Abstract
We conducted this retrospective study to investigate whether microwave ablation (MWA) of primary tumor sites plus epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) could improve survival in advanced non small cell lung cancer (NSCLC) with EGFR mutations. MWA was conducted at the primary tumor sites, followed by EGFR-TKIs in the MWA plus EGFR-TKIs group. EGFR-TKIs were administered until disease progression or intolerable toxicity. The primary endpoint was progression-free survival (PFS); secondary endpoints were overall survival (OS) and objective response rate (ORR). A total of 58 patients were recruited, including 34 in the MWA plus EGFR-TKIs group and 24 in the EGFR-TKIs group. No significant difference in ORR was observed with MWA treatment (61.8% vs. 45.8%, p = 0.230). Patients treated with MWA plus EGFR-TKIs failed to show superior survival in either PFS (13.2 months vs. 11.6 months, p = 0.640) or OS (39.8 months vs. 20.4 months, p = 0.288). MWA was not an independent prognostic factor for PFS or OS. MWA of primary tumor sites plus EGFR-TKIs demonstrated no survival advantage compared with EGFR-TKIs alone in advanced NSCLC patients with EGFR sensitive mutations. MWA should not be recommended for unselected patients with EGFR-sensitive mutations.
Keywords: epidermal growth factor receptor, tyrosine kinase inhibitors, non-small cell lung cancer, microwave ablation, progression free survival
INTRODUCTION
EGFR sensitive mutations, especially in-frame deletions in exon 19 and a point mutation in exon 21 (L858R), are present in 30–40% of non-small cell lung cancer (NSCLC) patients and respond well to EGFR-TKIs such as gefitinib and erlotinib. It is estimated that the ORR ranged from 60% to 83%, with a median PFS of 8 to 11 months. [1–5]
Patients who respond to EGFR-TKIs will develop resistance eventually. Several mechanisms had been clarified; among them, an EGFR T790M mutation in exon 20 [6–9] and c-MET [10, 11] amplifications have been explored widely, and account for 50% and 25% of all resistant mechanisms, respectively. What is more, PIK3CA mutation, [12] ERBB2 amplification, [13] HGF overexpression, [14] AXL activation, [15] epithelial-mesenchymal transition, and pathology type transformation, especially adenocarcinoma transformation into small cell lung cancer, have also been reported as causes of secondary resistance to EGFR-TKIs. [16–18]
According to the progression of EGFR-TKIs, secondary resistance can be clarified into three types: intracranial disease progression, development of asymptomatic oligometastases, and symptomatic disease progression. [19, 20] For the former two types, EGFR-TKIs could be continued after local therapy is administered. [21, 22]
For patients with intracranial progression, both whole-brain radiation therapy (WBRT) and stereotactic radiotherapy (SRT) could be treatment regimens. [20–22] For patients with oligometastases other than intracranial metastases, radiation therapy or thermal ablation could be applied. [20, 23–25] Ni et al. showed that patients with extra-central nervous system oligoprogressive disease had a median PFS of 8.8 months after microwave ablation (MWA), which was significantly different when compared with a transformation to platinum-based doublet chemotherapy. [25]
In previous studies, we verified that advanced NSCLC could benefit from a combination of MWA at primary tumor sites and platinum-based doublet chemotherapy. [26] What is more, a significant difference in PFS was also observed when compared with chemotherapy alone. [27] A recent phase II prospective, randomized, controlled clinical trial verified that local consolidative therapy with maintenance therapy for patients with three or fewer metastases from NSCLC (those who benefit from first-line systematic therapies) and demonstrated improved PFS when compared with maintenance therapy alone. The median PFS were 11.9 months and 3.9 months for consolidative plus maintenance therapy and maintenance therapy alone, respectively. [28] Local therapy including radiation and MWA in combination with systematic therapies improve survival in advanced NSCLC. Therefore, we conducted this retrospective study to determine whether advanced NSCLC patients with EGFR-sensitive mutations could benefit from MWA at primary tumor sites plus EGFR-TKIs when compared with EGFR-TKIs alone.
RESULTS
Patient characteristics
From January 25, 2010 to May 19, 2016, 58 patients were enrolled, of whom 34 were in the MWA plus EGFR-TKIs group and 24 were in the EGFR-TKIs group. In the MWA plus EGFR-TKIs group, 26 were women, 13 were aged 65 years or older, 33 had adenocarcinoma histology and an ECOG PS of 1, and 28 were nonsmokers. EGFR mutations included 18 19Del mutations and 19 exon 21 L858R mutations (including 3 patients with both 19Del mutations and L858R mutations). Nineteen patients were treated with EGFR-TKIs as a first-line therapy and 15 patients were treated with EGFR-TKIs as a post-first-line therapy. The primary tumor size ranged from 0.8 to 9.0 cm, with a mean of 3.7 cm. Most patients (23 patients, 67.6%) underwent ablation with two antennas and the typical ablation energy was 70 W (23 patients, 67.6%). In the EGFR-TKIs group, 13 were women aged 65 years or older, 23 had adenocarcinoma histology, 21 had an ECOG PS of 1, and 17 were nonsmokers. EGFR mutations included 16 19Del mutations and 10 exon 21 L858R mutations (including two patients with both mutations). Sixteen patients were treated with EGFR-TKIs as a first-line therapy, and 8 patients were treated with EGFR-TKIs as a post-first-line therapy. The primary tumor size ranged from 1.6 to 8.8 cm, with a mean of 3.9 cm. Baseline characteristics of the enrolled patients are shown in detail in Table 1.
Table 1. Baseline characteristics of 58 enrolled patients.
| MWA+EGFR-TKIs | EGFR-TKIs | ||||
|---|---|---|---|---|---|
| Number | Percent(%) | Number | Percent(%) | ||
| Gender | |||||
| Male | 8 | 23.5 | 11 | 45.8 | |
| Female | 26 | 76.5 | 13 | 54.2 | |
| Age | 58.9(29-85) | 62.0(38-79) | |||
| ≥65 | 13 | 38.2 | 11 | 45.8 | |
| <65 | 21 | 61.8 | 13 | 54.2 | |
| Smoking history | |||||
| Non-smokers | 28 | 82.4 | 17 | 70.8 | |
| Smokers | 6 | 17.6 | 7 | 29.2 | |
| ECOG | |||||
| 0 | 1 | 2.9 | 3 | 12.5 | |
| 1 | 33 | 97.1 | 21 | 87.5 | |
| Pathology | |||||
| Adenocarcinoma | 33 | 97.1 | 23 | 95.8 | |
| Non-adenocarcinoma | 1 | 2.9 | 1 | 4.2 | |
| Stage | |||||
| IIIB | 3 | 8.8 | 1 | 4.2 | |
| IV | 31 | 91.2 | 23 | 95.8 | |
| EGFR sensitive mutations | |||||
| 19Del | 18* | 39.4 | 16# | 66.7 | |
| L858R | 16 | 60.6 | 8 | 33.3 | |
| Primary tumor size | |||||
| Mean | 3.7 | 3.9 | |||
| Range | 0.8-9.0 | 1.6-8.8 | |||
| Primary tumor size | |||||
| ≥3.5cm | 15 | 44.1 | 12 | 50.0 | |
| <3.5cm | 19 | 55.9 | 12 | 50.0 | |
| Primary tumor site | |||||
| Right lung | 20 | 58.8 | 15 | 62.5 | |
| Left lung | 14 | 41.2 | 9 | 37.5 | |
| Primary tumor site | |||||
| Upper and middle lobe | 12 | 35.3 | 21 | 87.5 | |
| Lower lobe | 22 | 64.7 | 3 | 12.5 | |
| Power of MWA | |||||
| 60W | 11 | 32.4 | |||
| 70w | 23 | 67.6 | |||
| Time of MWA | |||||
| Mean | 11.5 minutes | ||||
| Range | 3-28.5 minutes | ||||
| EGFR-TKIs regimen | |||||
| 1st-line | 19 | 55.9 | 16 | 66.7 | |
| 2nd and post-2nd line | 15 | 44.1 | 8 | 33.3 | |
| EGFR-TKIs type | |||||
| Gefitinib | 32 | 94.1 | 18 | 75.0 | |
| Erlotinib | 2 | 5.9 | 6 | 25.0 | |
| Response | |||||
| CR+PR | 21 | 61.8 | 11 | 45.8 | |
| SD+PD | 13 | 38.2 | 13 | 54.2 | |
| Treatment post-TKIs | |||||
| No | 20 | 58.8 | 16 | 66.7 | |
| Yes | 14 | 41.2 | 8 | 33.3 | |
| Treatment post-TKIs | |||||
| Chemotherapy | 4 | 28.6 | 3 | 42.9 | |
| Previous TKIs | 8 | 57.1 | 4 | 57.1 | |
| Other TKIs | 2 | 14.3 | 0 | 0.0 | |
*Three patients had both 19Del and L858R mutations.
#Two patients had both 19Del and L858R mutations.
Response to MWA and EGFR-TKIs
Complete ablation was achieved in 29 (85.3%) of the 34 total patients in the MWA plus EGFR-TKIs group. The ORRs to EGFR-TKIs in the MWA plus EGFR-TKIs and EGFR-TKIs groups were 61.8% (21/34) and 45.8% (11/24) (p = 0.230), respectively.
The correlation between MWA and survival
Until November 19, 2016, with a median follow-up of 19.3 months (range, 6 to 52 months), 35 patients progressed, including 21 in the MWA plus EGFR-TKIs group and 14 in the EGFR-TKIs group. Sixteen patients died, of whom 9 and 7 were in the MWA plus EGFR-TKIs group and EGFR-TKIs group, respectively.
Patients treated with MWA plus EGFR-TKIs failed to show a survival advantage when compared with EGFR-TKIs only, with PFS durations of 13.2 months (95% confidence interval [CI], 9.0–17.5 months) and 11.6 months (95% CI, 4.7–18.5 months, p = 0.640), respectively (Figure 1). Patients with adenocarcinoma had longer PFS in comparison with non-adenocarcinoma NSCLC (13.2 months, 95% CI, 9.8–16.6 months vs. 0.4 months, 95% CI, 0.2–0.5 months, p = 0.000). The response to EGFR-TKIs had a tendency to predict PFS (CR + PR vs. PD + SD, 14.6 months [95% CI, 12.4–16.9 months] vs. 10.0 months [95% CI, 3.8–16.3 months], p = 0.067) (Table 2).
Figure 1. Kaplan-Meier estimates of PFS in 58 patients.
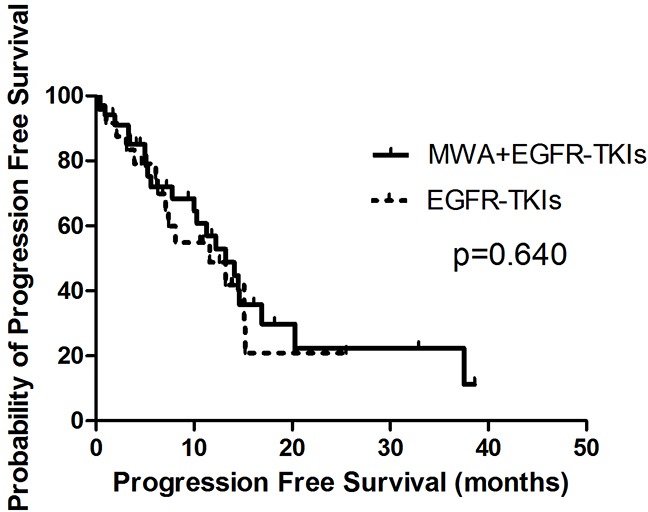
The median PFS of patients treated with MWA plus EGFR-TKIs was 13.2 months (95%CI, 9.0-17.5 months), and those received EGFR-TKIs was 11.6 months (95%CI, 4.7-18.5 months).
Table 2. Univariant analyses of progression free survival and overall survival.
| PFS(ms) | 95%CI | p | OS(ms) | 95%CI | p | ||
|---|---|---|---|---|---|---|---|
| Gender | 0.053 | 0.140 | |||||
| Male | 7.4 | 4.2-10.7 | 21.4 | 12.4-30.4 | |||
| Female | 14.1 | 12.1-16.1 | 39.8 | 13.8-65.7 | |||
| Age | 0.631 | 0.490 | |||||
| ≥65 | 14.1 | 9.8-18.4 | 23.5 | 18.0-29.0 | |||
| <65 | 12.2 | 7.9-16.4 | 32.5 | 24.4-40.6 | |||
| Smoking history | 0.403 | 0.540 | |||||
| Non-smokers | 13.2 | 10.2-16.1 | 31.8 | 24.6-38.9 | |||
| Smokers | 10.3 | 5.3-23.3 | 23.1 | 25.1-38.0 | |||
| ECOG | 0.373 | 0.720 | |||||
| 0 | 20.1 | 7.4-32.7 | 28.2 | 19.9-36.5 | |||
| 1 | 14.8 | 10.7-18.8 | 31.1 | 29.9-42.6 | |||
| Pathology | 0.000 | 0.000 | |||||
| Adenocarcinoma | 13.2 | 9.8-16.6 | 16.2 | 12.2-20.3 | |||
| Non-adenocarcinoma | 0.4 | 0.2-0.5 | 0.4 | 0.2-0.5 | |||
| Stage | 0.692 | 0.938 | |||||
| IIIB | 10.0 | 4.1-16.0 | 25.6 | 13.6-37.6 | |||
| IV | 13.1 | 10.2-16.1 | 31.2 | 24.5-37.9 | |||
| EGFR mutation | 0.599 | 0.385 | |||||
| 19Del | 11.6 | 6.2-17.0 | 36.3 | 29.9-41.6 | |||
| L858R | 13.2 | 10.6-15.7 | 27.7 | 19.9-35.5 | |||
| MWA | 0.640 | 0.288 | |||||
| No | 11.6 | 4.7-18.5 | 20.4 | 11.3-29.5 | |||
| Yes | 13.2 | 9.0-17.5 | 39.8 | 8.3-71.2 | |||
| EGFR-TKI regimen | 0.501 | 0.975 | |||||
| 1st-line | 14.1 | 10.1-18.2 | 30.7 | 23.0-38.4 | |||
| 2nd and post-2nd line | 12.2 | 3.6-20.8 | 31.1 | 22.0-40.3 | |||
| EGFR-TKI type | 0.981 | 0.936 | |||||
| Gefitinib | 13.2 | 10.3-16.1 | 30.3 | 23.4-37.2 | |||
| Erlotinib | 7.1 | 0.0-26.8 | 34.3 | 21.4-47.2 | |||
| Response | 0.067 | 0.042 | |||||
| CR+PR | 14.6 | 12.4-16.9 | 36.7 | 28.2-45.3 | |||
| SD+PD | 10.0 | 3.8-16.3 | 25.2 | 17.5-33.0 | |||
| Treatment post-TKIs | 0.147 | ||||||
| No | 5.0 | 3.1-7.0 | 11.4 | 7.7-15.1 | |||
| Yes | 7.8 | 2.9-12.7 | 39.7 | 5.5-74.0 |
OS was similar in both groups (MWA plus EGFR-TKIs group vs. EGFR-TKIs group, 39.8 months [95% CI, 6.3–71.2 months] vs. 20.4 months [95% CI, 11.3–29.5 months], p = 0.288; Figure 2). Patients with adenocarcinoma had better OS in comparison with non-adenocarcinoma NSCLC (16.2 months [95% CI, 12.2–20.3 months] vs. 0.4 months [95% CI, 0.2–0.5 months], p = 0.000). Patients who achieved an ORR (CR + PR vs. PD + SD, 36.7 months [95% CI, 28.2–45.3 months] vs. 25.2 months [95% CI, 17.5–33.0 months], p = 0.042) and received treatment after progression with EGFR-TKIs (treatment post-EGFR-TKIs vs. no treatment post-EGFR-TKIs, 39.7 months [95% CI, 5.5–74.0 months] vs. 11.4 months [95% CI, 7.7–15.1 months], p = 0.000) also showed a survival advantage (Table 2).
Figure 2. Kaplan-Meier estimates of OS in 58 patients.

The median OS of patients treated with MWA plus EGFR-TKIs was 39.8 months (95%CI, 6.3-71.2 months), and those received EGFR-TKIs was 20.4 months (95%CI, 11.3-29.5 months).
In the multivariate analyses of PFS and OS, MWA was not a significant prognostic factor, and the corresponding p-values were 0.753 (hazard ratio [HR], 1.132 [95% CI, 0.522–2.457]) (Table 3) and 0.976 (HR, 1.019 [95% CI, 0.300–3.464]) (Table 4), respectively.
Table 3. Multivariant analyses of progression free survival.
| 95%CI | ||||
|---|---|---|---|---|
| HR | Lower | Upper | p | |
| Gender | 1.996 | 0.823 | 4.842 | 0.127 |
| ECOG | 1.610 | 0.344 | 7.534 | 0.545 |
| Smoking history | 0.981 | 0.397 | 2.425 | 0.967 |
| MWA | 1.132 | 0.522 | 2.457 | 0.753 |
| Response | 0.619 | 0.295 | 1.301 | 0.206 |
Table 4. Multivariant analyses of progression free survival.
| 95%CI | ||||
|---|---|---|---|---|
| HR | Lower | Upper | p | |
| Gender | 2.082 | 0.622 | 6.966 | 0.234 |
| Age | 1.564 | 0.538 | 4.552 | 0.412 |
| EGFR mutations | 0.478 | 0.155 | 1.473 | 0.199 |
| MWA | 1.019 | 0.300 | 3.464 | 0.976 |
| Response | 0.280 | 0.087 | 0.900 | 0.033 |
| Post-EGFR-TKIs | 0.404 | 0.121 | 1.343 | 0.139 |
In order to explore the correlation between the number of tumor sites and the survival benefit of MWA, we divided patients into two groups, those with three or fewer tumor metastases and those with more than three tumor sites other than the primary tumor in advanced. No differences were observed in either group in PFS or OS (Figures 3, 4, 5, 6). For patients with more than three tumor metastases, the median PFS were 11.3 months (95% CI, 3.1–22.9 months) and 15.1 months (95% CI, 8.1–22.2 months) (p = 0.874) (Figure 3) for the MWA plus EGFR-TKIs group and the EGFR-TKIs group, respectively, and the corresponding OS were 28.4 months (95% CI, 18.1–38.6 months) and 18.2 months (95% CI, 15.1–21.2 months) (p = 0.859), respectively (Figure 4). For patients with three or fewer tumor metastases, the median PFS were 13.2 months (95% CI, 9.1–17.4 months) and 7.4 months (95% CI, 5.7–9.1 months) (p = 0.545) (Figure 5) for the MWA plus EGFR-TKIs group and the EGFR-TKIs group, respectively, and the corresponding OS were 38.0 months (95% CI, 28.8–47.2 months) and 23.3 months (95% CI, 12.2–34.3 months), respectively (p = 0.212) (Figure 6).
Figure 3. Kaplan-Meier estimates of PFS in 26 patients with metastatic sites more than 3.
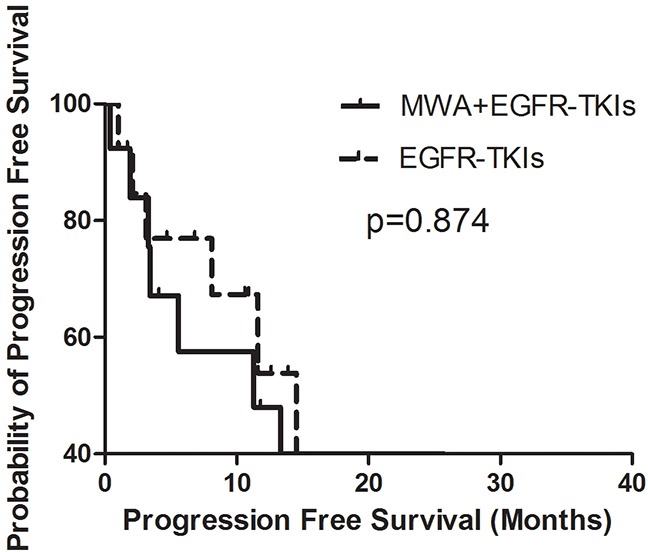
The median PFS of patients treated with MWA plus EGFR-TKIs was 11.3 months (95%CI, 3.1-22.9 months), and those received EGFR-TKIs was 15.1 months (95%CI, 8.1-22.2 months).
Figure 4. Kaplan-Meier estimates of OS in 26 patients with metastatic sites more than 3.
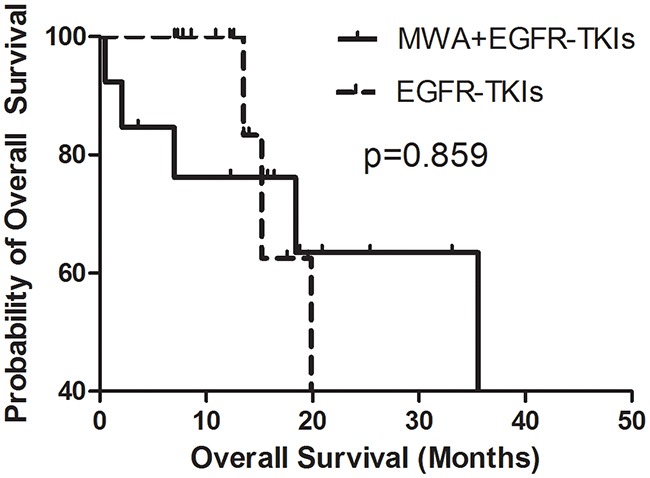
The median OS of patients treated with MWA plus EGFR-TKIs was 28.4 months (95%CI, 18.1-38.6 months), and those received EGFR-TKIs was 18.2 months (95%CI, 15.1-21.2 months).
Figure 5. Kaplan-Meier estimates of PFS in 32 patients with metastatic sites of 3 or fewer.
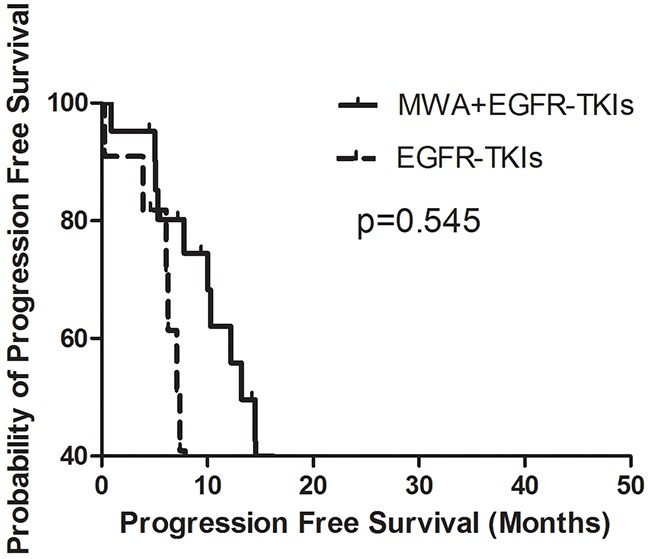
The median PFS of patients treated with MWA plus EGFR-TKIs was 13.2 months (95%CI, 9.1-17.4 months), and those received EGFR-TKIs was 7.4 months (95%CI, 5.7-9.1 months).
Figure 6. Kaplan-Meier estimates of OS in 32 patients with metastatic sites of 3 or fewer.
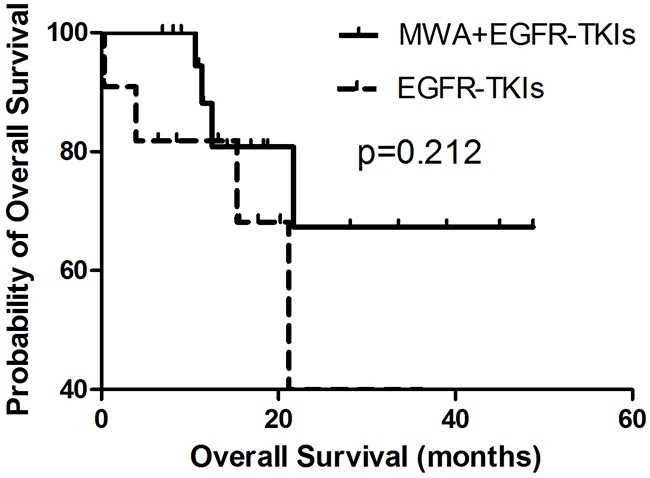
The median OS of patients treated with MWA plus EGFR-TKIs was 38.0 months (95%CI, 28.8-47.2 months), and those received EGFR-TKIs was 23.3 months (95%CI, 12.2-34.3 months).
DISCUSSION
EGFR-TKIs remain the standard of care for advanced NSCLC with EGFR-sensitive mutations and show a dramatic improvement in PFS. [1–5] However, all patients will develop resistance ultimately, even those who achieve complete or incomplete remission. [6–18]
Multiple studies explored the following treatment strategy after the failure of EGFR-TKIs. Based on the progression models, the therapies varied. [19, 20] For those with brain progression, SBRT or SRT was the first choice, and the EGFR-TKIs were continued. [20–22] For patients with local disease progression, local treatments including radiation or thermal ablation in combination with previous EGFR-TKIs were recommended [20, 23, 24, 33–35]. However, chemotherapy and transfer to a third EGFR-TKI were treatment options for those with widespread metastasis.
We firstly explored whether the combination of MWA at primary tumor sites and EGFR-TKIs could improve PFS when compared with EGFR-TKIs alone for advanced NSCLC with EGFR-sensitive mutations. No significant difference was observed in either PFS or OS in univariate and multivariate analyses, indicating that MWA and EGFR-TKIs did not have a synergistic effect, which was different from radiation plus EGFR-TKIs. Welsh et al. [21] showed that patients with EGFR-sensitive mutations and brain metastases had superior survival and ORRs when treated with EGFR-TKIs and SBRT. William et al. [22] verified that the median OS and intracranial PFS were longer in the group treated with RT upfront compared with the group treated with EGFR-TKIs upfront. What is more, Zeng et al. [36] showed that concomitant administration of gefitinib and WBRT was found to result in higher treatment response and disease control rates in patients with EGFR-sensitive mutations and NSCLC brain metastases compared with gefitinib alone. Hong et al. [33] found that in NSCLC patients with EGFR-sensitive mutations and skeletal metastasis progression, EGFR-TKIs continued in combination with radiation after treatment with EGFR-TKIs and disease progression resulted in a PFS of 5.6 to 8.0 months. Isolated bone failure without systemic disease progression is associated with better survival when treated with continuation of EGFR-TKIs plus local radiation. [24] Studies verified that EGFR-TKIs could modulate the radiation response. [37, 38] When combined with radiation, EGFR-TKIs promote a further reduction in the S-phase fraction, resulting in an accumulation of cells in G1 and G2. [37] What is more, TKIs enhance the induction of apoptosis, inhibit EGFR autophosphorylation and Rad51 expression, and improve radiosensitivity. [37] EGFR inhibition led to pronounced cellular senescence of irradiated cells. Moreover, cellular senescence is a prominent mechanism in radiosensitization. The senescence and radiosensitization were linked to an increase in residual radiation-induced DNA double-strand breaks irrespective of p53/p16 status. [39]
To explore the correlation between the number of tumor sites and the survival benefit of MWA, we divided patients into two groups, those with three or fewer tumor metastases and those with more than three tumor sites other than the primary tumor in advanced. No differences were observed in either group in PFS or OS. Gomez et al. [28] showed that advanced NSCLC patients without driver mutations after systematic treatments of platinum-based doublet chemotherapy and patients with driver mutations after EGFR-TKIs or ALK inhibitors had longer PFS when treated with consolidated treatments followed by maintenance treatments compared with maintenance treatments alone. An 8-month PFS was observed, which was significantly different. However, the study was restricted to those with oligometastases, which was defined as no more than three tumor sites. Chiang et al. [34] also showed that radical palliative thoracic RT was safe and might be beneficial for primary lung lesions in patients with metastatic NSCLC and controlled extrathoracic diseases. The median OS, PFS after RT, and OS after RT were 50 months, 15 months, and 18 months, respectively. Qin et al. [35] reported that when patients who responded to EGFR-TKIs received EGFR-TKIs and local radiofrequency hyperthermia, the PFS and OS were 22 months and 26 months, respectively. The difference in PFS indicated that local treatments, which did not focus on all tumor sites, did not have a survival advantage for patients with EGFR-sensitive mutations treated with MWA plus EGFR-TKIs.
In conclusion, for advanced NSCLC patients with EGFR-sensitive mutations, MWA at the primary tumor sites plus EGFR-TKIs failed to show a survival advantage when compared with EGFR-TKIs alone.
MATERIALS AND METHODS
Patient characteristics
Patients with pathologically verified advanced NSCLC and EGFR-sensitive mutations, i.e., an exon 19 deletion or exon 21 L858R point mutation, were recruited. Other inclusion criteria included an age no less than 18 years; Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 to 1; no local therapy at primary tumor sites, such as radiation, radiofrequency ablation (RFA), or I125 radioactive particle implantation; and adequate bone marrow, hepatic, and renal function. The exclusion criteria were as follows: serious issues with pulmonary and cardiovascular function such that MWA could not be risked; previous treatment with EGFR-TKIs or local therapies for primary tumors; baseline interstitial lung disease; cerebral hemorrhage, cerebral infarction, or coronary heart disease, especially unstable angina or myocardial infarction during the previous 6 months; and anti-platelet or anti-necrosis treatments during the past 1 week.
The study was approved by the ethics committee of Shandong Provincial Hospital affiliated with Shandong University prior to study enrollment. Written informed consent was obtained from all enrolled patients.
MWA procedure
All patients allocated to the MWA plus EGFR-TKIs group received MWA at primary tumor sites under computed tomography guidance. The procedure has been detailed in our previous reports. [25, 26, 29]
EGFR mutation testing
DNA was extracted from 4-μm formalin-fixedand parrffin-embedded,(FFPE) tumor slides by using the QIAamp DNA FFPE Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. The EGFR mutation testing procedure was described in detail in our previous study. [30] A classic S-curve and a Ct value >30 were considered to be a positive result, indicating the presence of a mutation.
EGFR-TKIs
Patients received 250 mg oral gefitinib or 150 mg erlotinib once daily until disease progression or intolerable toxicity. The interval between MWA and EGFR-TKIs therapy was 1 week.
The follow-up and response evaluation
MWA follow-up was performed every month for 3 months post-ablation and at 3-month intervals thereafter. The response to EGFR-TKIs was evaluated 1 month later and then at 2-month intervals.
The response to MWA was assessed according to a Chinese expert consensus [31]. The response to EGFR-TKIs was conducted according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [32].
Statistical analyses
SPSS version 17.0 (USA) was applied for statistical analyses. The primary endpoint was PFS; the secondary endpoint was overall survival (OS). PFS was calculated from the date of MWA to disease progression for both primary tumor sites and other tumor sites or death for those treated with MWA plus EGFR-TKIs. For patients who received EGFR-TKIs only, PFS was calculated from the start of treatment with EGFR-TKIs to disease progression or death. OS was calculated from the diagnosis of NSCLC to death from any cause. Both PFS and OS were analyzed using Kaplan-Meier univariate analyses and Cox regression multivariate analyses. Factors with p-values less than 0.5 in the univariate analyses besides MWA were examined in the Cox regression multivariate analyses. Chi-square test was used to test the correlation between the response to EGFR-TKIs and the treatment regimens. All tests were two-sided, and p-values less than 0.05 were considered to represent a significant difference.
Acknowledgments
The scientific guarantor of this publication is Xin Ye. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by Shandong Province medical and health science and technology development projects (2014WS0346). Zhigang Wei has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. This retrospective observational study was performed at one institution.
Footnotes
CONFLICTS OF INTEREST
No conflicts of interest exists.
REFERENCES
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med.2009; 361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA, Ladanyi M. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 11.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang WC, Yu CJ, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii A, Harada T, Iwama E, Ota K, Furuyama K, Ijichi K, Okamoto T, Okamoto I, Takayama K, Nakanishi Y. Hypermethylation of the CpG dinucleotide in epidermal growth factor receptor codon 790: implications for a mutational hotspot leading to the T790M mutation in non-small-cell lung cancer. Cancer Genet. 2015;208:271–278. doi: 10.1016/j.cancergen.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, Ohashi K, Janjigian YY, Spitzler PJ, Melnick MA, Riely GJ, Kris MG, Miller VA, et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano S, Takeuchi S, Nakagawa T, Yamada T. Ligand-triggered resistance to molecular targeted drugs in lung cancer: roles of hepatocyte growth factor and epidermal growth factor receptor ligands. Cancer Sci. 2012;103:1189–1194. doi: 10.1111/j.1349-7006.2012.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, Choi YJ, Choi CM, Kim SW, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Vasu VT, Mishra R, Singleton KR, Yoo M, Leach SM, Farias-Hesson E, Mason RJ, Kang J, Ramamoorthy P, Kern JA, Heasley LE, Finigan JH, et al. Bioinformatics-driven discovery of rational combination for overcoming EGFR-mutant lung cancer resistance to EGFR therapy. Bioinformatics. 2014;30:2393–2398. doi: 10.1093/bioinformatics/btu323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumour specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 19.Matikas A, Mistriotis D, Georgoulias V, Kotsakis A. Current and future approaches in the management of non-small-cell lung cancer patients with resistance to EGFR TKIs. Clin Lung Cancer. 2015;16:252–261. doi: 10.1016/j.cllc.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr, Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC, Camidge DR. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, Chang JY, Wefel JS, McGovern SL, Garland LL, Chen SS, Holt J, Liao Z, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor mutant none small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. 2016;95:673–679. doi: 10.1016/j.ijrobp.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, Pietanza MC, Azzoli CG, Rizvi NA, Krug LM, Miller VA, Kris MG, Riely GJ. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JA, Lee JY, Kim WS, Song JS, Rho JK, Choi CM, Lee JC. Clinical implications of isolated bone failure without systemic disease progression during EGFR-TKI treatment. Clin Lung Cancer. 2016;17:573–580. doi: 10.1016/j.cllc.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Ni Y, Bi J, Ye X, Fan W, Yu G, Yang X, Huang G, Li W, Wang J, Han X, Ni X, Wei Z, Han M, et al. Local microwave ablation with continued EGFR tyrosine kinase inhibitor as a treatment strategy in advanced non-small cell lung cancers that developed extra-central nervous system oligoprogressive disease during EGFR tyrosine kinase inhibitor treatment: a pilot study. Medicine (Baltimore) 2016;95:e3998. doi: 10.1097/MD.0000000000003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, Ni X, Wang J, Han X. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142. doi: 10.1007/s00270-014-0895-0. [DOI] [PubMed] [Google Scholar]
- 27.Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, Han X. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464. doi: 10.1007/s12032-014-0464-z. [DOI] [PubMed] [Google Scholar]
- 28.Gomez DR, Blumenschein GR, Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, Karam JA, Kavanagh BD, Tang C, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Ye X, Zheng A, Huang G, Ni X, Wang J, Han X, Li W, Wei Z. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763. doi: 10.1002/jso.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, Han X, Meng M, Ni Y. Advanced non small cell lung cancer: response to microwave ablation and EGFR status. Eur Radiol. 2017;27:1685–1694. doi: 10.1007/s00330-016-4474-4. [DOI] [PubMed] [Google Scholar]
- 31.Ye X, Fan W, Chen JH, Feng WJ, Gu SZ, Han Y, Huang GH, Lei GY, Li XG, Li YL, Li ZJ, Lin ZY, Liu BD, et al. Chinese expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors. Thoracic Cancer. 2015;6:112–121. doi: 10.1111/1759-7714.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Hong SH, Kim YS, Lee JE, Kim IH, Kim SJ, Han D, IeR Yoo, Chung YG, Kim YH, Lee KY, Kang JH. Clinical characteristics and continued epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor administration in EGFR-mutated non-small cell lung cancer with skeletal metastasis. Cancer Res Treat. 2016;48:1110–1119. doi: 10.4143/crt.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang Y, Yang JC, Hsu FM, Chen YH, Shih JY, Lin ZZ, Lan KH, Cheng AL, Kuo SH. The response, outcome and toxicity of aggressive palliative thoracic radiotherapy for metastatic non-small cell lung cancer patients with controlled extrathoracic diseases. PLoS One. 2015;10:e0145936. doi: 10.1371/journal.pone.0145936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Y, Sun Y, Liu Y, Luo Y, Zhu J. Pilot study of radiofrequency hyperthermia in combination with gefitinib in gefitinib-effective patients with advanced NSCLC. Thorac Cancer. 2016;7:422–427. doi: 10.1111/1759-7714.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng YD, Zhang L, Liao H, Liang Y, Xu F, Liu JL, Dinglin XX, Chen LK. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non-small-cell lung cancer: a retrospective study. Asian Pac J Cancer Prev. 2012;13:909–914. doi: 10.7314/apjcp.2012.13.3.909. [DOI] [PubMed] [Google Scholar]
- 37.Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM, Harari PM. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65:3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 38.Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, Nirodi CS. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res. 2007;67:5267–5274. doi: 10.1158/0008-5472.CAN-07-0242. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Morsbach F, Sander D, Gheorghiu L, Nanda A, Benes C, Kriegs M, Krause M, Dikomey E, Baumann M, Dahm-Daphi J, Settleman J, Willers H. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res. 2011;1:6261–6269. doi: 10.1158/0008-5472.CAN-11-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]


