ABSTRACT
Background. Tumor necrosis as well as tumor-associated macrophages (TAMs) in the tumor invasive front (TIF) have been suggested to have a prognostic value in selected solid tumors, inclusive hilar cholangiocarcinoma. However, little is known regarding their influence on tumor progression and prognosis in intrahepatic cholangiocarcinoma (iCC).
Methods. We analyzed surgically resected tumor specimens of human iCC (n = 88) for distribution and localization of TAMs, as defined by expression of CD68, formation of necrosis and extent of peritumoral fibrosis. Abundance of TAMs, tumor necrosis and grade of fibrosis were assessed immunohistochemically and histologically and correlated with clinicopathological characteristics, tumor recurrence and patients' survival. Statistical analysis was performed using SPSS software.
Results. Patients with tumors characterized by low levels of TAMs in TIF or necrosis showed a significantly decreased 1-, 3- and 5-y recurrence-free survival and a significantly decreased overall survival, when compared with patients with tumors showing high levels of TAMs in TIF or no necrosis. Patients with high density of TAMs in TIF showed significantly lower incidence of tumor recurrence, as well (p < 0.05). Absence of tumor necrosis and TAMs in TIF were confirmed as independent prognostic variables in the multivariate survival analysis (all p < 0.05).
Conclusions. High levels of TAMs in TIF or absence of histologic tumor necrosis are associated with a significantly improved recurrence-free and overall survival of patients with iCC. These results suggest TAMs and necrosis as valuable prognostic markers in routine histopathologic evaluation, and might indicate more individualized therapeutic strategies.
KEYWORDS: Intrahepatic cholangiocarcinoma, Tumor associated macrophages, TAMs, CD68, tumor necrosis, fibrosis, prognosis, patient outcome
Abbreviations
- HCC
hepatocellular carcinoma
- HC
hilar cholangiocarcinoma
- H&E
hematoxylin and eosin
- iCC
intrahepatic cholangiocarcinoma
- mAb
monoclonal antibody
- PDGRF
platelet-derived growth factor receptor
- TBS
tris-buffered saline
- TAMs
tumor-associated macrophages
- TCA
tumor central area
- TIF
tumor-infiltrating front
- TME
tumor microenvironment
- TT
tumorous tissue
- UICC
Union for International Cancer Control
- VEGF
vascular endothelial growth factor
Introduction
All classes of leukocytes can be detected within malignant tumors. Tumor-associated macrophages (TAMs) constitute up to 50% of this leukocyte cell population. TAMs influence tumor progression in various ways that depend on differences in tumor microenvironment (TME), histology and TAMs' polarization state.1 TAMs can be divided schematically into two phenotypes, M1 and M2, according to their effects on tumor. M1 polarization promotes inflammation and suppresses tumor progression by producing pro-inflammatory and antitumoral cytokines. In contrast, M2 state suppresses inflammation and antitumor immune response, resulting in the promotion of tumor progression.2,3 TME-tailored immunologic phenotype of infiltrating immune cells mediates an impaired tumor surveillance that facilitates tumor progression. It is TME, tumor-derived factors and escape mechanisms that foster tumor growth, neovascularization and metastasis. However, a constant interplay between TME, infiltrating immune cells and their polarization state plays a key role in this process.
TME can shape and reeducate the infiltrating immunologic components into a pro-tumoral phenotype. However, novel experimental research highlights a differential influence and functionality of localized tumor sites, i.e., tumor central area (TCA) and tumor invasive front (TIF), on related immunologic responses.4,5 TAMs localized in TCA or TIF can impact tumor progression in opposite manner.6 High abundance of TAMs is associated with an unfavorable prognosis in hepatocellular carcinoma (HCC), esophageal, ovarian and breast cancer.7-12 In contrast, M1-polarized TAMs suppress tumor progression in non-small cell lung cancer.13
There is emerging data linking hepatic macrophage heterogeneity to formation of histologic tumor necrosis and peritumoral fibrosis.14-18 Necrosis is associated with tumor recurrence in different tumor entities. Consequently, tumor necrosis has been included in pathological classifications and prognostic indices in primary solid organ malignancies.19,20 The formation of peritumoral fibrosis has been shown to impact patient outcome and prognosis of several inflammatory and haematopoietic disorders, as well.21,22
We have previously shown that formation of necrosis and TAMs in TIF impact patient survival and prognosis in hilar cholangiocarcinoma (HC).23 Moreover, TAMs association to formation of tumor necrosis is considerable in HC.24 However, the importance of TAMs and tumor necrosis remains uncertain in iCC. Scarce vascularization of this tumor entity may facilitate hypoxic conditions, and thus formation of necrosis might well characterize subsets of iCC, as well.25,26 The aim of this study was therefore to evaluate the relationship and potential association of infiltrating TAMs, tumor necrosis and peritumoral fibrosis with tumor growth, metastasis, recurrence and clinical prognosis in iCC.
Results
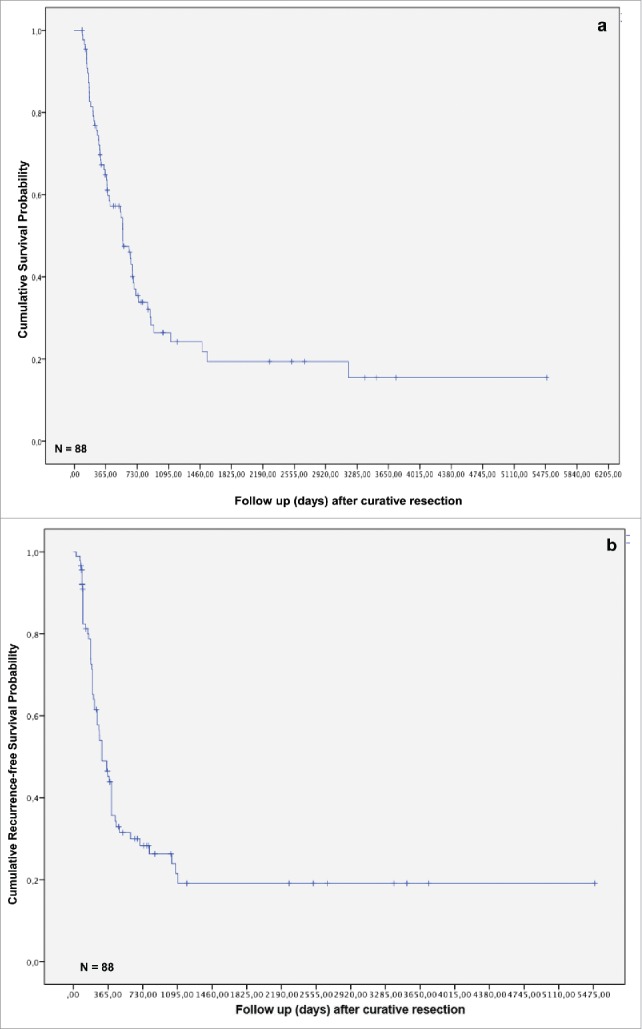
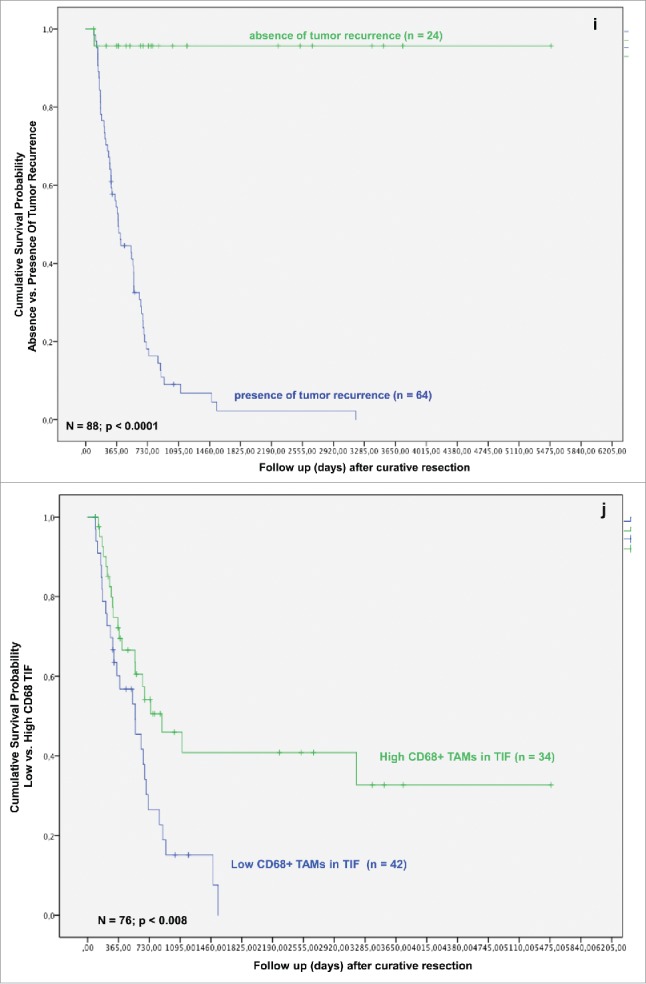
One-, three- and five-year overall and recurrence-free survival of our cohort was 61.0%, 27.2% and 18.8%, and 46.8%, 22.7% and 18.6%, respectively (Figs. 1A and B). Sixty (67.4%) of 88 patients died within the follow-up interval. Sixty-four patients (71.9%) developed tumor recurrence and 55 (61.8%) patients died for recurrence of iCC. Local tumor recurrence was seen in 19 (21.3%) patients, while 2 (2.2%) patients developed distant metastases without manifestation of local recurrence (Table 1). None of the patients with local recurrence had a metastatic spread of the tumor.
Figure 1.
(Continued)
Figure 1.
(Continued)
Figure 1.
Overall survival and recurrence-free survival of the patients included in the study.
Figure 1.
(Continued)
Table 1.
Clinicopathological characteristics of the patients included in the study.
| Clinicopathological characteristics |
||
|---|---|---|
| Variable | Value (%) | |
| No. of patients | 88 | |
| Gender | ||
| Male | 46 (52.3%) | |
| Female | 42 (47.7%) | |
| Patient age | ||
| ≤ 60 | 46 (52.3%) | |
| >60 | 42 (47.7%) | |
| Tumor nodules | ||
| Solitary | 30 (35.8%) | |
| Multiple | 58 (64.2%) | |
| Tumor diameter | ||
| Small | 28 (31.8%) | |
| Large | 60 (68.2%) | |
| Bilobular hepatic tumor involvement | ||
| Yes | 52 (59.1%) | |
| No | 36 (40.9%) | |
| Pathologic R-Category | ||
| R0 | 48 (54.5%) | |
| R1/R2 | 40 (45.5%) | |
| Distant metastases | ||
| With | 2 (2.2%) | |
| Without | 86 (97.8%) | |
| Angionvasion | ||
| Positive | 5 (5.6%) | |
| Negative | 83 (94.4%) | |
| Lymphangiosis carcinomatosa | ||
| Positive | 38 (43.8%) | |
| Negative | 50 (56.2%) | |
| Perineural sheath infiltration | ||
| Positive | 24 (28.1%) | |
| Negative | 64 (71.9%) | |
| Histologic differentiation | ||
| Well | 62 (70.1%) | |
| Moderate/Poor | 26 (29.9%) | |
| Pathologic T stage | ||
| T1/T2 | 35 (39.3%) | |
| T3/T4 | 53 (60.7%) | |
| Pathologic N stage | ||
| Positive | 34 (38.6%) | |
| Negative | 54 (61.4%) | |
| Tumor recurrence | ||
| With | 62 (70.8%) | |
| Without | 26 (29.2%) | |
| Local recurrence | ||
| With | 18 (20.2%) | |
| Without | 70 (79.8%) | |
Macrophage distribution in intrahepatic cholangiocarcinoma
TAMs showed a homogenous infiltration pattern in TCA and TIF (Fig. 2A). The majority of TAMs were located in direct contact to tumor cells. Interestingly, a high density of TAMs was observed at TIF in 42 patients (TIF score 2/high density/positive), while rest of tumor specimens (n = 34) displayed only a scarce or complete absence of infiltrating TAMs (TIF scores 0 and 1/low density/negative) (Fig. 2B).
Figure 2.
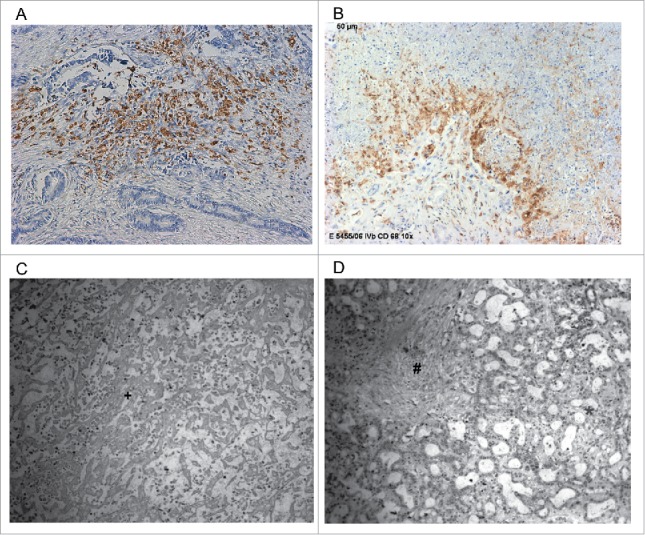
(A) Intrahepatic cholangiocarcinoma stained with mAb CD68 PG-M1 with a high abundance of TAMs in tumor central area (TCA). Original magnification: x 200. (B) Intrahepatic cholangiocarcinoma stained with mAb CD68 PG-M1 with a high abundance of TAMs in tumor-infiltrating front (TIF). Original magnification: x 200. (C, D) Histological tumor specimen with (C) necrosis and (D) fibrosis. *Vital tumor cell formations, +Tumor necrosis, #Tumor fibrosis.
Tumor-infiltrating macrophages associate with pathological parameters and tumor recurrence
Patients with iCC were divided into several groups either by “low” or “high” density of macrophages in TCAs (“TumorCD68-low group”; n = 50) and “TumorCD68-high group”; n = 38, respectively), or “low” or “high” density of TAMs in TIF (“Invasive frontCD68-low group” n = 34; and “Invasive frontCD68-high group”, n = 42, respectively).
High frequency of TAMs in TIF correlated with reduced incidence of lymphangiosis carcinomatosa (Table 2). Thirty (71.4%) patients in the Invasive frontCD68-high group did not have lymphangiosis carcinomatosa, whereas in 23 (67.6%) patients in the Invasive frontCD68-low group presence of lymphangiosis carcinomatosa was detected (p = 0.001). Thirty-four (81.0%) patients in the Invasive frontCD68-high group had a well- differentiated tumor (G1), when compared with 20 (58.8%) patients in the Invasive frontCD68-low group (p = 0.021). Similarly, 23 (81.3%) patients in the Invasive frontCD68-low group displayed an advanced T stage, when compared with 18 (43.9%) cases in the Invasive frontCD68-high group (p = 0.001).
Table 2.
Correlation of CD68-positiveTAMs in the tumor invasive front with clinicopathological characteristics of intrahepatic cholangiocarcinoma.
| Clinicopathological analysis | |||
|---|---|---|---|
| Variable | high CD68 | low CD68 | p |
| No. of patients | 42 | 34 | |
| Patient age, years | 0.356 ≤ 60 | 19 (45.2%) | 19 (55.9%) |
| >60 | 23 (54.8%) | 15 (44.1%) | |
| Gender | 0.951 | ||
| Male | 17 (40.5%) | 14 (41.2%) | |
| Female | 25 (59.5%) | 20 (58.8%) | |
| Tumor nodules | 0.062 | ||
| Solitary | 9 (21.4%) | 14 (41.2%) | |
| Multiple | 33 (78.6%) | 20 (58.8%) | |
| Tumor diameter | 0.064 | ||
| Small | 17 (40.5%) | 7 (20.6%) | |
| Large | 25 (59.5%) | 27 (79.4%) | |
| Bilobular hepatic tumor involvement | 0.101 | ||
| Yes | 11 (26.2%) | 15 (44.1%) | |
| No | 31 (73.8%) | 19 (55.9%) | |
| Distant metastases | 0.111 | ||
| With | 0 (0.00%) | 2 (5.9%) | |
| Without | 42 (100%) | 32 (94.1%) | |
| Angioinvasion | 0.197 | ||
| With | 2 (4.8%) | 0 (0.00%) | |
| Without | 40 (95.2%) | 34 (100%) | |
| Lymphangiosis carcinomatosa | 0.001 | ||
| Positive | 12 (28.6%) | 23 (67.6%) | |
| Negative | 30 (71.4%) | 11 (32.4%) | |
| Perineural sheath infiltration | 0.581 | ||
| Positive | 10 (23.8%) | 10 (29.4%) | |
| Negative | 32 (76.2%) | 24 (70.6%) | |
| Histological differentiation | 0.021 | ||
| Well/moderate | 34 (82.9%) | 20 (58.8%) | |
| Poor | 7 (17.1%) | 14 (41.2%) | |
| Pathological T stage | 0.001 | ||
| T1/T2 | 23 (56.1%) | 6 (18.8%) | |
| T3/T4 | 18 (43.9%) | 26 (81.3%) | |
| Pathological N stage | 0.744 | ||
| Positive | 15 (35.7%) | 13 (39.4%) | |
| Negative | 27 (64.3%) | 20 (60.6%) | |
| Tumor recurrence | 0.004 | ||
| With | 22 (52.4%) | 30 (88.2%) | |
| Without | 20 (47.6%) | 4 (11.8%) | |
| Local recurrence | 0.002 | ||
| With | 3 (7.1%) | 12 (35.3%) | |
| Without | 39 (92.9%) | 22 (64.7%) | |
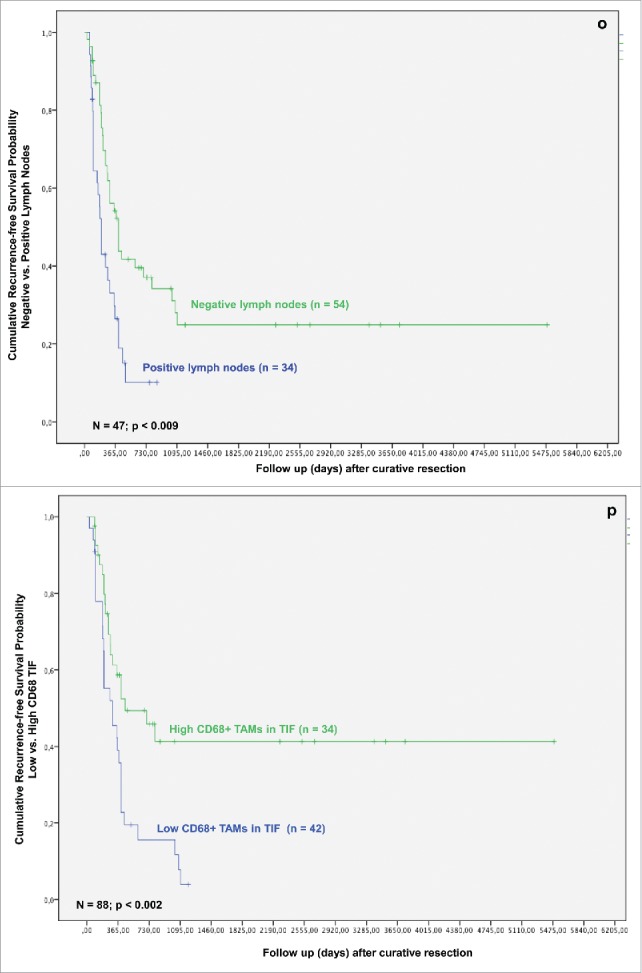
Furthermore, low abundance of TAMs in TIF correlated with a significantly increased overall tumor recurrence (Table 2). Thirty (88.2%) patients in Invasive frontCD68-low group displayed tumor recurrence (p = 0.001). In comparison, in the Invasive frontCD68-high group in only 22 (52.4%) cases a recurrent disease was apparent. Moreover, patients with high levels of TAMs in TIF showed a significantly reduced incidence of local tumor recurrence, as well. Thirty-nine (92.9%) patients in the Invasive frontCD68-high group showed a complete absence of local tumor recurrence, whereas 22 (63.7%) in the Invasive frontCD68-low group did not show local tumor recurrence (p = 0.002).
Tumor necrosis associates with angioinvasion and formation of multiple tumor nodules
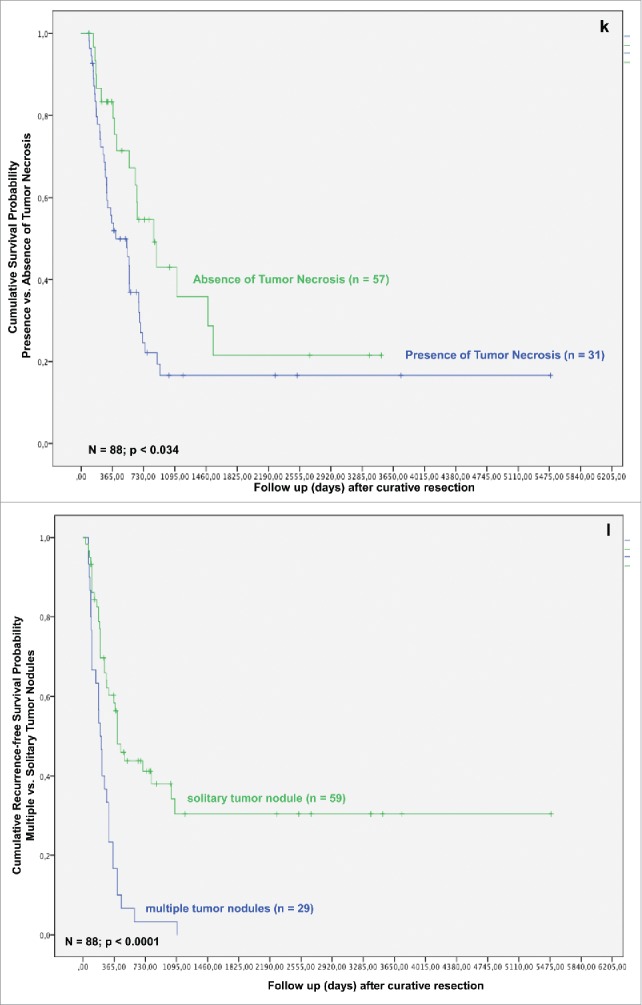
Tumor samples of 31 patients (34.8%) displayed no tumor necrosis (Necrosisnegative group), in 57 cases (64.7%), necrosis was evident (Necrosispositive group) (Fig. 2C). In the Necrosismild group, there were 34 (59.6%) patients. In comparison, the Necrosissevere group comprised 23 (40.4%) patients.
In the Necrosispositive group 4 (12.9%) patients displayed angioinvasion, whereas in the Necrosisnegative group in only one case angioinvasion was evident (p = 0.031) (Table 3). In the Necrosissevere group, 12 (52.2%) of 23 patients showed a formation of multiple tumor nodules, compared with only 9 (26.5%) cases in Necrosismild group (p = 0.048) (Table 3). The formation of histologic tumor necrosis showed a distinct trend to positively correlate with presence of TAMs in TCA or TIF, although without reaching statistical significance (p = 0.109 and p = 0.251, respectively) (Table 3). Patients with mild formation of histologic tumor necrosis showed a trend to reduced incidence of lymph node metastases, overall and local tumor recurrence (p = 0.078, p = 0.138 and p = 0.153, respectively; data not shown).
Table 3.
Correlation of histologic tumor necrosis with clinicopathological characteristics of intrahepatic cholangiocarcinoma.
| Clinicopathological analysis | |||
|---|---|---|---|
| Variable | Necrosis positive | Necrosis negative | p |
| No. of patients | 57 | 31 | |
| Patient age, years | 0.325 | ||
| ≤ 60 | 23 (40.4%) | 14 (45.2%) | |
| >60 | 34 (59.6%) | 17 (54.8%) | |
| Gender | 0.662 | ||
| Male | 17 (40.5%) | 14 (41.2%) | |
| Female | 25 (59.5%) | 20 (58.8%) | |
| Tumor nodules | 0.288 | ||
| Solitary | 25 (43.9%) | 10 (32.3%) | |
| Multiple | 32 (56.1%) | 21 (67.7%) | |
| Tumor diameter | 0.586 | ||
| Small | 17 (29.8%) | 11 (35.5%) | |
| Large | 40 (70.2%) | 20 (64.5%) | |
| Bilobular hepatic tumor involvement | 0.180 | ||
| Yes | 36 (63.2%) | 15 (48.4%) | |
| No | 21 (36.8%) | 16 (51.6%) | |
| Distant metastases | 0.658 | ||
| With | 1 (1.8%) | 1 (3.2%) | |
| Without | 56 (98.2%) | 30 (96.8%) | |
| Angioinvasion | 0.031 | ||
| With | 1 (1.8%) | 4 (12.9%) | |
| Without | 56 (98.2%) | 27 (87.1%) | |
| Lymphangiosis carcinomatosa | 0.093 | ||
| Positive | 29 (50.9%) | 10 (32.3%) | |
| Negative | 28 (49.1%) | 21 (67.7%) | |
| Perineural sheath infiltration | 0.371 | ||
| Positive | 18 (31.6%) | 7 (22.6%) | |
| Negative | 39 (68.4%) | 24 (77.4%) | |
| Histological differentiation | 0.308 | ||
| Well/moderate | 37 (67.0%) | 23 (76.7%) | |
| Poor | 20 (34.0%) | 8 (23.3%) | |
| Pathological T stage | 0.103 | ||
| T1/T2 | 19 (33.3%) | 16 (51.7%) | |
| T3/T4 | 38 (66.7%) | 15 (48.3%) | |
| Pathological N stage | 0.0.85 | ||
| Positive | 26 (45.6%) | 9 (26.7%) | |
| Negative | 31 (54.4%) | 22 (73.3%) | |
| Tumor recurrence | 0.368 | ||
| With | 42 (73.7%) | 20 (64.5%) | |
| Without | 15 (26.3%) | 11 (35.5%) | |
| Local recurrence | 0.195 | ||
| With | 14 (24.6%) | 4 (12.9%) | |
| Without | 43 (75.4%) | 27 (87.1%) | |
| CD68-positive TAMs in TCA | 0.109 | ||
| High | 29 (50.9%) | 21 (69.0%) | |
| Low | 28 (49.1%) | 10 (31.0%) | |
| CD68-positive TAMs in TIF | 0.251 | ||
| High | 25 (50.0%) | 10 (36.0%) | |
| Low | 25 (50.0%) | 16 (64.0%) | |
| Variable | Mild necrosis | Severe necrosis | p |
| No. of patients | 34 | 23 | |
| Multiple tumor nodules | 0.048 | ||
| With | 9 (26.5%) | 12 (52.2%) | |
| Without | 25 (73.5%) | 11 (47.8%) | |
Peritumoral fibrosis associates with angioinvasion and lymph node metastasis
Thirty-two specimens (36.3%) showed mild fibrosis. Strong formation of peritumoral fibrosis was apparent in 56 (63.6%) patients (Fig. 2D).
In the Fibrosisstrong group, 55 (98.9%) patients displayed absence of angioinvasion, whereas in the Fibrosismild group, only in 4 (12.8%) patients no angioinvasion was detected (p = 0.033). Interestingly, patients with strong formation of peritumoral fibrosis showed a significantly reduced incidence of lymph node involvement, as well (p = 0.037). Thirty-seven (69.8%) cases in the Fibrosisstrong group showed a complete absence of lymph node metastases, whereas 14 (46.7%) patients in the Fibrosismild group did not have positive lymph nodes.
Prognostic significance of CD68-positive macrophages and tumor necrosis in intrahepatic cholangiocarcinoma
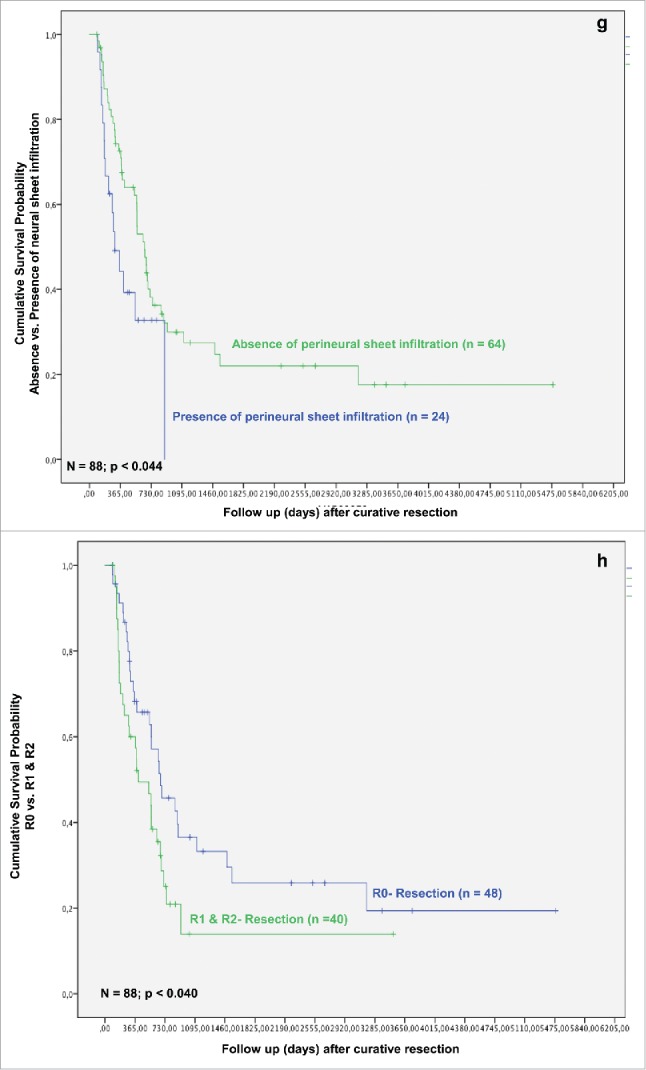
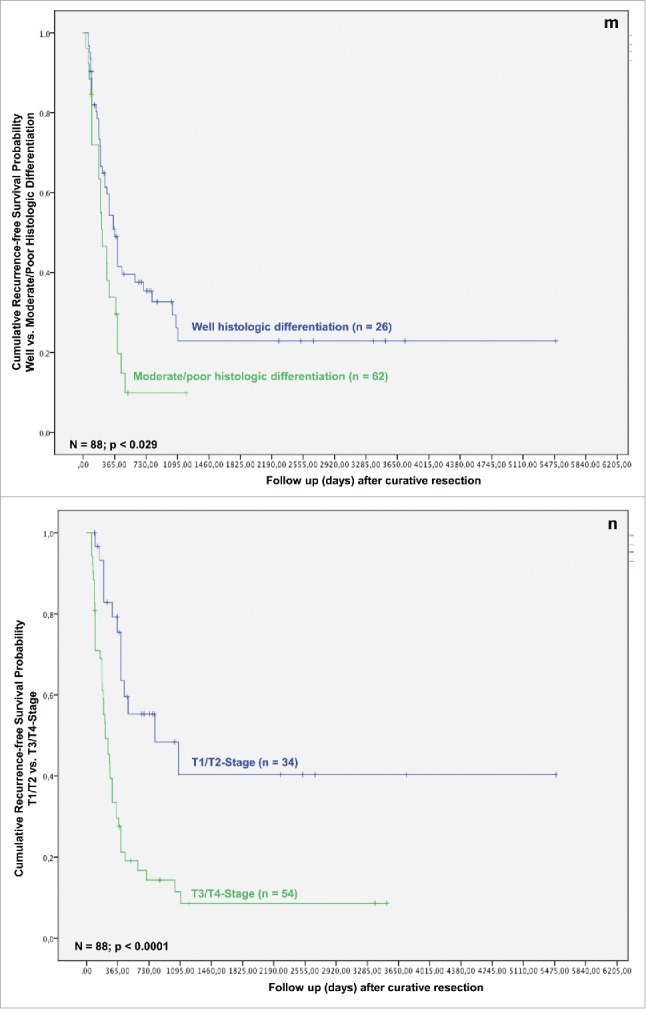
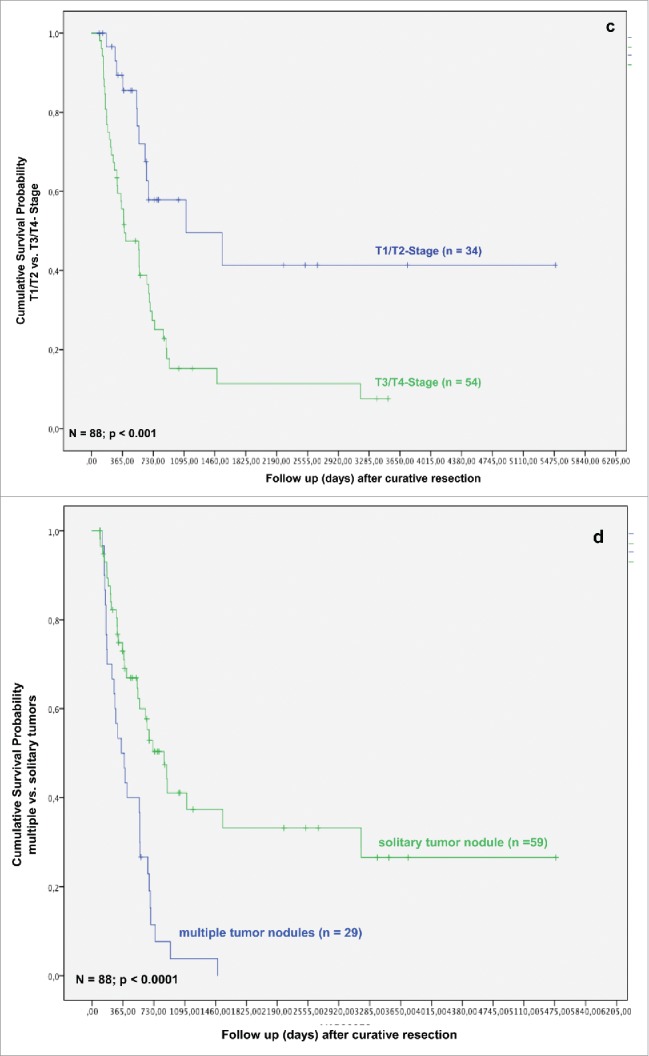
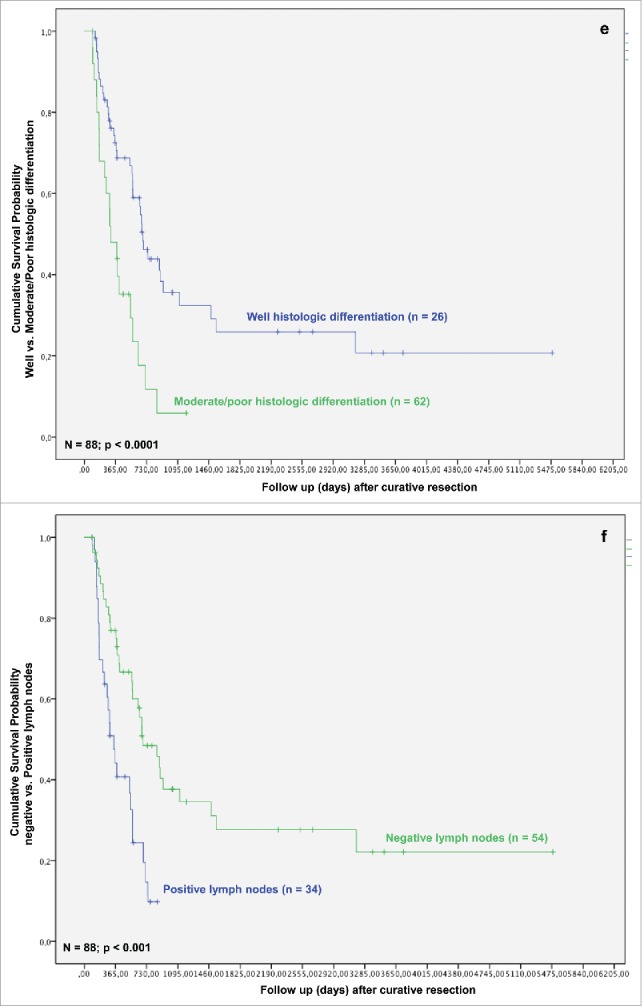
In the next step, we analyzed whether TAMs and tumor necrosis in addition to other clinicopathological parameters predict survival after resection for iCC. In the univariate analysis T-stage, multiple tumor nodules, histologic grading, nodal status, perineural sheet infiltration, R-category, overall tumor recurrence, TAMs in TIF and histologic tumor necrosis were associated with a statistically significant impact on patient survival after resection (Figs. 1C–K).
Figure 1.
(Continued)
As related to recurrence-free survival, in univariate analysis formation of multiple tumor nodules, bilobular hepatic tumor involvement, R category, histologic grading, T-stage, nodal status and TAMs in TIF were associated with a statistically significant impact (Figs. 1J–P).
Figure 1.
(Continued)
In multivariate analysis in regard of overall survival TAMs in TIF, histologic tumor necrosis, patient age, histologic grading and tumor recurrence were identified as independent prognostic factors for survival (all p < 0.05) (Table 4). When looking at recurrence-free survival, in multivariate analysis bilobular hepatic tumor involvement, perineural sheet infiltration and histologic grading exerted a significant impact on survival (all p < 0.05; Table 4). Other well-established common prognostic factors, e.g., lymph node involvement and local tumor recurrence did not exert prognostic significance in the current work.
Table 4.
Multivariate analysis of prognostic factors in patients with intrahepatic cholangiocarcinoma.
| Multivariate analysis (Overall Survival) | ||||
|---|---|---|---|---|
| Variable | Category | Odds ratio | p | Confidence interval |
| Histologic differentiation | well or moderate/ poor | 2.266 | 0.040 | 1.038–4.946 |
| T status | T1,T2/T3,T4 | 1.688 | 0.236 | 0.710–4.009 |
| Overall tumor recurrence | negative/positive | 0.011 | 0.0001 | 0.001–0.093 |
| Local tumor recurrence | negative/positive | 0.707 | 0.401 | 0.315–1.588 |
| CD68-positive TAMs in the tumor invasive front | high/low | 2.752 | 0.016 | 1.207–6.275 |
| Tumor necrosis | negative/positive | 0.244 | 0.002 | 0.100–0.596 |
| Multivariate analysis (Recurrence-free survival) | ||||
| Variable |
Category |
Odds ratio |
p |
Confidence interval |
| Tumor nodules | solitary/multiple | 0.301 | 0.001 | 0.149–0.606 |
| Perineural sheet infiltration | negative/positive | 0.370 | 0.008 | 0.178–0.772 |
| Histologic differentiation | well or moderate/ poor | 2.123 | 0.047 | 1.010–4.460 |
| Lymph node Involvement | negative/positive | 0.542 | 0.066 | 0.282–1.042 |
| CD68-positive TAMs in the tumor invasive front | high/low | 0.558 | 0.094 | 0.282–1.104 |
Influence of TAMs invasion, tumor necrosis and peritumoral fibrosis on overall and recurrence-free survival in intrahepatic cholangiocarcinoma
Based on the identification of TAMs in TIF and tumor necrosis as independent prognostic factors in both the univariate and multivariate analysis, we investigated their effect on patients' survival in iCC. The presence of TAMs in TIF affected patients' survival after resection for iCC. Univariate analysis revealed a significantly better survival in the Invasive frontCD68-high group (p = 0.003). The overall survival rates in the Invasive frontCD68-high group were 74.9%, 47.5% and 41.8% at 1-, 3- and 5-y following liver resection in comparison with 60.2%, 15.1% at 1-, 3-y following resection in the Invasive frontCD68-low group (p = 0.008) (Fig. 1J). None of the patients in the Invasive frontCD68-low group could reach 5-y survival after surgery. Moreover, a high density of CD68-positive cells in TIF correlated with a significantly improved recurrence-free survival, as well. 1-, 3- and 5-y recurrence-free survival rates in the Invasive frontCD68-high group were 57.9%, 41.6% and 41.6% compared with 38.8%, 8.7% in the Invasive frontCD68-low group, respectively (Fig. 1P). Again, none of the patients in the Invasive frontCD68-low group could reach 5-y recurrence-free survival after surgery. The differences were statistically significant (p = 0.002).
Figure 1.
(Continued)
Formation of histologic tumor necrosis affected patients' survival, as well. Univariate analysis revealed significantly better results for patients with absence of histologic tumor necrosis (p = 0.037). Kaplan–Meier method revealed that patients in the Necrosisnegative group have a significantly improved overall survival, when compared with Necrosispositive group (Fig. 1K) (p = 0.034).
Figure 1.
(Continued)
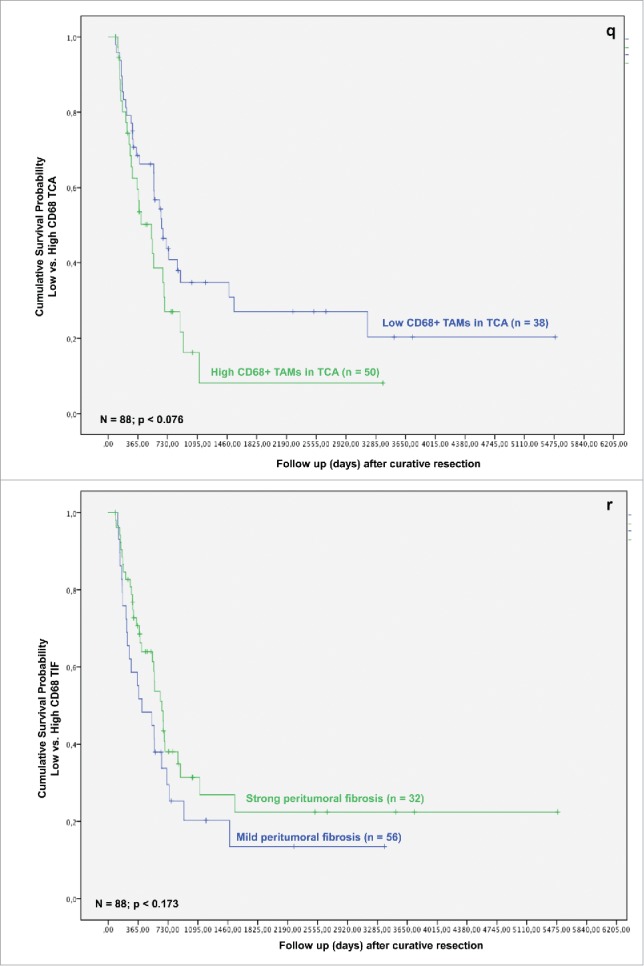
Overall and recurrence-free survival of patients with iCC were significantly better in patients with high levels of TAMs in the area of TIF and absence of tumor necrosis when compared with those with a high density of TAMs and presence of histologic necrosis in tumor. Formation of severe histologic tumor necrosis showed a trend to a reduced overall and recurrence-free survival, as well (p = 0.135 and p = 0.198, respectively; data not shown). Interestingly, low TAMs invasion in TCA and formation of strong peritumoral fibrosis showed distinct trends toward an improved overall and recurrence-free survival, though without reaching statistical significance (p = 0.076, p = 0.173 and p = 0.260, p = 0.321, respectively) (Figs. 1Q and R).
Figure 1.
(Continued)
Discussion
Analyzing immunoreactivity for TAMs, formation of tumor necrosis and peritumoral fibrosis in tumor samples from patients who underwent liver resection for iCC, we were able to show that formation of mild peritumoral fibrosis is a risk factor for lymph node involvement, low abundance of CD68 in TIF and histologic tumor necrosis are risk factors for tumor recurrence and formation of multiple tumor nodules, serve as independent prognostic factors for survival, and translate into poor overall as well as recurrence-free survival.
The significance of tumor-related immunologic responses with regard to disease progression and patient prognosis has long been recognized. In the field of solid organ transplantation, novel immunologic therapeutic and diagnostic tools have gained importance and been successful translated from bench to bedside.27,28 However, in clinical oncology only marginal practical approach on clinical daily basis has been implemented in recent years.29-31 Overwhelming experimental and clinical evidence suggests a key role of innate and adaptive immune system in tumor progression.32-34 Forasmuch, novel diagnostic and therapeutic strategies are urgently needed to tailor a personalized immunologic approach in clinical management of underlying oncological disease.
The tumor mass and microenvironment represent a multifaceted playground, where different cell types, including cancer cells, fibroblasts, endothelial, stromal and immune-competent cells are entangled into a constant interaction. Cholangiocarcinoma exhibits a desmoplastic nature, associated with a massive presence of TAMs. Cholangiocarcinoma-associated macrophages represent a poorly defined but very intriguing immune subset. Indeed, TAMs density significantly impacts prognosis, survival and development of metastasis in cholangiocarcinoma patients, suggesting a major role of macrophages in progression of this tumor entity. Additionally, TAMs polarization is influenced by both macrophage localization and TME signals. Hence, related to their localization in specific tumor areas, macrophages can exert opposing effects, either promoting or inhibiting tumor progression.2,35,36
In the present work, we hypothesized that based on TAMs localization in TCA or TIF a different impact on tumor progression and patient outcome may result. Our findings demonstrate that in a highly aggressive tumor entity like iCC, high TAMs frequencies in TIF associate with improved patient outcome. In contrast, we found that a high TAMs density in TCA reveals a strong tendency to a deteriorated survival. These results are similar to reports regarding colon, gastric, and endometrial cancers, which showed that higher densities of tumor-infiltrating macrophages in tumor-peripheral areas correlate with a favorable prognosis.37-39 Moreover, similar to our results, a high number of TAMs in tumor-central area were correlated with a worse prognosis in endometrial cancer.39
A possible explanation for these findings is that macrophage function may be altered by hypoxia and formation of necrosis, i.e., through increased expression of hypoxia-inducible factors like (HIF) 1-α and HIF2-α.40,41 Oxygen concentration and gradients vary between central and peripheral areas of solid tumors, affecting TAMs polarization state depending on their location. In particular, both HIF-2α accumulation in immune cells and hypoxia activate molecular mechanisms fostering M2 polarization, and thus promotion of tumor progression.41,42
In the present work, we furthermore propose histologic tumor necrosis as a simple diagnostic tool prognosticating patients' outcome after surgery for iCC. To date, there is only a scarce amount of data regarding the association between tumor necrosis and various clinicopathological characteristics in solid tumors. Recently published data suggest a prognostic value of histological tumor necrosis in solid organ malignant disease.19,20,43-45 Our group was the first to report on the prognostic value of histologic tumor necrosis in patients with HC.24 In the current work, tumor necrosis was associated with survival and was identified as independent prognosticator for iCC, as well.
Hepatic macrophages are central in the pathogenesis of chronic liver injury, but are crucially involved in maintaining homeostasis, as well.17 Tumor-promoting properties of TAMs may reflect, at least to some extent, normal features of resident macrophages rather than functions induced by TME.46 In most cases formation of tumor necrosis is related to creation of hypoxic conditions in aggressive and fast-growing tumors. The cause for necrosis formation in iCC and its mechanistic link to poor clinical outcome may be related to immunologic factors, such as infiltrating TAMs, because larger tumors did not associate with presence of necrosis in the current work. We found an outlined tendency to formation of histologic tumor necrosis and high TAMs densities in TCA and TIF, respectively. Moreover, these results are in correspondence with our previous findings linking macrophage density to formation of histologic tumor necrosis in patients with HC, and with novel experimental results highlighting a fundamental role of macrophages in creation of fibrosis and necrosis via death receptor, chemokine and fibrocystin/polyductin-dependent mechanisms.17,23,24,47,48
In conclusion, in the current study, we demonstrate for the first time the significance of tumor necrosis and its association with survival and patients' outcome after liver resection for iCC. Moreover, we show that presence of CD68-positive TAMs in TIF correlates with reduced tumor recurrence and serves as an independent prognostic factor for survival, as well. Thus, TAMs and formation of histological tumor necrosis may be crucially involved in the progression of this tumor entity, and their utilization as a diagnostic tool may deliver new approaches in the management of this cancer. Moreover, the current results confirm our findings in HC, which may implicate similar therapy regimes for both tumor entities.
Methods
Patients and tumor samples
A total of 88 patients who underwent major hepatectomy between January 1996 and December 2002 for iCC were included in the study. ICC was confirmed histopathologically and classified according to the Union for International Cancer Control (UICC) classification. Written-informed consent was obtained from all patients. This study was approved by the ethics committee of Charité – Universitätsmedizin Berlin (ID: 111 EA1/318/15).
In all included patients, liver surgery was performed in formally curative intent. None of the patients received neoadjuvant therapy before operation. None of the patients died in the postoperative course. Histological evaluation of all specimens was performed by an independent pathologist, without any knowledge of prognosis or clinicopathological variables.
Formalin-fixed, paraffin-embedded tumor samples with a representative sample of the tumor were retrieved from the files of the Institute of Pathology. Histological diagnosis of the primary tumor stage and nodal status were determined by hematoxylin and eosin (H&E) stained sections. The clinicopathological characteristics of the study population are depicted in Table 1.
Immunohistochemistry
All used protocols in regard to immunohistochemistry, histology, quantification of cell infiltrates, necrosis and peritumoral fibrosis have been described previously.23,24
In detail, formalin-fixed and paraffin-embedded tumor sections (5-µm thick) were dewaxed and rehydrated. Antigen retrieval was performed by heating the slides in 10 mM Tris buffer with 1 mM EDTA (pH 9) in a streamer for 20 min. Endogenous peroxidase activity was inhibited with 3% H2O2 for 5 min. After washing with Tris buffered saline (TBS) with tween, the endogenous biotin was suppressed by sequential incubations with 0.1% avidin and 0.01% biotin (Dako, Glostrup, Denmark) for 10 min each at room temperature. Additional non-specific binding sites were blocked with 3% skimmed milk powder for 30 min at room temperature. Tissue sections were incubated with the monoclonal mouse antibody anti-human CD68 Clone PG-M1 (1:50, Dako, Glostrup, Denmark) for 30 min at room temperature. The universal LSAB+ system-HRP (Dako, Glostrup, Denmark) and the DAB+ liquid substrate chromogen system (Dako, Glostrup, Denmark) was applied for the visualization of the antibody reaction. The biotinylated anti-rabbit, anti-mouse and anti-goat immunoglobulin and the streptavidin-conjugated horseradish peroxidase of the universal LSAB+ system was incubated consecutively for 20 min each. Sections were counterstained with hematoxylin. Specificity controls were performed without primary antibody.
Quantification of CD68 density
All specimens were evaluated by three independent researchers (GA, CD and IP), and an independent pathologist (KS), without any knowledge of prognosis or clinicopathological variables. TAMs were defined by their expression of CD68. For quantification of infiltrating TAMs, the whole tumor area was thoroughly investigated for presence of CD68-positive cells. The evaluation of TAMs density was performed as percentage of CD68 stained/positive cells related to total tumor cell amount.
The density of TAMs in TCA was semi-quantitatively classified into the following categories: 0, negative; 1, 1–25%; 2, 26–75%; and 3, >75%. For statistical analysis, TCA scores 0 and 1 were categorized as low density, and TCA scores 2 and 3 as high density of TAMs. The density of TAMs in TIF were semi-quantitatively classified into the following major categories: 0, negative; 1, 1–25% and 2, >25%. For statistical analysis, TIF scores 0 and 1 were categorized as low density or negative, TIF score 2 as high density (or positive).
Occurrence of tumor necrosis
The degree of necrosis was classified according to the amount of necrotic cells/areals of the slice in relation to vital tissue. The following degrees of necrosis were established: none (0%), 5%, 10%, 15%, 20%, 25%, 30%, 35% and 40%. The presence of histological tumor necrosis in the studied specimens was classified into two categories: 1, negative (< 5%) or 2, positive (> 5% necrosis). Patients were divided into two groups according to their “positive” or “negative” necrosis scores. In a second step, a further discrimination of the “positive” group into mild (Necrosismild group; 5–20% necrosis) and severe necrosis (Necrosissevere group; >20%) was undertaken.
Occurrence of peritumoral fibrosis
Degree of peritumoral fibrosis in the examined tumor specimens was classified into three categories: mild, moderate and high.
Patients were divided into two groups either by “mild” or “strong (moderate/high)” fibrosis scores (Fibrosismild group or Fibrosisstrong group).
Statistical analysis
Survival analysis, univariate analysis and Kaplan–Meier curves were generated with assistance of the SPSS software program (Version 23.0.0.0 / Year 2015). Comparison of categorical and continuous variables was performed using the Chi2-test and the Wilcoxon-test, respectively. Survival data were compared with the log rank-test. Variables with a significant influence on survival in the univariate analysis were entered into a cox regression analysis. A difference was considered significant for p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work presented in this paper was made possible by funding from the Berlin Institute of Health (BIH). Georgi Atanasov is participant of the BIH Charité Clinician Scientist Program funded by the Charité - Universitätsmedizin Berlin and the Berlin Institute of Health.
References
- 1.Takeya M, Komohara Y. Role of tumor-associated macrophages in human malignancies: Friend or foe? Pathol Int 2016; 66:491-505; PMID:27444136; https://doi.org/ 10.1111/pin.12440 [DOI] [PubMed] [Google Scholar]
- 2.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66(2):605-12; PMID:16423985; https://doi.org/ 10.1158/0008-5472.CAN-05-4005 [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polari- zation: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23(11):549-55; PMID:12401408; https://doi.org/ 10.1016/S1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 4.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G et al.. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol 2017; 66(1):102-15; PMID:27593106; https://doi.org/ 10.1016/j.jhep.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis CE, Harney AS, Pollard JW. The multifaceted role of perivascular macrophages in tumors. Cancer Cell 2016; 30(1):18-25; PMID:27411586; https://doi.org/ 10.1016/j.ccell.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oishi K, Sakaguchi T, Baba S, Suzuki S, Konno H. Macrophage density and macrophage colony-stimulating factor expression predict the postoperative prognosis in patients with intrahepatic cholangiocarcinoma. Surg Today 2015; 45(6):715-22; PMID:25052379; https://doi.org/ 10.1007/s00595-014-0989-y [DOI] [PubMed] [Google Scholar]
- 7.An T, Sood U, Pietruk T, Cummings G, Hashimoto K, Crissman JD. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol 1987; 128:52-60; PMID:3496796 [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle L, Brown NJ, Lewis CE. The role of tumor-associated macrophages in tumor progression: Implications for new anticancer therapies. J Pathol 2002; 196:254-65; PMID:11857487; https://doi.org/ 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Allavena P, Sica A. Tumor-associated macrophages as a prototypic type II polarized phagocyte population: Role in tumor progression. Eur J Cancer 2004; 40:1660-7; PMID:15251154; https://doi.org/ 10.1016/j.ejca.2004.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol Rep 2005; 14:425-31; PMID:16012726; https://doi.org/org/ 10.3892/or.14.2.425 [DOI] [PubMed] [Google Scholar]
- 11.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263-6; PMID:16439202; https://doi.org/ 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 12.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 2008; 84:623-30; PMID:18467655; https://doi.org/ 10.1189/jlb.1107762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010; 10:112; PMID:20338029; https://doi.org/ 10.1186/1471-2407-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T et al.. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 2014; 63:1960-71; PMID:24561613; https://doi.org/ 10.1136/gutjnl-2013-306294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 2009; 50:261-74; PMID:19554540; https://doi.org/ 10.1002/hep.22950 [DOI] [PubMed] [Google Scholar]
- 16.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015; 61:1066-79; PMID:25066777; https://doi.org/ 10.1002/hep.27332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 2014; 60:1090-6; PMID:24412603; https://doi.org/ 10.1016/j.jhep.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 18.Wermuth PJ, Jimenez SA. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Trans Med 2015; 4:2; PMID:25852818; https://doi.org/ 10.1186/s40169-015-0047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards CH, Mohammed Z, Qayyum T, Horgan PG, McMillan DC. The prognostic value of histological tumor necrosis in solid organ malignant disease: A systematic review. Future Oncol 2011; 7:1223-35; PMID:21992733; https://doi.org/ 10.2217/fon.11.99 [DOI] [PubMed] [Google Scholar]
- 20.Cornfield DB, Palazzo JP, Schwartz GF, Goonewardene SA, Kovatich AJ, Chervoneva I, Hyslop T, Schwarting R. The prognostic significance of multiple morphologic features and biologic markers in ductal carcinoma in situ of the breast: A study of a large cohort of patients treated with surgery alone. Cancer 2004; 100:2317-27; PMID:15160334; https://doi.org/ 10.1002/cncr.20260 [DOI] [PubMed] [Google Scholar]
- 21.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clin Infect Dis 2011; 52:889-900; PMID:21427396; https://doi.org/ 10.1093/cid/cir076 [DOI] [PubMed] [Google Scholar]
- 23.Valent P, Orazi A, Büsche G, Schmitt-Gräff A, George TI, Sotlar K, Streubel B, Beham-Schmid C, Cerny-Reiterer S, Krieger O et al.. Standards and impact of hematopathology in myelodysplastic syndromes (MDS). Oncotarget 2010; 1:483-96; PMID:21317447; https://doi.org/ 10.18632/oncotarget.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, Wiltberger G, Matia I, Prager I, Schierle K et al.. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer 2015; 15:790; PMID:26497197; https://doi.org/ 10.1186/s12885-015-1795-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanasov G, Schierle K, Hau HM, Dietel C, Krenzien F, Brandl A, Wiltberger G, Englisch JP, Robson SC, Reutzel-Selke A et al.. Prognostic significance of tumor necrosis in hilar cholangiocarcinoma. Ann Surg Oncol 2016; 24(2):518-25; PMID:27480355; https://doi.org/ 10.1002/jso.24249 [DOI] [PubMed] [Google Scholar]
- 25.Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford) 2005; 7:278-82; PMID:18333207; https://doi.org/ 10.1080/13651820500373028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi S, Borad MJ, Patel T, Gores GJ. Cholangiocarcinoma: Molecular pathways and therapeutic opportunities. Semin Liver Dis 2014; 34:456-64; PMID:25369307; https://doi.org/ 10.1055/s-0034-1394144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, Watanabe M, Aoyagi T, Suzuki T, Shimamura T et al.. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016; 64(2):632-43; PMID:2677371; https://doi.org/ 10.1002/hep.28459 [DOI] [PubMed] [Google Scholar]
- 28.Chenouard A, Chesneau M, Bui Nguyen L, Le Bot S, Cadoux M, Dugast E, Paul C, Malard-Castagnet S, Ville S, Guérif P et al.. Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transplant 2017; 17(6):1490-1501; PMID:27888555; https://doi.org/ 10.1111/ajt.14142 [DOI] [PubMed] [Google Scholar]
- 29.Löffler MW, Chandran PA, Laske K, Schroeder C, Bonzheim I, Walzer M, Hilke FJ, Trautwein N, Kowalewski DJ, Schuster H et al.. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J Hepatol 2016; 65(4):849-55; PMID:27397612; https://doi.org/ 10.1016/j.jhep.2016.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2012; 19(2):171-8; PMID:21874278; https://doi.org/ 10.1007/s00534-011-0437-y [DOI] [PubMed] [Google Scholar]
- 31.Higuchi R, Yamamoto M, Hatori T, Shimizu K, Imai K, Takasaki K. Intrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: Report of a case. Surg Today 2006; 36(6):559-62; PMID:16715430; https://doi.org/ 10.1007/s00595-006-3201-1 [DOI] [PubMed] [Google Scholar]
- 32.Dunn-Pirio AM, Vlahovic G. Immunotherapy approaches in the treatment of malignant brain tumors. Cancer 2016; 123(5):734-50; PMID:27875627; https://doi.org/ 10.1002/cncr.30371 [DOI] [PubMed] [Google Scholar]
- 33.Sultan M, Coyle KM, Vidovic D, Thomas ML, Gujar S, Marcato P. Hide-and-seek: The interplay between cancer stem cells and the immune system. Carcinogenesis 2016; 38(2):107-18; PMID:27866156; https://doi.org/ 10.1093/carcin/bgw115 [DOI] [PubMed] [Google Scholar]
- 34.Yosef N, Regev A. Writ large: Genomic dissection of the effect of cellular environment on immune response. Science 2016; 354(6308):64-8; PMID:27846493; https://doi.org/ 10.1126/science.aaf5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bij GJ, Bogels M, Oosterling SJ, Kroon J, Schuckmann DT, de Vries HE, Meijer S, Beelen RH, van Egmond M. Tumor infiltrating macrophages reduce development of peritoneal colorectal carcinoma metastases. Cancer Lett 2008; 262:77-86; PMID:18187256; https://doi.org/ 10.1016/j.canlet.2007.11.040 [DOI] [PubMed] [Google Scholar]
- 36.Mason RP, Antich PP, Babcock EE, Constantinescu A, Peschke P, Hahn EW. Non-invasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys 1994; 29:95-103; PMID:8175452; https://doi.org/ 10.1016/0360-3016(94)90231-3 [DOI] [PubMed] [Google Scholar]
- 37.Ohno S, Hiroyuki I, Dhar DJ, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival gastric cancer patients. Anticancer Res 2003; 23:5015-22; PMID:14981961 [PubMed] [Google Scholar]
- 38.Forssell J, Oberg A, Henriksson ML. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13(5):1472-9; PMID:17332291; https://doi.org/ 10.1158/1078-0432.CCR-06-2073 [DOI] [PubMed] [Google Scholar]
- 39.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, Kohchi C, Soma G, Inoue M. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res 2004; 24:3335-42; PMID:15515429 [PubMed] [Google Scholar]
- 40.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T et al.. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207:2439-53; PMID:20876310; https://doi.org/ 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda N, O'Dea EL, Doeden A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010; 24:491-501; PMID:20194441; https://doi.org/ 10.1101/gad.1881410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 2010; 120:2699-714; PMID:20644254; https://doi.org/ 10.1172/JCI39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards CH, Mohammed Z, Qayyum T, Horgan PG, McMillan DC. The prognostic value of histological tumor necrosis in solid organ malignant disease: A systematic review. Future Oncol 2011; 7:1223-35; PMID:21992733; https://doi.org/ 10.2217/fon.11.99 [DOI] [PubMed] [Google Scholar]
- 44.Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol 2010; 41:1749-57; PMID:20869096; https://doi.org/ 10.1016/j.humpath.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 45.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. Am J Clin Pathol 2012; 137:283-9; PMID:22261455; https://doi.org/ 10.1309/AJCPLBK9L9KDYQZP [DOI] [PubMed] [Google Scholar]
- 46.Finkernagel F, Reinartz S, Lieber S, Adhikary T, Wortmann A, Hoffmann N, Bieringer T, Nist A, Stiewe T, Jansen JM et al.. The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget 2016; 7(46):75339-52; PMID:27659538; https://doi.org/ 10.18632/oncotarget.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perks WV, Singh RK, Jones GW, Twohig JP, Williams AS, Humphreys IR, Taylor PR, Jones SA, Wang EC. Death receptor 3 promotes chemokine-directed leukocyte recruitment in acute resolving inflammation and is essential for pathological development of mesothelial fibrosis in chronic disease. Am J Pathol 2016; 186(11):2813-23; PMID:27664471; https://doi.org/ 10.1016/j.ajpath.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locatelli L, Cadamuro M, Spirlì C, Fiorotto R, Lecchi S, Morell CM, Popov Y, Scirpo R, De Matteis M, Amenduni M et al.. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 2016; 63(3):965-82; PMID:26645994; https://doi.org/ 10.1002/hep.28382 [DOI] [PMC free article] [PubMed] [Google Scholar]


