Abstract
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal (GI) disorder that is characterized by a combination of abdominal pain or discomfort, bloating and alterations in bowel movements. This review presents recent developments concerning the roles of diet and GI endocrine cells in the pathophysiology of IBS and of individual dietary guidance in the management of IBS. Patients with IBS typically report that food aggravates their IBS symptoms. The interactions between specific types of foodstuffs rich in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and GI endocrine cells induce changes in cell densities. Providing individual dietary guidance about a low FODMAP intake, high soluble-fiber intake, and changing the proportions of protein, fat and carbohydrates helps to reduce the symptoms experienced by patients with IBS and to improve their quality of life. These improvements are due to restoring the densities of the GI endocrine cells back to normal. The reported observations emphasize the role of GI endocrine cells in the pathophysiology of IBS and support the provision of dietary guidance as a first-line treatment for managing IBS.
Keywords: dietary guidance, enteroendocrine cells, fermentable oligosaccharides, disaccharides, monosaccharides and polyols, irritable bowel syndrome
1. Introduction
Irritable bowel syndrome (IBS)
IBS is a common chronic disorder of the gastrointestinal (GI) tract (1–4) that seems to have multifactorial causes (2). It is more common in women than in men, and occurs more often under the age of 50 years (5–11). IBS is reportedly the most commonly diagnosed disorder of the GI in general practice (12), being an even more common reason for seeking medical care than diabetes, hypertension or asthma (13–15). Typically, 12–14% of visits to general practitioners and 25–28% of referrals to gastroenterologist involve patients with IBS (4,13,15,16).
The estimated prevalence of IBS ranges between 5 and 20% of the population worldwide (1,2,4,6,8–10,17–22), with marked geographical variations (9). Using Rome II criteria, the prevalence is 11.5% in Europe (9), 12.1% in Canada (23), 4.7% in the USA (24), 6.9% in Australia (25), 34% in Egypt (26), 4% in India (27,28) and 4.6–5.6% in China (29). The prevalence of IBS in Scandinavian countries is as follows: 10.5% in Denmark (30), 14.5% in Sweden (31), 8% in the south of Norway (32) and 25% in the north of Norway (33).
IBS is mainly diagnosed clinically based on the presenting symptoms due to the lack of biochemical, histopathological or radiological diagnostic tests (3,4,34,35). The symptoms of IBS comprise abdominal pain or discomfort, abdominal bloating or distension and alterations in the stool (1,4,34,36). Based on the predominant bowel movements, patients with IBS are classified into diarrhea-predominant (IBS-D), constipation-predominant (IBS-C) and mixed diarrhea and constipation (37,38). Rome IV criteria are the currently used symptom-based diagnostic criteria (39).
It is known that IBS neither increases mortality (40) nor develops into serious diseases, such as cancer or inflammatory bowel disease (41,42). However, the morbidity associated with IBS can be as serious as that for major chronic diseases, such as congestive heart failure (43), hepatic cirrhosis (44), renal insufficiency and diabetes (11), with considerable costs to society (17,45–48). Patients with IBS tend to be less productive at work or school due to frequent absences (6,13,36,49,50), changing or losing jobs and turning down promotions more frequently (13,36). These patients have to pay high healthcare costs due to the need to undergo numerous diagnostic tests, frequent visits to the doctor, recurrent hospital admissions and the consumption of more medications than patients without IBS (36). IBS is therefore considered an economic burden for both the patients themselves and society as a whole (2,17,43,45,51–53). The quality of life is lower for patients with IBS than for healthy subjects (4,6,9,19–22,52) due to IBS negatively affecting several aspects of the life of patients, such as sleep, diet, work, leisure, travel, sexual activity and mood (depression or anxiety) (36).
Pathogenesis
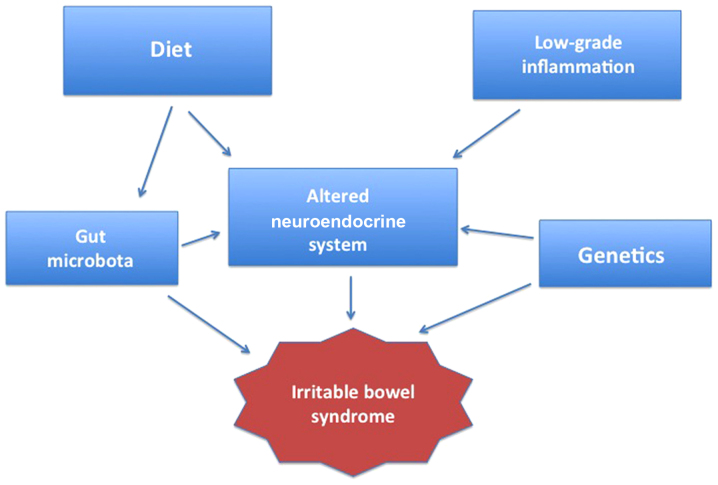
Several factors seem to be involved in the pathogenesis of IBS, including hereditariness, diet, mucosal low-grade inflammation, GI microbiota and abnormal endocrine cells in the GI tract (Fig. 1) (4,35,54–78). This review discusses the effects of applying individual dietary guidance on symptoms, quality of life and GI endocrine cells in patients with IBS.
Figure 1.
Factors considered to be involved in the pathogenesis of irritable bowel syndrome (IBS).
2. Diet
More than two-thirds of patients with IBS associate the development of their symptoms with the consumption of certain foodstuffs (71–73), such as milk and other dairy products, wheat products, caffeine, certain meats, cabbage, onion, peas, beans, tomatoes, hot spices and fried foods (13,52,71,79), as well as raw vegetables, raw broccoli, paprika, leeks, garlic and mushrooms (52). These foodstuffs are rich in the poorly absorbed rapidly fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) (13,52,80–82). The time delay between the consumption of food until the appearance of IBS symptoms varies, with 28% of patients experiencing symptoms within 15 min of eating, and 93% reporting that the symptoms become more severe between 15 min and 3 h (71).
FODMAPs
Carbohydrates constitute the largest source of energy for humans, ranging from 40 to 80% of the total energy requirements (81). There are two types of dietary carbohydrates, namely long-chain carbohydrates (starch, resistant starch and non-starch polysaccharides) and short-chain (sugars, polyols and oligosaccharides) (80). Long-chain carbohydrates provide benefits of fecal bulking, accelerating colonic transit and causing slight acidification of the luminal milieu (80). On the other hand, short-chain carbohydrates, also referred to as prebiotics, stimulate the growth of beneficial bacteria in the colon (Bifidobacteria and Lactobacillus) and include fructans (fructo-oligosaccharides and inulin) and galacto-oligosaccharides (80,83). Prebiotics help to reduce the risk of GI infections (84), improve laxation (85) and calcium absorption (86), preserve the GI mucosal barrier (87) and stimulate the immune system of the GI tract that may reduce the risk of colon cancer (demonstrated in animals) (88).
The microbiota in the colon ferments undigested long-chain carbohydrates and produces gases (carbon dioxi de, hydrogen and/or methane) and short-chain fatty acids (butyrate, which is an important source of energy for colonocytes and the colonic microbiota) (80,89–91). The poorly absorbed short-chain carbohydrates increase the volume of fluid in the bowel through osmosis (80,91,92), which results in a natural laxative effect in healthy individuals and diarrhea in patients with IBS (80). The gut microbiota in patients with IBS comprises fewer Lactobacillus and Bifidobacterium spp. and more Clostridium spp. that ferment FODMAPs and fiber and produce gas, leading to luminal distension with symptoms of abdominal pain and bloating (93–95).
The importance of both the presence of specific types of FODMAPs and the total content of FODMAPs in the diet should be noted (75,80,81). Some foods contain several types of FODMAPs; for example, white onion contains excess fructose, raffinose, nystose and kestose, which are particularly problematic for patients with IBS (81). The main clinical problems associated with the total content of a major FODMAP are likely to be due to fructans in vegetables and, to a lesser extent, to free fructose and sorbitol in fruits (81).
Dietary fiber
Dietary fiber is defined as the sum of indigestible polysaccharides and lignin (96). The various types of fiber are categorized based on their holding capacity of water into water-soluble and water-insoluble. Water-soluble fiber (with a high water-holding capacity) such as pectin, gums and psyllium accounts for 4 to 21% of the dietary fiber in cereal bran and 19–59% of that in legumes, vegetables and fruits. Oat is the grain that is richest in water-soluble fiber. Water-insoluble fiber (with a low water-holding capacity) is found in wheat, rye, rice and most other grains. Legumes and beans contain both water-soluble and water-insoluble fiber (96).
It has been reported that foods containing a higher proportion of dietary fiber, particularly water-soluble fiber, can help to prevent diseases, such as coronary heart disease, diabetes, irritable bowel disease and colon cancer and obesity (96, and refs therein).
Fiber has been used as a bulking agent in the treatment of IBS (74,96). Consuming water-soluble fiber tends to improve the symptoms of IBS compared to consuming water-insoluble fiber (74,97–99). Indeed, consuming water-insoluble fiber may actually worsen the symptoms of IBS (74,97) by causing increased bloating and abdominal discomfort (100). For example, it is recommended for patients with IBS to consume psyllium (mostly water-soluble fiber) rather than bran (water-insoluble fiber) in order to alleviate their symptoms (100).
Fat
The lipolysis of triglycerides comprising >12 carbon atoms is initiated in the stomach with the formation of emulsions of finely dispersed lipids that bind to gastric lipase. This process of fat digestion is completed in the duodenum by pancreatic lipase and releases fatty acids and monoglycerides. The lipid components (fatty acids and monoglycerides) then form water-soluble micelles with conjugated bile acids and are absorbed across the enterocyte membrane. The triglycerides are then reassembled and incorporated into chylomicrons and transported via the lymphatic system. On the other hand, medium-chain triglycerides (comprising 8–12 carbon atoms) are absorbed directly into the bloodstream without the need for luminal lipolysis and micelle formation. Under physiological conditions, no dietary fat enters the colon since fat is digested and completely absorbed in the small bowel.
Fat modulates the functions of the GI tract in healthy individuals (89). Different meals with different caloric contents activate several braking mechanisms in the GI tract at different rates (101–103). In healthy individuals, fat in the stomach slows gastric emptying, while in the duodenum it stimulates pyloric pressure (89,102) and increases biliopancreatic secretion (104), thus activating the gastroduodenal brake (89,105,106). When fat reaches the proximal small intestine, it promotes a jejunal brake to decrease biliopancreatic secretions (103,104) and slow the intestinal transit (107). Finally, an ileal brake is activated upon the arrival of fat at the ileum (108), which allows more time for fat digestion and absorption and thereby prevents it being lost into the colon (107). The jejunal and ileal brakes are mediated by different GI hormones (107,108).
Patients with IBS have abnormal lipid-dependent motor dysfunction that affects the small intestine, but not the colon (109). These patients also exhibit increased small intestine sensitivity to lipid exposure, which induces symptoms of bloating (89,109), fullness and nausea at lower nutrient loads, and enhances GI sensitivity to mechanical distension (89). In addition, intraluminal fat in the small intestine of patients with IBS impairs gas transit and results in the development of gas retention (bloating) and abdominal distension, particularly in the jejunum (109). Lipids also exacerbate rectal hypersensitivity (89,110) and increase the perception of rectal distension in patients with IBS (89,111), causing pain in patients with IBS-C patients, but urgency in patients with IBS-D (89).
Some patients with IBS reportedly relate the development of their symptoms to the consumption of fat-rich meals. However, no consistent differences in dietary fat consumption have been observed between patients with IBS and healthy controls (52,89).
Protein
Almost 20% of the dietary protein enters the distal colon and undergoes putrefaction by colonic bacteria to produce ammonia, amines, phenols and sulfides (112,113). Ammonia is essential for bacterial metabolism and protein synthesis. Branched-chain fatty acids (isovalerate and isobutyrate) (113,114) and short-chain fatty acids (butyrate) (113,115) are produced in the distal part of the colon in the absence of carbohydrate fermentation (which occurs at the proximal colon) (113). Another product of protein putrefaction is sulfur-containing gas (hydrogen sulfide), which has a foul odor (112). The products of protein putrefaction are potentially harmful, phenols are carcinogenic in other systems and hydrogen sulfide is toxic to the epithelium. However, only the malodorous flatus is of concern to patients with IBS, and no definitive effects of malabsorbed protein on intestine motility or visceral hypersensitivity have been identified (112).
Gluten is a mixture of two proteins (gliadin and glutenin) that is found in wheat, barley and rye (112). Consuming gluten activates the immune system so as to change the function of the mucosal barrier that increases intestinal permeability, a condition known as celiac disease that presents with symptoms mimicking IBS. A gluten-free diet usually reduces bowel frequency in patients with IBS-D who are positive for human leukocyte antigen (HLA)-DQ2/8 due to the reduction in intestinal permeability (116). In addition, a gluten-free diet improves the symptoms of IBS due to the associated reduced intake of FODMAPs in wheat rather than of gluten in foods with gluten as a common component (117).
Food chemicals
Natural chemicals, such as amines, glutamates and salicylates occur in foods. Salicylates are found in plants whereas amines and glutamates are products of protein breakdown in animal meat (118). Food additives, such as glutamates are used as flavor enhancers and benzoates, sulfites, and nitrates are used as preservatives (118). These bioactive chemicals interact with the GI luminal chemoreceptors and influence the function of the enteric nervous system of the GI tract (112). A diet that is low in these chemicals may be beneficial to patients with IBS, whereas there is no evidence of the benefits of reducing caffeine or ethanol consumption (112).
3. Gastrointestinal endocrine cells
General
The GI endocrine cells are scattered among the epithelial cells lining the GI lumen (61,119–121). They comprise almost 1% of all epithelial cells in the GI tract and are considered to be the largest endocrine organ in the body (121–123). All epithelial cell types in the GI tract (including GI endocrine cells) originate from pluripotent stem cells with an endodermal origin (124–133). Gastrointestinal stem cells differentiate into endocrine cells over a period of 2–6 days (134,135). The GI endocrine cells project specialized microvilli into the GI lumen to sense the luminal contents (mainly nutrients) and release specific hormones into the lamina propria (61,119,120,136–145).
There are at least 15 different types of GI endocrine cells in the GI tract releasing different types of hormones (2,127). The types of released hormones depend on the types of sensed nutrients: protein and fat trigger the release of serotonin; somatostatin, ghrelin, polypeptide YY (PYY) and carbohydrates suppress ghrelin release; and carbohydrates and fat trigger the release of oxyntomodulin (enteroglucagon) (2,93,146). Particular GI endocrine cells are located either in specific parts of the GI tract or throughout the GI tract (93,127,146,147). Cells producing gastrin and ghrelin are found in the stomach, those producing secretin, cholecystokinin and gastric inhibitory peptide are found in the duodenum, those producing oxyntomodulin (enteroglucagon) and PYY are located in the lower small and large intestines, and those producing serotonin and somatostatin are found throughout the GI tract (93,127,146,147). The functions of the different hormones are summarized in Table I (2).
Table I.
Functions of the hormones of the gastrointestinal endocrine cells.
| Hormones | Function |
|---|---|
| Gastrin | Stimulates gastric acid secretion and histamine release; trophic action on gastric mucosa and stimulates contraction of lower esophageal sphincter and antrum |
| Ghrelin | Increases appetite and feeding; stimulates gastric and intestinal motility |
| Secretin | Stimulates pancreatic bicarbonate and fluid secretion; inhibits gastric emptying; and inhibits contractile activity of small and large intestine |
| CKK | Inhibits gastric emptying; stimulates gall bladder contraction, intestinal motility and pancreatic exocrine secretion; stimulates growth; and regulates food intake |
| GIP | Belongs to incretins. Inhibits gastric acid secretion |
| Oxyntomodulin (enteroglucagon) | Inhibits gastric and pancreatic secretions |
| PYY | Major 'ileal brake' mediator. Delays gastric emptying; inhibits gastric and pancreatic secretion. Anti-diarrheal effect by stimulating the absorption of water and electrolytes |
| Serotonin | Stimulates gastric antrum, small intestinal and colonic motility |
| Somatostatin | Inhibits intestinal contraction, gut exocrine and neuroendocrine secretions |
CKK, cholecystokinin; GIP, gastric inhibitory peptide; PYY, peptide YY.
Several functions of the GI tract, such as motility, secretion, absorption, microcirculation, local immune defense, cell proliferation and food intake, are regulated by the interactions of GI endocrine cells with themselves and with the enteric nervous system, independent of the central nervous system but also communicating and integrated with it (2,119,146,148–151). The GI endocrine cells release their hormones that exert their effects via endocrine signaling (through the bloodstream to distant targets), paracrine or autocrine signaling (locally), synaptic signaling or neuroendocrinally (being released from synapses into the bloodstream) (2,93,146,148).
GI endocrine cells in IBS
There is increasing evidence of an altered neuroendocrine system, namely the GI endocrine cells, being a cause of IBS, since the densities of different types of GI endocrine cells in the different parts of the GI tract are abnormal in patients with IBS (4,35,61–70). Such alterations are responsible for abnormal functions of the GI tract, such as visceral hypersensitivity, dysmotility and abnormal secretion, all of which are characteristics of IBS (35).
4. Individual dietary guidance
Several studies have shown that a low-FODMAP diet can improve the symptoms experienced by patients with IBS (78,90,91,152–157) and that these patients tend to comply with consuming such a diet; one study found that >75% of patients were compliant (75). However, consuming such a diet over a long period of time is associated with several complications, such as a lack of nutrients (52), and changes in the fecal microbiota (158,159). Many patients with IBS make a conscious choice to avoid certain foodstuffs, some of which belong to the FODMAPs group. However, they also tend to 'unknowingly' consume other foodstuffs that are rich in FODMAPs and avoid food sources that are important to their health (52). A Norwegian study on food intolerance and IBS found that 62% of the included subjects limited or excluded some foodstuffs from their daily meals, while 12% made drastic changes in their diet that could result in nutritional deficiencies in the long term (72). Patients with IBS tend to have low intakes of calcium, potassium, magnesium, vitamin A, vitamin B12 and vitamin B2 (52,160–162). Avoiding such adverse effects of the long-term consumption of a low-FODMAP diet therefore requires the administration of dietary guidance (75).
Administering individual dietary guidance
Educating patients can facilitate changes in their behavior for the purpose of disease management and prevention (163). Patients with IBS are particularly interested in learning about dietary modifications, coping strategies and the causes of the disease (163). Dietary guidance can be administered individually, whereby patients are provided with information via one-to-one consultations; this approach is useful due to the tolerance to different FODMAPs, varying widely between individual patients (164). When being provided with individual dietary guidance, patients are scheduled to attend several relatively short sessions, administered by a physician, nurse or nutritionist, to receive information concerning IBS and the appropriate foodstuffs they should consume to reduce their symptoms. The main emphasis should be on consuming foodstuffs that are low in FODMAPs and water-insoluble fiber, and changing the proportions of protein (increase), fat (decrease) and carbohydrates (decrease). Providing several sessions of consultation gives the patients reassurance and confidence while they are receiving dietary guidance. Using a daily diary to register the daily consumption of food and fluids and the accompanying IBS symptoms (if any) helps in identifying which foodstuffs worsen the symptoms experienced by the patients (52,165,166).
Effect of individual dietary guidance on symptoms and quality of life of patients with IBS
Individual dietary guidance helps patients with IBS to choose dietary items that are low in FODMAPs and water-insoluble fiber (166). The effects of this approach on symptoms and the quality of life of patients with IBS have been assessed using several questionnaires, such as the Birmingham IBS Symptom Questionnaire, the Irritable Bowel Syndrome - Quality of Life (IBS-QOL) questionnaire, and the Short-Form Nepean and Dyspepsia Index (SF-NDI) quality-of-life questionnaire (52,166). The Birmingham IBS symptom questionnaire is disease-specific and measures the symptoms experienced by IBS patients, namely pain, diarrhea and constipation (167). IBS symptoms, as assessed by the Birmingham IBS symptom questionnaire, improve significantly after receiving dietary guidance, particularly the symptoms of pain (52,166) and diarrhea (166), but not constipation (52,166).
The IBS-QOL questionnaire is IBS-specific and assesses physical and psychosocial functioning as a result of IBS (168,169). The SF-NDI questionnaire is a disease-specific questionnaire that assesses the health-related quality of life and was constructed and validated primarily in patients with dyspepsia (170). However, a validated version of the questionnaire translated into Norwegian was demonstrated to perform well in patients with IBS (171). The total scores, as assessed by the IBS-QOL and SF-NDI questionnaires, showed significant improvements in the quality of life of patients with IBS after they received dietary guidance (52,166).
Individual dietary guidance helps to reduce IBS symptoms, improving the quality of life of patients with IBS (52,165,166), and ensures that they have an adequate intake of necessary vitamins and minerals, thus avoiding multiple nutrition deficiencies (52,166).
Effects of individual dietary guidance on GI endocrine cells
At the cellular level, changing the diet through individual dietary guidance changes the densities of different GI endocrine cells in different parts of the GI tract (172–175), as shown in Figs. 2–5. Chromogranin A (CgA) is considered to be a general marker of endocrine cells (176–178). As previously demonstrated, the densities of CgA-immunoreactive cells are abnormal in the stomach, duodenum, ileum and colon of patients with IBS, and dietary guidance tends to change these densities toward the values measured in healthy control subjects (173,179,180). These changes are considered to be brought about by changes in different endocrine cells in the respective parts of the GI tract after providing dietary guidance. For example, in the stomach, the densities of several endocrine cells (gastrin, ghrelin, serotonin and somatostatin) changed toward the values measured in healthy control subjects after providing dietary guidance, but only somatostatin cell density showed a significant change in the gastric corpus (181). The densities of serotonin cells in the duodenum and ileum changed significantly (172,175), and the density of somatostatin cells in the duodenum changed significantly after providing dietary guidance toward that measured in healthy control subjects (175). After providing dietary guidance, the densities of serotonin and PYY cells tend to normalize in different segments of the colon, with the density of somatostatin cells increasing in the rectum (174).
Figure 2.
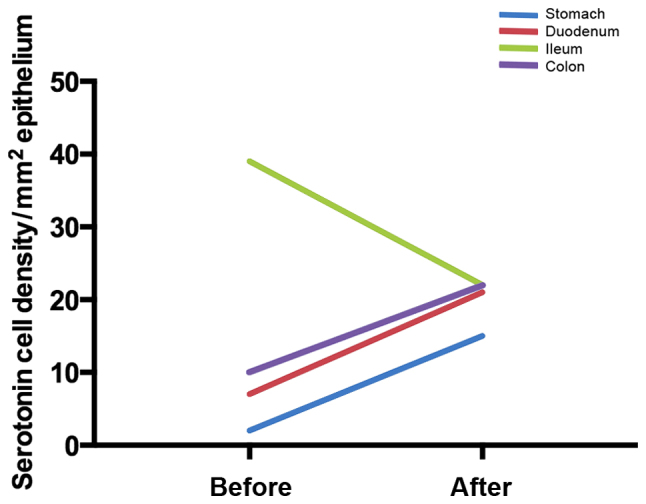
Mean serotonin cell densities in different parts of the gastrointestinal (GI) tract in patients with irritable bowel syndrome (IBS) before and after receiving dietary guidance.
Figure 3.
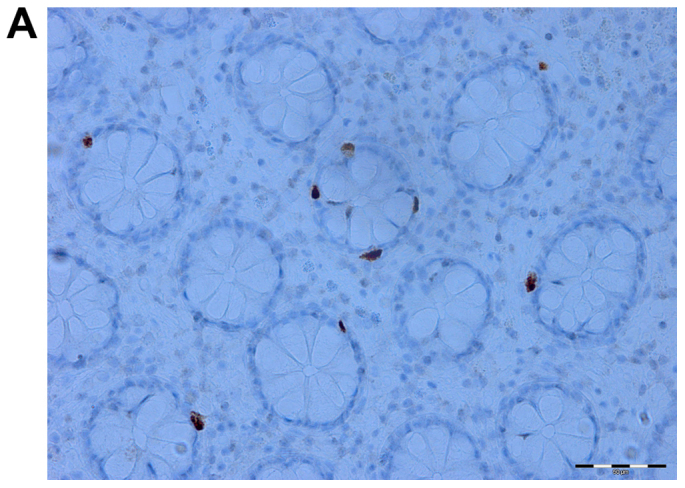
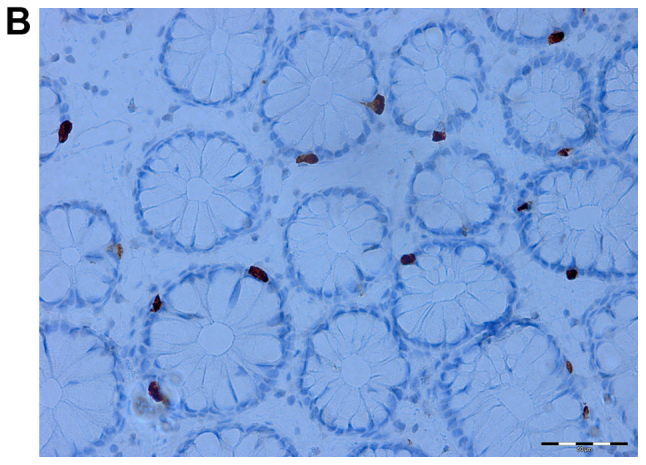
Serotonin cells in the colon of an irritable bowel syndrome (IBS) patient before (A) and after (B) receiving dietary guidance.
Figure 4.
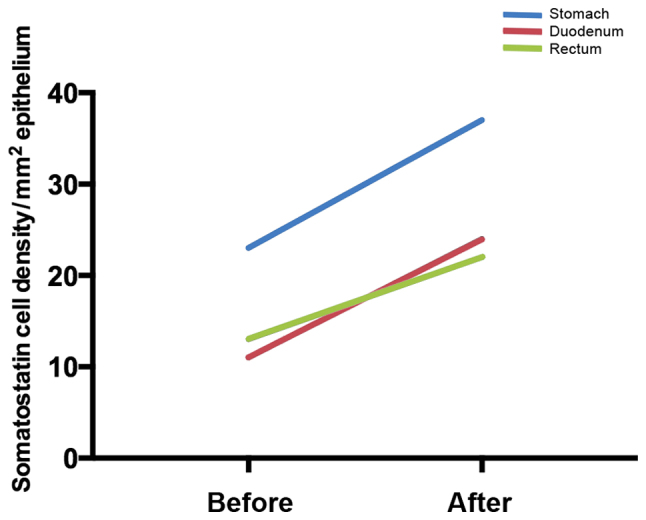
Mean somatostatin cell densities in different parts of the gastrointestinal (GI) tract in patients with irritable bowel syndrome (IBS) before and after receiving dietary guidance.
Figure 5.
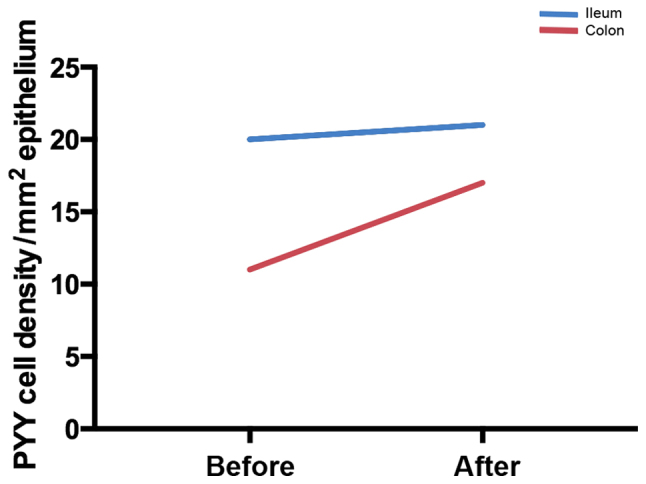
Mean peptide YY (PYY) cell densities in the ileum and colon of patients with irritable bowel syndrome (IBS) before and after receiving dietary guidance.
The observation that dietary guidance can alter the densities of several GI endocrine cells in patients with IBS, particularly serotonin, PYY and somatostatin cells, to approach those in healthy control subjects suggests that these changes underlie the reduction in pain and the improvement in diarrhea as quantified using the Birmingham IBS symptom questionnaire, and consequently the improvement in the quality of life of these patients.
5. Conclusion
Patients with IBS typically report that food aggravates their IBS symptoms. The interactions between specific types of foodstuffs and GI endocrine cells result in changes in these cell densities. Providing individual dietary guidance about a low FODMAP intake, high soluble-fiber intake, and changing the proportions of protein, fat and carbohydrates helps to reduce the symptoms experienced by IBS patients and to improve their quality of life. This improvement is accompanied by changes in the densities of the GI endocrine cells where some densities are restored back to the normal values. These observations emphasize on the role of GI endocrine cells in the pathophysiology of IBS, and support the provision of dietary guidance as a first-line treatment for managing IBS (76).
Acknowledgments
The studies conducted by the authors and cited in this review were supported by grants from Helse-Vest (grant no. 911976) and Helse-Fonna (grant no. 40415).
References
- 1.Thompson WG. A world view of IBS. In: Camilleri M, Spiller RC, editors. Irritable Bowel Syndrome: Diagnosis and Treatment. WB Saunders; Edinburgh: 2002. pp. 17–26. [Google Scholar]
- 2.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T, editors. Irritable Bowel Syndrome. Nova Science Publishers; New York, NY: 2012. [Google Scholar]
- 3.Camilleri M, Heading RC, Thompson WG. Clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407–1430. doi: 10.1046/j.1365-2036.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- 4.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i37.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kay L, Jørgensen T, Jensen KH. The epidemiology of irritable bowel syndrome in a random population: prevalence, incidence, natural history and risk factors. J Intern Med. 1994;236:23–30. doi: 10.1111/j.1365-2796.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. U.S. householder survey of functional gastro-intestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy TM, Jones RH, Hungin AP, O'flanagan H, Kelly P. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43:770–774. doi: 10.1136/gut.43.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everhart JE, Renault PF. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology. 1991;100:998–1005. doi: 10.1016/0016-5085(91)90275-P. [DOI] [PubMed] [Google Scholar]
- 9.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50:M184–M189. doi: 10.1093/gerona/50A.4.M184. [DOI] [PubMed] [Google Scholar]
- 11.Gralnek IM, Hays RD, Kilbourne AM, Chang L, Mayer EA. Racial differences in the impact of irritable bowel syndrome on health-related quality of life. J Clin Gastroenterol. 2004;38:782–789. doi: 10.1097/01.mcg.0000140190.65405.fb. [DOI] [PubMed] [Google Scholar]
- 12.Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000;46:78–82. doi: 10.1136/gut.46.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review) Int J Mol Med. 2012;29:723–731. doi: 10.3892/ijmm.2012.926. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology. 1987;92:1282–1284. doi: 10.1016/S0016-5085(87)91099-7. [DOI] [PubMed] [Google Scholar]
- 15.Schuster MM. Defining and diagnosing irritable bowel syndrome. Am J Manag Care. 2001;7(Suppl 8):S246–S251. [PubMed] [Google Scholar]
- 16.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol Hepatol. 2014;8:435–443. doi: 10.1586/17474124.2014.888952. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–1741. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WG, Heaton KW. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79:283–288. [PubMed] [Google Scholar]
- 19.Bordie AK. Functional disorders of the colon. J Indian Med Assoc. 1972;58:451–456. [PubMed] [Google Scholar]
- 20.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey RF, Salih SY, Read AE. Organic and functional disorders in 2000 gastroenterology outpatients. Lancet. 1983;1:632–634. doi: 10.1016/S0140-6736(83)91802-0. [DOI] [PubMed] [Google Scholar]
- 22.Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. doi: 10.1023/A:1013208713670. [DOI] [PubMed] [Google Scholar]
- 24.Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., III A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–2824. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 25.Boyce PM, Koloski NA, Talley NJ. Irritable bowel syndrome according to varying diagnostic criteria: are the new Rome II criteria unnecessarily restrictive for research and practice? Am J Gastroenterol. 2000;95:3176–3183. doi: 10.1111/j.1572-0241.2000.03197.x. [DOI] [PubMed] [Google Scholar]
- 26.Abdulmajeed A, Rabab MA, Sliem HA, Hebatallah NE. Pattern of irritable bowel syndrome and its impact on quality of life in primary health care center attendees, Suez governorate, Egypt. Pan Afr Med J. 2011;9:5. doi: 10.4314/pamj.v9i1.71177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoshal UC, Abraham P, Bhatt C, Choudhuri G, Bhatia SJ, Shenoy KT, Banka NH, Bose K, Bohidar NP, Chakravartty K, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28. [PubMed] [Google Scholar]
- 28.Makharia GK, Verma AK, Amarchand R, Goswami A, Singh P, Agnihotri A, Suhail F, Krishnan A. Prevalence of irritable bowel syndrome: a community based study from northern India. J Neurogastroenterol Motil. 2011;17:82–87. doi: 10.5056/jnm.2011.17.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Hou X. A review of the irritable bowel syndrome investigation on epidemiology, pathogenesis and pathophysiology in China. J Gastroenterol Hepatol. 2011;26(Suppl 3):88–93. doi: 10.1111/j.1440-1746.2011.06641.x. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen S, Jensen TH, Henriksen SL, Haastrup PF, Larsen PV, Søndergaard J, Jarbøl DE. Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scand J Gastroenterol. 2015;50:162–169. doi: 10.3109/00365521.2014.983157. [DOI] [PubMed] [Google Scholar]
- 31.Kjellström L, Molinder H, Agréus L, Nyhlin H, Talley NJ, Andreasson A. A randomly selected population sample undergoing colonoscopy: prevalence of the irritable bowel syndrome and the impact of selection factors. Eur J Gastroenterol Hepatol. 2014;26:268–275. doi: 10.1097/MEG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 32.Vandvik PO, Lydersen S, Farup PG. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand J Gastroenterol. 2006;41:650–656. doi: 10.1080/00365520500442542. [DOI] [PubMed] [Google Scholar]
- 33.Breckan RK, Asfeldt AM, Straume B, Florholmen J, Paulssen EJ. Prevalence, comorbidity, and risk factors for functional bowel symptoms: a population-based survey in Northern Norway. Scand J Gastroenterol. 2012;47:1274–1282. doi: 10.3109/00365521.2012.688215. [DOI] [PubMed] [Google Scholar]
- 34.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 35.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77–81. doi: 10.1159/000007593. [DOI] [PubMed] [Google Scholar]
- 37.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Clinical Services Committee of The British Society of Gastroenterology: Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tillisch K, Labus JS, Naliboff BD, Bolus R, Shetzline M, Mayer EA, Chang L. Characterization of the alternating bowel habit subtype in patients with irritable bowel syndrome. Am J Gastroenterol. 2005;100:896–904. doi: 10.1111/j.1572-0241.2005.41211.x. [DOI] [PubMed] [Google Scholar]
- 39.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016 Feb 19; doi: 10.1053/j.gastro.2016.02.032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Harvey RF, Mauad EC, Brown AM. Prognosis in the irritable bowel syndrome: a 5-year prospective study. Lancet. 1987;1:963–965. doi: 10.1016/S0140-6736(87)90304-7. [DOI] [PubMed] [Google Scholar]
- 41.García Rodríguez LA, Ruigómez A, Wallander MA, Johansson S, Olbe L. Detection of colorectal tumor and inflammatory bowel disease during follow-up of patients with initial diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2000;35:306–311. doi: 10.1080/003655200750024191. [DOI] [PubMed] [Google Scholar]
- 42.Nørgaard M, Farkas DK, Pedersen L, Erichsen R, de la Cour ZD, Gregersen H, Sørensen HT. Irritable bowel syndrome and risk of colorectal cancer: a Danish nationwide cohort study. Br J Cancer. 2011;104:1202–1206. doi: 10.1038/bjc.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead WE, Burnett CK, Cook EW, III, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41:2248–2253. doi: 10.1007/BF02071408. [DOI] [PubMed] [Google Scholar]
- 44.Schmulson MRG, Kershenobich D. Los pacientes con trastornos funcionales digestivos (TFD) tienen major compromiso de la calidad de vida (CV) evaluadas por el SF-36 comparados con pacientes con hepatitis C y pancreatitis cronica. Rev Gastroenterol Mex. 2000;65(Suppl):50–51. In Spanish. [Google Scholar]
- 45.Martin R, Barron JJ, Zacker C. Irritable bowel syndrome: toward a cost-effective management approach. Am J Manag Care. 2001;7(Suppl 8):S268–S275. [PubMed] [Google Scholar]
- 46.Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265–269. doi: 10.1007/s11894-009-0039-x. [DOI] [PubMed] [Google Scholar]
- 47.American Gastroenterological Association . The burden of gastrointestinal diseases. Bethesda, MD: 2001. [Google Scholar]
- 48.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 49.Li FX, Patten SB, Hilsden RJ, Sutherland LR. Irritable bowel syndrome and health-related quality of life: a population-based study in Calgary, Alberta. Can J Gastroenterol. 2003;17:259–263. doi: 10.1155/2003/706891. [DOI] [PubMed] [Google Scholar]
- 50.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163:265–274. doi: 10.1001/archinte.163.3.265. [DOI] [PubMed] [Google Scholar]
- 52.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5:1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 53.Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 54.Saito YA. The role of genetics in IBS. Gastroenterol Clin North Am. 2011;40:45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456–2469. doi: 10.3748/wjg.v20.i10.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grover M, Camilleri M, Smith K, Linden DR, Farrugia G. On the fiftieth anniversary. Postinfectious irritable bowel syndrome: mechanisms related to pathogens. Neurogastroenterol Motil. 2014;26:156–167. doi: 10.1111/nmo.12304. [DOI] [PubMed] [Google Scholar]
- 57.Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K, et al. Pathogenesis of irritable bowel syndrome - review regarding associated infection and immune activation. Digestion. 2013;87:204–211. doi: 10.1159/000350054. [DOI] [PubMed] [Google Scholar]
- 58.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 59.Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol. 2014;20:2470–2481. doi: 10.3748/wjg.v20.i10.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 61.El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (Review) Int J Mol Med. 2009;24:727–732. doi: 10.3892/ijmm_00000285. [DOI] [PubMed] [Google Scholar]
- 62.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60–65. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 64.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Low-grade inflammation in the rectum of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2013;7:1081–1085. doi: 10.3892/mmr.2013.1320. [DOI] [PubMed] [Google Scholar]
- 65.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. doi: 10.3109/00365521.2010.503965. [DOI] [PubMed] [Google Scholar]
- 67.El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Rep. 2012;6:1223–1225. doi: 10.3892/mmr.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Salhy M, Rauma J. Low density of ghrelin cells in the oxyntic mucosa correlated to slow gastric emptying in patients with type 1 diabetes. Mol Med Rep. 2009;2:893–896. doi: 10.3892/mmr_00000188. [DOI] [PubMed] [Google Scholar]
- 69.El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–3513. doi: 10.1007/s10620-010-1169-6. [DOI] [PubMed] [Google Scholar]
- 70.El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–1244. doi: 10.3892/mmr.2013.1325. [DOI] [PubMed] [Google Scholar]
- 71.Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 72.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome - etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–672. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 73.Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892–1897. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 74.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–1214. doi: 10.1016/j.jada.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 76.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26(Suppl 3):128–131. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- 77.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Roest RH, Dobbs BR, Chapman BA, Batman B, O'Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 79.Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099–1104. doi: 10.1136/gut.30.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, Gibson PR, Muir JG. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24:154–176. doi: 10.1111/j.1365-277X.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 81.Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, Gibson PR. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC) J Agric Food Chem. 2009;57:554–565. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- 82.Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem. 2007;55:6619–6627. doi: 10.1021/jf070623x. [DOI] [PubMed] [Google Scholar]
- 83.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 84.Gibson GR, McCartney AL, Rastall RA. Prebiotics and resistance to gastrointestinal infections. Br J Nutr. 2005;93(Suppl 1):31–34. doi: 10.1079/BJN20041343. [DOI] [PubMed] [Google Scholar]
- 85.Szajewska H, Ruszczyński M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367–372. doi: 10.1016/j.jpeds.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 86.Cashman K. Prebiotics and calcium bioavailability. Curr Issues Intest Microbiol. 2003;4:21–32. [PubMed] [Google Scholar]
- 87.Kleessen B, Blaut M. Modulation of gut mucosal biofilms. Br J Nutr. 2005;93(Suppl 1):35–40. doi: 10.1079/BJN20041346. [DOI] [PubMed] [Google Scholar]
- 88.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O'Riordan M, O'Sullivan GC, Pool-Zobel B, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–96. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 89.Feinle-Bisset C, Azpiroz F. Dietary lipids and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:737–747. doi: 10.1038/ajg.2013.76. [DOI] [PubMed] [Google Scholar]
- 90.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 91.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 92.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 93.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (Review) Int J Mol Med. 2014;34:363–371. doi: 10.3892/ijmm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Talukder S. Effect of dietary fiber on properties and acceptance of meat products: a review. Crit Rev Food Sci Nutr. 2015;55:1005–1011. doi: 10.1080/10408398.2012.682230. [DOI] [PubMed] [Google Scholar]
- 97.Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245–251. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 98.Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2005;18:CD003460. doi: 10.1002/14651858.CD003460.pub2. [DOI] [PubMed] [Google Scholar]
- 99.Akehurst R, Kaltenthaler E. Treatment of irritable bowel syndrome: a review of randomised controlled trials. Gut. 2001;48:272–282. doi: 10.1136/gut.48.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Quigley EM. Task Force on the Management of Functional Bowel Disorders American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–S26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 101.Parr NJ, Grime JS, Baxter JN, Critchley M, Mackie CR. Small bowel resistances and the gastroduodenal brake. Gut. 1987;28:950–954. doi: 10.1136/gut.28.8.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White CM, Poxon V, Alexander-Williams J. Effects of nutrient liquids on human gastroduodenal motor activity. Gut. 1983;24:1109–1116. doi: 10.1136/gut.24.12.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vidon N, Chaussade S, Merite F, Huchet B, Franchisseur C, Bernier JJ. Inhibitory effect of high caloric load of carbohydrates or lipids on human pancreatic secretions: a jejunal brake. Am J Clin Nutr. 1989;50:231–236. doi: 10.1093/ajcn/50.2.231. [DOI] [PubMed] [Google Scholar]
- 104.Vidon N, Pfeiffer A, Franchisseur C, Bovet M, Rongier M, Bernier JJ. Effect of different caloric loads in human jejunum on meal-stimulated and nonstimulated biliopancreatic secretion. Am J Clin Nutr. 1988;47:400–405. doi: 10.1093/ajcn/47.3.400. [DOI] [PubMed] [Google Scholar]
- 105.Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483–486. doi: 10.1136/gut.32.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boyd KA, O'Donovan DG, Doran S, Wishart J, Chapman IM, Horowitz M, Feinle C. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol Gastrointest Liver Physiol. 2003;284:G188–G196. doi: 10.1152/ajpgi.00375.2002. [DOI] [PubMed] [Google Scholar]
- 107.Lin HC, Zhao XT, Wang L. Jejunal brake: inhibition of intestinal transit by fat in the proximal small intestine. Dig Dis Sci. 1996;41:326–329. doi: 10.1007/BF02093823. [DOI] [PubMed] [Google Scholar]
- 108.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake - inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salvioli B, Serra J, Azpiroz F, Malagelada JR. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101:1853–1857. doi: 10.1111/j.1572-0241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 110.Simrén M, Abrahamsson H, Björnsson ES. Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol. 2007;5:201–208. doi: 10.1016/j.cgh.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 111.Caldarella MP, Milano A, Laterza F, Sacco F, Balatsinou C, Lapenna D, Pierdomenico SD, Cuccurullo F, Neri M. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am J Gastroenterol. 2005;100:383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 112.Gibson PR, Barrett JS, Muir JG. Functional bowel symptoms and diet. Intern Med J. 2013;43:1067–1074. doi: 10.1111/imj.12266. [DOI] [PubMed] [Google Scholar]
- 113.Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 114.Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- 115.Blachier F, Mariotti F, Huneau JF, Tomé D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. 2007;33:547–562. doi: 10.1007/s00726-006-0477-9. [DOI] [PubMed] [Google Scholar]
- 116.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O'Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Talley NJ. Dietary modification as a treatment for irritable bowel syndrome. Gastroenterol Hepatol. 2012;8:552–554. [PMC free article] [PubMed] [Google Scholar]
- 118.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–667. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 119.Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39–48. doi: 10.1016/S0047-6374(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 120.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of 'taste' in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells - how it interacts with enteroendocrine cells. Adv Nutr. 2012;3:8–20. doi: 10.3945/an.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227–240. doi: 10.1007/BF00220741. [DOI] [PubMed] [Google Scholar]
- 124.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 125.Sandstrom O, El-Salhy M. Age-related changes in neuroendocrine system of the gut. A possible role in the pathogenesis of gastrointestinal disorders in the elderly. Minireview based on a doctoral thesis. Ups J Med Sci. 2001;106:81–97. doi: 10.3109/2000-1967-161. [DOI] [PubMed] [Google Scholar]
- 126.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–75. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 129.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 130.Fontaine J, Le Lièvre C, Le Douarin NM. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo? Gen Comp Endocrinol. 1977;33:394–404. doi: 10.1016/0016-6480(77)90055-7. [DOI] [PubMed] [Google Scholar]
- 131.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 132.Rawdon BB, Andrew A. Origin and differentiation of gut endocrine cells. Histol Histopathol. 1993;8:567–580. [PubMed] [Google Scholar]
- 133.Hoffman J, Kuhnert F, Davis CR, Kuo CJ. Wnts as essential growth factors for the adult small intestine and colon. Cell Cycle. 2004;3:554–557. doi: 10.4161/cc.3.5.858. [DOI] [PubMed] [Google Scholar]
- 134.Höcker M, Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Ann N Y Acad Sci. 1998;859:160–174. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- 135.Inokuchi H, Fujimoto S, Kawai K. Cellular kinetics of gastrointestinal mucosa, with special reference to gut endocrine cells. Arch Histol Jpn. 1983;46:137–157. doi: 10.1679/aohc.46.137. [DOI] [PubMed] [Google Scholar]
- 136.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;209:309–335. doi: 10.1007/978-3-642-24716-3_14. [DOI] [PubMed] [Google Scholar]
- 137.Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657–R666. doi: 10.1152/ajpregu.00345.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 139.Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem. 2008;64:349–356. doi: 10.1007/BF03174091. [DOI] [PubMed] [Google Scholar]
- 140.San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest. 2009;56(Suppl):209–217. doi: 10.2152/jmi.56.209. [DOI] [PubMed] [Google Scholar]
- 141.Rudholm T, Wallin B, Theodorsson E, Näslund E, Hellström PM. Release of regulatory gut peptides somatostatin, neurotensin and vasoactive intestinal peptide by acid and hyperosmolal solutions in the intestine in conscious rats. Regul Pept. 2009;152:8–12. doi: 10.1016/j.regpep.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 142.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 143.Buchan AM. Nutrient tasting and signaling mechanisms in the gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–G1107. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- 144.Montero-Hadjadje M, Elias S, Chevalier L, Benard M, Tanguy Y, Turquier V, Galas L, Yon L, Malagon MM, Driouich A, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shooshtarizadeh P, Zhang D, Chich JF, Gasnier C, Schneider F, Haïkel Y, Aunis D, Metz-Boutigue MH. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept. 2010;165:102–110. doi: 10.1016/j.regpep.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 146.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci. 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 147.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El-Salhy M. The possible role of the gut neuroendocrine system in diabetes gastroenteropathy. Histol Histopathol. 2002;17:1153–1161. doi: 10.14670/HH-17.1153. [DOI] [PubMed] [Google Scholar]
- 149.Debas HT, Mulvihill SJ. Neuroendocrine design of the gut. Am J Surg. 1991;161:243–249. doi: 10.1016/0002-9610(91)91139-A. [DOI] [PubMed] [Google Scholar]
- 150.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 151.McConalogue K, Furness JB. Gastrointestinal neurotransmitters. Baillieres Clin Endocrinol Metab. 1994;8:51–76. doi: 10.1016/S0950-351X(05)80226-5. [DOI] [PubMed] [Google Scholar]
- 152.Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohn's Colitis. 2009;3:8–14. doi: 10.1016/j.crohns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 153.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 154.Shepherd SJ1, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 155.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 156.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 157.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–1083. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 159.Hustoft TN, Hausken T, Ystad SO, Valeur J, Brokstad K, Hatlebakk JG, Lied GA. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- 160.Böhn L, Störsrud S, Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil. 2013;25:23–30. doi: 10.1111/nmo.12001. [DOI] [PubMed] [Google Scholar]
- 161.Ligaarden SC, Lydersen S, Farup PG. Diet in subjects with irritable bowel syndrome: a cross-sectional study in the general population. BMC Gastroenterol. 2012;12:61. doi: 10.1186/1471-230X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Williams EA, Nai X, Corfe BM. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterol. 2011;11:9. doi: 10.1186/1471-230X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Halpert A, Dalton CB, Palsson O, Morris C, Hu Y, Bangdiwala S, Hankins J, Norton N, Drossman D. National Survey on Patient Educational Needs in IBS and Development and Validation of the Patient Educational Needs Questionnaire (PEQ): What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ) Am J Gastroenterol. 2007;102:1972–1982. doi: 10.1111/j.1572-0241.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 164.El-Salhy M, Gundersen D. Diet in irritable bowel syndrome. Nutr J. 2015;14:36. doi: 10.1186/s12937-015-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Salhy M, Lillebø E, Reinemo A, Salmelid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotics administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterol Insights. 2010;2:1–6. doi: 10.4081/gi.2010.e6. [DOI] [Google Scholar]
- 166.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep. 2013;8:845–852. doi: 10.3892/mmr.2013.1565. [DOI] [PubMed] [Google Scholar]
- 167.Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30. doi: 10.1186/1471-230X-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/A:1018831127942. [DOI] [PubMed] [Google Scholar]
- 169.Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 170.Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15:207–216. doi: 10.1046/j.1365-2036.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 171.Arslan G, Lind R, Olafsson S, Florvaag E, Berstad A. Quality of life in patients with subjective food hypersensitivity: applicability of the 10-item short form of the Nepean Dyspepsia Index. Dig Dis Sci. 2004;49:680–687. doi: 10.1023/B:DDAS.0000026318.81635.3b. [DOI] [PubMed] [Google Scholar]
- 172.Mazzawi T, El-Salhy M. Dietary guidance and ileal enteroendocrine cells in patients with irritable bowel syndrome. Exp Ther Med. 2016;12:1398–1404. doi: 10.3892/etm.2016.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. 2014;10:2322–2326. doi: 10.3892/mmr.2014.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur J Clin Nutr. 2016;70:175–181. doi: 10.1038/ejcn.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mazzawi T, El-Salhy M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp Biol Med. 2017 doi: 10.1177/1535370217699537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 177.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 178.Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins - widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58:95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- 179.Mazzawi T, El-Salhy M. Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int J Mol Med. 2016;37:1247–1253. doi: 10.3892/ijmm.2016.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. 2015;2015:823897. doi: 10.1155/2015/823897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. 2015;69:519–524. doi: 10.1038/ejcn.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]