Abstract
Background
The aim of this study was to evaluate the diagnosis value of an immunohistochemical (IHC) panel of three antibodies for the diagnosis of gastrointestinal stromal tumors (GISTs).
Material and methods
In 80 consecutive GISTs without lymph node metastases, the IHC examinations were performed using the antibodies CD117 (c-KIT), DOG-1 and c-theta (PKCθ) protein. The diagnostic value of PKCθ in c-KIT/DOG-1 negative GISTs has been explored in fewer than 10 Medline-indexed papers.
Results
The c-KIT, PKCθ and DOG-1 positivity was noted in 92.50% (n = 74), 90% (n = 72) and 76.25% (n = 61) of the cases, respectively. All of the C-KIT negative cases (n = 6) were also DOG-1 negative but displayed PKCθ positivity. All of the DOG-1 positive cases (n = 61) also expressed c-KIT. No correlation between the examined markers and clinicopathological parameters was noted.
Conclusions
The PKCθ sensitivity is similar to c-KIT and superior to DOG-1 sensitivity. All of the c-KIT/DOG-1 negative GISTs seem to express PKCθ. For a proper diagnosis of GIST, the c-KIT/DOG-1/PKCθ panel should be used, with possible therapeutic but not prognostic value.
Keywords: c-theta, protein kinase, GIST, diagnosis, CD117, Pathology Section
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract, with a 10-15 per million per year global incidence [1]. It is more frequently diagnosed in the fifth to seventh decades with an approximately equal gender distribution. The most frequent location is the stomach (55-60%), followed by small intestine (30-35%), colorectal segments (4-6%) and esophagus ( < 1%); rarely, GISTs may develop in the mesentery or retroperitoneum, when they are classified as extra-gastrointestinal GISTs (E-GIST) [2, 3].
Morphologically, GISTs may display several types of architecture. The most frequent type is the spindle cell architecture (70%), followed by epithelioid (20%) and mixed type (10%). In daily practice, the diagnosis of GIST is mainly based on the immunohistochemical (IHC) marker c-KIT (CD117). Due to its possible positivity in other tumors, such as melanomas, adenoid cystic carcinomas, Merkel cell carcinomas, Kaposi sarcomas, liposarcomas or leiomyomas/leiomyosarcomas, additional markers such DOG-1 (discovered on GIST-1) are used in most of the pathology laboratories [4, 5]. Those tumors that are negative for both of these markers, although they have KIT or PDGFRA mutations, are difficult to diagnose and remain unrecognized, although they could respond to Imatinib [6–10]. For this reason, new proteins are proposed to support the GIST diagnosis.
One of the relatively new markers described that are displayed by the c-KIT negative GISTs is the protein kinase C-θ (PKCθ), which is also known as c-theta protein. It is a serine-threonine protein kinase involved in T-cell activation and survival, skeletal muscle signal transduction and differentiation, nerve-muscle interaction, neuronal differentiation, cell proliferation, cancer cell-stroma interaction, transcription and apoptosis [6–10]. As the exact role of PKCθ in GIST is unknown, this IHC marker has not yet been approved for the daily diagnosis of GISTs.
The aim of the study was to analyze the diagnostic sensitivity of the c-KIT, DOG-1 and PKCθ expression in GIST and to perform a review of the 15 representative papers indexed in the Medline database (published between June 2004 and March 19, 2017) in the field of the supposed diagnostic value of the PKCθ [6–20]. Only six of these papers took into account the three markers [7, 9, 14–16, 18]. The other nine [6, 8, 10, 11–13, 17, 19, 20] were focused on correlation between c-KIT and PKCθ, without taking into account the DOG-1 expression.
RESULTS
Correlation between the IHC markers and clinicopathological factors
The median age of the patients ranged between 19 and 80 years (61.58±11.84 years). The other clinicopathological characteristics are shown in Table 1. All the cases had no lymph node metastasis and were negative for desmin. Distant metastases were identified in liver (n = 5) and peritoneum (n = 6).
Table 1. Clinicopathological characteristics of patients.
| Variable | n=80 |
|---|---|
| Age (years) | 61.58±11.84 (range 19-80 years) |
| Gender: Male/Female | 35/45 (1:1.28) |
| Tumor size (Median: 6.47±4.67 cm, range 0.4 to 21 cm) | |
| ≥5 cm | 45 |
| <5cm | 35 |
| Mitoses (50HPF) (Median: 8.43±14.02, range 0 to 89) | |
| ≥5 | 29 |
| <5 | 51 |
| Tumor location | |
| Stomach | 35 |
| Small intestine | 25 |
| Colorectum | 6 |
| E-GIST | 14 |
| Histological pattern | |
| Spindle cell | 64 |
| Epithelioid cell | 2 |
| Mixed | 14 |
| Risk group | |
| Very low | 10 |
| Low | 21 |
| Intermediate | 16 |
| High | 33 |
| Ki67 index | |
| Low (≤5%) | 60 |
| High (>5%) | 20 |
| Local invasion | |
| present | 14 |
| absent | 66 |
| Distant metastases | |
| present | 11 |
| absent | 69 |
| Necrosis | |
| present | 32 |
| absent | 48 |
The positive rates of c-KIT and DOG-1 in GISTs were 92.50% (74/80) and 76.25% (61/80) respectively. PKCθ positive staining was detected in 72/80 (90%) cases.
The expression of c-KIT and PKCθ has no significant correlation with clinicopathological parameters including gender, age, tumor size, mitotic rate, tumor location, histological type, risk degree, local invasion or presence of distant metastasis or intratumoral necrosis (Table 2).
Table 2. Correlation of the immunohistochemical expression of c-KIT, DOG-1 and PKCθ with the clinicopathological parameters (NA=non-available).
| n | c-KIT | DOG-1 | PKC-theta | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | + | OR (CI:95%) | p | - | + | OR (CI:95%) | p | - | + | OR (CI:95%) | p | ||
| Gender | |||||||||||||
| Male | 35 | 5 | 30 | 7.33 (0.81-66.00) | 0.08 | 9 | 26 | 1.21 (0.43-3.40) | 0.79 | 1 | 34 | 0.15 (0.01-1.36) | 0.07 |
| Female | 45 | 1 | 44 | 10 | 35 | 7 | 38 | ||||||
| Age (years) | |||||||||||||
| ≤45 | 8 | 0 | 8 | 0.60 (0.03-11.67) | 0.98 | 2 | 6 | 1.07 (0.19-5.84) | 0.98 | 0 | 8 | 0.44 (0.02-8.44) | 1 |
| >45 | 72 | 6 | 66 | 17 | 55 | 8 | 64 | ||||||
| Tumor size | |||||||||||||
| ≥5 cm | 45 | 4 | 41 | 1.61 (0.27-9.34) | 0.69 | 11 | 34 | 1.09 (0.38-3.09) | 0.95 | 5 | 40 | 1.33 (0.29-6.00) | 1 |
| <5cm | 35 | 2 | 33 | 8 | 27 | 3 | 32 | ||||||
| Mitotic rate (50HPF) | |||||||||||||
| High (≥5) | 29 | 2 | 27 | 0.87 (0.14-5.07) | 0.95 | 5 | 24 | 0.55 (0.17-1.72) | 0.41 | 2 | 27 | 0.55 (0.10-2.95) | 0.49 |
| Low (<5) | 51 | 4 | 47 | 14 | 37 | 6 | 45 | ||||||
| Tumor location | |||||||||||||
| Stomach | 35 | 2 | 33 | NA | 0.09 | 10 | 25 | NA | 0.02 | 3 | 32 | NA | 0.61 |
| Small intestine | 25 | 1 | 24 | 3 | 22 | 3 | 22 | ||||||
| Colorectum | 6 | 2 | 4 | 4 | 2 | 1 | 5 | ||||||
| E-GIST | 14 | 1 | 13 | 2 | 12 | 1 | 13 | ||||||
| Histological pattern | |||||||||||||
| Spindle cell type | 64 | 4 | 60 | NA | 0.53 | 15 | 49 | NA | 0.66 | 6 | 58 | NA | 0.82 |
| Epithelioid cell type | 2 | 0 | 2 | 0 | 2 | 0 | 2 | ||||||
| Mixed type | 14 | 2 | 12 | 4 | 10 | 2 | 12 | ||||||
| Risk group | |||||||||||||
| Very low | 10 | 1 | 9 | NA | 0.80 | 3 | 7 | NA | 0.95 | 3 | 7 | NA | 0.14 |
| Low | 21 | 1 | 20 | 5 | 16 | 1 | 20 | ||||||
| Intermediate | 16 | 2 | 14 | 4 | 12 | 1 | 15 | ||||||
| High | 33 | 2 | 31 | 7 | 26 | 3 | 30 | ||||||
| Ki67 index | |||||||||||||
| Low | 60 | 2 | 58 | 0.13 (0.02-0.82) | 0.03 | 13 | 47 | 0.64 (0.20-2.01) | 0.54 | 7 | 53 | 2.50 (0.28-21.75) | 0.40 |
| High | 20 | 4 | 16 | 6 | 14 | 1 | 19 | ||||||
| Local invasion | |||||||||||||
| positive | 14 | 2 | 12 | 2.58 (0.42-15.74) | 0.28 | 2 | 12 | 0.48 (0.09-2.37) | 0.49 | 1 | 13 | 0.64 (0.07-7.53) | 0.69 |
| negative | 66 | 4 | 62 | 17 | 49 | 7 | 59 | ||||||
| Distant metastasis | |||||||||||||
| present | 11 | 0 | 11 | 0.42 (0.02-8.06) | 0.58 | 2 | 9 | 0.67 (0.13-3.45) | 0.64 | 1 | 10 | 0.88 (0.09-7.98) | 0.91 |
| absent | 69 | 6 | 63 | 17 | 52 | 7 | 62 | ||||||
| Necrosis | |||||||||||||
| present | 32 | 3 | 29 | 1.55 (0.29-8.22) | 0.67 | 8 | 24 | 1.12 (0.39-3.19) | 0.95 | 2 | 30 | 0.46 (0.08-2.47) | 0.37 |
| absent | 48 | 3 | 45 | 11 | 37 | 6 | 42 | ||||||
Out of all the examined clinicopathological parameters, DOG-1 was only correlated with tumor location. Although the DOG-1 positive cases have predominated, significant positivity was noted for the GIST that involved the small intestine or retroperitoneal area (Table 2).
Correlation between the four examined IHC markers
The value of the Ki67 index was directly correlated with c-KIT expression without correlation with DOG-1 or PKCθ (Table 2).
Out of the examined cases, 70% (56/80) expressed all the three examined markers: c-KIT, DOG-1 and PKCθ. All of the 61 DOG-1 positive cases and 13 of the 19 DOG-1 negative GISTs (68.42%) displayed c-KIT positivity (p = 0.0001).
PKCθ was expressed in 66 out of the 74 c-KIT positive GISTs and 56 out of the 61 DOG-1 positive cases (89.18% and 91.80% respectively). All of the six DOG-1 negative/ c-KIT negative cases expressed PKCθ. From the 13 DOG-1 negative/c-KIT positive cases, 10 cases (76.92%) displayed diffuse PKCθ positivity. All of the eight PKCθ negative GISTs were positive for c-KIT; five out of eight cases also expressed DOG-1 (Figure 1).
Figure 1. Correlation between c-KIT, DOG-1 and PKCθ expression revealed by Venn-diagram.

DISCUSSION
In patients with GIST, the previously published papers showed a c-KIT positivity rate of 80-100%, in line with the present study [8, 19, 22, 23]. The c-KIT negative cases were reported to be more frequently located on the stomach (96% of all negative GISTs) and displaying epithelioid or spindle cell-type architecture [7, 19]. In the present study, regardless of the tumor's location, the two epithelioid-type GISTs were c-KIT negative.
DOG-1 is a transmembrane protein located on the 11q13 chromosome that was reported to be IHC-expressed in 57-96% of GISTs [9, 14, 23]. Its expression is directly correlated with c-KIT positivity [14]; all of the DOG-1 positive cases expressed c-KIT in our material but not all of the c-KIT positive GISTs were also positive for DOG-1. Usually, DOG-1 does not mark other tumors, such as leiomyomas/leiomyosarcomas, melanomas, schwannomas, malignant peripheral nerve sheath tumors, inflammatory fibroid polyps, small cell carcinomas, Merkel cell carcinomas or seminomas [7, 15, 24]. Uncommonly, DOG-1 sporadic positivity was reported for renal tubes, eccrine glands and hair follicles. Some tumors such as dermatofibrosarcomas, uterine-type retroperitoneal leiomyomas (8%), peritoneal leiomyomatoses (23%), leiomyosarcomas and other soft tissue tumors with histiocytic or lipomatous differentiation, carcinomas of the esophagus (60%), stomach (26%) and colorectal segments (5%), basal cell carcinomas (6%), squamous cell carcinomas (21%), hepatocellular carcinomas, adenoid cystic carcinomas, synovial sarcomas (16%) and desmoplastic melanomas (1%) also displayed DOG-1 positivity [9, 15, 23–26].
PKCθ was previously reported to be expressed in the interstitial cells of the Cajal lineage, Auerbach's plexus, T-cells, mast cells, endothelial cells, lymphoid organs, nervous system, skeletal muscle, and 72-100% of GISTs, without positivity for other c-KIT negative soft tissue tumors, desmoid tumors or carcinomas [6, 8–10, 14, 20]. It is important to mention that the c-KIT positive non-GIST tumors, such as small or large cell carcinomas, renal chromophobe cell carcinomas, thymic carcinomas or seminomas, did not display PKCθ positivity [6, 10]. However, the referenced studies [6, 10, 20] only included 26-48 GISTs and 1-10 cases from the non-sarcomatous tumors. Weak PKCθ expression was reported by other researchers in 25-33% of leiomyomas, 6-28% of leiomyosarcomas, 33% of malignant peripheral nerve sheath tumors/Ewing sarcomas, 10-57% of gastrointestinal schwannomas (especially in Verocay bodies), 15% of desmoid tumors, more than one-third of melanomas and 10% of adenoid cystic carcinomas [6, 7, 9, 15, 19, 20].
In our study, all of the three IHC markers were expressed in 70% of GISTs and the sensitivity of the polyclonal c-KIT and PKCθ was nearly identical: 92.50% versus 90%, similar to some of the literature data [9]. The sensitivity of both markers was superior to the K9 clone of DOG-1 (76.25%). Other authors proved a similar sensitivity of c-KIT and DOG-1 but admitted a slightly greater c-KIT positivity for the tumors localized on the colorectal segments [23, 27] and a relatively higher sensitivity of the clone SP31 versus the commercial K9 clone of DOG-1 that was used in the present study (95% versus 90-94%) [9, 15]. The heterogeneity of the studies, the small number of examined cases and the paucity of the used clones induce discrepant results, with even a higher DOG-1 or PKCθ sensitivity, compared with c-KIT, being proved [6, 10, 15, 27]. The differences can also be explained by the predominance of the DOG-1 positive cases in the tumors of the small intestine and E-GISTs, proved by the present material. In other studies, the gastrointestinal GISTs predominated [10]. Moreover, none of our cases showed lymph node metastases.
The PKCθ diagnostic value seems to be important for the c-KIT negative cases [7, 9]. A 70-100% PKCθ positivity was previously reported in c-KIT negative GISTs regardless of KIT or PDGFRA status [8, 19, 20]. PKCθ also marked the PDGFRA mutant GISTs (for exons 12 or 18) that are negative for c-KIT and even the cases with myxoid stroma [6, 19]. Overexpression of the PKCθ gene and PKCθ expression at the RNA level were also displayed in GIST samples but not in other c-KIT positive non-GIST soft tissue sarcomas or other tumors [10, 20].
Although a correlation between c-KIT and DOG-1 was proved by most of the studies, discrepant results were founded for c-KIT negative cases. The c-KIT negative GISTs are 36-100% DOG-1 positive [15], express either DOG-1 or PKCθ or show double positivity for DOG-1 and PKCθ [7, 9] but can also be negative for both DOG-1 and PKCθ (2/5 cases) [9]. The uncommon DOG-1 negativity was proved in only 3-4% of c-KIT negative GISTs; they are usually wild type KIT/PDGFRA mutant cases [9]. To our knowledge, the diagnostic value of PKCθ in double c-KIT/DOG-1 negative GISTs was shown in only three papers: 2/26 [7], 1/5 [9] and 1/1 c-KIT negative GISTs [16]. In all of these cases, PKCθ positivity was proved, as in this material, which comprised the largest reported case series of c-KIT/DOG-1 negative GISTs displaying PKCθ positivity (6/6 c-KIT negative GISTs selected from 80 GISTs). In one of the studies, the molecular examinations performed in c-KIT negative GISTs showed mutations in: KIT exon 11 for DOG-1 negative/PKCθ negative GISTs (two cases), PDGFRA exon 18 for DOG-1 negative/PKCθ positive (one case) or DOG-1 positive/PKCθ positive GISTs (one case), and PDGFRA exon 12 for DOG-1 positive/PKCθ positive GISTs (one case) [9]. The PKCθ negative GISTs showed c-KIT mutations in exon 11 regardless of the other IHC markers [19].
Similar PKCθ gene expression levels were proved when detected with a goat polyclonal or a mouse monoclonal antibody [20]. It may be mandatory to prove the PKCθ positivity to confirm the diagnosis of GIST, but its negativity is uninformative [20] because all of the PKCθ negative GISTs display c-KIT and CD34 positivity [6], with/without DOG-1 expression [9], similar to our data.
In the present study, no one of the three examined markers proved to be an indicator of prognosis. They proved to be valuable as diagnostic tools only. The main limitations of this study are the small number of cases and absence of cases with lymph node metastases.
As the possible role of PKCθ in protecting T-cells from apoptosis and promoting activation of the immune cells’ inflammation, as well as in promoting multidrug resistance (MDR), was previously proved [10, 28, 29], we conclude that PKCθ might be a novel therapeutic target for the immune therapy of GISTs or a potential indicator of resistance to Imatinib. In c-KIT/DOG-1 negative GISTs, the PKCθ expression should be checked for a complex differential diagnosis.
MATERIALS AND METHODS
Tissue samples
The present retrospective study included 80 formalin-fixed and paraffin-embedded tissue samples of primary GISTs. The surgically removed GIST specimens from consecutive cases were retrospectively collected from the Department of Pathology of the Clinical County Emergency Hospital of Tirgu-Mures, Romania, from 2003 to 2015. No neoadjuvant chemotherapy was given prior to resection. The approval of the Ethical Committee of the University of Medicine and Pharmacy of Tirgu-Mures, Romania, was obtained for retrospective evaluation of the cases.
The morphological diagnosis of GIST was confirmed by two pathologists, the histological pattern was identified and the mitotic index was calculated. The main prognostic factors, such as size, mitotic index and anatomical location, were analyzed based on the NIH's modified consensus classification [21].
Immunohistochemical analysis
For IHC analyses, tissue microarray (TMA) blocks were performed, containing three representative areas of each GIST tissue (3 mm diameter core). The IHC stains were performed using the antibodies c-KIT (rabbit polyclonal, DAKO Glostrup, Denmark, dilution 1:500), DOG-1 (mouse monoclonal, clone K9, Novocastra, Newcastle, UK, dilution 1:50), Ki67 (MIB-1 clone, DAKO, dilution 1:500), and PKCθ (polyclonal, ABCAM, dilution 1:200) according to the instructions of the manufacturer. The developing was performed with DAB (diaminobenzidine) solution (Novocastra). For the negative controls, incubation was conducted with the omission of specific antibodies [30].
The cut-off value used was 5% for Ki67. The c-KIT, DOG-1 and PKCθ marked the cell cytoplasm with/without membrane positivity. In line with the previous studies, cases with positivity in a few single cells were considered negative (Figure 2); positive cases (Figure 3) showed focal or diffuse unequivocal positivity in several cell clusters or more than 10% of the tumor cells [7, 10]. The IHC assessment was performed independently by two pathologists.
Figure 2.
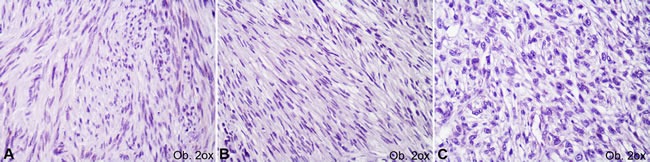
Negative immunoexpression of c-KIT (A), DOG-1 (B) and PKCθ (C) in gastrointestinal stromal tumors.
Figure 3.

Positive immunoexpression of c-KIT (A), DOG-1 (B) and PKCθ (C) in gastrointestinal stromal tumors.
Statistical analysis
Statistical analysis was performed using the GraphPad InStat 3 software and two-sided tests. A p-value < 0.05 with 95% confidence interval was considered statistically significant.
Acknowledgments
The English Proofread Editing Service polished the English-language manuscript.
Footnotes
FUNDING
This work was partially supported by the University of Medicine and Pharmacy of Tirgu-Mures, Romania, in the joint project with Studium Prospero Foundation and Hungarian Science Academy, research projects frame 136/2017.
CONFLICT OF INTERESTS
No conflict of interests are declared.
REFERENCES
- 1.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 2.Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Klieser E, Pichelstorfer M, Weyland D, Kemmerling R, Swierczynski S, Dinnewitzer A, Jager T, Kiesslich T, Neureiter D, Illig R. Back to the start: evaluation of prognostic markers in gastrointestinal stromal tumors. Mol Clin Oncol. 2016;4:763–773. doi: 10.3892/mco.2016.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 5.Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motegi A, Sakurai S, Nakayama H, Sano T, Oyama T, Nakajima T. PKC theta, a novel immunohistochemical marker for gastrointestinal stromal tumors (GIST), especially useful for identifying KIT-negative tumors. Pathol Int. 2005;55:106–112. doi: 10.1111/j.1440-1827.2005.01806.x. [DOI] [PubMed] [Google Scholar]
- 7.Kang GH, Srivastava A, Kim YE, Park HJ, Park CK, Sohn TS, Kim S, Kang DY, Kim KM. DOG-1 and PKC-θ are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol. 2011;24:866–875. doi: 10.1038/modpathol.2011.11. [DOI] [PubMed] [Google Scholar]
- 8.Lee HE, Kim MA, Lee HS, Lee BL, Kim WH. Characteristics of KIT-negative gastrointestinal stromal tumors and diagnostic utility of protein kinase C theta immunostaining. J Clin Pathol. 2008;61:722–729. doi: 10.1136/jcp.2007.052225. [DOI] [PubMed] [Google Scholar]
- 9.Rios-Moreno MJ, Jaramillo S, Pereira Gallardo S, Vallejo A, Mora M, Garcia-Escudero A, Amerigo J, Gonzalez-Campora R. Gastrointestinal stromal tumors (GISTs): CD117, DOG-1 and PKCθ expression. Is there any advantage in using several markers? Pathol Res Pract. 2012;208:74–81. doi: 10.1016/j.prp.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Blay P, Astudillo A, Buesa JM, Campo E, Abad M, Garcia-Garcia J, Miquel R, Marco V, Sierra M, Losa R, Lacave A, Brana A, Balbin M, et al. Protein kinase C theta is highly expressed in gastrointestinal stromal tumors but not in other mesenchymal neoplasias. Clin Cancer Res. 2004;10:4089–4095. doi: 10.1158/1078-0432.CCR-04-0630. [DOI] [PubMed] [Google Scholar]
- 11.Zhu MJ, Ou WB, Fletcher CD, Cohen PS, Demetri GD, Fletcher JA. KIT oncoprotein interactions in gastrointestinal stromal tumors: therapeutic relevance. Oncogene. 2007;26:6386–6395. doi: 10.1038/sj.onc.1210464. [DOI] [PubMed] [Google Scholar]
- 12.Ou WB, Zhu MJ, Demetri GD, Fletcher CD, Fletcher JA. Protein kinase C-theta regulates KIT expression and proliferation in gastrointestinal stromal tumors. Oncogene. 2008;27:5624–5634. doi: 10.1038/onc.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Smyrk TC, Young WF, Jr, Stratakis CA, Carney JA. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol. 2010;34:53–64. doi: 10.1097/PAS.0b013e3181c20f4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YN, Jung HR, Hwang I. Clinicopathological and immunohistochemical features of gastointestinal stromal tumors. Cancer Res Treat. 2010;42:135–143. doi: 10.4143/crt.2010.42.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatima N, Cohen C, Siddiqui MT. DOG-1 utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. Cancer Cytopathol. 2011;119:202–208. doi: 10.1002/cncy.20149. [DOI] [PubMed] [Google Scholar]
- 16.Kim KH, Nelson SD, Kim DH, Choi KU, Kim SJ, Min KW, Jang KS, Paik SS, Oh YH, Chae SW, Sohn JH, Kim HJ, Cho YK, et al. Diagnostic relevance of overexpressions of PKC-θ and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases. Anticancer Res. 2012;32:923–937. [PubMed] [Google Scholar]
- 17.Valadao M, Braggio D, Santos AF, Pimenta-Inada HK, Linhares E, Gonçalves R, Romano S, Vilhena B, Small I, Cubero D, Cruz F, Oliveira AT, Martinho O, et al. Involvement of signalling molecules in the prediction of response to imatinib treatment in metastatic GIST patients. J Surg Res. 2012;178:288–293. doi: 10.1016/j.jss.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Jin MS, Zou YB, Gao JN, Li XB, Peng F, Wang HY, Wu ZD, Wang YP, Duan XM. Diagnostic significance of DOG-1 and PKC-θ expression and c-Kit/PDGFRA mutations in gastrointestinal stromal tumours. Scand J Gastroenterol. 2013;48:1055–1065. doi: 10.3109/00365521.2013.816770. [DOI] [PubMed] [Google Scholar]
- 19.Kim KM, Kang DW, Moon WS, Park JB, Park CK, Sohn JH, Jeong JS, Cho MY, Jin SY, Choi JS, Kang DY. PKCtheta expression in gastrointestinal stromal tumor. Mod Pathol. 2006;19:1480–1486. doi: 10.1038/modpathol.3800673. [DOI] [PubMed] [Google Scholar]
- 20.Duensing A, Joseph NE, Medeiros F, Smith F, Hornick JL, Heinrich MC, Corless CL, Demetri GD, Fletcher CD, Fletcher JA. Protein Kinase C theta (PKCtheta) expression and constitutive activation in gastrointestinal stromal tumors (GISTs) Cancer Res. 2004;64:5127–5131. doi: 10.1158/0008-5472.CAN-04-0559. [DOI] [PubMed] [Google Scholar]
- 21.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Han H, Wang J, Zhang B, Shao Y, Zhang L, Wang H, Wang H, Wu Y, Li X, Li R, Tian Y. Diagnosis value of CD117, PDGFRA alone or in combination DOG-1, as biomarkers for gastrointestinal stromal tumors. Ann Transl Med. 2015;3:308. doi: 10.3978/j.issn.2305-5839.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen M, Wang ZF, Lasota J. DOG-1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. doi: 10.1097/PAS.0b013e3181a90e1a. [DOI] [PubMed] [Google Scholar]
- 24.Turdean SG, Gurzu S, Jung I, Neagoe RM, Sala D. Unexpected maspin immunoreactivity in Merkel cell carcinoma. Diagn Pathol. 2015;10:206. doi: 10.1186/s13000-015-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung I, Gurzu S, Turdean S, Ciortea D, Sahlean DI, Golea M, Bara T. Relationship of endothelial area with VEGF-A, COX-2, maspin, c-KIT, and DOG-1 immunoreactivity in liposarcomas versus non-lipomatous soft tissue tumors. Int J Clin Exp Pathol. 2015;8:1776–1782. [PMC free article] [PubMed] [Google Scholar]
- 26.Ciortea CD, Jung I, Gurzu S, Kovecsi A, Turdean SG, Bara T. Correlation of angiogenesis with other immunohistochemical markers in cutaneous basal and squamous cell carcinomas. Rom J Morphol Embryol. 2015;56:665–670. [PubMed] [Google Scholar]
- 27.Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG-1-1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 28.Gill PK, Gescher A, Gant TW. Regulation of MDR1 promoter activity in human breast carcinoma cells by protein kinase C isozymes alpha and theta. Eur J Biochem. 2001;268:4151–4157. doi: 10.1046/j.1432-1327.2001.02326.x. [DOI] [PubMed] [Google Scholar]
- 29.Marrocco V, Fiore P, Benedetti A, Pisu S, Rizzuto E, Musaro A, Madaro L, Lozanoska-Ochser B, Bouche M. Pharmacological inhibition of PKCθ counteracts muscle disease in a mouse model of duchenne muscular dystrophy. EBioMedicine. 2017;16:150–161. doi: 10.1016/j.ebiom.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Stack MS. An update on immunohistochemistry in translational cancer research. Cancer Transl Med. 2015;1:115–122. [Google Scholar]


