Abstract
Diabetic nephropathy (DN) is a serious and one of the most common microvascular complications of diabetes. There is accumulating evidence to indicate that advanced glycation end products (AGEs), senescent macroprotein derivatives formed at an accelerated rate under conditions of diabetes, play a role in DN. In this study, we found that the serum and urine levels of C-X-C motif chemokine ligand 9 (CXCL9) were significantly elevated in patients with DN compared with healthy controls. Based on an in vitro model of mouse podocyte injury, AGEs decreased the proliferation of podocytes and increased the expression of CXCL9 and C-X-C motif chemokine receptor 3 (CXCR3), and promoted the activation of signal transducer and activator of transcription 3 (STAT3). The knockdown of CXCL9 by the transfection of mouse podoyctes with specific siRNA significantly increased the proliferation and decreased the apoptosis of the podoyctes. Moreover, the levels of inflammatory factors, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 were also decreased in the podoyctes transfected with siRNA-CXCL9, accompanied by the increased expression of nephrin and podocin, and decreased levels of Bax/Bcl-2 and activated caspase-3. The knockdown of CXCL9 also led to the inactivation of the Janus kinase 2 (JAK2)/STAT3 pathway. Importantly, the use of the JAK2 inhibitor, AG490, and valsartan (angiotensin II receptor antagonist) attenuated the injury induced to mouse podoyctes by AGEs. On the whole, and to the best of our knowledge, this study demonstrates for the first time that AGEs exert pro-apoptotic and pro-inflammatory effects in mouse podoyctes through the CXCL9-mediated activation of the JAK2/STAT3 pathway. Thus, our data provide a potential therapeutic target for DN.
Keywords: diabetic nephropathy, advanced glycation end products, podocyte, C-X-C motif chemokine ligand 9, Janus kinase-2/signal transducer and activator of transcription 3
Introduction
As one of the most common causes of end-stage renal disease, diabetic nephropathy (DN) accounts for the disability and high mortality rate in patients with diabetes. The incidence of DN in patients with type 1 or 2 diabetes within 20–25 years of the onset of the disease is approximately 25–40% (1). Although the prevention and treatment of DN are attracting increasing attention from researchers, the current therapeutic options are far from satisfactory as the intensive therapy of blood glucose and pressure is often difficult to maintain and may increase the risk of hypoglycemia and/or hypotension in patients with diabetes (2). Therefore, the development of novel therapeutic strategies that may specifically target DN is urgently required.
Podocytes are one of the most important components of the glomerular filtration barrier which has specific cytobiological traits and physiological functions. It has been shown that the loss of podocytes is an early characteristic of DN that predicts its progressive course (3), and decreased nephrin expression is observed during the early stages of DN, playing an important role in accelerating the development of DN (4). DN has been reported to be an immunological renal disease; the presence of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, interferon-γ (INF-γ) and monocyte-chemoattractant protein-1 (MCP-1) has been reported in patients with diabetes and in animal diabetic models (5–7). However, the exact role of podocytes in the inflammatory response has not yet been fully determined.
Various elements, including advanced glycation end products (AGEs), reactive oxygen species (ROS), protein kinase C (PKC) and the renin-angiotensin system (RAS), are considered to play a role in the development and progression of DN (8). AGE formation in the kidneys may contribute to the progressive alteration in the renal architecture and loss of renal function in patients, resulting in basement membrane thickening and mesangial expansion, hallmarks of DN (9). The activation of AGEs in podocytes leads to multiple pathophysiological effects, including hypertrophy with cell cycle arrest and apoptosis, altered migration and in the generation of pro-inflammatory cytokines (10). Previous studies have reported that AGEs reduce podocyte adhesion via the upregulation of integrin-linked kinase (ILK) expression, which occurs partly through the activation of the RAS in podocytes (11). AGEs are also potent stimulators of chemokine production, including IL-8 [also known as C-X-C motif chemokine ligand (CXCL)8], MCP-1 (CCL2), INF-γ inducible protein 10 (IP-10/CXCL10), macrophage inflammatory protein-1α (MIP-1α/CCL3) and RANTES (CCL5) (7). The downregulation of CXCL9 and its receptor, CXCR3, suppresses the loss of renal function and may be a potential therapeutic target for human immune-mediated nephritis (12). However, the role of CXCL9 in AGE-induced podocyte injury has not yet been reported to date, at least to the best of our kowledge.
The activation of the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway is an important mechanism through which hyperglycemia contributes to renal damage (13). Similarly, JAK/STAT activation has been reported in rat glomerular cells exposed to high glucose (14) and may be important in glomerular transforming growth factor-β (TGF-β) activation in early DN (15). Moreover, angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers, including valsartan, which prevent the progression of DN, also prevent JAK/STAT activation in glomerular cells from diabetic rats (16).
In this study, we examined the effect of AGEs on the proliferation and apoptosis of podocytes and on the expression of CXCL9 and the JAK2/STAT3 signaling pathway. We also investigated whether AGE-induced podocytes injury occurs through the CXCL9-mediated activation of the JAK2/STAT3 pathway.
Materials and methods
Patient samples
Serum and urine samples were obtained from 45 patients with DN admitted to Tongji Hospital, Shanghai, China and subjects in the control group were 45 healthy volunteers. Ethical approval for the study was provided by the independent Ethics Committee of Tongji Hospital. Written informed consent was obtained from all participants in this study. All the experimental procedures were carried out in accordance with the Helsinki Declaration, 1975.
Cell culture and treatment
Mouse podocytes were obtained from the Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China) and cultured in RMPI-1640 supplemented with 10% fetal bovine serum (FBS), 100X penicillin-streptomycin solution and 10 U/ml IFN-γ, and incubated in a humidified atmosphere at 33°C with 5% CO2. Following proliferation to 80% confluence, the podocytes were cultured in the above-mentioned medium without 10 U/ml IFN-γ and incubated in a humidified atmosphere at 37°C with 5% CO2 for 10–14 days. To establish the injury model, the podocytes were seeded at 80% confluence in complete medium (Invitrogen Life Technologies, Carlsbad, CA, USA) containing 10% FBS. After 24 h, the medium was changed to serum-free medium with AGEs (10, 50, 100 and 150 mg/l) at the indicated time points, respectively. In addition to AGE (150 mg/l) induction, podocytes were also treated with 10 µM AG490 or 20 µM valsartan (as a positive control) for 48 h.
Cell proliferation assay
Mouse podocytes (1×103/well) were plated in 96-well plates. Following treatment with AGEs for 12, 24, 48 and 72 h, 10% cell counting kit-8 (CCK-8, CK04; Dojindo Molecular Technologies, Kumamoto, Japan) diluted in serum-free RMPI-1640 was mixed in each well for a further 1 h. The absorption of each sample was measured at a 450 nm wavelength using a Labsystems MK3 microplate reader (Thermo Fisher Scientific, Inc., Rockford, IL, USA) to detect cell viability according to the manufacturer's instructions. Cells not treated with AGEs served as the control group.
Transfection with small interfering RNA (siRNA)
siRNA targeting CXCL9 (siRNA-CXCL9; Sangon Biotech Co., Ltd., Shanghai, China) was used to knockdown CXCL9 mRNA expression. The cells were transfected with siRNA (40 nM) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. Non-specific siRNA (Sangon Biotech Co., Ltd.) was used as a negative control (NC), and the selective silencing of CXCL9 was confirmed by real-time PCR. The cells were analyzed at 48 h following transfection.
Cell apoptosis assay
The analysis of cell apoptosis was performed using flow cytometry and the Annexin V apoptosis detection kit (eBioscience, San Diego, CA, USA). Briefly, the mouse podocytes were plated in 6-well plates at a density of 1×105 cells/well and incubated with 195 µl Annexin V and 5 µl propidium iodide (PI) for 15 min in the dark at 4°C. The early apoptotic cells are represented in the lower right quadrant of the FACS histogram, and the late apoptotic cells, which were stained with FITC and PI, emit red-green fluorescence and are represented in the upper right quadrant of the FACS histogram.
Real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions. The complementary DNA was synthesized using a cDNA synthesis kit (Thermo Fisher Scientific, Inc.). The conditions for cDNA synthesis were as follows: 37°C for 60 min, followed by 85°C for 5 min and 4°C for 5 min. Real-time PCR was performed using SYBR-Green (Takara Biotechnology Co., Ltd., Dalian, China) and data collection was conducted using an ABI 7500 Real-Time PCR system (Applied Biosystems Life Technologies, Foster City, CA, USA). Primers were list as follows: CXCL9 forward, 5′-CACTTCGCTGCTATCTAATTGG-3′ and reverse, 5′-TAGGCACTGTGGAAGATTTAGG-3′; CXCR3 forward, 5′-ACCATTACTGTGCCTTAGC-3′ a nd reverse, 5′-TATTTGCCTCTCCCTCTTCTC-3′; STAT3 forward, 5′-GACTCAAAGCCACCTCATTC-3′ and reverse, 5′-GCCTTGCCTTCCTAAATACC-3′; and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-ATCACTGCCACCCAGAAG-3′ and reverse, 5′-TCCACGACGGACACATTG-3′. GAPDH was used an internal control for normalization. The real-time PCR cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 45 sec, and a final extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec and 60°C for 15 sec. Gene expression was calculated using the 2−ΔΔCt method.
Western blot analysis
Mouse podocytes were seeded at a density of 5×105 cells/well in 6-well plates, cultured overnight and then treated with AGEs for 3 or 48 h. Total proteins were isolated from the mouse podocytes and were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto polyvinylidene fluoride membranes (Roche Diagnostics, Mannheim, Germany). The membranes were first incubated with rabbit monoclonal anti-p-STAT3 (ab76315; 1:1,000; Abcam, Cambridge, MA, USA), anti-JAK2 (#3230; 1:1,000), anti-p-JAK2 (#3771; 1:1,000) (both from Cell Signaling Technology, Danvers, MA, USA), podocin (ab181143; 1:10,000; Abcam) and anti-GAPDH (#5174; 1:1,500; Cell Signaling Technology) antibodies, and mouse monoclonal anti-STAT3 (ab119352; 1:1,000) and anti-Bcl-2 (ab117115; 1:400) (both from Abcam) antibodies; as well as rabbit polyclonal anti-CXCL9 (sc-50302; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-CXCR3 (ab181013; 1:600; Abcam), anti-Bax (sc-493; 1:300; Santa Cruz Biotechnology, Inc.), anti-caspase-3 (ab44976; 1:500) and anti-nephrin (ab58968; 1:500) antibodies (both from Abcam). The blots were then incubated with goat anti-mouse or anti-rabbit secondary antibodies (A0208 and A0216; 1:1,000; Beyotime Institute of Biotechnology, Haimen, China) and visualized using enhanced chemiluminescence (ECL; Thermo Fisher Scientific). GAPDH antibody was used as an internal control. The blotting bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
The TNF-α, IL-6 and CXCL9 levels present in the mouse podocytes or in the serum and urine of patients with DN were determined using commercially available murine-specific sandwich ELISA kit following the manufacturer's instructions.
Statistical analysis
Data are expressed as the means ± SD of triplicate samples. All results were confirmed in at least 3 time-independent experiments. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical analysis was performed using unpaired a two-tailed Student's t-test and one-way ANOVA tests. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
CXCL9 levels in the serum and urine of patients with DN
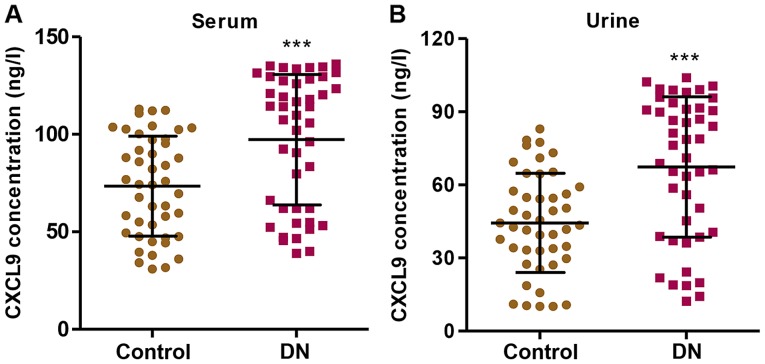
In order to examine the role of CXCL9 in DN, we first measured the levels of CXCL9 in both the serum and urine of patients with DN (n=45) and healthy controls (n=45). The CXCL9 concentration was significantly increased in the patients with DN compared with the healthy controls in both serum and urine (Fig. 1). Importantly, a higher concentration of CXCL9 was observed in the serum than in urine. These results suggest that CXCL9 plays an important role in DN; thus CXCL9 was further investigated in subsequent experiments.
Figure 1.
CXCL9 levels in serum and urine of diabetic patients. CXCL9 levels in (A) serum and (B) urine of patients with diabetic nephropathy (DN) were measured by enzyme-linked immunosorbent assay (ELISA). ***P<0.001 vs. control (healthy subjects).
Treatment with AGEs inhibits the proliferation of podocytes
To further investigate the association of CXCL9 with DN, we established an in vitro DN model of mouse podocyte injury induced by AGEs. We found that AGEs at various concentrations (10, 50, 100 and 150 mg/l) significantly inhibited the proliferation of mouse podocytes in a concentration- and time-dependent manner (Fig. 2). After 72 h of incubation, the proliferation of podocytes treated with AGEs (10, 50, 100 and 150 mg/l) was suppressed by 13.31±0.11%, 22.7±0.17%, 43.8±0.25% and 54.6±0.41%, respectively. These findings indicated that treatment with AGEs inhibited the proliferation of podocytes in a concentration- and time-dependent manner.
Figure 2.
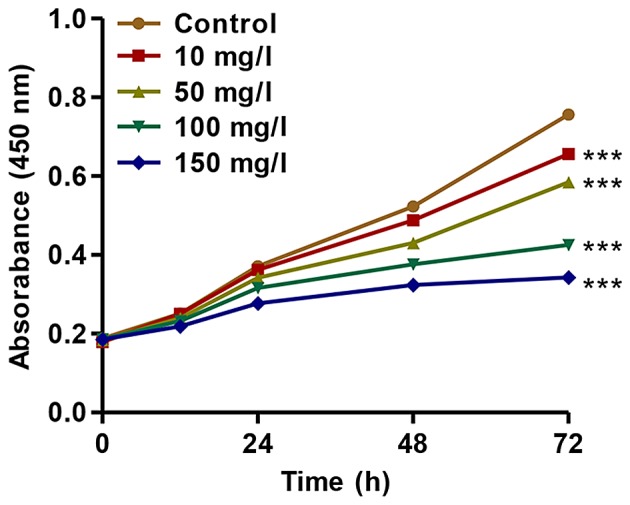
Effect of advanced glycation end products (AGEs) on the proliferation of podocytes. The proliferation of podocytes was measured by cell counting kit-8 (CCK-8) assay at the indicated time points. ***P<0.001 vs. control.
Effect of AGEs on the expression of CXCL9 and CXCR3, and STAT3 activation
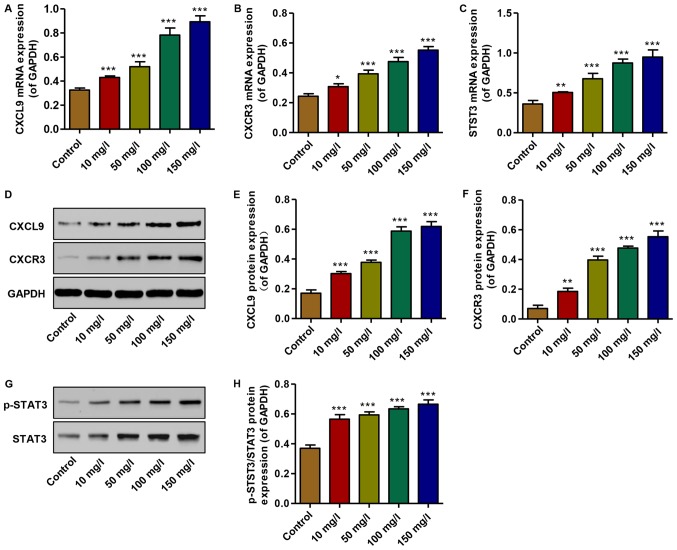
To examine the effects of AGEs on the expression of CXCL9 and its receptor, and STAT3 activation in vitro, real-time PCR and western blot analysis were performed. The mRNA expression levels of CXCL9, CXCR3 and STAT3 were increased in the podocytes treated with AGEs (10, 50, 100 and 150 mg/l) in a concentration-dependent manner compared with the controls (Fig. 3A–C). Similarly, the protein expression levels of CXCL9 and CXCR3 were increased, accompanied by the activation of STAT3; an increase in the levels of p-STAT3/STAT3 was observed in the AGE-treated podocytes in a concentration-dependent manner compared with the controls (Fig. 3D–H). Thus, AGEs at 150 mg/l were therefore used in the subsequent experiments. These data demonstrate that increased levels of CXCL9 and CXCR3, and STAT3 activation may contribute to the AGE-induced inhibition of podocyte proliferation.
Figure 3.
Effect of advanced glycation end products (AGEs) on the expression of CXCL9 and CXCR3 and STAT3 activation. (A–C) The mRNA levels of CXCL9, CXCR3 and STAT3 were measured by real-time PCR. (D–H) The protein levels of CXCL9, CXCR3, p-STAT3 and STAT3 were measured by western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs. control.
Knockdown of CXCL9 increases the proliferation and inhibits the apoptosis of podocytes
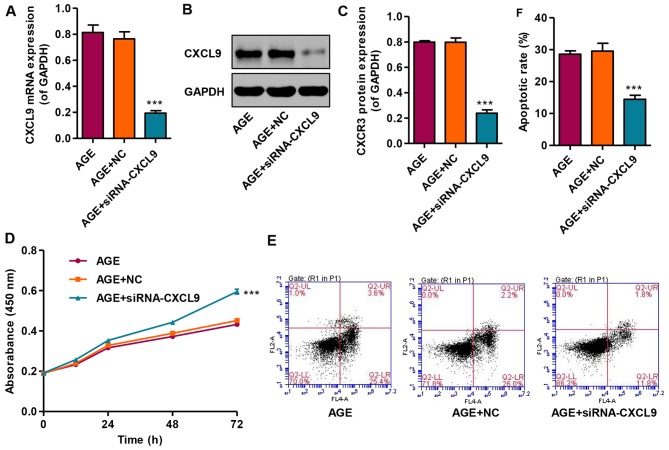
In order to examine the effects of CXCL9 on podocytes in vitro, CXCCL9 was knocked down by siRNA in podocytes. The results revealed that transfection with siNRA-CXCL9 significantly decreased the expression of CXCL9 in the AGE-treated podocytes at both the mRNA and protein level (Fig. 4A–C). In addition, the effects of CXCL9 on podocyte proliferation and apoptosis were also measured by CCK-8 assay and flow cytometry, respectively. Transfection with siNRA-CXCL9 significantly increased the proliferation and decreased the apoptosis of the AGE-treated podocytes compared with the cells treated with AGEs alone (Fig. 4D–F). However, the AGE-treated podocytes transfected with nonspecific siRNA (NC) exhibited no significant changes in CXCL9 expression, proliferation and apoptosis compared with the podocytes treated with AGEs alone. These results indicate that CXCL9 is involved in the proliferation and apoptosis of AGE-treated mouse podocytes.
Figure 4.
Effect of CXCL9 knockdown on the proliferation and apoptosis of podocytes. (A) The mRNA level of CXCL9 was measured by real-time PCR. (B and C) The protein level of CXCL9 was measured by western blot analysis. (D) The proliferation of podocytes was measured by cell counting kit-8 (CCK-8) assay at the indicated time points. (E and F) The apoptosis of podocytes was measured by flow cytometry. ***P<0.001 vs. AGE.
Knockdown of CXCL9 inhibits the release of the TNF-α and IL-6 inflammatory factors
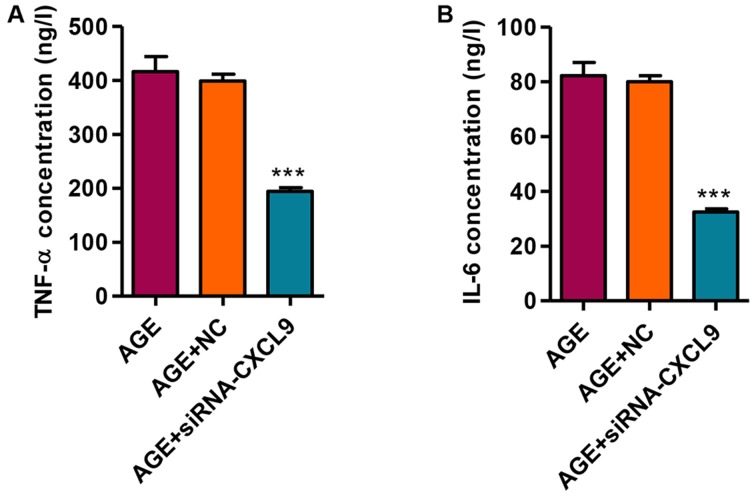
We then measured the secretion levels of TNF-α and IL-6 in response to transfection with siRNA-CXCL9 in the AGE-treated podocytes. Following the transfection of the AGE-treated podocytes with siRNA-CXCL9 for 48 h, the secretion levels of TNF-α and IL-6 were significantly decreased (Fig. 5). These findings thus suggest that the downregulation of CXCL9 exerts an anti-inflammatory effect in AGE-damaged podocytes.
Figure 5.
Effect of CXCL9 knockdown on the release of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). The levels of (A) TNF-α and (B) IL-6 were measured by enzyme-linked immunosorbent assay (ELISA). ***P<0.001 vs. AGE.
Effect of CXCL9 knockdown on protein expression and JAK2/STAT3 activation
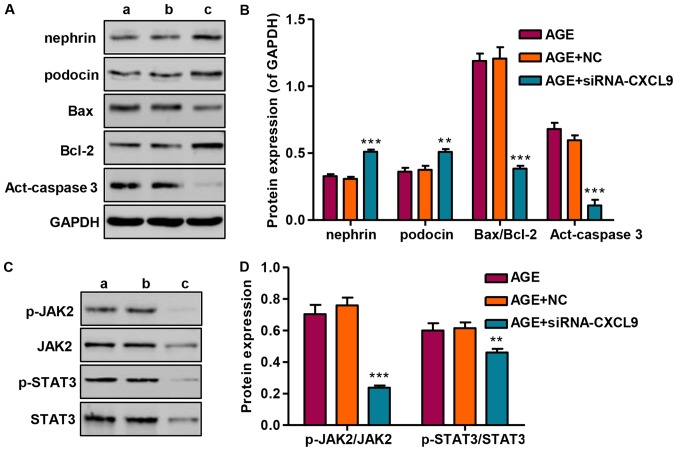
Following transfection of the podocytes with siRNA-CXCL9 for 48 h, the expression levels of nephrin and podocin were significantly increased, while the Bax/Bcl-2 ratio and activated caspase-3 (act-caspase-3) were significantly suppressed (Fig. 6A and B). As we had found that STAT3 was activated in the AGE-treated podocytes, the levels of STAT3 and upstream JAK2 signaling were also measured by western blot analysis in the podocytes transfected wtih siRNA-CXCL9. Our results revealed that siRNA-CXCL9 significantly suppressed the activation of JAK2 and STAT3, as evidenced by decreased levels of p-JAK2/JAK2 and p-STAT3/STAT3, in the AGE-treated podocytes transfected with siRNA-CXCL9 (Fig. 6C and D). These results thus suggest that the protective effects against AGE-induced damage to podocytes exerted by the downregulation CXCL9 are partially associated with the inhibition of JAK2/STAT3 activation in podocytes.
Figure 6.
Effect of CXCL9 knockdown on protein expression and Janus kinase-2/signal transducer and activator of transcription 3 (JAK2/STAT3) activation. (A and B) The protein expression levels of podocyte markers and apoptosis-associated proteins were measured by western blot analysis. (C and D) The activation of JAK2 and STAT3 was measured by western blot analysis. lane a, AGE; lane b, AGE + NC; lane c, AGE + siRNA-CXCL9. **P<0.01 and ***P<0.001 vs. AGE.
AG490 and valsartan attenuate the AGE-induced apoptosis of podocytes
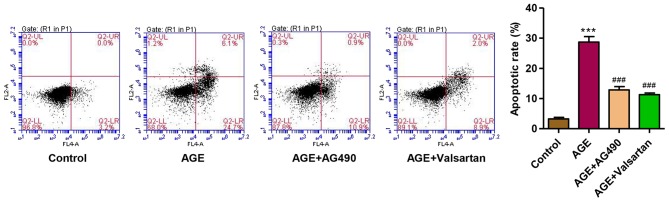
Considering the role of JAK2/STAT3 signaling in AGE-induced damage to podocytes, the JAK2 inhibitor, AG490, was used. Valsartan, an angiotensin II receptor antagonist, has long been proven to exert an anti-proteinuria effect and to decrease podocytes damage in DN (37). Therefore, with valsartan as a control, we observed that following treatment of the podocytes with AGEs for 48 h, the apoptotic rate was significantly increased. However, treatment with AG490 and valsartan markedly attenuated the apoptosis of the podocytes induced by treatment with AGEs (Fig. 7). These findings suggest that the activation of JAK2/STAT3 is implicated in AGE-induced podocyte apoptosis.
Figure 7.
Effect of AG490 and valsartan on the apoptosis of podocytes. The apoptosis of podocytes was measured by flow cytometry. ***P<0.001 vs. control; ###P<0.001 vs. advanced glycation end products (AGEs).
Effect of AG490 and valsartan on JAK2/STAT3 activation and protein expression
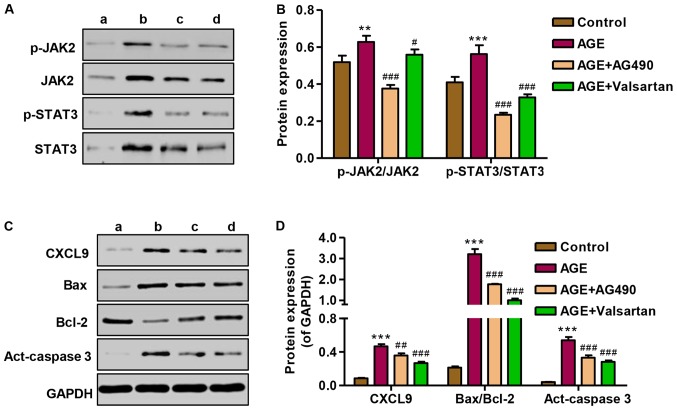
To investigate the role of AG490 in the apoptosis of podocytes induced by AGEs, the expression of CXCL9, Bax/Bcl-2 and activated caspase-3, as well as JAK2/STAT3 signaling was measured by western blot analysis. Following treatment of the podocytes with AGEs, the promoting effect of AGEs on JAK2 and STAT3 signaling in podocytes was markedly attenuated by treatment with AG490 and valsartan (Fig. 8A and B). The expression levels of CXCL9, Bax/Bcl-2 and activated caspase-3 were significantly increased following treatment with AGE; however, treatment with AG490 and valsartan markedly attenuated the overexpression of CXCL9, Bax/Bcl-2 and activated caspase-3 induced by AGE treatment (Fig. 8C and D). These results indicate that the AGE-induced apoptosis of podocytes is associated with the CXCL9-mediated activation of JAK2/STAT3 signaling.
Figure 8.
Effect of AG490 and valsartan on protein expression and Janus kinase-2/signal transducers and activator of transcription 3 (JAK2/STAT3) activation. (A and B) The activation of JAK2 and STAT3 was measured by western blot analysis. (C and D) The protein expression levels of CXCL9 and apoptosis-associated proteins were measured by western blot analysis. lane a, control; lane b, AGE; lane c, AGE + AG490; lane d, AGE + Valsartan. ***P<0.001 vs. control; #P<0.05, ##P<0.01 and ###P<0.001 vs. advanced glycation end products (AGEs).
Discussion
CXC chemokines are particularly important for leukocyte infiltration in inflammatory diseases. It has been demonstrated that inflammation is one of the potential pathogenic mechanisms responsible for the development of DN (17). However, to date, there are limited data available on inflammation related to CXC chemokines in human DN. In this study, we measured the serum and urine levels of the CXC chemokine, CXCL9, in 45 patients wth DN and 45 healthy controls by ELISA. The serum and urine levels of CXCL9 in the patients with DN were significantly elevated compared with those in the controls. In agreement with our findings, a previous study found that the levels of 3 CXC chemokines, CXCL5, CXCL8 and CXCL9, were significantly increased in the urine and serum of patients with DN (18). These results suggest CXCL9 may be involved in the progression of DN.
In patients with type 1 and 2 diabetes, the serum and tissue levels of AGEs have been shown to be significantly increased compared with the healthy control subjects (19), and the tissue levels of AGEs in diabetic renal disease have been shown to be twice those of patients with only diabetes and no renal disease (20), suggesting that AGEs are responsible for the progression of DN. A reduction in podocyte density is an important determinant of progressive DN and precedes the development of renal dysfunction and albuminuria in diabetic patients and in animal models of diabetes (21,22). Chen et al (23) reported that AGEs induced podocytes apoptosis in a dose-dependent manner and Chuang et al (24) showed that AGEs activated FOXO4, leading to the apoptosis of podocytes, which was similar to our findings in that AGEs inhibited the proliferation of mouse podocytes in a dose- and time-dependent manner.
Accumulating evidence from animal models supports the notion that CXCL9 and its receptor, CXCR3, which is highly expressed in Th1 CD4+ cells, play a critical role in the recruitment of T cells, macrophages and dendritic cells during the development of chronic renal injury (25). An increase in CXCL9 protein levels was detected in streptozotocin-injected mice and showed its pronociceptive properties (26). Activated and resting CXCR3 macrophages express CXCR3 during kidney disease and are therefore central to inducing renal injury (12). In the present study, we found that CXCL9, as well as CXCR3, was significantly increased in response to AGEs in podocytes in a dose-dependent manner. Furthermore, STAT3 signaling was also activated by AGE treatment. After binding to their receptors, CXCL9 activates JAKs, which in turn leads to the tyrosine phosphorylation of STAT3 (27). Previous studies have reported that the increased expression of STAT3 reduces the IFN-α induction of CXCL9 mRNA in myeloid cells (28); in vitro, in human bronchial epithelial cells, IL-13 augmented IL-27-induced CXCL9 expression, which appeared to be due to augmented STAT1 activation and reduced STAT3 activation (29). The different stimulators, including AGEs, IL-27 and IFN-α may interpret the contradictory findings.
In this study, to further investigate the role of CXCL9 in AGE-induced podocyte damage, siRNA-CXCL9 was used to transfected into the podocytes. We found that CXCL9 downregulation significantly increased the proliferation and decreased the apoptosis of podocytes, and decreased the levels of the inflammatory factors, TNF-α and IL-6. In line with the experimental data, patients with type 2 diabetes have 3–4-fold greater serum levels of TNF-α compared with non-diabetic patients, and that urinary TNF-α excretion correlates well with the clinical markers of DN and the progression of the disease (30). Experimental studies have indicated that IL-6 overexpression in diabetic kidneys correlates with kidney hypertrophy and albumin excretion (31,32). Nephrin and podocin are two recently discovered podocyte-specific proteins, pivotal in establishing podocyte slit membrane structure and in the maintenance of an intact filtration barrier (33). In the present study, nephrin and podocin expression levels were markedly increased following transfection of the AGEs-treated podocytes with siRNA-CXCL9.x
Furthermore, the apoptosis-associated protein expression of Bax/Bcl-2 and activated caspase-3 was decreased, which is consistent with our apoptosis analysis measured by flow cytometry. In the present study, siRNA-CXCL9 triggered an effect opposite to that of the AGEs, whereby JAK2 and STAT3 activation was significantly suppressed in podocytes. Similar data by other investigators have indicated that increases in JAK2 and STAT3 gene expression occur in patients with DN compared with healthy controls (34). Lu et al (35) reported a mouse with reduced capacity of STAT3 activation showing less proteinuria, macrophage infiltration and inflammation at an early stage of DN. Total glucosides of paeony (TGP) significantly inhibited DN progression and these protective effects are associated with the ability of TGP to inhibit the JAK2/STAT3 pathway (13). AG490, a JAK2 specific inhibitor, was used in this study to determine the role of JAK2/STAT3 signaling in AGE-induced podocyte damage. Our results revealed that AG490 significantly inhibited JAK2 and STAT3 activation and the apoptosis of podocytes and the expression of CXCL9, Bax/Bcl-2, and activated caspase-3. Podocyte STAT3 activation can result in more severe nephropathy independent of upstream JAK signaling, or at least in changes in upstream JAK signaling. Thus, the activation of JAK2 and STAT3 in podocytes is important in the pathogenesis of DN (36). In addition, valsartan has been shown to exert protective effects against the progression of DN (37) and exerts a similar effect as AG490, suggesting that JAK2/STAT3 signaling is also implicated in the protective effects of valsartan against AGE-induced podocyte damage.
In conclusion, our study demonstrated that CXCL9 synthesis was upregulated in patients with DN and in AGE-treated mouse podocytes, and both CXCL9 downregulation and JAK2 inhibitor treatment significantly inhibited the decrease in proliferation and apoptosis induced by AGEs, as well as inflammatory factor secretion and JAK2/STAT3 activation. Our data may aid in the understanding of the multifactorial nature of DN, and suggest that CXCL9 may be a novel therapeutic target in the treatment of DN.
References
- 1.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 2.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev. 2010;3:101–108. doi: 10.4161/oxim.3.2.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 4.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 5.Koike N, Takamura T, Kaneko S. Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF-α stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 2007;80:1721–1728. doi: 10.1016/j.lfs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–S82. doi: 10.1681/ASN.2004110961. [DOI] [PubMed] [Google Scholar]
- 7.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamagishi S, Imaizumi T. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–2299. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- 9.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645–F659. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 10.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C, Zheng Z, Shi C, Liu X, Ye Z, Lou T. Advanced glycation end-products reduce podocyte adhesion by activating the renin-angiotensin system and increasing integrin-linked kinase. Exp Ther Med. 2013;6:1494–1498. doi: 10.3892/etm.2013.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, Lu B, Farber J, Luster AD, Kelley VR. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Wu YG, Su J, Zhang JJ, Zhang P, Qi XM. Total glucosides of paeony regulates JAK2/STAT3 activation and macrophage proliferation in diabetic rat kidneys. Am J Chin Med. 2012;40:521–536. doi: 10.1142/S0192415X12500401. [DOI] [PubMed] [Google Scholar]
- 14.Banes AK, Shaw S, Jenkins J, Redd H, Amiri F, Pollock DM, Marrero MB. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:F653–F659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB. Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in tgf-beta and fibronectin synthesis in mesangial cells. Diabetes. 2002;51:3505–3509. doi: 10.2337/diabetes.51.12.3505. [DOI] [PubMed] [Google Scholar]
- 16.Jiao B, Wang YS, Cheng YN, Gao JJ, Zhang QZ. Valsartan attenuated oxidative stress, decreased MCP-1 and TGF-β1 expression in glomerular mesangial and epithelial cells induced by high-glucose levels. Biosci Trends. 2011;5:173–181. doi: 10.5582/bst.2011.v5.4.173. [DOI] [PubMed] [Google Scholar]
- 17.Chung AC, Lan HY. Chemokines in renal injury. J Am Soc Nephrol. 2011;22:802–809. doi: 10.1681/ASN.2010050510. [DOI] [PubMed] [Google Scholar]
- 18.Higurashi M, Ohya Y, Joh K, Muraguchi M, Nishimura M, Terawaki H, Yagui K, Hashimoto N, Saito Y, Yamada K. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with type 2 diabetic nephropathy. J Diabetes Complications. 2009;23:178–184. doi: 10.1016/j.jdiacomp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Galler A, Müller G, Schinzel R, Kratzsch J, Kiess W, Münch G. Impact of metabolic control and serum lipids on the concentration of advanced glycation end products in the serum of children and adolescents with type 1 diabetes, as determined by fluorescence spectroscopy and nepsilon-(carboxymethyl)lysine ELISA. Diabetes Care. 2003;26:2609–2615. doi: 10.2337/diacare.26.9.2609. [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, Sivitz W, Monnier VM. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC., III Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: Prevention by lipoic acid treatment. BMC Nephrol. 2006;7:6. doi: 10.1186/1471-2369-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang L, Yang JW, Liu C. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol. 2008;28:1014–1022. doi: 10.1159/000148209. [DOI] [PubMed] [Google Scholar]
- 24.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int. 2007;72:965–976. doi: 10.1038/sj.ki.5002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holdsworth SR, Tipping PG. Leukocytes in glomerular injury. Semin Immunopathol. 2007;29:355–374. doi: 10.1007/s00281-007-0097-9. [DOI] [PubMed] [Google Scholar]
- 26.Zychowska M, Rojewska E, Pilat D, Mika J. The role of some chemokines from the CXC subfamily in a mouse model of diabetic neuropathy. J Diabetes Res. 2015;2015:750182. doi: 10.1155/2015/750182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang JS, Lee YH, Chuang LY, Guh JY, Hwang JY. Cinnamaldehyde and nitric oxide attenuate advanced glycation end products - induced the JAK/STAT signaling in human renal tubular cells. J Cell Biochem. 2015;116:1028–1038. doi: 10.1002/jcb.25058. [DOI] [PubMed] [Google Scholar]
- 28.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 29.Xie M, Mustovich AT, Jiang Y, Trudeau JB, Ray A, Ray P, Hu H, Holguin F, Freeman B, Wenzel SE. IL-27 and type 2 immunity in asthmatic patients: Association with severity, CXCL9, and signal transducer and activator of transcription signaling. J Allergy Clin Immunol. 2015;135:386–394. doi: 10.1016/j.jaci.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro JF, Mora C, Muros M, García J. Urinary tumour necrosis factor-α excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant. 2006;21:3428–3434. doi: 10.1093/ndt/gfl469. [DOI] [PubMed] [Google Scholar]
- 31.Navarro JF, Milena FJ, Mora C, León C, García J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–570. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- 32.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, Mathieson PW. Co-localization of nephrin, podocin, and the actin cytoskeleton: Evidence for a role in podocyte foot process formation. Am J Pathol. 2002;161:1459–1466. doi: 10.1016/S0002-9440(10)64421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brosius FC, III, Alpers CE. New targets for treatment of diabetic nephropathy: What we have learned from animal models. Curr Opin Nephrol Hypertens. 2013;22:17–25. doi: 10.1097/MNH.0b013e32835b3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu TC, Wang ZH, Feng X, Chuang PY, Fang W, Shen Y, Levy DE, Xiong H, Chen N, He JC. Knockdown of Stat3 activity in vivo prevents diabetic glomerulopathy. Kidney Int. 2009;76:63–71. doi: 10.1038/ki.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brosius FC, III, He JC. JAK inhibition and progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24:88–95. doi: 10.1097/MNH.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen B, Hou XH, Guan GJ, Liu G, Liu HY, Li XG. Effects of mycophenolate mofetil, valsartan and their combined therapy on preventing podocyte loss in early stage of diabetic nephropathy in rats. Chin Med J (Engl) 2007;120:988–995. [PubMed] [Google Scholar]