Abstract
Microglia cells are the major reservoir of HIV-1 (HIV) within the CNS. However, current models using transformed cell lines are not representative of primary microglia and fetal brain samples for isolation of primary human microglia (HMG) are increasingly difficult to obtain. Here, we describe a monocyte-derived microglia (MMG) cell model of HIV infection that recapitulates infection of primary HMG. CD14+ cells isolated from healthy donors were cultured with M-CSF, beta-nerve growth factor, GM-CSF, and CCL2, and compared to HMG. MMG and HMG cells were infected with HIV and viral replication was detected by p24 antigen. Both MMG and HMG cells were found to acquire spindle shape with few branched or unbranched processes at their ends during the second week in culture and both were found to be CD11b+/CD11c+/CD14+/CD45+/CD195+/HLADRlow/CD86low/CD80+. Whereas hT-Hμglia and HMC3 transformed cell lines are deficient in human microglia signature genes (C1Q, GAS6, GPR34, MERTK, PROS1, and P2RY12), MMG cells expressed all of these genes. Additionally, MMG expressed all the microglia signature miRNA (miR-99a, miR125b-5p, and miR-342-3p). Both MMG and HMG produced ROS and phagocytosed labeled zymosan particles upon PMA stimulation. MMG and HMG infected with HIV produced equivalent levels of HIV p24 antigen in culture supernatants for 30 days post-infection. Thus, we have developed and characterized a microglia cell model of HIV infection derived from primary monocytes that recapitulates the phenotypic and molecular properties of HMG, is superior to transformed cell lines, and has similar HIV replication kinetics to HMG.
Keywords: Monocyte-derived microglia, Human microglia, MMG, HMG, HIV-1
Introduction
Microglia cells represent a central population of brain macrophages that function in brain homeostasis and play an important role in inflammation on exposure to pathologic conditions including toxins, pathogens, and neurological diseases such as human immunodeficiency virus type-1 (HIV) associated neurological disorders (HAND) (Howell et al. 2010; Lee et al. 2010; Politis et al. 2011; Streit 1996; Streit 2001). Until recently, the origin of microglia cells has been the subject of considerable controversy. Although advances in mouse brain studies have convincingly demonstrated the myeloid origin of microglia generated from primitive macrophages in the yolk sac during embryogenesis (Davoust et al. 2008; Ginhoux et al. 2010; Ling et al. 1980), the origin and turnover of microglia cells in humans is still debated. Microglia progenitors are thought to be of mesodermal/mesenchymal origin (Chan et al. 2007). It is postulated that during early postnatal development a subpopulation of monocytes enters the brain, differentiates into microglia cells, and subsequently exist as a stable population in the adult brain. However, in the intact adult brain, the blood-brain barrier restricts this monocyte movement, which may lead to their differentiation into microglia (Ajami et al. 2007; Mildner et al. 2007). Damage to the blood-brain barrier can permit monocytic migration in the adult brain. It appears that microglia cells further differentiate since there is evidence supporting the existence of at least two types of microglia populations. The first population has a resting ramified phenotype, while the second population represents the activated amoeboid morphology (Rezaie et al. 2005; Sievers et al. 1994). Microglia are required for clearance of apoptotic cells and cellular debris including myelin and amyloid deposits and play an important role in normal brain development (Napoli and Neumann 2009; Neumann et al. 2009).
The central nervous system (CNS) is susceptible to infection with lentiviruses (Clements and Zink 1996) and in the case of HIV, perivascular macrophages and microglia are the primary cells in the brain infected with HIV (Gabuzda et al. 1986; Gonzalez-Scarano and Martin-Garcia 2005; Lackner et al. 1991; Lane et al. 1996; Wiley et al. 1986). HIV trafficking across the blood-brain barrier is poorly understood. Some have hypothesized that HIV invasion into the perivascular region is through infected CD4+ T cells and monocytes (Haase 1986; Peluso et al. 1985). Direct contact of HIV-infected macrophages and CD4+ cells can transmit HIV to other brain cell types including perivascular macrophages and microglia (Cosenza et al. 2002; Fischer-Smith et al. 2004; Williams and Hickey 2002), and establish a productive infection (Strizki et al. 1996). Also, the activation of macrophages and microglia cells by HIV or exposure to viral proteins leads to the release of toxins, which are thought to be responsible for subsequent neuron and astrocytic dysfunction associated with HAND (Genis et al. 1992; Giulian et al. 1990; Kaul et al. 2001; Lipton and Gendelman 1995).
Numerous models have been established to study microglia. However, microglia research using human cells has been limited due to the restricted availability of primary sources of human microglia including aborted fetal tissue or postmortem brain tissue. The limited availability of tissue for isolation of primary microglia cells has resulted in the majority of HIV-related research being conducted using mouse embryonic cells which are not permissive to HIV infection or microglia cell lines that can be easily grown and manipulated (Bassett et al. 2012; Hinojosa et al. 2011) (Hinze and Stolzing 2011; Noto et al. 2010). However, continuous cell line models including HMO6 (Janabi et al. 1995), HMC3 (Janabi et al. 1995) and hTERT-immortalized glial (hT-Hμglia) cells (Janabi et al. 1995; Wires et al. 2012) have been shown to have numerous morphologic, genotypic, and functional differences from primary microglia due to their genetic manipulation and long-term culture. These cell lines have limited value for the study of HIV pathogenesis because they have been genetically transformed, have a high multiplication rate, and are poorly permissive to HIV replication. The importance of microglia cells in the pathogenesis of HAND and as a likely reservoir of virus in persons on fully suppressive combination antiretroviral therapy (cART) makes the development of an in vitro model of primary microglia cells a high priority.
Recently, Leone et al. and Etemand et al. showed that human blood-derived monocytes can be cultured to obtain human microglia-like cells (M-MG) using astrocyte-conditioned medium (ACM) (Leone et al. 2006) or serum-free media supplemented with macrophage colony-stimulating factor (M-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), nerve growth factor (NGF), and monocyte chemoattractant protein-1 (MCP1) or CCl2 factors (Etemad et al. 2012). However, until recently, few markers had been identified that differentiate microglia cells from macrophages and other cell types within the CNS, and neither model rigorously identified the derived cells as microglia.
Recently, Butovsky et al. identified a unique genetic and microRNA signature for human fetal and adult microglia including highly or unique expression of P2ry12, Gpr34, Mertk, C1qa, Pros1, and Gas6, and the enriched expression of miR-342-3p and high expression of miR-99a and miR-125b-5p (Butovsky et al. 2014). They have also identified an important role for TGFβ-1 in differentiation of microglia cells under both in vitro and in vivo conditions (Butovsky et al. 2014).
Here, we present the derivation of microglia cells from primary human monocytes and show that in addition to expressing the previously known cellular markers of primary microglia, these monocyte-derived microglia (MMG) also express all of the unique genetic and microRNA microglia signatures identified by Butovsky et al. (Butovsky et al. 2014). Additionally, we show that MMG are permissive to HIV and exhibit the same replication kinetics as primary microglia derived from fetal tissue.
Materials and methods
Ethics statement
HIV seronegative donors were enrolled for venous blood draw using a protocol that was reviewed and approved by the Human Research Protection Program of the University of California, San Diego, in accordance with the requirements of the Code of Federal Regulations on the Protection of Human subjects (45 CFR 46 and 21 CFR 50 and 56). Written informed consent was obtained from all blood donors prior to their participation.
Cell culture and reagents
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of HIV seronegative donors by density gradient centrifugation over Ficoll–Paque Plus (GE Healthcare). CD14+ monocytes cells were isolated from PBMCs using human CD14 microbeads beads (Miltenyi Biotech).
Monocyte-derived macrophages (MDM) were generated in vitro by culturing CD14+ monocytes in RPMI 1640 (Gibco) supplemented with 10 % (v/v) charcoal dextran-treated, heat-inactivated FBS (Gemini Bio-Products), 1 % antibiotics (10,000 units/ml penicillin, 10,000 μg/ml streptomycin, Gibco), and 10 ng/ml macrophage colony-stimulating factor (Peprotech) for 10 days at 37 °C, 5 % CO2. MMG cells were generated from CD14+ monocytes using methods modified from those originally described by Etemand et al. (Etemad et al. 2012). CD14+ monocytes isolated from healthy human donor blood were cultured in RPMI-1640 GlutaMax (Gibco) supplemented with 1 % antibiotics (10,000 units/ml penicillin 10,000 μg/ml streptomycin, Gibco) and mixture of human recombinant cytokines including M-CSF (10 ng/ml; Peprotech), beta-nerve growth factor (NGF-β 10 ng/ml; R&D Systems), and CCL2 (100 ng/ml; Peprotech). Every third day, cells were supplemented with fresh media containing M-CSF, GM-CSF, NGF-β, and CCL2. Cells were cultured for 10 days. Most of the characterization experiments were performed on day 10.
Human microglia isolation and cell culture
Normal human fetal brain tissue was obtained from the University of Washington and approved by the Human Research Protection Program of the University of California, San Diego (Project # 150172XX) in accordance with the requirements of the Code of Federal Regulations on the Protection of Human subjects (45 CFR§46.102 (f)). Human fetal brain microglia were isolated as previously described (D’Souza et al. 1995; Lambert et al. 2008). Briefly, fetal brain tissue was minced and treated with DNase1/trypsin and passed through a nylon mesh. Cells were plated at a density of ~7 × 106 cells/ml in a T75 tissue culture flask in high-glucose DMEM supplemented with 10 % AB-human serum (MP Biomedicals) and M-CSF (10 ng/ml; Peprotech). After 10–14 days, flasks were shaken and floating microglia cells were collected. These human fetal microglia cells (HMG) were plated in 6-well tissue culture plates at a density of 1 × 106 cells/ml in high-glucose DMEM supplemented with 10 % AB-human serum (MP Biomedicals) and M-CSF (10 ng/ml; Peprotech).
Phase contrast microscopy
The morphology of MMG and HMG prior to or after HIV infection was assessed by phase contrast microscopy using an Olympus 1X71 inverted microscope equipped with a ×20/0.45NA LUCPLFLN objective. Images were acquired using DP72 Image Acquisition Interface (Olympus).
Immunofluorescence microscopy
Cells were fixed in Dulbecco’s phosphate-buffered saline supplemented with 4 % (w/v) paraformaldehyde for 10 min and treated with a permeabilization/blocking containing 3 % bovine serum albumin (Sigma-Aldrich) and 0.1 % (v/v) Triton X-100 (Sigma-Aldrich) for 1 h. Following permeabilization and blocking, cells were incubated with primary antibodies: polyclonal goat anti-Iba-1 (Abcam), polyclonal rabbit anti-integrin alpha M/CD11b (Novus Biologicals) and monoclonal mouse anti-CD68/SR-D1 for 1 h at 20–22 °C. Cells were then probed with donkey anti-goat Alexa Fluor (AF) 568, donkey anti-rabbit AF488, and donkey anti mouse AF647 (all Molecular Probes, Life technologies). Cells were nuclear stained and mounted using Prolong Gold Antifade mountant with 4′, 6-diamidino-2-phenylindole (DAPI) from Molecular Probes, Life technologies. Labeled cells were visualized using an Olympus Fluoview FV-1000 confocal imaging system on a 1X81 platform equipped with a U Plan Fluorite ×60/1.42 NA oil differential interference objective (Olympus).
Flow cytometry
Flow analysis of freshly isolated monocytes differentiated into MMG was performed after 10–12 days in culture. Fetal microglia cells were isolated from 10-day-old brain cultures and cultured for an additional 2 days. Expression of specific surface molecules and intracellular molecules was studied using flow cytometry. Cells were detached using a scraper and staining was performed according to manufacturer’s protocol. Expression of CD11c, CD11b, CD195, CD14, CD45, HLA-DR, CD80, and CD86 was measured by incubating the MMG and HMG cells with fluorescein isothiocyanate (FITC)-anti-CD11c, AF488-anti CD11b, allophycocyanin (APC)-anti CD80, PerCP-anti-HLA-DR, phycoerythrin-Cy7-anti-CD86 and PerCP-anti-CD14 (all Biolegend), eFluor450-anti-CD45, and APC-anti-CD195 (all eBioscience) on ice for 30 min. Intracellular CD68 was detected using BD Cytofix/CytoPerm kit with PE-anti-CD68 according to manufacturer’s protocol (BD Biosciences). Corresponding labeled isotype control or FMO controls were included in all experiments. For cell viability analysis of HIV-infected MMG, cells were stained with fixable viability dye eFluor506 (eBioscience). The cells were fixed and resuspended in phosphate-buffered saline (PBS) for flow cytometry using BD FACSCanto RUO-ORANGE analyzer. Data were analyzed using FlowJo software.
Phagocytosis
The phagocytic activity of MMG and HMG was investigated by means of prelabeled zymosan particles as a phagocytosis pathogen using Cytoselect™ 96-well phagocytosis assay (Cell Biolabs). Briefly, MMG and HMG cells were seeded at a density of 50,000 cells/well in a 96-well plate. Cells were allowed to differentiate for 10 days under standard culture conditions (humidified chamber, 5 % CO2, 37 °C). Prior to incubation with zymosan particles, cells were stimulated with phorbol-myristate-acetate (PMA) (50 ng) for 2 h. Following 2 h incubation with prelabeled zymosan particles, cells were processed according to manufacturer’s instructions and the phagocytic index of these cells was determined by reading absorbance at 405 nm.
Determination of reactive oxygen species production
Production of reactive oxygen species (ROS) in MMG and HMG was determined by detecting the conversion of 2′,7′-dichlorofluorescein diacetate (H2DCFDA) into fluorescent 2′,7′-dichlorofluorescein (DCF) using the OxiSelect™ Intracellular ROS assay kit (Cell Biolab). MMG and HMG cells were seeded at a density of 50,000 cells/well in a 96-well plate. Cells were allowed to differentiate for 10 days under standard culture conditions (humidified chamber, 5 % CO2, 37 °C). On day 10, cells were incubated with H2DCFDA for 1 h. Following incubation with H2DCFDA, cells were stimulated with ATP (100 μM) or PMA (50 ng) for 2 h. TBHP-stimulated cells were used as positive control. ROS generation by MMG and HMG cells was analyzed by reading fluorescence with a fluorometric plate reader at 480 nm/530 nm.
RNA isolation and real-time PCR
Total RNAwas isolated using RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol and 20–40 ng RNAwas used in 20–40 μl of reverse transcription reaction (high-capacity cDNA Reverse Transcription Kit; Applied Biosystems). Five nanograms of RNA was used in 10 μl reverse transcription reaction with specific miRNA probes (Applied Biosystems). qPCR reactions were performed in triplicate. mRNA or miRNA levels were normalized with GAPDH and U6snRNA, respectively, by the formula 2−ΔCt, where ΔCt = CtgeneX/miRX – Ctgapdh/U6. All qRT-PCR data were performed in triplicate and the data are presented as mean ± SD.
Virus preparation and infection
HIV-1Ba-L obtained through the NIH AIDS Research and Reference Reagent Program from Dr. Suzanne Gartner and Dr. Robert Gallo (Gartner et al. 1986) was grown in primary culture of PBMC. Virus stocks were prepared as previously described (Campbell and Spector 2008). The 50 % tissue culture infective dose (TCID50) was determined using the Spearman-Karber (Japour et al. 1993) method using the Alliance HIV-1 p24 antigen ELISA (Perkin Elmer Life Sciences). Cells were infected at a multiplicity of infection (MOI) of 0.1 for 8 h. For virus replication analysis, p24 expression was measured in the culture supernatants by ELISA (Perkin Elmer Life Sciences).
Statistical analysis
Comparison between groups was performed using the paired, two-tailed, Student’s t test, or Wilcoxon rank test for non-parametric comparisons. Differences with a p value <0.05 were considered statistically significant.
Results
The morphology of monocyte-derived microglia mimics that of fetal human microglia in culture
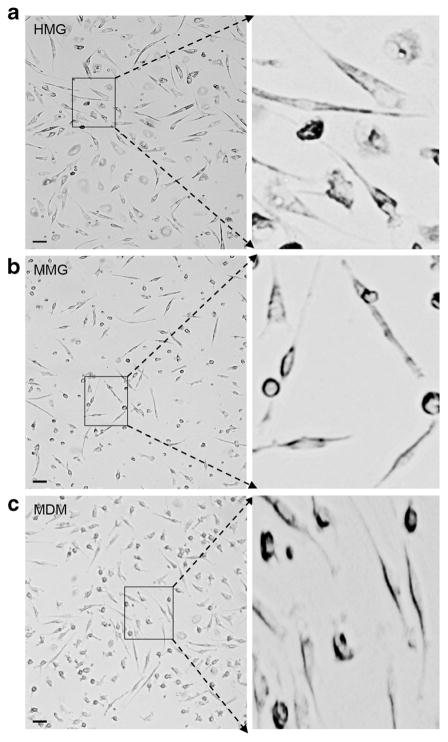
Morphological changes in microglia cells are associated with their role in CNS disease (Hanisch and Kettenmann 2007; Kreutzberg 1996). In vitro cultured primary HMG have been previously reported to acquire rod, spindle, or amoeboid morphology (Kettenmann et al. 2011). Here, we compared the morphology of CD14+ MMG with fetal brain-derived HMG. Since human microglia show morphologic similarity to human macrophages, MDM cells derived from the CD14+ monocytes of same donor were used as control (Fig. 1c). CD14+ monocytes were cultured in the presence of recombinant human growth factors M-CSF, GM-CSF, NGF-β, and CCL2 for 12 days to generate MMG cells. CD14+ monocytes were cultured in the presence of M-CSF for 12 days to generate MDMs, Aborted fetal brain tissue obtained at 90 to 145 days gestation was used as the source of primary microglia cells. These cells were cultured in vitro in presence of M-CSF for 10–14 days at which time the cell morphology was compared between the HMG, MMG, and MDM by phase contrast microscopy (Fig. 1a–c). After differentiation, MMG acquire spindle shape with reduced cell body and appear morphologically similar to HMG (Fig. 1a, b). An enlarged view of these phase images demonstrates that MMG and HMG show a reduction in the central body and have developed branched or ramified cell processes (Fig. 1a, b) consistent with previous reports of primary microglia (Kettenmann et al. 2011; Leone et al. 2006).
Fig. 1.
Phase contrast images of monocyte-derived microglia (MMG) and human fetal brain-derived microglia (HMG) cells. a MMG cells were generated in vitro by culturing CD14+ cells in the presence of macrophage colony-stimulating factor (MCSF), granulocyte macrophage colony-stimulating factor (GMCSF), beta-nerve growth factor (NGF-β), and CCL2 for 10–12 days. b HMG cells were isolated from 120- to 145–day-old fetal brain and cultured in high-glucose DMEM supplemented with 10 % AB-human and M-CSF for 10–12 days. c MDM were generated in vitro by culturing CD14+ cells in the presence of macrophage colony-stimulating factor (MCSF). Enlarged view of each cell type is presented on the right. Representative images of MDM, MMG, and HMG cells derived using monocytes from three independent healthy human donor bloods and fetal brain tissues, respectively. Scale bar indicates 10 μM
Identification of microglia cells in culture
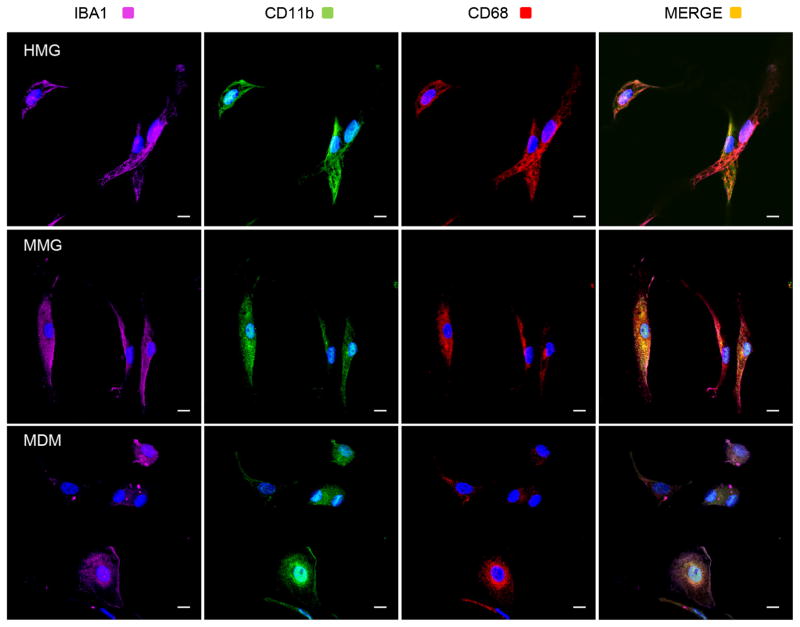
HMG are identified by a variety of markers including: αMβ2 integrin/CD11b or complement receptor 3 (CR3) (Akiyama and McGeer 1990; Sedgwick et al. 1991) which has a role in phagocytosis (Lee et al. 2009; Ma et al. 2003; Rotshenker 2009); Iba1, a calcium binding protein reported to have role in calcium homeostasis, membrane ruffling, and phagocytosis (Imai et al. 1996; Imai and Kohsaka 2002; Ito et al. 1998); (Ohsawa et al. 2000; Ohsawa et al. 2004); and CD68, a glycoprotein found in the cytoplasm (Chen et al. 2002; Davoust et al. 2008; Sedgwick et al. 1991). In our initial set of experiments, we used each of these markers to identify microglia cells in MMG and HMG cultures by immunofluorescence microscopy. MMG derived as described above were fixed, permeabilized, and stained with antibody to CD11b (green), Iba1 (magenta), CD68 (red), and nuclear stain, DAPI (blue) (Fig. 2, middle). Similarly, primary HMG were isolated from fetal brain samples and analyzed by immunofluorescence microscopy (Fig. 2, top). Since human macrophages commonly express most of the human microglia markers, MDM cells derived from CD14+ monocytes of the same donor were used as control (Fig. 2, bottom). Both MMG and HMG stained positive for each of the three markers (Fig. 2). Additionally, the level of expression of IBA1, CD11b, and CD68 was found to be very similar between MMG and HMG cells. Thus, the cell morphology and expression pattern of key human microglia marker proteins in MMG closely resembles with primary HMG cells.
Fig. 2.
MMG cells express human microglia characteristic markers Iba1, CD11b, and CD68. MDM, MMG, and HMG cells were cultured in vitro as described earlier for 12 days. After 12 days, cells were fixed, permeabilized, and then stained with DAPI (blue) and antibody to Iba1 (magenta), CD11b (green), and CD68 (red). Representative images of MDM, MMG, and HMG cells derived using monocytes from three independent healthy human donor bloods and fetal brain tissues, respectively. Merged image for Iba1, CD11b, CD68, and DAPI is shown on the right. Scale bar indicates 10 μm
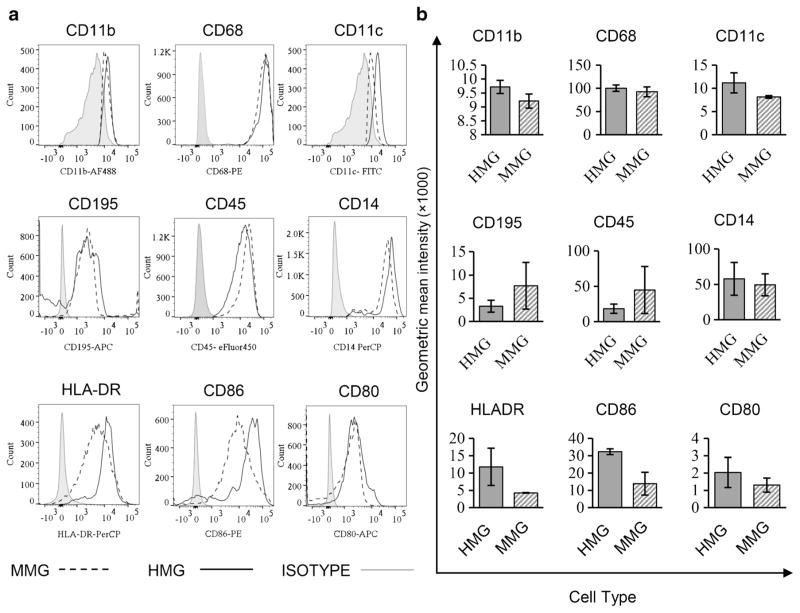
To further compare other surface molecules that have been associated with microglia, we examined the expression of CD11c, CD11b, CD195, CD14, CD45, HLA-DR, CD86, and CD80, and intracellular molecules CD68 in MMG and HMG cells (Fig. 3a). While MDM cells have relatively low CD11b expression compared to HMG cells (data not shown), MMG express similar levels of CD11b compared to HMG as well as CD45, a protein tyrosine phosphatase and an inhibitory receptor for CD22 that regulates the TNFα production by microglia (Mott et al. 2004) (P > 0.05; Fig. 3a, b). CD14, another co-receptor expressed by microglia and known to function in inflammatory responses via TLR4 (Fassbender et al. 2004; Reed-Geaghan et al. 2009) and CCR5 were similarly expressed on MMG and HMG (P > 0.05; Fig. 3a, b). In contrast, we did find that MDM have higher CCR5 surface expression compared to both HMG and MMG (P < 0.05, data not shown).
Fig. 3.
MMG cells express the same surface and intracellular marker proteins as HMG cells. MMG and HMG were cultured in vitro for 12 days and the percentage of cells expressing CD195, CD11c, CD14, CD45, HLA-DR, CD80, CD86 surface markers, and CD11b and CD68 intracellular markers was estimated by flow cytometry and data was analyzed using FlowJo. a Representative histograms showing expression of CD195, CD11c, CD14, CD45, HLA-DR, CD80, CD86 CD11b surface markers, and CD68 intracellular markers in HMG and MMG cells. The gray bar indicates the negative staining control (FMO/isotype) using the corresponding isotype control antibody. b Relative expression of CD195, CD11c, CD14, CD45, HLA-DR, CD80, CD86, CD11b surface markers, and CD68 intracellular markers in MMG and HMG cells is presented as geometric mean intensity. Data presented are mean ± SD after normalization using isotype values from three independent healthy human donor blood-derived MMG and three independent fetal brain tissue-derived HMG cells
To further characterize MMG and HMG, we examined activation markers including HLA-DR, CD80, and CD86. MMG and HMG were found to be HLA-DRlow CD80low (Fig. 3a, b); CD86 expression was not statistically different between the two microglia cell types (Fig. 3a, b). Therefore, both MMG and HMG were found to have a HLADRlow/CD86low/CD80low profile.
Monocyte-derived microglia cells express human microglia signature genes
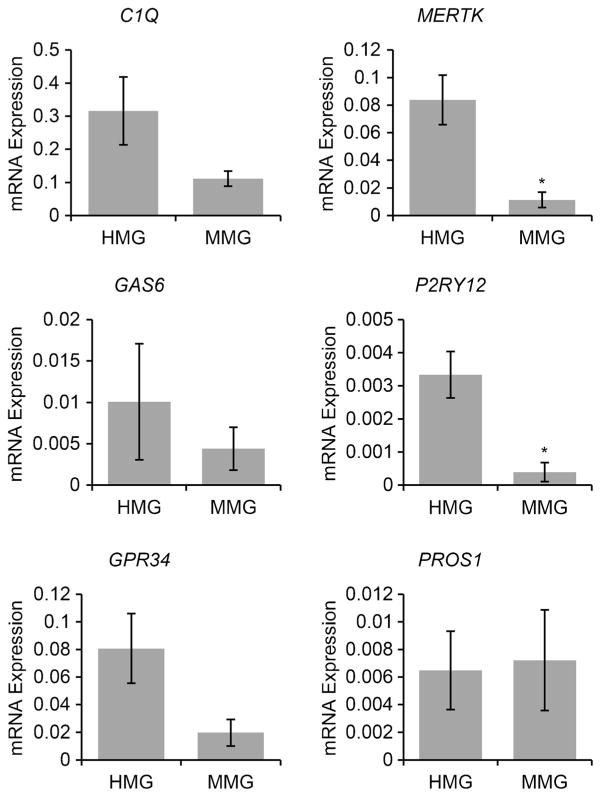
Human microglia cells have been shown to express unique molecular signatures (Butovsky et al. 2014). To further establish that MMG closely resemble HMG, we compared the expression of microglial signature genes C1Q, GAS6, GPR34, PROS1, P2RY12, and MERTK in both microglia cell types. RNA was isolated from cultured cells and analyzed by qPCR. As shown in Fig. 4, MMG express similar levels of molecular signature genes C1Q, GAS6, GPR34, and PROS1 (P > 0.05 for each comparison). However, MMG were found to express lower levels of MERTK and P2RY12 compared to HMG (P < 0.05). Conversely, it should be noted that the expression of MERTK and P2RY12 was significantly higher in MMG than that found in hT-Hμglia and HMC3 cell lines (P < 0.005, Fig. S1). We also compared expression of microglial signature genes between CD14+ monocytes and MMG derived from the same donor monocytes (Fig. S2). We observed significant enrichment of C1QA, MERTK, PROS1, and GPR34 signatures following differentiation of monocytes into MMG (p < 0.05, p < 0.05, p < 0.005, p < 0.05, Fig. S2).
Fig. 4.
Human microglia signature genes are present in MMG cells. Human healthy donor blood was used to isolate MMG from monocytes. MMG were generated in vitro by culturing CD14+ cells in the presence of M-CSF, GM-CSF, NGF-β, and CCL2 for 10–12 days. HMG cells were isolated from 90- to 145-day-old human fetal brain tissue. qPCR analysis of human microglia signature genes in MMG (n = 3) and HMG cells (n = 3) for c1q, gas6, gpr34, pros1, p2ry12, and mertk genes. Gene expression was normalized against GAPDH using ΔCt (n = 3). Microglia signature gene expression is compared between MMG cells and HMG cells derived from three independent healthy human donor bloods and fetal brain tissues, respectively. Data are presented as ± SD, n = 3; *P < 0.05
Monocyte-derived microglia cells express signature microglial miRNAs
In addition to specifically enriched gene expression, HMG express a unique set of miRNAs including miR-99a, miR-125b-5p, and miR-342-3p (Butovsky et al. 2014). MDM and MMG share many biochemical markers which makes it difficult to distinguish these cell populations in culture. Therefore, we first compared expression of these miRNAs in MDM and MMG. Comparing MMG to MDM, expression of miR-99a, miR-342-3p, and miR-125-b-5p were increased four-fold, three-fold, and two-fold, respectively (P < 0.005, P < 0.05, P < 0.05, data not shown). Additionally, MMG are significantly enriched for signature miRNA miR-99a, miR-342-3p, and miR-125-b-5p compared to their parent CD14+ monocytes (p < 0.05, p < 0.05, p = 0.005, Fig. S3). Thus, human microglia signature miRNA expression is enriched in MMG cells compared to MDM derived from the same donor CD14+ monocytes. We next compared the expression of these miRNAs between HMG and MMG cells (Fig. 5). Both HMG and MMG had similar miRNA signature profiles (Fig. 5). Expression did not differ statistically for miR-99a and miR-342-3p (P > 0.05); however, primary HMG were found to express higher levels of miR-125b-5p compared to MMG (P < 0.05). Thus, although there are some variations when compared to HMG, MMG cells express all the human microglia signature miRNAs (Fig. 5).
Fig. 5.
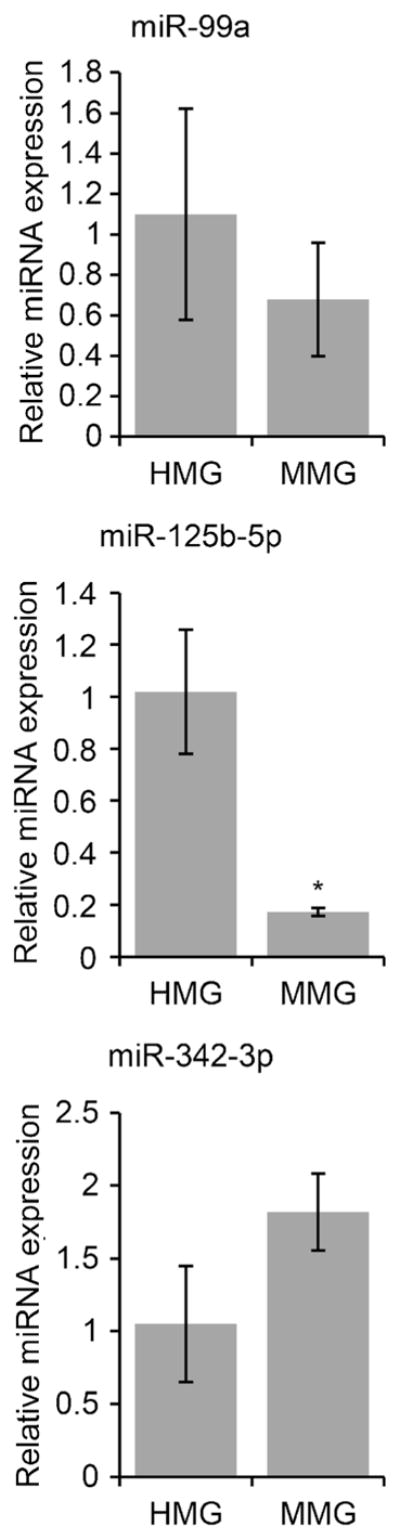
Human microglia signature miRNA are present in MMG cells. HMG cells were isolated from 90- to 145-day-old human fetal brain tissue. MMG cells were generated in vitro by culturing CD14+ cells from healthy human donor blood in the presence of MCSF, GMCSF, NGF-β, and CCL2 for 10–12 days. RNA extracted from primary HMG and MMG cells was analyzed for human signature miRNA miR-99a, miR-125b-5p, and miR-342-3p expression by qPCR. miRNA expression level was normalized against U6 miRNA using ΔCt (n = 3). miRNA levels were compared between HMG and MMG cells derived from three independent healthy human donor bloods and fetal brain tissues, respectively. Data are presented as mean ± SD n = 3. *P < 0.05
Monocyte-derived microglia are functionally active
Expression of human microglia characteristic proteins IBA1, GLUT5 (glucose transporter, Type 5), and SOD (superoxide dismutase) were analyzed in MMG and HMG cells by immunoblotting. Glial fibrillary acidic protein (GFAP), marker protein for astrocytes, was analyzed to check for the presence of astrocytes in HMG culture, and GAPDH expression was determined as control. Both MMG and HMG were shown to express all the characteristic proteins except GFAP (Fig. 6a). Having established the expression of microglia characteristic marker proteins in MMG and HMG cells, we examined the function of these cells using ROS production and phagocytosis (Fig. 6b, c). Both MMG and HMG cells were found to be functional and responsive to stimulation. Upon treatment with PMA or ATP, both microglia cell types produced ROS as detected by fluorescence measurement (DCFDA) at 480 nm/530 nm (Fig. 6b). Moreover, these cells also showed significant similarity in their phagocytic activity (Fig. 6c). Both MMG and HMG cells were able to phagocytose zymosan particles, and PMA stimulation increased phagocytic activity of MMG and HMG (Fig. 6c).
Fig. 6.
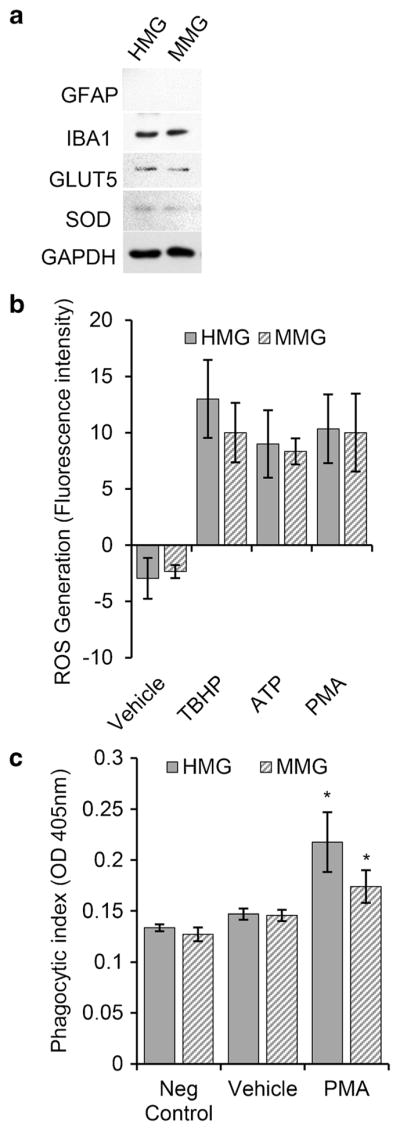
MMG and HMG cells are functional in culture. a Western blot analysis of the protein levels of different glial cell markers. MMG and HMG cells were cultured in vitro for 10 days. At day 10, cells were lysed and analyzed for endogenous glial fibrillary acidic protein (GFAP), IBA1, glucose transporter type 5 (GLUT5), superoxide dismutase (SOD), and GAPDH. b MMG and HMG cells produce ROS upon stimulation. ROS generation was determined by detecting the conversion of H2DCFDA into DCF with a fluorescence plate reader. Unstimulated MMG and HMG cells were compared with the protein kinase C activator, phorbol-myristate-acetate (PMA, 50 nM) and ATP (100 μM) stimulated cells. Tert-butyl hydrogen peroxide (TBHP, 50 μM) was used as a positive control. c Quantification of phagocytic response in MMG and HMG cells. The phagocytic ability of MMG and HMG cells in response to PMA activation was measured using prelabled zymosan (Saccharomyces cerevisiae) particles. The engulfed particles were detected using a colorimetric 96-well assay
Monocyte-derived microglia support long-term HIV-1 infection
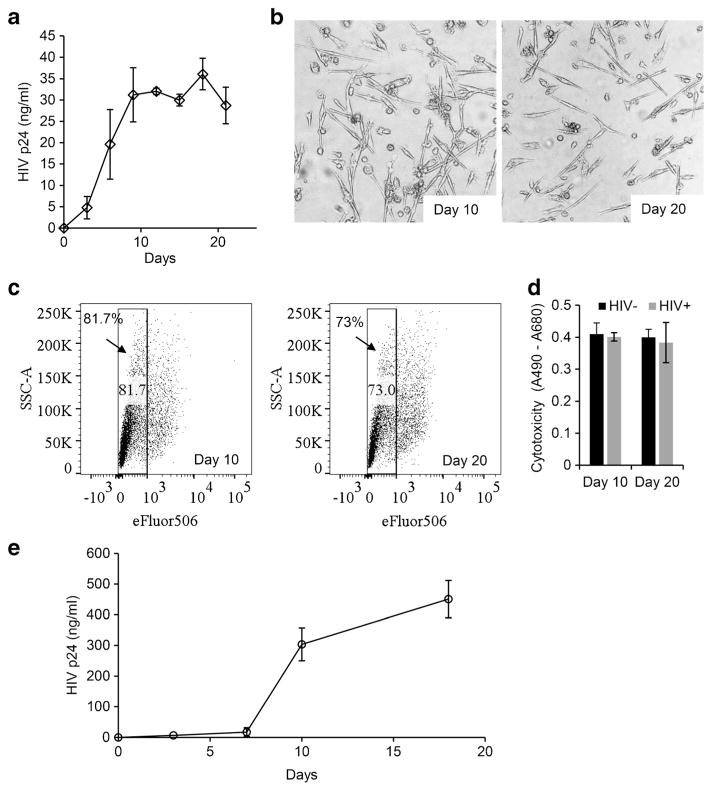
Microglia cells are thought to be the major site of HIV infection and to serve as a persistent viral reservoir within the CNS. Therefore, our next set of experiments was designed to examine the ability of HIV to infect MMG and the viability of infected MMG during prolonged infection in culture. MMG were infected with HIV-1Ba-L at a MOI of 0.1 and p24 antigen was determined over 20 days post-infection. MMG were found to be susceptible to HIV infection (Fig. 7a) and showed more than 70 % viability at the end of the 20 days (Fig. 7b, c). Also, no difference was observed in extracellular LDH release between uninfected and HIV-infected MMG cells (Fig. 7d). We further analyzed the infectivity of the virions released from infected MMG cells in PHA-stimulated PBMC cells (Fig. 7e). Culture supernatant equivalent to 1 ng p24 antigen derived from HIV-infected MMG cells (n = 2) infected over day 20 was used to infect 1 million PHA activated PBMC cells. Culture supernatants collected at days 0, 3, 7, 10, and 18 post-infection showed an increase in p24 antigen production over time (Fig. 7e). Thus, MMG cells support long-term HIV infection and release infectious virions into culture supernatants.
Fig. 7.
MMG supports HIV infection. a MMG cells were generated in vitro by culturing CD14+ cells from healthy human donor blood in the presence of M-CSF, GM-CSF, NGF-β, and CCL2 for 10 days. On day 10, MMG cells were infected with HIVBa-L at MOI 0.1. At days 0, 3, 6, 9, 12, 15, 18, and 21 post-infection, extracellular release of HIV p24 antigen into the culture supernatant was analyzed by p24 antigen capture ELISA. Data presented as mean ± SD, n = 3. b Representative phase contrast images of MMG cells at days 10 and 20 post-infection (n = 2). c MMG cells cultured for 10 days and then exposed to HIV. Cell viability was analyzed on days 10 and 20 post-infection by staining with viability dye eFluor506 and analyzed by flow cytometry. d MMG cells were analyzed for cytotoxicity post-HIV exposure. Extracellular release of LDH was measured at days 10 and 20 post-infection (n = 3). e Culture supernatants were collected from MMG cells exposed to HIV for 20 days. Culture supernatant equivalent to 1 ng p24 antigen was used to infect 106 PHA stimulated PBMCs. Culture supernatants were collected at days 0, 3, 7, 10, and 18 post-infection and analyzed for p24 using a p24 antigen capture ELISA
HIV infection kinetics in MMG and HMG cells
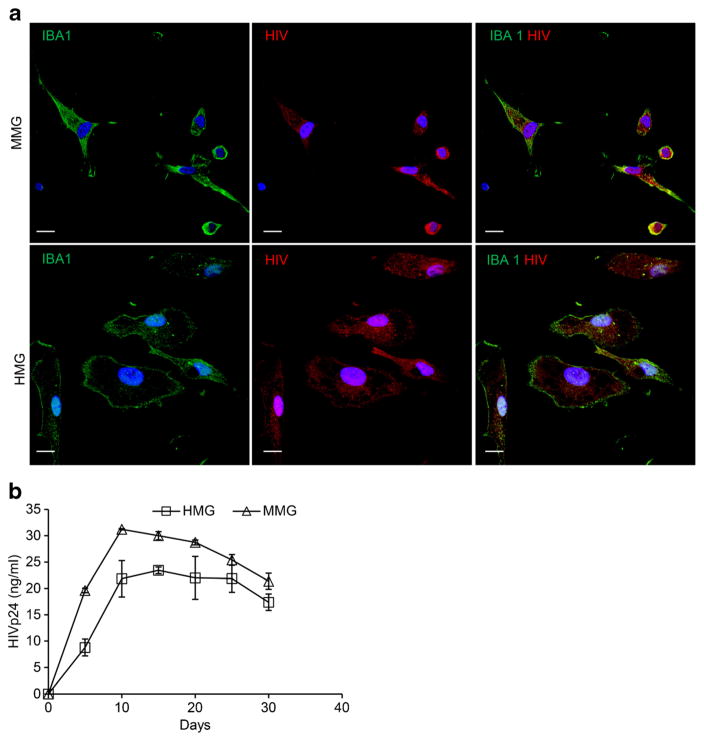
Having established that MMG are permissive to HIV infection and survive for prolonged periods of time, we next compared the replication kinetics between MMG and HMG. MMG and HMG cells were infected with HIV-1Ba-L at a MOI of 0.1 and p24 antigen was determined up to 30 days post-infection. At 20 days post-infection, intracellular p24 could be detected in the majority of MMG and HMG by immunofluorescence (Fig. 8a). HIV replication kinetics was compared between MMG and HMG cells by measuring extracellular release of p24 antigen in the culture supernatants using p24 antigen capture ELISA. Replication kinetics were found to be similar in MMG compare to HMG (Fig. 8b). Thus, MMG cells can support productive long-term HIV infection and can be used as a model for acute and persistent infections.
Fig. 8.
MMG and HMG cells show similar HIV infection kinetics. a Representative fluorescence microscopy images of HIV-infected MMG and HMG cells which were fixed, permeabilized, and then stained with antibody to IBA1 (green) and HIV p24 and p17 (red). Scale bar indicates 10 μm. b 0.5 million MMG and HMG cells were cultured in vitro for 10 days and then infected with HIVBa-L at MOI 0.1. Culture supernatants from HIV-infected MMG and HMG cells were collected at days 0, 5, 10, 15, 20, 25, and 30 post-infection and analyzed for p24 expression by p24 antigen capture ELISA. HIVBa-L infection profile was compared between MMG and HMG cells for 30 days post-infection. Data are presented as mean ± SD, n = 3
Discussion
The CNS is an important reservoir of HIV infection and strategies to eradicate HIV from persons on cART will require clearance of virus from cells within the brain. While astrocytes, oligodendrocytes, and neurons have been suggested as potential sites of HIV replication (Bagasra et al. 1996; Saito et al. 1994), there is a general consensus that within the CNS microglia are the major HIV reservoir (Gonzalez-Scarano and Martin-Garcia 2005; Ioannidis et al. 1995). However, studies of HIV infection of microglia cells have been severely limited by the difficulty in obtaining fetal and adult brain tissue for isolation of primary HMG (Bassett et al. 2012; Hinojosa et al. 2011). Thus, given the significance of microglia cells, there is an urgent need for a suitable cellular model that sufficiently recapitulates human microglia function and molecular signature to elucidate the role of microglia in HIV-associated CNS dysfunction and the potential success of approaches for viral eradication.
While human microglia cell models are often characterized using markers commonly expressed by macrophages and microglia cells, they have not been shown to express the unique human microglia molecular and miRNA signatures (Butovsky et al. 2014). Also, the available human immortalized cell lines show important differences in their cytokine profile and morphology due to transformation and repeated passaging in culture (Stansley et al. 2012). Moreover, although microglia cells are thought to be a long-lived reservoir of HIV, to date, no model has been shown to support a persistent infection with HIV. Thus, any findings obtained solely through the examination of interactions between HIV and currently available models of microglia cells should be viewed with caution.
In the research presented here, we set out to derive primary microglia cells from human peripheral blood monocytes that would recapitulate the morphology and function of primary HMG and express the unique molecular signatures that characterize microglia. We further required that these in vitro-derived MMG exhibit the same HIV replication kinetics and be capable of maintaining a persistent infection.
To establish that the MMG described here met each of these objectives, we first showed that the morphology of MMG appeared similar to HMG and using immunohistochemistry demonstrated that the MMG were positive for CD11b, CD68, and Iba-1. We further characterized MMG as CD11c+/CD11b+/CD68+/CD195+/low/CD45+/CD14+/HLADR+/low/CD86+/low/CD80+ cells. Thus, MMG cells express all immunological characteristics specifically identified previously for human microglia cells (McGeer et al. 1993; Williams et al. 1992). Moreover, the MMG expressed the unique genetic markers P2ry12, Mertk, Gas6, Gpr34, Pros1, and C1qa which further distinguish microglia from monocytes, and other CNS and immune cells (Butovsky et al. 2014). The expression of these signature genes is a distinct advance over commonly used microglia cell lines including HMC3 and hT-hμglia that fail to express these microglia-specific genes (Butovsky et al. 2014). Finally, to further confirm that MMG are representative of primary microglia in culture, we showed that miRNAs miR-99a, miR-125b-5p, and miR-342-3p specifically identified as distinct for HMG were present in MMG (Butovsky et al. 2014). Though we observed some variation between MMG and HMG cells for Mertk and P2ry12 and miR-125b-5p expression in our qPCR analysis, MMG show significant expression levels for both immunological and human microglia-specific markers unlike cell line models expressing the microglia immunological characteristics but lack the genetic signatures of human microglia (Butovsky et al. 2014).
Having established phenotypically and genotypically that MMG have a similar profile as HMG, we evaluated two characteristic effector functions of human microglia—ROS production and phagocytosis in MMG cells. Our data regarding ROS generation and phagocytosis further indicates that MMG display similar capacity to phagocytose zymosan particles and generate ROS upon PMA stimulation as the primary human microglia. In addition to phenotypic and functional analysis, we further analyzed these cells for HIV replication kinetics. Because HIV infection of the CNS has been associated with macrophage tropic strains (He et al. 1997; Lavi et al. 1997; Shieh et al. 1998), we infected MMG and HMG cells with HIV-1BaL that is known to infect macrophages. Similar HIV replication kinetics was observed in both cell populations. Additionally, MMG were able to support a persistent HIV infection, and infectious virions were released in culture supernatants. These later stages of infection are similar to what has been observed in macrophages (Gendelman et al. 1988; Orenstein et al. 1984).
In summary, we have shown that MMG derived from primary human monocytes recapitulate many of the unique characteristics of primary fetal brain-derived HMG and can support a persistent infection with HIV. The use of these cells has the potential to greatly facilitate studies designed to understand the pathogenesis of HIV within microglia and the contribution of these cells to HAND. Additionally, persistently infected MMG can serve as an in vitro model to investigate strategies for the elimination of virus from the CNS. Finally, the MMG described here have the potential to provide a model for other microbial infections and neurological diseases that involve microglia cells.
Supplementary Material
Acknowledgments
This research was supported in part by 1R01 NS077874 and 1R01 NS077874 from the National Institute of Neurological Disorders and Stroke and the International Maternal Perinatal Adolescent AIDS Clinical Trials (IMPAACT) Network. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by NIAID (U01 AI068632) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; contract number N01-DK-9-001/HHSN267200800001C). We thank Terrance Robinson, Rodney Trout, and Byungho Wang for technical assistance and Gang Zhang for isolation and culture of primary human microglia.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s13365-016-0472-1) contains supplementary material, which is available to authorized users.
Compliance with ethical standards HIV seronegative donors were enrolled for venous blood draw using a protocol that was reviewed and approved by the Human Research Protection Program of the University of California, San Diego, in accordance with the requirements of the Code of Federal Regulations on the Protection of Human subjects (45 CFR 46 and 21 CFR 50 and 56). Written informed consent was obtained from all blood donors prior to their participation. Normal human fetal brain tissue was obtained from the University of Washington and approved by the Human Research Protection Program of the University of California, San Diego (Project # 150172XX), in accordance with the requirements of the Code of Federal Regulations on the Protection of Human subjects (45 CFR§46.102 (f)).
Conflict of interest The authors declare that they have no conflict of interest.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bassett T, Bach P, Chan HM. Effects of methylmercury on the secretion of pro-inflammatory cytokines from primary microglial cells and astrocytes. Neurotoxicology. 2012;33:229–234. doi: 10.1016/j.neuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Spector SA. CCL2 increases X4-tropic HIV-1 entry into resting CD4+ T cells. J Biol Chem. 2008;283:30745–30753. doi: 10.1074/jbc.M804112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- Clements JE, Zink MC. Molecular biology and pathogenesis of animal lentivirus infections. Clin Microbiol Rev. 1996;9:100–117. doi: 10.1128/cmr.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995;15:7293–7300. doi: 10.1523/JNEUROSCI.15-11-07293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Etemad S, Zamin RM, Ruitenberg MJ, Filgueira L. A novel in vitro human microglia model: characterization of human monocyte-derived microglia. J Neurosci Methods. 2012;209:79–89. doi: 10.1016/j.jneumeth.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Haase AT. Pathogenesis of lentivirus infections. Nature. 1986;322:130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze A, Stolzing A. Differentiation of mouse bone marrow derived stem cells toward microglia-like cells. BMC Cell Biol. 2011;12:35. doi: 10.1186/1471-2121-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol. 2010;69:1017–1033. doi: 10.1097/NEN.0b013e3181f3a5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Reichlin S, Skolnik PR. Long-term productive human immunodeficiency virus-1 infection in human infant microglia. Am J Pathol. 1995;147:1200–1206. [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- Japour AJ, Mayers DL, Johnson VA, Kuritzkes DR, Beckett LA, Arduino JM, Lane J, Black RJ, Reichelderfer PS, D’Aquila RT, et al. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. The RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Antimicrob Agents Chemother. 1993;37:1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Desbarats J, Arbour N, Hall JA, Olivier A, Bar-Or A, Antel JP. Dendritic cell differentiation signals induce anti-inflammatory properties in human adult microglia. J Immunol. 2008;181:8288–8297. doi: 10.4049/jimmunol.181.12.8288. [DOI] [PubMed] [Google Scholar]
- Lane JH, Tarantal AF, Pauley D, Marthas M, Miller CJ, Lackner AA. Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am J Pathol. 1996;149:1097–1104. [PMC free article] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie JA, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Yang CS, Fang KM, Tzeng SF. Role of ciliary neurotrophic factor in microglial phagocytosis. Neurochem Res. 2009;34:109–117. doi: 10.1007/s11064-008-9682-0. [DOI] [PubMed] [Google Scholar]
- Leone C, Le Pavec G, Meme W, Porcheray F, Samah B, Dormont D, Gras G. Characterization of human monocyte-derived microglia-like cells. Glia. 2006;54:183–192. doi: 10.1002/glia.20372. [DOI] [PubMed] [Google Scholar]
- Ling EA, Penney D, Leblond CP. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the ‘ameboid cells’ present in the corpus callosum of postnatal rats. J Comp Neurol. 1980;193:631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto D, Takahashi K, Miyake S, Yamada M. In vitro differentiation of lineage-negative bone marrow cells into microglia-like cells. Eur J Neurosci. 2010;31:1155–1163. doi: 10.1111/j.1460-9568.2010.07152.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113(Pt 17):3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844–856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- Orenstein JM, Schulof RS, Simon GL. Ultrastructural markers in acquired immune deficiency syndrome. Arch Pathol Lab Med. 1984;108:857–859. [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA, Piccini P. Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum Brain Mapp. 2011;32:258–270. doi: 10.1002/hbm.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J Mol Neurosci. 2009;39:99–103. doi: 10.1007/s12031-009-9186-7. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh JT, Albright AV, Sharron M, Gartner S, Strizki J, Doms RW, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers J, Parwaresch R, Wottge HU. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia. 1994;12:245–258. doi: 10.1002/glia.440120402. [DOI] [PubMed] [Google Scholar]
- Stansley B, Post J, Hensley K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J Neuroinflammation. 2012;9:115. doi: 10.1186/1742-2094-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Strizki JM, Albright AV, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel JP, Yong VW. Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992;51:538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Wires ES, Alvarez D, Dobrowolski C, Wang Y, Morales M, Karn J, Harvey BK. Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol. 2012;18:400–410. doi: 10.1007/s13365-012-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.