695–1703. doi: 10.1128/AAC.46.6.1695-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chunchanur SK, Nadgir SD, Halesh LH, Patil BS, Kausar Y, Chandrasekhar MR. Detection and antifungal susceptibility testing of oral Candida dubliniensis from human immunodeficiency virus-infected patients. Indian J Pathol Microbiol. 2009;52(4):501–504. doi: 10.4103/0377-4929.56138. [DOI] [PubMed] [Google Scholar]
- 17.Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull. 1998;21(9):990–992. doi: 10.1248/bpb.21.990. [DOI] [PubMed] [Google Scholar]
- 18.Bang KH, Kim YK, Min BS, et al. Antifungal activity of magnolol and honokiol. Arch Pharm Res. 2000;23(1):46–49. doi: 10.1007/BF02976465. [DOI] [PubMed] [Google Scholar]
- 19.Chang B, Lee Y, Ku Y, Bae K, Chung C. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Med. 1998;64(4):367–369. doi: 10.1055/s-2006-957453. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Liao K, Wang D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS One. 2015;10(2):e0117695. doi: 10.1371/journal.pone.0117695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 22.Jin Y, Yip HK, Samaranayake YH, Yau JY, Samaranayake LP. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003;41(7):2961–2967. doi: 10.1128/JCM.41.7.2961-2967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuribara H, Kishi E, Kimura M, Weintraub ST, Maruyama Y. Comparative assessment of the anxiolytic-like activities of honokiol and derivatives. Pharmacol Biochem Behav. 2000;67(3):597–601. doi: 10.1016/s0091-3057(00)00401-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin YR, Chen HH, Lin YC, Ko CH, Chan MH. Antinociceptive actions of honokiol and magnolol on glutamatergic and inflammatory pain. J Biomed Sci. 2009;16:94. doi: 10.1186/1423-0127-16-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko CH, Chen HH, Lin YR, Chan MH. Inhibition of smooth muscle contraction by magnolol and honokiol in porcine trachea. Planta Med. 2003;69(6):532–536. doi: 10.1055/s-2003-40654. [DOI] [PubMed] [Google Scholar]
- 27.Chen LC, Liu YC, Liang YC, Ho YS, Lee WS. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J Agric Food Chem. 2009;57(16):7331–7337. doi: 10.1021/jf901477g. [DOI] [PubMed] [Google Scholar]
- 28.Park J, Lee J, Jung E, et al. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur J Pharmacol. 2004;496(1–3):189–195. doi: 10.1016/j.ejphar.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 29.Yang YL, Lo HJ. Mechanisms of antifungal agent resistance. J Microbiol Immunol Infect. 2001;34(2):79–86. [PubMed] [Google Scholar]
- 30.Xiang MJ, Liu JY, Ni PH, et al. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013;13(4):386–393. doi: 10.1111/1567-1364.12042. [DOI] [PubMed] [Google Scholar]
- 31.Vermitsky JP, Self MJ, Chadwick SG, et al. Survey of vaginal-flora Candida species isolates from women of different age groups by use of species-specific PCR detection. J Clin Microbiol. 2008;46(4):1501–1503. doi: 10.1128/JCM.02485-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corsello S, Spinillo A, Osnengo G, et al. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol. 2003;110(1):66–72. doi: 10.1016/s0301-2115(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 33.Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43(5):2155–2162. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauffman CA. Candiduria. Clin Infect Dis. 2005;41(Suppl 6):S371–S376. doi: 10.1086/430918. [DOI] [PubMed] [Google Scholar]
- 35.Fidel PL, Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80–96. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller MA, Jones RN, Castanheira M. Regional data analysis of Candida non-albicans strains collected in United States medical sites over a 6-year period, 2006–2011. Mycoses. 2014;57(10):602–611. doi: 10.1111/myc.12206. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller MA, Diekema DJ, Gibbs DL, et al. Global Antifungal Surveillance Group Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46(2):515–521. doi: 10.1128/JCM.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badiee P, Alborzi A, Davarpanah MA, Shakiba E. Distributions and antifungal susceptibility of Candida species from mucosal sites in HIV positive patients. Arch Iran Med. 2010;13(4):282–287. [PubMed] [Google Scholar]
- 39.Thompson GR, 3rd, Patel PK, Kirkpatrick WR, et al. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):488–495. doi: 10.1016/j.tripleo.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jabra-Rizk MA, Johnson JK, Forrest G, Mankes K, Meiller TF, Venezia RA. Prevalence of Candida dubliniensis fungemia at a large teaching hospital. Clin Infect Dis. 2005;41(7):1064–1067. doi: 10.1086/432943. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan DJ, Moran GP, Pinjon E, et al. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res. 2004;4(4–5):369–376. doi: 10.1016/S1567-1356(03)00240-X. [DOI] [PubMed] [Google Scholar]
- 42.Yan Z, Young AL, Hua H, Xu Y. Multiple oral Candida infections in patients with Sjogren’s syndrome–prevalence and clinical and drug susceptibility profiles. J Rheumatol. 2011;38(11):2428–2431. doi: 10.3899/jrheum.100819. [DOI] [PubMed] [Google Scholar]
- 43.Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol. 2009;47(7):681–689. doi: 10.3109/13693780802549594. [DOI] [PubMed] [Google Scholar]
- 44.Uppuluri P, Pierce CG, López-Ribot JL. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 2009;4(10):1235–1237. doi: 10.2217/fmb.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee PK, Chandra J. Candida biofilm resistance. Drug Resist Updat. 2004;7(4–5):301–309. doi: 10.1016/j.drup.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71(8):4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother. 2002;49(6):889–891. doi: 10.1093/jac/dkf045. [DOI] [PubMed] [Google Scholar]
- 50.Nett J, Lincoln L, Marchillo K, et al. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51(2):510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016;18(5):310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langford ML, Hasim S, Nickerson KW, Atkin AL. Activity and toxicity of farnesol towards Candida albicans are dependent on growth conditions. Antimicrob Agents Chemother. 2010;54(2):940–942. doi: 10.1128/AAC.01214-09. [DOI] [PMC free article] [PubMed] [Google Scholar]