ABSTRACT
Renal insufficiency is a frequent cancer-associated problem affecting more than half of all cancer patients at the time of diagnosis. To minimize nephrotoxic effects the dosage of anticancer drugs are reduced in these patients, leading to sub-optimal treatment efficacy. Despite the severity of this cancer-associated pathology, the molecular mechanisms, as well as therapeutic options, are still largely lacking. We here show that formation of intravascular tumor-induced neutrophil extracellular traps (NETs) is a cause of kidney injury in tumor-bearing mice. Analysis of clinical biomarkers for kidney function revealed impaired creatinine clearance and elevated total protein levels in urine from tumor-bearing mice. Electron microscopy analysis of the kidneys from mice with cancer showed reversible pathological signs such as mesangial hypercellularity, while permanent damage such as fibrosis or necrosis was not observed. Removal of NETs by treatment with DNase I, or pharmacological inhibition of the enzyme peptidylarginine deiminase 4 (PAD4), was sufficient to restore renal function in mice with cancer. Tumor-induced systemic inflammation and impaired perfusion of peripheral vessels could be reverted by the PAD4 inhibitor. In conclusion, the current study identifies NETosis as a previously unknown cause of cancer-associated renal dysfunction and describes a novel promising approach to prevent renal failure in individuals with cancer.
KEYWORDS: Cancer, DNase I, GSK484, kidney injury, neutrophil extracellular traps
Introduction
Insufficient kidney function is a frequent and fatal condition in cancer patients. Two European studies performed during the last decade revealed that more than 50% of adult patients with solid cancer are affected by renal insufficiency at the time of diagnosis.1-3 These two studies calculated glomerular filtration rate (GFR) based on serum creatinine. GFR estimations can also be based on measurement of cystatin C in serum. In agreement, a study using the cystatin C method found a reduction in GFR in 40% of cancer patients.4 Decreased GFR, a hallmark of impaired kidney function, has been reported to correlate with mortality in cancer patients in several independent studies.5-7 Besides contributing directly to mortality, decreased renal function interferes with anticancer therapy. A majority of anticancer drugs used in the clinic are excreted predominantly via the urine and a suboptimal filtration rate of the kidneys therefore constitutes a risk for accumulation of cytotoxic compounds in the kidneys and subsequent nephrotoxic effects.3 As a consequence of renal insufficiency dose-reduction of the drug is often required, which obstructs successful treatment and contributes to malignant progression and mortality. The only available treatment option for renal insufficiency in the clinic is dialysis and novel therapeutic approaches are therefore needed. Despite the clinical impact of renal insufficiency it is still not fully clarified why cancer patients experience failure of organs, which are not directly affected by either primary or secondary tumor growth. We recently demonstrated that hypoperfusion of the kidney vasculature as well as renal inflammation, both prominent hallmarks of renal failure, can be caused by intravascular formation of neutrophil extracellular traps (NETs) in tumor-bearing mice.8 However, the effect of tumor-induced formation of NETs on kidney function has never been addressed.
Formation of NETs (NETosis) was first described in 2004 as a mechanism used by neutrophils to fight severe bacterial infections.9 During NETosis, which can occur via different mechanisms, neutrophils release their chromatin and granule contents including anti-bacterial proteases such as myeloperoxidase and neutrophil elastase and create a web-like structure with high concentration of anti-bacterial factors that trap and kill infectious agents.10 In recent years, NETs have been suggested to also play a role in numerous non-infectious pathologies such as atherosclerosis, systemic lupus erythematosus (SLE), diabetes, thrombosis and cancer.11-17 Demers and colleagues reported that neutrophils from mice with cancer are more prone to undergo NETosis due to G-CSF produced by the tumor cells and suggested NETs as a potential cause of cancer-associated deep vein thrombosis (DVT).11 We could recently confirm a central role for G-CSF in tumor-induced NETosis of circulating neutrophils, using an anti-G-CSF antibody.8 In agreement, mice with subcutaneous B16 melanoma tumors expressing negligible amounts of G-CSF did not display significant intravascular NETosis. Additional tumor-derived factors such as IL-8, a potent chemotactic factor for myeloid cells, has also been implicated in cancer-associated NETosis.18
Recent reports have provided further evidence for a role of NETs in cancer-associated pathology in humans.19 Two studies show a correlation between NETs and a hypercoagulable state in cancer patients.20,21 Furthermore, tumor-induced NETs were identified as indirect promoters of the actual malignancy by polarization of neutrophils toward a pro-tumorigenic phenotype in patients with intestinal cancer.21 In another study, NETs induced by postsurgical stress in cancer patients with liver metastases correlated with reduced disease-free survival.22 This is in line with an earlier study by Cools-Lartigue, showing that infection-induced NETs sequester circulating tumor cells and promote metastasis.23 This finding may provide a mechanistic explanation for the clinical observation that postsurgical infections in cancer patients are associated with worse outcome, independent of the direct problems caused by the infection. Accumulating evidence thus indicate that tumor-induced NETosis plays an important role in disease outcome and represents a potential therapeutic target in patients with cancer.
Renal hypoperfusion and associated ischemia is a main contributor to renal failure.24 Based on our previous data showing that tumor-induced NETs impair perfusion of peripheral vessels and promote systemic inflammation, we hypothesized that intravascular NETosis could be a previously unknown cause of renal insufficiency in individuals with cancer. In this study, we show that mice with cancer display clinical hallmarks of kidney injury. This condition could be reverted by removal of NETs, either by treatment with DNase I or with an inhibitor of the enzyme peptidylarginine deiminase 4 (PAD4). The findings in the current study may have important therapeutic implications, both for prevention of cancer-associated organ failure and for enabling optimal dosing of chemotherapeutic drugs.
Results
Mice with cancer show signs of renal insufficiency
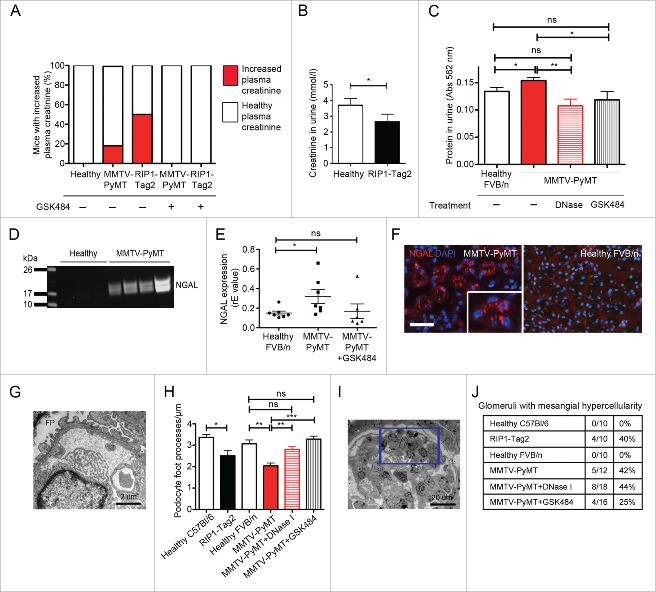
We have recently shown that mice with mammary carcinoma (MMTV-PyMT) and pancreatic neuroendocrine tumors (RIP1-Tag2) display systemic inflammation and impaired vascular perfusion of distant organs unaffected by tumor growth, such as kidney and heart.8 To address whether these tumor-induced systemic effects translate into renal insufficiency, we analyzed several clinical parameters for renal function. An impaired GFR is characterized by an accumulation of creatinine in the blood, and a decreased excretion of creatinine via the urine. Indeed, we found that plasma creatinine levels were elevated in subgroups of both MMTV-PyMT and RIP1-Tag2 mice, indicating renal dysfunction in these individuals (Fig. 1A, three left bars; 17% of MMTV-PyMT, 50% of RIP1-Tag2 mice). The RIFLE-criteria have been derived to classify renal insufficiency in patients and a parameter used to grade the severity of damage is the increase in serum creatinine. Three categories have been identified based on increased serum creatinine levels compared with healthy conditions: Risk (1.5-fold increase), Injury (2-fold increase) and Failure (3-fold increase).25 All MMTV-PyMT and RIP1-Tag2 mice with increased plasma creatinine had at least twice as high creatinine value compared with the healthy littermates (except for one mouse with slightly less than a 2-fold increase). These results indicate that the analyzed mice display renal insufficiency comparable to kidney injury, but have not yet developed renal failure. RIP1-Tag2 mice also showed significantly decreased urine creatinine levels, further supporting an impaired kidney function in tumor-bearing mice (Fig. 1B). Due to limited urine volumes, total protein level in urine was used as a read-out for kidney function in MMTV-PyMT mice instead of creatinine measurements, which requires larger volumes. Significantly higher total protein levels were detected in urine from MMTV-PyMT mice compared with healthy littermates (Fig. 1C), indicating renal insufficiency.
Figure 1.
Mice with cancer show clinical signs of renal insufficiency. (A) Plasma creatinine levels were measured as an indicator of renal function (healthy, n = 17; MMTV-PyMT, n = 12; RIP1-Tag2 mice, n = 8; MMTV-PyMT + GSK484, n = 20; RIP1-Tag2 + GSK484, n = 3). Healthy plasma creatinine level is defined as the span between the highest and lowest creatinine value in all non-tumor-bearing mice. All mice with plasma creatinine above the highest value in the healthy group are defined as mice with increased plasma creatinine levels. (B) Urine creatinine concentration was measured in RIP1-Tag2 mice and healthy littermates (healthy, n = 11; RIP1-Tag2, n = 25). (C) Total protein levels in urine from MMTV-PyMT mice and healthy littermates were analyzed using the BCA assay (healthy, n = 21; MMTV-PyMT, n = 35; MMTV-PyMT + DNase I, n = 10; MMTV-PyMT + GSK484, n = 15). (D) Western blot for NGAL in urine from MMTV-PyMT mice and healthy littermates. (E) Analysis of NGAL mRNA in the kidney by qPCR. Each data point corresponds to one individual mouse. (F) Immunostaining of kidney sections from MMTV-PyMT mice (left panel) and healthy littermates (right panel). (G) Electron microscopy analysis was used to analyze podocyte foot processes (FP) in kidney glomeruli from healthy C57BL/6, n = 10 (2 mice); RIP1-Tag2, n = 14 (2 mice); healthy FVB/n, n = 10 (2 mice); MMTV-PyMT, n = 10 (2 mice); MTV-PyMT + DNase I, n = 15 (3 mice); MMTV-PyMT + GSK484, n = 15 (3 mice). (H) Quantification of the number of podocyte foot processes/μM in glomeruli from MMTV-PyMT mice (untreated or treated with DNase I or GSK484) and healthy littermates. (I) Mesangial hypercellularity (indicated by square) in kidneys from MMTV-PyMT mice. (J) Quantification of the number of glomeruli with signs of mesangial hypercellularity. * = p < 0.05, ** = p < 0.01 and *** = p < 0.005. Scale bar corresponds to 100 μm in F.
While creatinine is a relatively late indicator of renal dysfunction, NGAL (neutrophil gelatinase associated lipocalin) is an early marker for kidney exposure to harmful stimuli, such as ischemia. NGAL is expressed and secreted by kidney tubule epithelium and the levels correlate to the severity of renal impairment.26,27 Western blot revealed the presence of NGAL in urine from tumor-bearing mice, while no NGAL was detected in urine from healthy littermates (Fig. 1D). Expression analysis of kidney tissue using qPCR showed a significant increase in NGAL transcript levels in MMTV-PyMT mice compared with healthy littermates, confirming that the elevated NGAL in urine is kidney derived (Fig. 1E). Immunostaining of kidneys from tumor-bearing mice confirmed the localization of NGAL to renal tubules, characteristic for what has been reported following ischemic tissue damage (Fig. 1F). This expression pattern was not found in kidneys from healthy littermates.
Histological analysis by light microscopy revealed no obvious alteration in the kidneys of tumor-bearing mice (data not shown). However, analysis of kidney tissue by electron microscopy (EM) revealed pathological signs with decreased number of podocyte foot processes/μm in the glomeruli, a clinically used indicator of pathologic foot process widening and fusion (Fig. 1G and H). Podocytes are essential components of the kidney filter and alteration in the architecture of foot processes is an early sign of kidney damage. 50 to 150 podocyte foot processes from at least 5 glomeruli were counted in each kidney. Segmental mild mesangial hypercellularity, a relatively late inflammatory response to kidney injury, was observed in all analyzed tumor bearing individuals. In RIP1-Tag2 mice, 40% (4 out of 10) of the glomeruli were affected, while the corresponding number in MMTV-PyMT mice was 42% (5 out of 12). None of the glomeruli (0 out of 10 in C57BL/6 and 0 out of 10 in FVB/n) from healthy mice displayed this phenotype (Fig. 1I and J). No gross histopathological signs, such as necrosis or fibrosis, were found, indicating that the tissue damage could still be in a reversible state in these models.
Based on both the functional biomarker measurement and kidney histology we conclude that mice with cancer display signs of kidney injury.
Neutrophil extracellular traps cause tumor-induced kidney injury
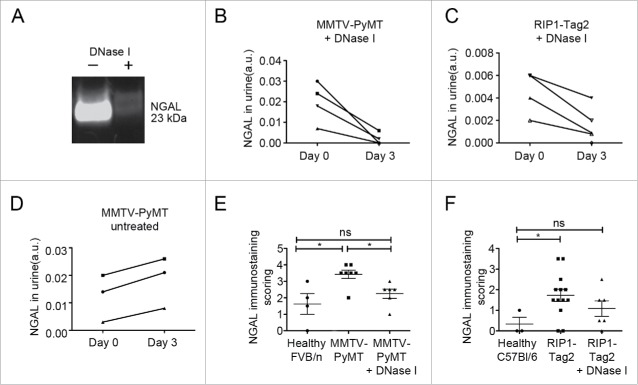
To investigate if NETosis was involved in the observed tumor-induced kidney injury, MMTV-PyMT mice with mammary carcinoma and RIP1-Tag2 mice with pancreatic neuroendocrine tumors were treated with DNase I for 3 d. DNase I treatment is an established method to dissolve NETs and we have previously demonstrated that it restores vascular function and suppresses systemic inflammation in tumor-bearing mice.8 MMTV-PyMT mice treated with DNase I displayed significantly lower total protein levels in urine compared with untreated ones (Fig. 1C). In addition, a striking decrease in urinary NGAL was observed after DNase I treatment, both in MMTV-PyMT and RIP1-Tag2 mice, indicating that NETosis indeed contributes to renal insufficiency in mice with cancer (Fig. 2A–C). In contrast, NGAL levels in urine increased during the same time in all untreated tumor-bearing mice (Fig. 2D). Immunostaining for NGAL in the kidney revealed normalized levels in DNase I treated MMTV-PyMT and RIP1-Tag2 mice, which could not be distinguished from their healthy littermates (Fig. 2E and F). Furthermore, DNase I contributed to better preservation of podocyte foot processes already after 3 d of treatment (Fig. 1H). In contrast, no effect on mesangial hypercellularity was observed after DNase I treatment (Fig. 1J). It is likely that a longer treatment period is required to observe such effects. However, daily treatment with DNase I for an extended period is not possible since the mice are immunocompetent and neutralizing antibodies against the bovine DNase I protein will appear within a week (data not shown).
Figure 2.
Treatment with DNase Isuppresses signs of renal insufficiency. (A) Representative Western blot for NGAL in urine from an MMTV-PyMT mouse before (day 0) and after DNase I treatment (day 3). (B) Western blot quantifications of NGAL in urine from MMTV-PyMT mice treated with DNase I for 3 d (n = 4). (C) Western blot quantifications of NGAL in urine from RIP1-Tag2 mice treated with DNase I for 3 d (n = 4). (D) Western blot quantifications of NGAL urine levels in untreated MMTV-PyMT mice analyzed day 0 and day 3 (n = 3). Scoring of immuostaining for NGAL in kidney sections from untreated or DNase I-treated MMTV-PyMT (E) and RIP1-Tag2 (F) mice. Each data point corresponds to one individual mouse. * = p < 0.05.
In conclusion, we found that DNase I treatment of tumor-bearing mice to remove NETs reverted signs of kidney injury.
Pharmacological inhibition of PAD4 prevents kidney injury in mice with cancer
An alternative approach to remove NETs is by inhibiting the activity of PAD4, an enzyme needed for NETs to form. PAD4 is required for citrullination of histones, a process that mediates chromatin decondensation in the nucleus before NET formation.28 Mice deficient for PAD4 lack the capacity to form NETs.29,30 Specific inhibitors of PAD4 were recently developed and proven efficient in preventing both human and mouse NETosis in vitro.31 Since these inhibitors are low molecular weight compounds they can be administered for extended periods without inducing a neutralizing immune reaction. Furthermore, DNase I can degrade NETs, but does not prevent formation of new NETs. To address whether PAD4 inhibition could suppress cancer-associated kidney injury, MMTV-PyMT mice were treated with the PAD4 inhibitor GSK48431 at 4 mg/kg daily for one week. This dose suppressed the elevated number of neutrophils undergoing NETosis in peripheral blood in mice with cancer (Fig. 3A; illustrating a granulocyte with externalized DNA and Fig. 3B; quantification of granulocytes with externalized DNA). We could not detect elevated plasma creatinine levels in any MMTV-PyMT or RIP1-Tag2 mice after treatment with GSK484 during a week (Fig. 1A, two right bars). In parallel, the total protein level in urine from MMTV-PyMT mice was significantly reduced compared with untreated tumor-bearing mice, further supporting an improved functional status of the kidneys after GSK484 treatment (Fig. 1C). In agreement with our previously published data using DNase I,8 treatment of MMTV-PyMT mice with GSK484 to prevent NETosis restored vascular perfusion of the kidneys (Fig. 3C and D).
Figure 3.
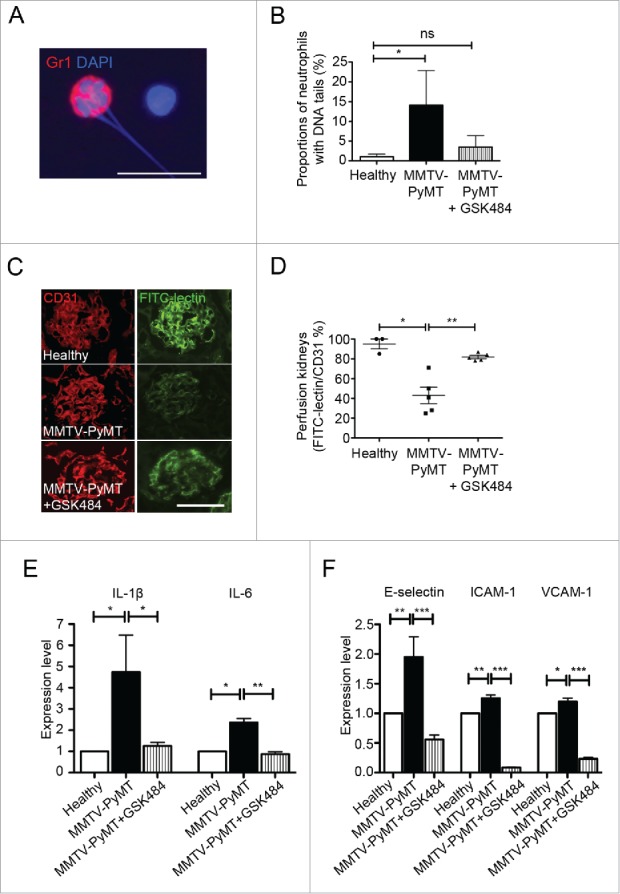
Pharmacological inhibition of PAD4 by GSK484 treatment improves perfusion and suppresses renal inflammation in kidneys from tumor-bearing mice. (A) Detection of neutrophils with extracellular DNA-tails in cytospin preparations of whole blood from MMTV-PyMT mice, by immunostaining for the neutrophil-associated marker Gr1 and DNA-staining with Hoechst. (B) Quantification of neutrophils with extracellular DNA-tails in peripheral blood from untreated and GSK484-treated MMTV-PyMT mice and healthy littermates (healthy, n = 4; MMTV-PyMT, n = 3; MMTV-PyMT + GSK484, n = 3). (C) FITC-lectin perfusion was used to analyze functionality of the renal vasculature in MMTV-PyMT mice with or without treatment with the PAD4-inhibitor. (D) Perfusion was quantified after immunostaining for the endothelial marker CD31, as the ratio FITC-lectin+/CD31+ area. Each data point corresponds to one individual mouse. (E) Kidney expression of the pro-inflammatory cytokines IL-1β and IL-6 was determined by qPCR. (F) Similarly, qPCR was used to measure the expression of the adhesion molecules E-selectin, ICAM-1 and VCAM-1 in kidneys. * = p < 0.05, ** = p < 0.01 and *** = p < 0.005. Scale bars correspond to 10 μm in A and 100 μm in B.
In a previous screen, we found transcriptional upregulation of IL-1ß, IL-6, CXCL1, E-selectin, ICAM-1 and VCAM-1 in kidney tissue from MMTV-PyMT mice with mammary carcinoma.8 This tumor-induced elevated expression of IL-1ß and IL-6, pro-inflammatory cytokines closely associated with renal failure, was suppressed to levels seen in healthy mice after GSK484 treatment (Fig. 3E). Moreover, the elevated renal expression in tumor-bearing mice of the endothelial-specific activation marker E-selectin, as well as the adhesion molecules ICAM-1 and VCAM-1, was significantly suppressed by GSK484 treatment during a week (Fig. 3F). For ICAM-1 and VCAM-1 the expression was reduced to levels below those seen in mice without tumors (Fig. 3F). Furthermore, treatment with GSK484 for one week resulted in normalization of podocyte foot processes in the kidneys of MMTV-PyMT mice (Fig. 1H). There was also a tendency to suppressed mesangial hypercellularity after 7 d treatment with the PAD4 inhibitor (Fig. 1J), which was not seen with DNase I treatment during 3 d.
In summary, we conclude that pharmacological inhibition of PAD4 prevents tumor-induced NETosis in vivo and can antagonize kidney injury in mice with mammary carcinoma or pancreatic neuroendocrine tumors.
Discussion
Renal dysfunction is a frequent and complicating factor in cancer therapy, since it can interfere with optimal dosing of chemotherapeutic drugs. In the current study, we show that two well established and widely used transgenic mouse tumor models, the RIP1-Tag2 pancreatic insulinoma and MMTV-PyMT metastatic mammary carcinoma models, display clinical signs of renal insufficiency with a severity corresponding to kidney injury in patients. We recently showed that vascular function in distant organs (kidney and heart) in mice with cancer is impaired due to tumor-induced formation of NETs.8 Whether this NET-induced reduction in vascular function is translated to a clinically detectable dysfunction of kidneys or heart has however not been addressed until now. In the current study, we show that these extracellular DNA traps, formed in individuals with cancer, are systemic mediators of kidney injury. Short-term treatment with DNAse I, an established strategy to remove NETs, improved kidney function and reverted clinical signs of kidney injury in mice with cancer. These data suggest that DNase I could be a potential therapeutic agent to suppress tumor-induced NETosis and associated organ failure in cancer patients. DNase I is already in clinical use for treatment of cystic fibrosis since 1993.32 For this condition, DNase I is administered by aerosol inhalation to degrade extracellular DNA and reduce viscosity of the pathologic secretions accumulated in the lungs due to chronic inflammation. Moreover, systemic DNase I injection has been performed in patients with the autoimmune disease SLE without any reported side-effects.33 The use of DNase I as a drug to prevent NETosis in cancer patients should therefore be a safe and realistic strategy. Nevertheless, recombinant protein drugs are relatively costly to produce and require species-specific reagents to avoid neutralizing immune reactions. We therefore analyzed the potential of the newly developed PAD4-inhibitor GSK484 to suppress tumor-induced NETosis in vivo. Moreover, a chemical inhibitor of NET formation would enable analysis of long-term suppression of NETosis in immunocompetent mice. This is currently not possible since recombinant mouse DNase I is not commercially available. Administration of GSK484 at a dose of 4 mg/kg daily during one week reverted signs of kidney dysfunction in tumor-bearing mice to the same extent as DNase I treatment, without any detectable signs of toxicity. With respect to suppressing mesangial hyperproliferation, a relatively late response to kidney injury, GSK484 was more efficient than DNase I. Possibly this reflects the longer treatment period with GSK484 (one week) compared with DNase I (3 d), which may be required to attenuate this particular response. GSK484 treatment could also suppress the tumor-induced systemic inflammation, previously reported to be dependent on NET formation.8 The reason for the strong reduction in ICAM-1 and VCAM-1 expression in the kidney after PAD4 inhibition is currently not known.
Is PAD4-inhibition a realistic therapeutic option in the clinic to prevent tumor-induced NETosis and associated organ dysfunction? Or do we put ourselves at risk for severe infections if we use NET-inhibiting drugs? While one study report that PAD4-deficient mice are more susceptible for bacterial infections due to lack of NETs in a model for necrotizing fasciitis,29 another study concluded that PAD4-mediated NETosis is not required for immunity against influenza infection.30 Potentially these data could reflect different requirements for NETs as a defense mechanism during bacterial and virus infections. However, the role of PAD4-mediated NETosis in bacterial infections was recently challenged by Martinod and colleagues, reporting that lack of PAD4 did not impair the capacity to fight infection caused by cecal ligation and puncture but instead protected against septic chock, a life-threatening complication from sepsis.34 Importantly, PAD4-deficiency does not seem to impair other anti-bacterial immune responses of the neutrophil, such as phagocytosis or secretion of anti-bacterial peptides.29,34 More research is needed to fully determine the potential risks with clinical applications of PAD4 inhibitors such as GSK484.
Prevention of tumor-induced NETosis could be beneficial for cancer patients from several aspects. One potential advantage of targeting NETosis in cancer is their reported involvement in DVT, a major cause of morbidity and mortality in cancer patients.35 NETs have also been described as promoters of metastasis in situations where infection or inflammation, induced for example by surgery, puts an extra strain on the individual with cancer.22,23 Data from our own group show that tumor-induced intravascular NETs promote endothelial activation in distant organs.8 The endothelium serves as an essential barrier regulating trafficking of immune cells, but also for extravasating tumor cells attempting to invade and colonize a secondary organ.36 While our data demonstrate increased activation of the endothelium in heart and kidney vasculature in tumor-bearing mice,8 this is likely a systemic effect that occurs also in organs representing common sites for metastasis, such as liver and lung. Whether tumor-induced NETosis promotes metastasis by facilitating extravasation of tumor cells remains to be shown. In a recent study by Park et al., DNase I-coated nanoparticles were reported to reduce metastasis in a mouse breast cancer model, suggesting that tumor-induced NETs do promote metastasis.37 However, enhanced tumor cell extravasation was not proposed as the underlying mechanism in that study, but instead increased local invasion.
In the current study, we show for the first time that tumor-induced NETosis contributes to kidney injury in two distinct transgenic mouse models of cancer. This is important considering that renal insufficiency is a frequent and potentially fatal condition in cancer patients. We also demonstrate that the condition can be reverted by therapeutic targeting of NETs with either DNase I or with a chemical PAD4 inhibitor. Based on these findings we suggest that prevention of NETosis in cancer patients should be further explored as a therapeutic approach with the potential to counteract systemic effects of cancer such as DVT, metastasis and organ failure.
Methods
Animals
Animal experiments were conducted with formal approval by the local ethics committee (Dnr C127/13, C77/13, C129/15) and performed in accordance with the United Kingdom Coordinating Committee on Cancer Research guidelines for welfare of animals with experimental neoplasia. The study includes two transgenic mouse models, the MMTV-PyMT mouse model for mammary carcinoma (FVB/n background) and the RIP1-Tag2 mouse model for pancreatic neuroendocrine carcinoma (C57BL/6 background). Littermates lacking the transgene were used as healthy controls.
DNase I treatment
DNase I (10 U in 100 μL 0.9% NaCl; Fermentas, EN0521) was administered by intra-peritoneal injection daily for 3 d.
GSK484 treatment
Mice were treated daily by intra-peritoneal injections of the PAD4 inhibitor GSK484 (4 mg/kg; Cayman Chemicals, Cat nr 17488). GSK484 was dissolved in 99.9% ethanol at a concentration of 25 mg/mL to generate a stock solution and further diluted 1:50 in 0.9% NaCl shortly before injection of 200 μL/mouse.
FITC-lectin perfusion
Mice were anaesthetized by intra-peritoneal injection of avertin (2%). FITC-conjugated lectin (100 μg in 100 μL PBS) (Vector Laboratories, Lycopersicon Esculentum, FL-1171) was administered by retro-orbital injection and allowed to circulate for 2–3 min followed by heart perfusion with 10 mL PBS (pH 7.4), and 10 mL PFA (2%) for fixation. Kidneys were dissected and incubated in sucrose (30%) over night at 4°C. Tissues were frozen in OCT cryomount (Histolab, 45830) and sectioned for analysis by immunostaining. The degree of perfusion was derived from the ratio FITC-lectin-positive area/CD31-positive area, as determined by Image J.
Urine sampling/preparation
Urine was collected from mice by light palpation of the bladder. Urine samples were centrifuged for 5000g, 5 min to remove debris.
Blood sampling and plasma preparation
Mice were anaesthetized by intra-peritoneal injection of avertin (2% in PBS) and blood was sampled by cardiac puncture using citrate (0.00169 M) as anticoagulant. Platelet poor plasma was prepared by spinning at 150g, 15 min to remove larger cells, followed by a second centrifugation at 1100g, 10 min to remove platelets.
Cytospin preparations and analysis of neutrophils with extracellular DNA tails
Citrated blood samples were centrifuged for 150g, 15 min to isolate blood cells. Erythrocytes were lysed by a brief incubation with ammonium chloride solution (0.15 M) and remaining cells were used for cytospin preparations by centrifugation at 500 rpm, 6 min. Slides with blood cells were stained with an antibody against Gr1 (BD Biosciences, 553123, 1:300) to identify neutrophils, Hoechst (Molecular Probes, H3570) to identify DNA and mounted with Fluoromount-G (Southern Biotech, 0100-01).
Western blot to detect NGAL in urine
Western blot was performed using the NuPAGE system (Life Technologies). Equal volumes of urine supernatant were loaded onto the gels. Proteins were transferred to an Immobilon-FL membrane (Millipore, IPFL00010), which was blocked in LI-COR blocking buffer (LI-COR Biosciences, 927-40000). NGAL was detected by incubation with an anti-Lipocalin-2 antibody (Abcam, ab63929; diluted 1:500) over night at 4°C, followed by incubation with a secondary IRDye 800CW donkey anti-rabbit antibody (LI-COR Biosciences, 926-32213; diluted 1:10 000). Imaging was performed using the Odyssey Infrared Imaging System 2.1 (LI-COR Biosciences).
RNA extraction and qPCR for expression analysis
For expression analysis by qPCR mice were sacrificed by cervical dislocation and organs were snap frozen in ice-cold isopentane. Frozen tissues were homogenized and RNA was extracted using the RNeasy Midi Kit (Qiagen, 75144), according to instructions from the manufacturer. The cDNA synthesis was performed using the iScript DNA synthesis kit (BioRad, 170-8891) and the KAPA SYBR FAST qPCR kit (KAPA Biosystems, KK4608) was used for the PCR reaction. For PCR primer sequences, see Supplemental methods.
Immunostainings
Immunostainings were performed for NGAL and CD31. Cryosections were fixed for 10 min in ice-cold methanol, washed in PBS and unspecific staining was blocked by incubation with 3% BSA. Staining for NGAL was performed using the following antibodies and dilutions: rabbit anti-Lipocalin-2 (Abcam, ab63929; diluted 1:500), Alexa594-conjugated goat anti-rabbit (Molecular Probes, A11012; diluted 1:1000). Staining for CD31 was performed using the following antibodies and dilutions: rat anti-CD31 (BD Biosciences, 553370; diluted 1:1000) and Cy3-conjugated donkey anti-rat (Jackson Immunoresearch, 712-165-150; 1:1000). Finally, tissue sections were counterstained with Hoechst (Molecular Probes, H3570) to visualize nuclei. A Nikon Eclipse 90i microscope with the NIS Elements 3.2 software was used for imaging, and analysis was performed using the Image J 1.44 Software (NIH). Scoring of NGAL staining in kidneys was performed by grading of staining from 0 to 5, where 0 = no expression and 5 = very high expression.
Histology
Kidneys were fixed in 4% PFA for histological analysis and sectioned at 5 μm thickness. Tissue sections were stained with Hematoxylin & Eosin, Periodic Acid Schiff and Sirius Red and examined by conventional light microscopy by a certified pathologist and a specifically trained researcher, who were blinded to the treatment and outcome data.
Electron microscopy
Kidneys were dissected from mice sacrificed by cervical dislocation and fixed in 2.5% glutaraldehyde over night at 4°C (healthy, n = 2; MMTV-PyMT, n = 2; MMTV-PyMT + DNase I, n = 3; MMTV-PyMT + GSK484, n = 3). The tissue was embedded using the agar 100 resin kit (Agar Scientific Ltd., UK), and 50–60 nm thin sections were stained in uranyl acetate and lead citrate. Imaging was performed in a Technai G2 Electron Microscope (FEI company, NL) with an ORIUS™ SC200 CCD camera (Gatan Inc., USA). Analysis was done by a certified pathologist and a specifically trained researcher, who were blinded to the treatment and outcome data.
Protein measurement in urine
Protein levels in urine were evaluated using the BCA Protein Assay (ThermoScientific, 23225). Urine was diluted 20× before measurement to minimize background signal and the levels were expressed as relative absorbance.
Creatinine quantifications
Creatinine was analyzed on a BS380 instrument (Mindray, Shenzhen, China) by an enzymatic method using reagents (8L24) from Abbott Laboratories (Abbott Park, IL, USA). The total coefficient of variation (CV) was 1.5% for creatinine at 87 μmol/L.
Statistical analysis
Statistical analysis was performed using the software Prism (GraphPad Software Inc.). The nonparametric two-tailed Mann–Whitney test was used to measure statistical differences between various groups. Significance indicated in figures; * p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.005.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by The Swedish Cancer Society (#11 0653), Ruth and Nils-Erik Stenbäck Foundation and the Foundation for Proteoglycan Research to AKO and by Magnus Bergvall Foundation and The Swedish Society of Medicine to JC.
References
- 1.Janus N, Launay-Vacher V, Byloos E, Machiels JP, Duck L, Kerger J, Wynendaele W, Canon JL, Lybaert W, Nortier J et al.. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer 2010; 103:1815-21; PMID:21063408; https://doi.org/ 10.1038/sj.bjc.6605979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: lessons from the IRMA study group. Semin Nephrol 2010; 30:548-56; PMID:21146120; https://doi.org/ 10.1016/j.semnephrol.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, Morere JF, Beuzeboc P, Deray G, Renal Insufficiency and Cancer Medications (IRMA) Study Group . Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 2007; 110:1376-84; PMID:17634949; https://doi.org/ 10.1002/cncr.22904 [DOI] [PubMed] [Google Scholar]
- 4.Larsson A, Flodin M, Hansson LO, Carlsson L. Patient selection has a strong impact on cystatin C and modification of diet in renal disease (MDRD) estimated glomerular filtration rate. Clin Biochem 2008; 41:1355-61; PMID:18674527; https://doi.org/ 10.1016/j.clinbiochem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 5.Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, Chung W, Oh KH, Jung JY. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol 2011; 33:121-30; PMID:21242672; https://doi.org/ 10.1159/000323740 [DOI] [PubMed] [Google Scholar]
- 6.Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, Wong G. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014; 63:23-30; PMID:23993153; https://doi.org/ 10.1053/j.ajkd.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 7.Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer treatments. ESMO Open 2016; 1:e000091; PMID:27843635; https://doi.org/ 10.1136/esmoopen-2016-000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, Olsson AK. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res 2015; 75:2653-62; PMID:26071254; https://doi.org/ 10.1158/0008-5472.CAN-14-3299 [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532-5; PMID:15001782; https://doi.org/ 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 10.Yipp BG, Kubes P. NETosis: how vital is it? Blood 2013; 122:2784-94; PMID:24009232; https://doi.org/ 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- 11.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012; 109:13076-81; PMID:22826226; https://doi.org/ 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107:15880-5; PMID:20798043; https://doi.org/ 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015; 21:815-9; PMID:26076037; https://doi.org/ 10.1038/nm.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010; 107:9813-8; PMID:20439745; https://doi.org/ 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT et al.. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 2014; 114:947-56; PMID:24425713; https://doi.org/ 10.1161/CIRCRESAHA.114.303312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015; 349:316-20; PMID:26185250; https://doi.org/ 10.1126/science.aaa8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cedervall J, Zhang Y, Olsson AK. Tumor-induced netosis as a risk factor for metastasis and organ failure. Cancer Res 2016; 76:4311-5; PMID:27402078; https://doi.org/ 10.1158/0008-5472.CAN-15-3051 [DOI] [PubMed] [Google Scholar]
- 18.Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A, Rodriguez-Paulete A et al.. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res 2016; 22:3924-36; PMID:26957562; https://doi.org/ 10.1158/1078-0432.CCR-15-2463 [DOI] [PubMed] [Google Scholar]
- 19.Cedervall J, Olsson A-K. Immunity gone astray - NETs in Cancer. Trends Cancer 2016; 2(11):633-634; https://doi.org/ 10.1016/j.trecan.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Sun W, Cui W, Li X, Yao J, Jia X, Li C, Wu H, Hu Z, Zou X. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol 2015; 8:14075-86; PMID:26823721 [PMC free article] [PubMed] [Google Scholar]
- 21.Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS, Bazolli B, Lu B, Penna G, Rescigno M. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun 2016; 7:11037; PMID:26996437; https://doi.org/ 10.1038/ncomms11037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 2016; 76:1367-80; PMID:26759232; https://doi.org/ 10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013; 123:3446-58; PMID:23863628; https://doi.org/ 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darmon M, Ciroldi M, Thiery G, Schlemmer B, Azoulay E. Clinical review: specific aspects of acute renal failure in cancer patients. Crit Care 2006; 10:211; PMID:16677413; https://doi.org/15312219 10.1186/cc4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure -definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 2004; 8:R204-12; PMID:15312219; https://doi.org/ 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 2008; 52:595-605; PMID:18725016; https://doi.org/ 10.1053/j.ajkd.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 27.Cernaro V, Bolignano D, Donato V, Lacquaniti A, Buemi A, Crasci E, Lucisano S, Buemi M. NGAL is a precocious marker of therapeutic response. Curr Pharm Des 2011; 17:844-9; PMID:21375495; https://doi.org/ 10.2174/138161211795428939 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA et al.. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184:205-13; PMID:19153223; https://doi.org/ 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010; 207:1853-62; PMID:20733033; https://doi.org/ 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One 2011; 6:e22043; PMID:21779371; https://doi.org/ 10.1371/journal.pone.0022043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH et al.. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015; 11:189-91; PMID:25622091; https://doi.org/ 10.1038/nchembio.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson AH. Human recombinant DNase in cystic fibrosis. J R Soc Med 1995; 88 Suppl 25:24-9; PMID:7776324 [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JC Jr., Manzi S, Yarboro C, Rairie J, McInnes I, Averthelyi D, Sinicropi D, Hale VG, Balow J, Austin H et al.. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999; 8:68-76; PMID:10025601; https://doi.org/ 10.1191/096120399678847380 [DOI] [PubMed] [Google Scholar]
- 34.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015; 125:1948-56; PMID:25624317; https://doi.org/ 10.1182/blood-2014-07-587709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002; 4:465-73; PMID:12407439; https://doi.org/ 10.1038/sj.neo.7900263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cedervall J, Dimberg A, Olsson AK. Tumor-induced local and systemic impact on blood vessel function. Mediators Inflamm 2015; 2015:418290; PMID:26770016; https://doi.org/ 10.1155/2015/418290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH et al.. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016; 8:361ra138; PMID:27798263; https://doi.org/ 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]