ABSTRACT
Chordoma is a rare tumor of notochordal origin, currently principally treated by surgery and/or irradiation. Here, we describe the clinical outcome of 3 consecutive patients with metastatic and locally advanced chordoma, treated with different immunotherapeutic approaches. All patients presented fast growing tumors and failure of standard therapies. One was treated with a tumor-based vaccine, the 2 others with anti-PD1 antibodies, all with impressive clinical and radiological responses. We therefore propose that chordoma is an immunogenic tumor and thus that translational and clinical research is necessary to develop rationally designed immunotherapy approaches.
KEYWORDS: chordoma, brachyury, immunotherapy, immune checkpoint inhibitors, melanoma antigens, neoantigen, tumor vaccine
Introduction
Arising from the embryonic notochord, chordoma is a rare tumor most commonly localized in the clivus, sacrum and spine. Chordoma is characterized by its slow growth, however, local invasiveness with spread to the surrounding tissues can occur. Metastasis is a rare event, occurring mainly in lymph nodes (LNs), lungs, liver and bones. Surgery is the cornerstone of chordoma treatment, the goal being en bloc resection. Radiotherapy, including proton therapy, is a valuable alternative for unresectable tumors or after incomplete surgery. Chemotherapy is considered ineffective and therapies targeting recently identified oncogenic pathways have not met expectations.1,2 No treatment is approved for local relapse after irradiation or for metastatic disease. Here, we describe 3 clinical cases that reveal the previously unsuspected potential of immunotherapy for chordoma.
Case description
Case 1
A 67-year-old female patient was diagnosed in 2004 with a C3 vertebral conventional chordoma, with classical epithelioid syncytial features (Fig. 1A). Immunohistochemistry (IHC) showed strongly positive nuclear brachyury expression throughout the tumor (Fig. 1B) as well as strong and diffuse cytoplasmic positivity for EGFR, weak positivity for CD117 and nuclear positivity for p53 in 30 to 50% of the nuclei (not shown). The initial tumor was partially resected with anterior C3 corpectomy with iliac bone graft reconstruction. The patient was then operated twice every year for local relapses that gradually invaded C2 and C4. In January 2007, she received local irradiation (46 Gy), followed by additional proton therapy (74 Gy). In January 2009, after a relapse-free period of 2 years, imaging showed right cervical LN metastases, which were surgically removed. Multiple progressions were then sequentially treated with imatinib (September 2009), right cervical LN dissection (January 2010), everolimus (November 2011), and erlotinib combined with cetuximab (April to July 2012), the latter allowing disease stabilization for 4 months. Multiple new metastases appeared in the parotid gland, paralaryngeal area, left cervical LNs, and subhyoid bone over the next 3 years, all treated by local irradiation or surgery. Pazopanib was introduced in June 2014 for 6 months, followed by local radiotherapy of an intra-orbital mass. In February 2015, a major change in tumor behavior was observed with the rapid onset of multiple large-sized (2 to 5 cm) subcutaneous metastases (scalp, thorax, left thigh). Considering the tumor kinetics, a sarcoma chemotherapy regimen was chosen. Four cycles of doxorubicin and ifosfamide were administered, leading to a dissociated response with total disappearance of some metastases. No limiting toxicity was identified with these previously mentioned therapies, which were all stopped due to disease progression. Unexpectedly, concomitant vitiligo was observed, suggesting a shared immune response to both tumor cells and melanocytes. In this context, we hypothesized that this immune response could be amplified using an immune checkpoint inhibitor (ICI). At that time, the patient had a painful submandibular bulk lesion and a complete palsy of the right facial nerve caused by tumor infiltration (Fig. 1C). Pembrolizumab 200 mg (compassionate use) was introduced in early November 2015, leading to a rapid clinical improvement within 6 weeks, shrinkage of the tumor bulk, and recovery of the facial palsy (Fig. 1D). The favorable outcome was confirmed by MRI assessment (Fig. 1E). The disease was controlled for 6 months with concurrent vitiligo expansion.
Figure 1.
Clinical course of patient 1. A: HE staining (400x). B: IHC staining for the brachyury protein (DAB, 400x). C: Baseline clinical features and D: resolution of the right facial palsy and shrinkage of the tumor located in the jaw after 6 cycles of pembrolizumab. E: T2 weighted MRI obtained before immunotherapy and during follow-up. Note the multilobulated multifocal tumor (dashed yellow contours) at the floor of the mouth and in the C2 vertebral body (solid yellow contours). F: PD-L1 staining before (left panel) and after (right panel) pembrolizumab initiation (DAB, 400x). G: PD1 staining before (left panel) and after (right panel) pembrolizumab initiation (DAB, 400x).
Case 2
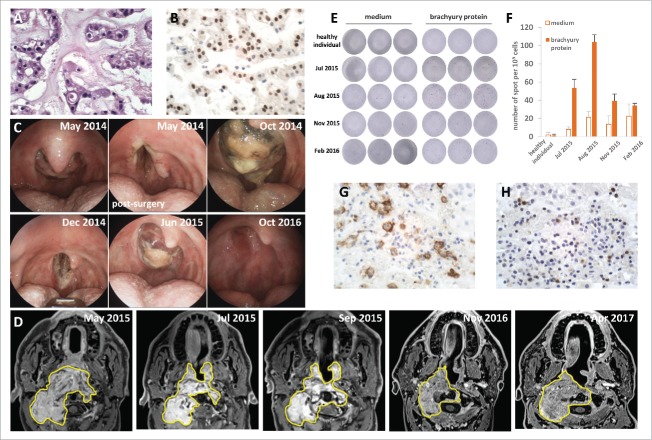
A 49-year-old male patient was diagnosed in August 2010 with clival chordoma. The chondroid subtype was suggested by the clinical presentation and the presence of hyalinized extracellular matrix (Fig. 2A). A subtotal resection was performed, immediately followed by adjuvant radiotherapy (74 Gy). Nuclear staining for brachyury (Fig. 2B) and membrane and cytoplasmic staining for CD117 (not shown) and S100 (not shown) was observed. After an event-free period of 2 years, multiple local progressions occurred, starting December 2012, all treated by surgery at increasingly shorter intervals. Imatinib was administered early 2014 with no clinical benefit. Surgery was performed again on November 2014. A subsequent progression in July 2015 was not suitable for surgery due to previous interventions and proximity of vascular structures. At that time, a small biopsy was performed and the patient was included in a pilot study evaluating the efficacy of MVX-ONCO-1 (NCT02193503), a personalized cell-based cancer immunotherapy combining the subcutaneous implantation of irradiated autologous tumor cells and biocompatible capsules containing allogeneic cells genetically engineered to release standardized GM-CSF over 7 d. This patient was the only chordoma patient included in this pilot study. The immunization was repeated 5 times over a 9-week period. Treatment was completed in September 2015 and no maintenance treatment was planned in the protocol.3-5 The patient provided the investigators with informed consent before entering the study. No systemic toxicity was observed. The patient developed a positive delayed type hypersensitivity reaction to his autologous tumor cells upon immunization. Endoscopic control performed 2 months after immunization showed not only tumor control in a previously progressing tumor with high growth kinetics but also complete disappearance of the mucosal involvement with no relapse after 19 months of follow-up (Fig. 2C). Serial MRIs of the facial bones showed a long-term reduction of the remaining tumor mass (Fig. 2D).
Figure 2.
Clinical course and brachyury-specific T cell response of patient 2. A: HE staining, (400x), B: IHC staining for the brachyury protein (DAB, 400x). C: endoscopic pictures at different time points during the course of the patient's disease. From left to right and top to bottom: May 2014, 4th relapse; May 2014, post-surgical debulking; October 2014, 5th relapse; December 2014, post-surgical debulking; June 2015, 6th relapse before biopsy; October 2016, no detectable clinical relapse 12 months after the end of the vaccination protocol. D: Contrast-enhanced T1 weighted MRI performed, from left to right and top to bottom: May 2015, before immunotherapy and surgery; July 2015, immediately after surgical biopsy and before immunotherapy; September 2015 during immunotherapy; and November 2016 and April 2017 after immunotherapy. Note a large tumor mass (yellow contours) leading to near total obstruction of the pharynx with invasion of the C2 vertebral body and the paravertebral musculature. Note the reduction of the intrapharyngeal mass after biopsy and the long-term stability of the remaining tumor mass. E and F: IFN- γ secretion after stimulation with brachyury protein or medium (negative control) analyzed by ELISPOT (E) and quantified (F). Total PBMC (105 per well) from a healthy individual (negative control) and from patient 2 at 4 different time points (July 2015: before vaccination, August 2015: during vaccination, November 2015: 2 months after end of vaccination, February 2016: 5 months after end of vaccination) were incubated for 24 h in the presence of purified brachyury protein (1 μg, Acris). A 4-fold increase in IFN-γ secretion in response to stimulation with brachyury protein as compared with medium can be observed in July and August 2015. IHC staining for PD-L1 (G) and PD1 (H) before MVX-ONCO-1 therapy initiation (DAB, 400x).
Case 3
A 47-year-old female patient was diagnosed in 2000 with a chondroid, petro-clival chordoma. Besides the histological features (presence of physaliferous cells, Fig. 3A, S100 and cytokeratin positivity, not shown) and clinical presentation that are typical of skull base chordoma, IHC showed strong nuclear positivity for brachyury (Fig. 3C), which gradually became negative with tumor dedifferentiation (Fig. 3B and D). The tumor was subtotally resected, and the patient received additional adjuvant proton therapy. A second partial resection was performed in November 2013 and imatinib was administered during 4 months, but was interrupted because of interstitial pneumonia. A third surgery was attempted in July 2014, but follow-up imaging showed massive skull base invasion including the basilar and left carotid arteries. From December 2014 to March 2016, tumor growth was stabilized with pazopanib that had to be stopped due to progression and onset of multiple transient ischemic attacks. Nivolumab 3 mg/kg (compassionate use) was started in April 2016 with a rapid major clinical improvement (resolution of headache, no recurrent ischemic stroke). This very favorable outcome was confirmed by the radiological response depicted in Fig. 3E. The clinical response lasted for 9 months until progression in January 2017, leading to discontinuation of nivolumab.
Figure 3.
Clinical course of patient 3. A: HE staining at diagnosis (400x, May 2000). B: HE staining after dedifferentiation (July 2014), C: IHC staining for the brachyury protein at diagnosis (DAB 400x, May 2000). D: IHC staining for the brachyury protein after dedifferentiation (DAB 400x, July 2014). E: Contrast-enhanced T1 weighted MRI images obtained before (April 2016) and during (May, July and October 2016) immunotherapy. Note the large tumor (contour) with extensive skull base invasion and encasement of the carotid arteries and cavernous sinuses. Carotid arteries are indicated by arrows. (F) IHC staining for PD-L1 (DAB 400x, July 2014) and (G) PD1 (DAB 400x, July 2014).
Molecular and immunological analysis
To elucidate the mechanisms underlying the responses observed in our patients, we undertook several exploratory analyses.
Immune response against common melanoma antigens
We first focused on patient 1, for whom vitiligo suggested a cross-reactive immune response against host melanocytes induced by the release of tumor antigens after chemotherapy-mediated tumor cell killing. Given the close embryological origin of the notochord and skin, some epitopes are likely to be shared by chordoma cells and melanocytes, and expression of melanoma-associated antigens by chordoma has indeed been reported.6 Thus, we investigated whether peripheral CD8+ T cells displayed specificity for common melanoma antigens (Melan-A, MAGE-A1, A3, A4, NY-ESO-1, gp100, tyrosinase and TRP2). ELISPOT analysis of overlapping long peptides covering these antigens did not show any IFN-γ secretion, suggesting that other epitopes are the target of this complex immune reaction.
Mutational load assessment
As described for various immunogenic tumors, response to ICI positively correlates with the tumor mutational load, clinical benefit being mediated by the enhancement of immune responses to neoantigens.7-9 Thus, mutation rate in the 3 chordoma samples was explored by sequencing genes involved in tumorigenicity (Ion AmpliSeqTM Comprehensive Cancer Panel). No mutation was found among the selected panel of 400 genes, suggesting a very low mutational load in the tested samples,10,11 and challenging the concept of neoepitopes as an essential prerequisite for efficient immunotherapy.
Immune response to the brachyury antigen
We observed an intense nuclear brachyury staining in all 3 patients, which disappeared after dedifferentiation in patient 3. This prompted us to investigate T cell responses to the brachyury protein. A brachyury-specific T cell response was detectable by ELISPOT at 2 different time points for patient 2 (Fig. 2E and F). This alone does not explain the favorable clinical outcome, but is probably a surrogate marker of a complex immune response. Brachyury-specific T cell responses were not detected in patient 1, whereas patient 3 could not be tested due to unavailability of PBMC.
PD1/PD-L1 status
Upon progression in April 2016, a subcutaneous metastasis was analyzed in patient 1, showing strong upregulation of PD-L1 (Fig. 1F) while PD1 was negative before and after immunotherapy (Fig. 1G). PD-L1/PD1 staining before therapy in patient 2 (Fig. 2G and H, respectively) and 3 (Fig. 3F and G, respectively) showed negativity to low expression of PD1 on both patients and low to important patchy expression for PD-L1.
Discussion
At first sight, chordoma is not a promising candidate for immunotherapy, considering the abundance of extracellular matrix and the low mutational burden,12 a commonly accepted criterion suggesting limited tumor immunogenicity.9 However, as extensively described in the context of melanoma,13 the rapid onset of vitiligo after chemotherapy for patient 1 was suggestive of a cross-reactive immune reaction to both melanocytes and tumor cells. To our knowledge such an autoreactive cutaneous immune reaction has not been reported in chordoma patients with or without metastatic skin lesions, in itself a relatively rare clinical presentation. This observation triggered the hypothesis that this chemotherapy-induced immune response could be boosted using an ICI, indeed resulting in a major clinical benefit for the patient. To strengthen the hypothesis that chordoma could potentially be responsive to immunotherapy, 2 additional consecutive chordoma patients were exposed to immune manipulation using either ICI or a tumor-based vaccine delivered in the context of a clinical trial. This resulted in a dramatic clinical benefit for both patients, without apparition of vitiligo, suggesting that the latter is not a pre-requisite for the efficacy of immunotherapy in chordoma.
To get insight into the mechanisms mediating these clinical responses, we investigated both cross-reactivity with melanoma antigens and tumor mutational load, neither of which provided an explanation for the effectiveness of ICI therapy. The detection of a brachyury-specific T cell response at 2 different time points for patient 2, although not explaining by itself the major clinical effect observed, suggests that immune responses to tumor antigens can be elicited in chordoma. Brachyury, a transcription factor expressed by many solid cancers including chordoma,14,15 is indeed being targeted in vaccination trials in chordoma patients16 that might be worth combining with ICI. The nature of the immune response mediating the impressive clinical effects observed in our patients needs to be further investigated.
Recent studies revealed the expression of PD1 and PD-L1 by a fraction of chordoma tumor cells and infiltrating lymphocytes, with preliminary data suggesting prognostic significance.17-19 The expression of PD-L1 is a dynamic and transient process in reaction to an inflammatory state,20 and its relation to ICI checkpoint responses is an area of active investigation.21 PD-L1 expression is associated with clinical response to PD1 blockade in certain solid tumors.22 However, of the 2 patients treated with anti-PD1 antibodies, only patient 1 presented a high PD-L1 expression, both patients however responding to treatment. Thus, PD1/PD-L1 expression is probably not a definitive predictor of the efficacy of immunotherapy in chordoma.
Conclusions
Further research is urgently warranted to elucidate how the outcome of chordoma could be improved using immunotherapy. Several critical issues are unresolved such as the biologic background of the abundant extracellular matrix, the steps of antigen presentation or the identification of chordoma epitopes. Meanwhile, based on the clinical observations reported here, we advocate the immediate start of clinical trials using nonspecific immune stimulation with checkpoint inhibitors, personalized cell-based immunization, or both. These results will clearly open new avenues for the therapeutic intervention of locally advanced and metastatic chordoma.
Disclosure of potential conflicts of interest
NM is founder and CSO of MaxiVAX SA; MaxiVAX SA is the sponsor of the clinical trial NCT02193503. The other authors report no conflict of interest.
Acknowledgements
Research in the laboratory of tumor immunology is supported by l'Association Marietta, le Fond Lionel Perrier, l'Association Frederic Fellay, Fond'action and la Ligue genevoise contre le Cancer.
Ethical consideration
All patients provided their inform consent to report their case description.
Author contributions
DM, NM and PYD formulated the hypothesis; DM, NM, BNL and PYD were responsible for treatment and follow-up; RV performed ELISPOT assays; DA performed IHC staining; MB provided radiological analysis; TMK performed molecular biology analysis; DM, VD and PYD drafted the manuscript. All authors reviewed and provided comments on the manuscript.
References
- 1.Lebellec L, Aubert S, Zaïri F, Ryckewaert T, Chauffert B, Penel N. Molecular targeted therapies in advanced or metastatic chordoma patients: Facts and hypotheses. Crit Rev Oncol Hematol 2015; 95(1):125-31; PMID:25682222; https://doi.org/ 10.1016/j.critrevonc.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 2.Stacchiotti S, Gronchi A, Fossati P, Akiyama T, Alapetite C, Baumann M, Blay JY, Bolle S, Boriani S, Bruzzi P, et al.. Best practices for the Management of Local-regional Recurrent Chordoma. A Position Paper by the Chordoma Global Consensus Group. Ann Oncol 2017; 28(6):1230-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lathuilière A, Mach N, Schneider B. Encapsulated Cellular Implants for Recombinant Protein Delivery and Therapeutic Modulation of the Immune System. Int J Mol Sci 2015; 16(5):10578; PMID:26006227; https://doi.org/ 10.3390/ijms160510578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mach N, Vernet R, Belkouch MC, Luy P, Ancrenaz V, Teta P, Blazek N, Grandjean N, Wasem J, Grogg J, et al.. MVX-ONCO-1 phase 1 final results of the first personalized cell-based immunotherapy using cell encapsulation technology. Ann Oncol 2016; 27(suppl_6):1058P-1058P; https://doi.org/ 10.1093/annonc/mdw378.12 [DOI] [Google Scholar]
- 5.Migliorini D, Vernet R, Belkouch MC, Luy P, Blaser S, Ancrenaz V, Blazek N, Grandjean N, Wasem J, Janin B, et al.. MVX-ONCO-1: First in man, Phase I clinical trial combining encapsulation cell technology and irradiated autologous tumor cells for personalized cell-based immunotherapy. Safety, feasibility and clinical outcome results. Eur J Cancer 51(suppl_3):S114; https://doi.org/ 10.1016/S0959-8049(16)30332-X [DOI] [Google Scholar]
- 6.Schwab JH, Boland PJ, Agaram NP, Socci ND, Guo T, O'Toole GC, Wang X, Ostroumov E, Hunter CJ, Block JA, et al.. Chordoma and chondrosarcoma gene profile: implications for immunotherapy. Cancer Immunol Immunother 2009; 58(3):339-49; PMID:18641983; https://doi.org/ 10.1007/s00262-008-0557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al.. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372(26):2509-20; https://doi.org/ 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015; 348(6230):124-128; PMID:25765070; https://doi.org/ 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al.. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371(23):2189-2199; https://doi.org/ 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa R, Carneiro BA, Agulnik M, Rademaker AW, Pai SG, Villaflor VM, Cristofanilli M, Sosman JA, Giles FJ. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget 2017; 8(5):8910-8920; PMID:27852042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, Yaeger R, Segal NH, Varghese AM, Reidy-Lagunes DL, et al.. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J Clin Oncol 2016; 34(18):2141-2147; PMID:27022117; https://doi.org/ 10.1200/JCO.2015.65.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zehir A, Nafa K, Zhou NY, Berger MF, Casanova J, Sadowska J, Lu C, Allis CD, Gounder M, et al.. Genomic Aberrations Frequently Alter Chromatin Regulatory Genes in Chordoma. Gene Chromosome Canc 2016; 55(7):591-600; https://doi.org/ 10.1002/gcc.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et al.. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016; 152(1):45-51; PMID:26501224; https://doi.org/ 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
- 14.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest 2010; 120(2):533-544; PMID:20071775; https://doi.org/ 10.1172/JCI38379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, Costarelli L, Litzinger M, Hamilton D, Huang B, et al.. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res 2012; 18(14):3868-3879; PMID:22611028; https://doi.org/ 10.1158/1078-0432.CCR-11-3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heery CR, Singh BH, Rauckhorst M, Marte JL, Donahue RN, Grenga I, Rodell TC, Dahut W, Arlen PM, Madan RA, et al.. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol Res 2015; 3(11):1248-1256; PMID:26130065; https://doi.org/ 10.1158/2326-6066.CIR-15-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Shen J, Gao Y, Liao Y, Cote G, Choy E, Chebib I, Mankin H, Hornicek F, Duan Z. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget 2015; 6(13):11139-11149; PMID:25871477; https://doi.org/ 10.18632/oncotarget.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou MX, Peng AB, Lv GH, Wang XB, Li J, She XL, Jiang Y. Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am J Transl Res 2016; 8(7):3274-3287; PMID:27508049 [PMC free article] [PubMed] [Google Scholar]
- 19.Mathios D, Ruzevick J, Jackson CM, Xu H, Shah S, Taube JM, Burger PC, McCarthy EF, Quinones-Hinojosa A, Pardoll DM, et al.. PD-1, PD-L1, PD-L2 expression in the chordoma microenvironment. J Neurooncol 2015; 121(2):251-259; PMID:25349132; https://doi.org/ 10.1007/s11060-014-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discovery 2015; 5(9):915-919; PMID:26272491; https://doi.org/ 10.1158/2159-8290.CD-15-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. ClinCancer Res 2014; 20(19):5064-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515(7528):568-571; PMID:25428505; https://doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]