Abstract
Objective
This purpose of this study was to describe and demonstrate CrossCheck, a multimodal data collection system designed to aid in continuous remote monitoring and identification of subjective and objective indicators of psychotic relapse.
Methods
Individuals with schizophrenia-spectrum disorders received a smartphone with the monitoring system installed along with unlimited data plan for 12 months. Participants were instructed to carry the device with them and to complete brief self-reports multiple times a week. Multi-modal behavioral sensing (i.e., physical activity, geospatial activity, speech frequency and duration) and device use data (i.e., call and text activity, app use) were captured automatically. Five individuals who experienced psychiatric hospitalization were selected and described for instructive purposes.
Results
Participants had unique digital indicators of their psychotic relapse. For some, self-reports provided clear and potentially actionable description of symptom exacerbation prior to hospitalization. Others had behavioral sensing data trends (e.g., shifts in geolocation patterns, declines in physical activity) or device use patterns (e.g., increased nighttime app use, discontinuation of all smartphone use) that reflected the changes they experienced more effectively.
Conclusion
Advancements in mobile technology are enabling collection of an abundance of information that until recently was largely inaccessible to clinical research and practice. However, remote monitoring and relapse detection is in its nascency. Development and evaluation of innovative data management, modeling, and signal-detection techniques that can identify changes within an individual over time (i.e. unique relapse signatures) will be essential if we are to capitalize on these data to improve treatment and prevention.
Introduction
The terms used to describe people with schizophrenia reflect our broad misconceptions about psychotic illness; phrases like “he’s delusional” or “she hears voices” suggest a stable and unchanging condition (Corrigan et al., 2000). However, longitudinal research has shown that psychosis is seldom a fixed or constant state. Even in the context of a severe mental illness like schizophrenia, the frequency, severity, and effect of psychotic symptoms may vary within individual over the span of months, weeks, or even days (Ben-Zeev et al., 2012; Oorschot et al., 2012). Psychosis in schizophrenia may be affected by both external conditions (e.g., location, social environment) and internal states (e.g., affective states, fatigue, substance-induced intoxication) (Ben-Zeev et al., 2011; Freeman et al., 2015; Swendsen, Ben-Zeev & Granholm, 2011). Clinically, people with schizophrenia vacillate between periods of full or partial remission and episodes of symptom exacerbation and psychotic relapse (Strauss et al., 2010). Psychotic relapses are associated with severe problems including homelessness, violent behavior, incarceration, self-injury, and suicide (Coid et al., 2016; Hawthorne et al., 2012; Hor & Taylor, 2010; Koyanagi, Stickley,& Haro 2015). Relapses are also a major driver of expensive healthcare costs including emergency services and psychiatric hospitalizations (Ascher-Svanum et al., 2010; Sun et al., 2007).
Psychotic relapse may be preceded by early warning signs including changes in one’s energy level, cognition, social functioning, psychomotor and physical activity, perception, and sleep patterns (Birchwood et al., 2000; Gleeson et al., 2010; Spaniel et al., 2016). Time-sensitive detection of these changes would be highly beneficial as evidence from randomized controlled trials suggests that even after the onset of early warning signs, illness management and pharmacological treatments can be deployed in time to prevent full psychotic relapses and hospitalization (Morriss et al., 2013).
Clinical evaluation approaches such as interviews or comprehensive assessment batteries do not allow for continuous and sensitive monitoring of gradually emerging warning signs. Pragmatically, they are costly, require highly-trained professionals, and are time, resource, and labor-intensive. Furthermore, they require direct contact for administration, which is dependent on clinic office hours and patients’ motivation and ability to ambulate to in-person meetings. Conceptually, these methods rely on individuals’ capacity to accurately recall their symptoms and psychotic experiences retrospectively, a task that is challenging for nonclinical populations and may be especially difficult for people with psychiatric disabilities or cognitive impairment. Given that clinical assessments are typically conducted at treatment facilities, individuals may also intentionally under-report (e.g. to avoid treatments, stigma, embarrassment) or hyper-endorse (e.g. to receive benefits, faster care, “white coat” effects) symptoms or behavioral problems. Therefore, the information gathered from patients in these settings may not be representative of their actual experience in their real-world environments, i.e., they have limited ecological validity (Trull & Ebner-Priemer, 2013). Finally, the information yielded from existing clinical evaluation approaches serves as a periodic “snapshot” rather than a time-varying record of one’s behavior and functioning. These data offer little for sensitive detection of risk patterns that may emerge within individuals longitudinally. In the context of these practical and methodological barriers, it is not surprising that clinicians and healthcare systems are often left to contend with the devastating outcomes of psychotic relapses only after the fact rather than when early warning signs appeared.
With these challenges in mind, our multidisciplinary team set out to develop a novel technology-assisted paradigm for monitoring and detection of early warning signs that could capture multi-dimensional indicators of one’s clinical status and functioning, as they occur in real-time and in real-world environments. To do so, we harnessed cutting-edge smartphone and sensor technology to develop CrossCheck, a multi-modal data collection and reporting system designed to aid in remote monitoring and identification of behavioral changes, symptom exacerbation, and possible increase in risk for relapse in people with schizophrenia.
This paper provides an overview of the CrossCheck system and describes several scenarios in which different mobile data elements (i.e., self-report, behavioral sensing, device use over time) showed promise in signal detection and identification of early warning signs of subsequent psychotic relapse. Participants were drawn from an ongoing study involving a year-long deployment of CrossCheck among individuals with schizophrenia who are at increased risk for relapse. The objective of the paper is to introduce readers to a new approach for continuous remote monitoring and to stimulate discussion about the potential of using ubiquitous smartphone technology to support individualized relapse detection and prevention. For people living with serious mental illness hospitalizations are often demoralizing, disempowering and traumatizing (Cohen, 1994; Frueh, et al., 2005; Morrison, Frame, & Larkin 2003). They affect one’s sense of self and confidence (Roe, 2005). Hospitalizations take people away from their families, communities, and work. For many, staying healthy, autonomous, and out of the hospital is a primary recovery goal (Liberman & Kopelowicz, 2005; Meuser, et al., 2006). Thus, providing individuals with empowering tools to gain mastery over their symptoms and relapses through self-monitoring and self-reporting information is consistent with the principles of psychiatric rehabilitation and recovery.
Method
This study was approved by the Committee for Protection of Human Subjects at Dartmouth College and Institutional Review Board at North Shore-Long Island Jewish Health System. CrossCheck system development and deployment were supported by NIH’s EUREKA (Exceptional Unconventional Research Enabling Knowledge Acceleration) program. The multi-institutional CrossCheck research team is comprised of investigators who bring diverse sets of expertise from clinical psychology and psychiatry, public health, computer science, electrical engineering, biostatistics, and the emerging interdisciplinary field of Mobile Health (mHealth). The program of research involved staged development of a comprehensive smartphone data collection system, iterative laboratory-based system testing and refinement, micro-trials with research staff, and brief (i.e., 1 or 2 week) usability/feasibility field trials with people with schizophrenia (Ben-Zeev et al., 2016). Once the system was deemed technically reliable and acceptable, the project advanced to year-long deployments of CrossCheck in the context of a randomized controlled trial (RCT) that is currently underway.
Procedures
Participants in the ongoing RCT are recruited from several treatment programs at a large psychiatric hospital in New York. Recruitment flyers are posted at the study site with the research coordinator’s phone number for direct contact. On-site research staff members also review the hospital’s electronic medical record (EMR) regularly to identify potential participants. Potential candidates are identified and their clinicians are contacted by the investigative team. Clinicians are asked to describe the study to their patients and refer those who provided authorization to be contacted. Candidates receive a detail explanation of the study and description of the CrossCheck system before undergoing eligibility screening. Participants are required to meet the following inclusion criteria: 1) Chart diagnosis of schizophrenia, schizoaffective disorder or psychosis not otherwise specified; 2) 18 years or older; and 3) an inpatient psychiatric hospitalization, daytime psychiatric hospitalization, outpatient crisis management, or short-term psychiatric hospital emergency room visit within 12 months. Individuals are excluded if they: 1) Have hearing, vision, or motor impairment that make it impossible to operate a smartphone (determined using a demonstration smartphone for screening); and 2) Have a reading level below the 6th grade (determined using the reading section of the Wide Range Achievement Test - 4th Edition); and 4) Are unable to provide informed consent (pass a competency screener). After completion of informed consent, participants are then asked to complete a demographic questionnaire and undergo a comprehensive baseline assessment of symptoms and functioning. Participants are then randomized to either receive the CrossCheck system in addition to their standard care, or treatment as usual. All participants are asked to return at 3, 6, 9 and 12 months for administration of an in-person assessment battery comprised of semi-structured clinical interviews and questionnaires. Research staff review participants’ EMR data at these intervals and document events that may reflect relapses, including ER visit for psychiatric reasons or psychiatric hospitalization.
Participants allocated to the CrossCheck arm receive a Samsung Galaxy S5 Android smartphone with the monitoring system installed along with unlimited data/call/text plan for 12 months. If participants already own a mobile phone, research staff members offer to migrate participants’ personal phone numbers and contacts to the study smartphone so that it can be used as their primary device. Once they receive a study smartphone, participants are asked to carry it with them as they go about their day and to charge it in the room with them when they slept.
Description of the CrossCheck System
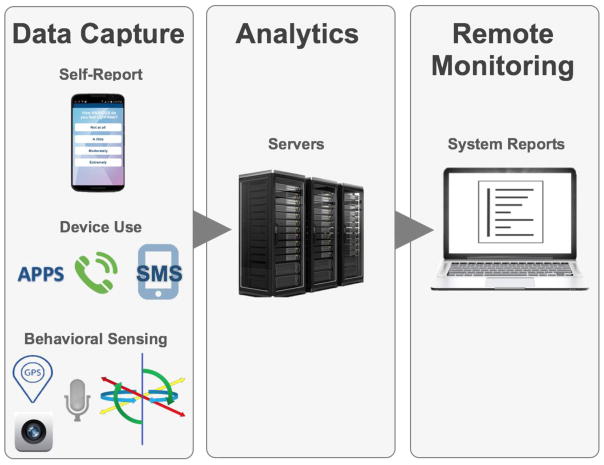
CrossCheck administers Ecological Momentary Assessment digital self-report questionnaires for “active” assessment (i.e., the participant is active in that they are asked to respond to questions administered on the smartphone touchscreen). The system also collects multi-modal behavioral sensing and device use data continuously (Figure 1). These forms of data collection are “passive”, i.e., CrossCheck stores and classifies data with no prompting, indication, or response burden placed on the user. While passive data collection is invisible, it is not covert; participants receive complete explanation of all CrossCheck monitoring functions during the informed consent process and are encouraged to contact research staff throughout their participation if they have any questions or concerns. Research staff encourage participants to carry the smartphone with them throughout the day to ensure the system collects valid data continuously. Study staff also call participants if it appears that there is a problem with passive data collection (e.g., location services have been deactivated, battery is not being charged) or data integrity (accelerometers suggest the device is perpetually stationary indicating the participant is not taking it with them).
Figure 1.
Overview of the CrossCheck System.
Ecological Momentary Assessment (EMA)
The smartphone software administers a 10-item self-report measure every Monday, Wednesday, and Friday. The user interface is simple and intuitive. Each question appears on a single screen with touchscreen response buttons underneath. Response options range from 0- “Not at all” to 3- “Extremely”. Once a user selects and taps on a response, their answer is recorded and the next question appears on a new screen. Questions are framed as single sentences with the key word in each question written in capital letters to draw the user’s attention to the most relevant word quickly (Ben-Zeev et al., 2012). The measure focuses on symptoms of psychosis (e.g., hallucinations, persecutory ideation), general mental health (e.g., stress, depression, hopefulness, calmness, clarity of thought), and functioning (e.g., socialization, sleep) (see Table 1 for complete list of questions). Items can be used as individual indicators or tallied as a single composite score which ranges from (-15 to +15). A lower composite score indicates higher risk, as it suggests greater severity of symptoms and poorer functioning (e.g., more hallucinations, reduced ability to think clearly, fewer social interactions).
Table 1.
Complete list of EMA questions
| Have you been feeling CALM? |
| Have you been SOCIAL? |
| Have you been bothered by VOICES? |
| Have you been SEEING THINGS other people can’t see? |
| Have you been feeling STRESSED? |
| Have you been worried about people trying to HARM you? |
| Have you been SLEEPING well? |
| Have you been able to THINK clearly? |
| Have you been DEPRESSED? |
| Have you been HOPEFUL about the future? |
Options: 0= Not at all; 1= A little; 2= Moderately; 3= Extremely
Behavioral Sensing
Physical Activity: Multi-axial accelerometers embedded in the device combined with Google Activity Recognition API (Application Programming Interface) are used to determine whether the user is on foot, sedentary, in a vehicle, on a bicycle, or unknown. Data are collected continuously. CrossCheck generates an activity rating every 10 seconds when the user is moving, or every 30 minutes if the device is stationary (i.e., labeled as sedentary). The system computes the duration of stationary vs. active periods for each day. Geospatial Activity: The embedded Global Positioning System (GPS), WiFi, and cellular tower location services provide an optimized location estimate. Receivers are activated every 10 minutes. GPS calculates the participants’ location using signals sent by satellites. The WiFi and Cellular tower receivers on the phone scan access points in its vicinity and record location. GPS and the other localization systems run independently; if GPS-derived location is unavailable the system uses the secondary indicators as the entry. Once a signal is received, the participant’s location is geo/time-stamped. The next time the positioning system is activated, it records the participant’s location. The system calculates the following set of features derived from the location estimation: distance traveled, standard deviation of distances, duration of time spent at the primary location (i.e. where participants spend most of their time), maximum displacement from the primary location, and location entropy. Speech Frequency and Duration: The smartphone microphone is activated every three minutes to capture ambient sound. CrossCheck uses speech detection software to identify periods in which human speech was present. To protect participant privacy, we use a speech detection system that does not record raw audio on the device, but instead, destructively processes the data in real-time to extract, classify, and store features that are useful to infer the presence of human speech but not enough to reconstruct conversation content. The data collection software remains active for the time human speech is present, logging start and end times. Speech frequency (i.e., number of individual “events” separated by 5 minutes or more of no human speech) and speech duration (length in minutes of each individual “event”) are calculated and stored continuously.
Device Use
Telecommunication: CrossCheck records the number and duration of incoming and outgoing phone calls and number of incoming and outgoing text messages (i.e., Short Message Service or SMS). In both cases, the spoken content of conversations and written content of text exchanges is not recorded to preserve user privacy. App Use: The system records the number of times an individual launched apps (i.e., software applications installed on the smartphone) daily. We calculate the total number of apps used daily and a subtotal for three app categories; social media (e.g., Facebook, Twitter, Instagram), activity (e.g., health and fitness, transportation and navigation, finance), and entertainment (e.g., YouTube, Netflix, Games). Phone Unlock: All study smartphones are programmed to lock after 30 seconds of user inactivity (e.g., typing, scrolling, repositioning). Once locked, the individual needs to type in their code on the touchscreen to unlock the device and access the smartphone features (e.g., emergency 911 calls are the only function that is accessible when the device is locked). CrossCheck records the number of times the device is unlocked and the total duration of unlocked time per day. Periods in which the smartphone is “unlocked” indicate that the participant is actively using smartphone functions of one kind or another.
Data Transmission and Review
All data are temporarily stored on the smartphone and are uploaded automatically via internet connection to a remote secure study server when participants recharge the smartphone battery. Once data are received they are processed and added to the individual’s existing data set. Incoming data are summarized in the form of an automated digital daily report that is sent to to the investigative team for regular quality checks and ongoing review of individual participant’s clinical status. Checks were performed weekly to identify problems including the inspection of long intervals of missing or invariant data which may be a product of sensor malfunctions, data upload or transmission failures, or user disengagement. If cases were identified, the research team would then contact participants to inquire if there were any technical issues experienced (e.g., GPS deactivated, microphone malfunctioned, lost phone, uninstalled app, malfunctioning charging cable) and would provide support as needed (e.g., replacement of hardware, reinstallation of software). In order to be comparable between days, sensor data on a given day is excluded if the sensor was active for less than 19 hours during the day (e.g. device was off, GPS disabled, microphone used for other applications). Thus, the availability of sensor feature data varies by individual and by feature. We present sensor data as daily aggregated values and available data percentages are reported for each sensor feature.
Clinical experts on the research team evaluate the content of participants’ responses to EMA items and sensor-indicated patterns. In the earlier stages of this ongoing study, if an individual demonstrated worrying patterns over several reports (e.g. self-reported worsening of symptoms, not leaving their primary location for days at a time, longer periods away from human speech) they were placed on a watch list which three members of the investigative team monitored closely and discussed as part of a weekly clinical meeting that was designed to determine whether there was a need to reach out to them and/or their providers. As more data from participants accumulated over the course of the study, this process became automated. If individuals have a risk score that exceeds a pre-determined threshold, the study clinical team reaches out to them directly for a phone check-in. Based on the outcome of their conversation, the investigators decide whether there is a need to contact their outpatient providers to trigger more intensive outreach and services.
Selection of Participants
For the current manuscript, we inspected visualizations of the EMA responses and several features of the sensor stream data from participants who experienced a hospitalization. Five participant data sets were selected based on their ability to demonstrate the wide range of possibilities for detecting relapse events using CrossCheck.
Results
Five participant data sets were curated from the CrossCheck arm of the study for instructive purposes. These individuals were selected because they experienced psychiatric hospitalizations during the study period (i.e., an objective indicator of relapse) and their smartphone data were viewed by our research team as particularly useful for demonstrating different patterns and considerations for preliminary modeling and data interpretation. Hospitalization periods are used as reference points and are represented in all figures by blue shading. Gaps in data following hospitalization represent the periods that participants were staying at inpatient units where the use of smartphones was prohibited or where sensor data was poor or not provided by the participant (e.g., turned location services off). Multi-modal data collection resumed when participants were discharged and started using the study device again.
Participant 1: Self-reported symptom exacerbation and decline in functioning prior to relapse
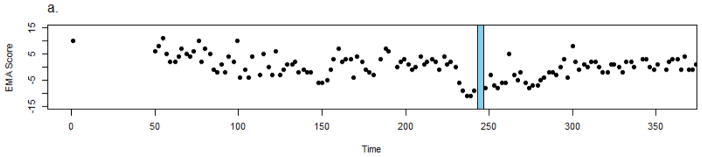
Participant 1 is a 32 y.o. multi-racial Hispanic female with a diagnosis of schizoaffective disorder who was enrolled in the CrossCheck arm for 243 days before being hospitalized for exacerbation of psychotic symptoms, conflicts at work/socially, and feeling unsafe at home as she had active suicidal ideation. She reported feeling bullied by her neighbors and thought that they were trying to force her out of her current job. She said she could hear them talking about her through the walls of her apartment. Once hospitalized, she reported hearing clinical staff talk about her through the walls. She later acknowledged the voices were not real.
Following a hospitalization of five days, she was discharged. Figure 2 shows the trajectory of her self-reported EMA composite score (lower scores indicate poorer functioning). EMA data were available approximately 84% of days (out of possible 3 days per week administered throughout their participation). The data suggest an initial gradual decline in EMA score spanning approximately 100 days, improvement and stabilization for approximately 80 days, followed by a sharp decline in EMA scores over the course of two and a half weeks before hospitalization (all EMA composite scores below zero). Following discharge, the participant re-engaged in mobile self-monitoring. While there was on overall trend of self-reported improvement following discharge, notably almost all EMA scores remained below zero for approximately 50 days.
Figure 2.
Crosscheck data for Participant 1.
Participant 2: No change in self-report but major change in sensor data prior to relapse
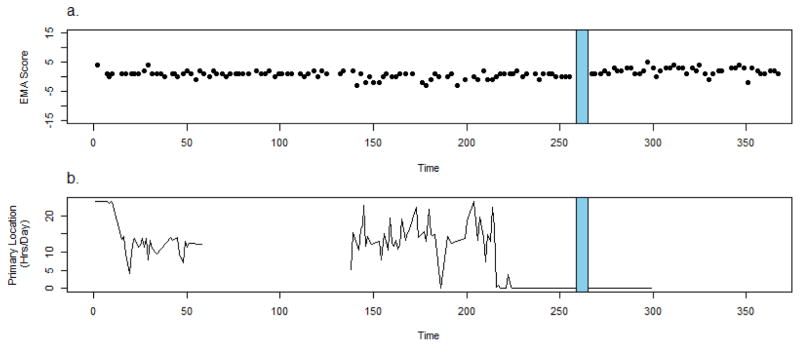
Participant 2 is a 35 y.o. African-American female with a diagnosis of schizoaffective disorder who was hospitalized after 259 days of CrossCheck data collection following worsening depression, paranoia, and nonadherence to her treatment regimen (gleaned from clinic notes). She reported believing that people were following her with the intent of harming her and her family members prior to hospitalization. The participant believed people planted cameras in her environment and were poisoning her food so she stopped eating and drinking, leading to considerable weight loss. The participant reported she had stopped taking her medication three weeks prior to relapse, but could not explain why.
The participant’s EMA scores do not vary much prior to relapse and there were no self-reported changes in her clinical status or behavior (EMA available approximately 89% of the days administered) (Figure 3a). However, passively collected geospatial data recorded for this period indicate major behavioral changes. Figure 3b shows that during the first 220 days of monitoring most days the participant spent a substantial portion of her time (average 12 hours per day) in a single location (identified as “primary” by the CrossCheck system, location data were available for 40%). Approximately 40 days prior to hospitalization the participant ceases (in all but 1 day) to spend any time at the primary location. The juxtaposition of EMA and behavioral sensing data demonstrates that while self-report may be sufficient and appropriate for remote monitoring of clinical status in some (e.g., participant 1), it is less useful for others. For individuals who do not articulate changes via self-report, additional data sources may be necessary and potentially more fruitful for identification of meaningful changes.
Figure 3.
Crosscheck data for Participant 2.
It is possible that this participant may have been less invested in reflecting about her condition during self-assessments. She may have had poor insight to illness or limited awareness of changes in her status as they occur. It is also possible that the items in the EMA questionnaire were not well-suited for her specific symptom manifestation. However, based on her recollections of the period preceding her relapse, a more likely explanation is that in the context of her intensified persecutory ideation, this participant may have been apprehensive about openly reporting changes in her thinking, functioning, and behavior.
Participant 3: Self-report and sensor indicators prior to relapse
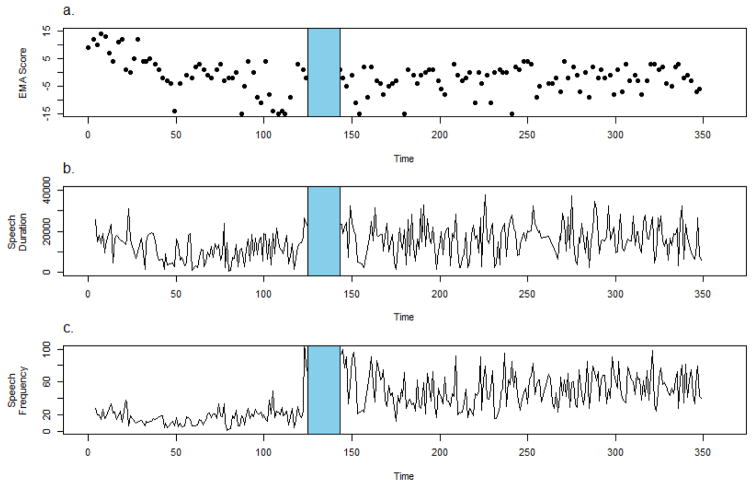
Participant 3 is a 19 y.o. African-American male with a diagnosis of schizoaffective disorder who was hospitalized after 125 days of CrossCheck monitoring following an episode of substance abuse and intense religious delusions and hallucinations. The participant reported seeing a sprit who instructed him to threaten his sister (who is of a different religion which he believed to be evil) and to destroy her possessions. He set fire to her dolls and threatened her with a knife.
The participant’s EMA data suggest a deterioration in his clinical status prior to hospitalization (Figure 4a). The participant reports doing well at the beginning of the data collection period (Maximum EMA score = 15), but then experienced a significant decline over the course of 3 months (Minimum EMA score= -15). EMA data were available for approximately 91% of days administered. Sensor data show a decline in both speech frequency and duration over the course of approximately 50 days, an increase over 70 days, followed by a salient upswing in speech duration and frequency in the final week prior to hospitalization (Figures 4b and 4c). Audio sensor data were available for 87% of days.
Figure 4.
Crosscheck data for Participant 3.
It is possible that when this participant initially experienced deterioration he withdrew from others, but when his symptoms became worse he engaged more (e.g., more agitated, confrontational, threating interactions). Given that the CrossCheck speech detection system does not distinguish between the primary user’s voice and those in their immediate vicinity, we cannot conclude that the individual was not alone; it is possible that the increase in speech frequency and duration prior to hospitalization reflects an increase in the participants’ vocal response (captured by the microphone) to the hallucinated “spirit”. This interpretation is further supported by the fact that the participant’s EMA scores suggest an increase in the frequency of hallucinations during the final weeks before hospitalization.
Participant 4: Self-report, sensor data, and device use indicators prior to relapse
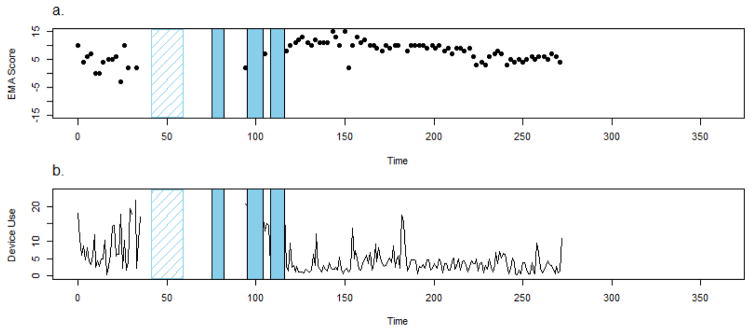
Participant 4 is a 27 y.o. white female with a diagnosis of psychosis not otherwise specified who reported multiple hospitalizations over the course of 3 months. The first hospitalization did not take place at the primary study site and the participant’s self-report could not be corroborated with EMR data (depicted in figure 5 with hashed lines). Following discharge, the participant reported losing her study smartphone. She was then hospitalized a second time for 7 days at the primary study site. Following discharge she received a replacement device by on-site study staff but was hospitalized the very next day for an additional 9 days at the primary study site (corroborated by EMR notes). Following discharge, she reengaged in the study briefly, but was hospitalized a fourth time for 7 days at the primary study site. The participant described feeling “possessed” and “cursed” during this tumultuous period. She reported believing she had telepathic abilities and feeling convinced that others can implant and extract her thoughts. The participant stated that she stopped taking her medications for a while because she thought prayer was sufficient to help ward off spirits.
Figure 5.
Crosscheck data for Participant 4.
The participant’s EMA data show a self-reported symptomatic and functional deterioration over the first 40 days using CrossCheck (Figure 5a). EMA data were available for approximately 70% of the days administered). Sensor data also suggested declines in physical activity (64% available), geospatial activity (75% available), and speech frequency and duration (60% available). Most striking, however, is this participant’s device use pattern (available 74% of days). Four days before her first hospitalization, the participant stopped using the device altogether and all forms of data upload desist. From a remote monitoring perspective, the participant “goes dark” (Figure 5b). Following her first discharge, she remains unmonitored because she lost the device and did not receive a replacement smartphone for several weeks following her second discharge. She then used CrossCheck for only one day (Figure 5a and 5B) but was hospitalized a third time and once again “goes dark”. Following her third discharge, she reengages in the study “reappears” for several days. CrossCheck data upload is ended abruptly with her fourth hospitalization. Following her final discharge, the participant immediately resumed CrossCheck use; data was uploaded continuously and the participant remained out of the hospital (Figure 5). This participant demonstrates that in some instances, the participant’s willingness and/or ability to maintain remote monitoring may also convey important information about their clinical status.
Participant 5: Atypical device use prior to relapse
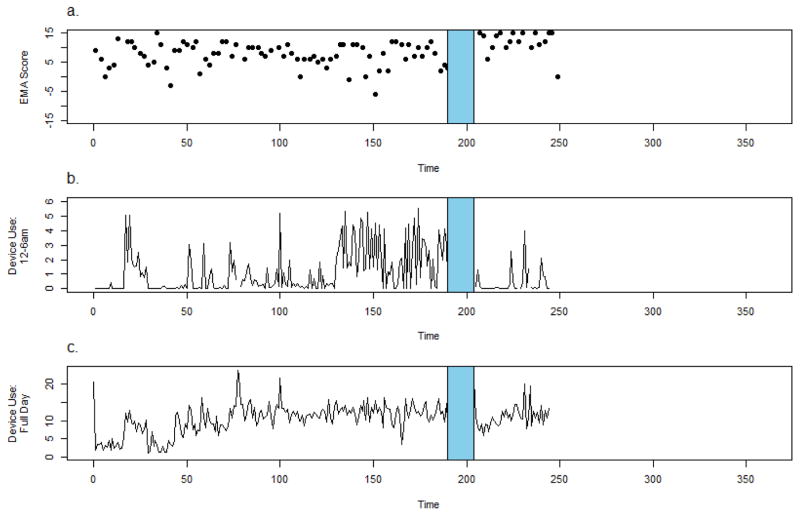
Participant 5 is a 19 y.o. female Hispanic American-Indian with a diagnosis of schizophrenia who was hospitalized after 190 days of CrossCheck data collection following worsening depression, poor sleep and homicidal ideation in the context of nonadherence with her medication regimen. The participant was admitted following an outpatient therapy session in which she described thinking about killing a young child and a dog.
CrossCheck EMA measures are administered during daytime hours so as not to disturb participants’ sleep. However, sensor and device use data are collected continuously, giving us unique observations of the nighttime hours that would otherwise be inaccessible. Examining data collected during different times of the day can shed light on individuals’ sleep/wake/activity schedules. Figure 6b depicts device “unlock duration” between midnight and 6 AM (device use data were available for 93% of days, and approximately 91% available for EMA). Unlocking the device precedes intentional smartphone use by the participant (e.g., online searches, watching video, playing games). For the first 150 days of her participation in monitoring, the participant’s’ device was typically locked during these nighttime hours. Approximately 60 days before her hospitalization, we see an increase in device “unlock duration” after midnight signaling device use during this time. Comparatively, device use data for the day did not change markedly during this time (Figure 6c).
Figure 6.
Crosscheck data for Participant 5.
Combined, these patterns suggest a behavioral change; the participant was more active at night prior to their relapse. Following her discharge from the hospital, the participant resumed participation in the study and nighttime activity dropped.
Discussion
Worldwide, the majority of people with psychotic disorders now own mobile phones, and many are open to using them as instruments that support mHealth approaches that can improve their wellbeing, illness-management, and recovery (Firth et al., 2015). This paper described CrossCheck-- a novel smartphone data collection system that combines active self-reporting and passive behavioral sensing as a method for remote monitoring of people with psychosis.
To our knowledge, the 12-month data collection period described in this paper constitutes the longest reported deployment of an mHealth smartphone app among people with psychosis to date. Further, this paper is also the first to report on preliminary attempts to explore the relationships between smartphone-captured within-person behavioral patterns and psychotic relapse events (Firth & Torous, 2015).
Our preliminary findings point to several directions. First, it is apparent that some people with psychosis are willing and able to engage in multi-modal illness monitoring using smartphones for extended periods of time (up to a year). Not all participants in the ongoing CrossCheck study complete a full year of data collection, adhere to the study protocol (i.e., carry the smartphone with them, charge the battery, complete self-reports regularly), or experience hospitalizations. Participants were selected for this paper because they were hospitalized and had data sets which were useful in demonstrating different applications of the CrossCheck system. It was striking to see that these individuals continued to use the smartphone and generate informative and potentially actionable data even as their clinical status was deteriorating. Moreover, once discharged from the hospital, they resumed their use of the smartphone and re-engaged with illness monitoring. These early findings bode well for the acceptability and feasibility of CrossCheck and similar mobile systems among some people with severe psychiatric disabilities.
Second, while it is already known that there is tremendous biological, behavioral, and clinical heterogeneity in people with schizophrenia-spectrum disorders (Carpenter & Kirkpatrick, 1988; Tsuang, & Faraone, 1995; Joyce et al., 2005; Pulver et al. 2000) this study demonstrates that there is also significant heterogeneity in the digital traces of their psychotic relapses. In some, self-reported digital ratings painted a clear and potentially actionable picture. In others, passively sensed and device use data were more useful in identifying changes in their behavior and functioning. Participants had unique daily data values (e.g., minutes proximal to human speech) and data trends (e.g., increase vs. decrease in daily exposure to human speech over several weeks) before hospitalization. It is likely that there are different relapse-related patterns across individuals. Longitudinal examination of whether mobile data trends that were present before one relapse re-appear before subsequent relapses will help illuminate whether some people have consistent digital relapse signatures. For individuals with recurring patterns, time-sensitive notifications and prompts to the user and/or their clinical team when the data suggest these patterns are reemerging may have clinical utility.
The potential of leveraging integrated mobile data collection to improve detection, treatment, prevention, and rehabilitation is exciting but this novel field is not without its unique challenges. The validity of sensor data is highly dependent on how people use their devices; For example, an individual who does not carry their mobile device with them regularly will produce data that may suggest they are periodically socially isolative (i.e., minimal human speech captured by the microphone, reduced text and call activity) or physically inactive (e.g., limited accelerometery data suggesting sedentary behavior) when this is not the case. People have different battery charging patterns or may share their device with others in their household; both scenarios would produce instances when data collected were not representative of the intended individual’s behavior. Storing, securing, managing and making sense of the enormous amount of data that mobile devices can collect is not trivial. These innovative data collection approaches necessitate new data modeling strategies that can control for “noise”, construct/extract useful elements, and identify data features or patterns that may be actionable. Ultimately systems like CrossCheck may not generate clinically useful data for everyone. Future research with larger samples will allow us to examine whether there are individual demographic, clinical, behavioral, or device use variables that can suggest for whom smartphone-enabled data collection is likely to be useful and for whom these remote monitoring and detection methods are less suitable.
Limitations
First, the data sets presented in this paper were drawn from a study that is still underway. We selected demonstrative cases and decided to report on preliminary findings because of the state of the field; approximately one third of people with schizophrenia already use smartphones that are capable of hosting systems similar to CrossCheck (Firth et al., 2015) and many more are expected to gain access to smartphones in the near future (Ben-Zeev, 2016). Researchers are actively exploring multi-modal smartphone data collection techniques as methods for supporting mental health research and intervention (e.g., Abdullah et al., 2016; Faurholt-Jepsen et al., 2014; Saeb et al., 2015; Torous et al., 2015). Insurers, digital health purveyors, and private sector technology companies have already begun to offer multi-modal smartphone monitoring systems to the general population with promises of clinical utility. With this backdrop of heightened activity and rapid commercial development, we thought it would be informative for readers to learn about the current state-of-the-science, as it pertains to illness monitoring of people with schizophrenia-spectrum disorders and identification of psychotic relapse. Second, although participants are instructed not to loan their devices to others, we cannot verify that they were the only individuals who used the smartphone. If someone from their household picked up the device to move it from one room to another, the accelerometers would log this movement even if the device was locked. Thus it is possible that some data are not representative of the intended user’s behavior. Finally, sensed data may not always capture the intended construct. For example, the smartphone speech detection system may not be fully capable of distinguishing live human speech from television-generated audio, making it difficult to determine with complete accuracy whether a participant was actually in a social environment or alone (Ben-Zeev et al. 2015).
Conclusions and Implications for Practice
Advancements in mobile software and hardware are enabling collection of an abundance of data that until recently were completely inaccessible to clinical research and practice. The current work illustrates the potential of this data to identify patterns in behavior associated with symptomatic decline. As data collection and follow-up for this study concludes, inferential modeling of sensor stream features may allow us to understand critical behavioral patterns that underlie symptomatic decline and clinical relapse events. Future predictive modeling implemented in mHealth applications in real time may be able to assist clinicians in identifying the start of symptom decline and relapse patterns earlier than previously possible. These new sources of information are beginning to enhance our understanding of the dynamic nature of psychosis and the nuanced behavioral manifestations of chronic illness. However, remote monitoring and relapse detection via mobile technology is in its nascency. While these developments in assessment and monitoring technology are exciting, we caution against prematurely concluding that they will lead to substantive improvements in treatment or prevention. The science in this area is evolving and how best to use these data is yet to be determined. Development and evaluation of innovative data management, modeling, and signal-detection techniques that identify changes within an individual over time (i.e. unique digital relapse signatures) will be essential if we are to create opportunities for novel interventions that can capitalize on intensive data influx to improve individual outcomes via context-informed just-in-time mHealth interventions.
References
- Abdullah S, Matthews M, Frank E, Doherty G, Gay G, Choudhury T. Automatic detection of social rhythms in bipolar disorder. JAMIA. 2016;(3):538–43. doi: 10.1093/jamia/ocv200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher-Svanum H, Zhu B, Faries DE, Salkever D, Slade EP, Peng X, Conley RR. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D. Mobile Health for All: Public-Private Partnerships Can Create a New Mental Health Landscape. JMIR Mental Health. 2016;3(2):e26. doi: 10.2196/mental.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, Ellington K, Swendsen J, Granholm E. Examining a cognitive model of persecutory ideation in the daily life of people with schizophrenia: a computerized experience sampling study. Schizophrenia Bulletin. 2011;37(6):1248–1256. doi: 10.1093/schbul/sbq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, McHugo G, Xie H, Dobbins K, Young MA. Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and a nonclinical comparison group. Schizophrenia Bulletin. 2012;38(3):396–404. doi: 10.1093/schbul/sbr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, Wang R, Abdullah S, Brian R, Scherer EA, Mistler LA, Hauser M, Kane J, Campbell AT, Choudhury T. Mobile Behavioral Sensing in Outpatients and Inpatients with Schizophrenia. Psychiatric Services. 2016;67(5):558–561. doi: 10.1176/appi.ps.201500130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, Scherer EA, Wang R, Xie H, Campbell AT. Next-generation psychiatric assessment: Using smartphone sensors to monitor behavior and mental health. Psychiatric Rehabilitation Journal. 2015;38(3):218–226. doi: 10.1037/prj0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Spencer E, McGovern D. Schizophrenia: early warning signs. Advances in Psychiatric Treatment. 2000;6:93–101. [Google Scholar]
- Carpenter WT, Jr, Kirkpatrick B. The heterogeneity of the long-term course of schizophrenia. Schizophrenia Bulletin. 1988;14(4):645. doi: 10.1093/schbul/14.4.645. [DOI] [PubMed] [Google Scholar]
- Cohen LJ. Psychiatric hospitalization as an experience of trauma. Archives of Psychiatric Nursing. 1994;8(2):78–81. doi: 10.1016/0883-9417(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Coid JW, Ullrich S, Bebbington P, Fazel S, Keers R. Paranoid ideation and violence: meta-analysis of individual subject data of 7 population surveys. Schizophrenia Bulletin. 2016;42(4):907–915. doi: 10.1093/schbul/sbw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan PW, River LP, Lundin RK, Uphoff-Wasowski K, Campion J, Mathisen J, Goldstein H, Bergman M, Gagnon C, Kubiak MA. Stigmatizing attributions about mental illness. Journal of Community Psychology. 2000;28:91–102. [Google Scholar]
- Faurholt-Jepsen M, Vinberg M, Frost M, Christensen E, Bardram J, Kessing L. Smartphone Data as an Electronic Biomarker of Illness Activity in Bipolar Disorder. Psychiatry Research. 2014;217(1–2):124–127. [Google Scholar]
- Freeman D, Emsley R, Dunn G, Fowler D, Bebbington P, Kuipers E, … Garety P. The stress of the street for patients with persecutory delusions: a test of the symptomatic and psychological effects of going outside into a busy urban area. Schizophrenia Bulletin. 2014;41(4):971–979. doi: 10.1093/schbul/sbu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh BC, Knapp RG, Cusack KJ, Grubaugh AL, Sauvageot JA, Cousins VC, … Hiers TG. Special section on seclusion and restraint: Patients’ reports of traumatic or harmful experiences within the psychiatric setting. Psychiatric Services. 2005;56(9):1123–1133. doi: 10.1176/appi.ps.56.9.1123. [DOI] [PubMed] [Google Scholar]
- Firth J, Cotter J, Torous J, Bucci S, Firth JA, Yung AR. Mobile phone ownership and endorsement of “mHealth” among people with psychosis: a meta-analysis of cross-sectional studies. Schizophrenia Bulletin. 2015;42(2):448–455. doi: 10.1093/schbul/sbv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J, Torous J. Smartphone Apps for Schizophrenia: A Systematic Review. JMIR mHealth uHealth. 2015;3(4):e102. doi: 10.2196/mhealth.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JF, Rawlings D, Jackson HJ, McGorry PD. Early warning signs of relapse following a first episode of psychosis. Schizophrenia Research. 2005;80:107–11. doi: 10.1016/j.schres.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Hor K, Taylor M. Review: Suicide and schizophrenia: a systematic review of rates and risk factors. Journal of Psychopharmacology. 2010;24(4 suppl):81–90. doi: 10.1177/1359786810385490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne WB, Folsom DP, Sommerfeld DH, Lanouette NM, Lewis M, Aarons GA, … Jeste DV. Incarceration among adults who are in the public mental health system: Rates, risk factors, and short-term outcomes. Psychiatric Services. 2012;63(1):26–32. doi: 10.1176/appi.ps.201000505. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Hutton SB, Mutsatsa SH, Barnes TR. Cognitive heterogeneity in first-episode schizophrenia. The British Journal of Psychiatry. 2005;187(6):516–522. doi: 10.1192/bjp.187.6.516. [DOI] [PubMed] [Google Scholar]
- Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuidical self-injury in England: results from a national survey. PloS one. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman RP, Kopelowicz A. Recovery from schizophrenia: a concept in search of research. Psychiatric Services. 2005 doi: 10.1176/appi.ps.56.6.735. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Frame L, Larkin W. Relationships between trauma and psychosis: a review and integration. British Journal of Clinical Psychology. 2003;42(4):331–353. doi: 10.1348/014466503322528892. [DOI] [PubMed] [Google Scholar]
- Morriss R, Vinjamuri I, Faizal MA, Bolton CA, McCarthy JP. Training to recognise the early signs of recurrence in schizophrenia. Cochrane Database of Systemic Reviews. 2013;2:CD005147. doi: 10.1002/14651858.CD005147.pub2. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Meyer PS, Penn DL, Clancy R, Clancy DM, Salyers MP. The illness management and recovery program: Rationale, development, and preliminary findings. Schizophrenia Bulletin. 2006;32(suppl 1):S32–S43. doi: 10.1093/schbul/sbl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Bentall R, Delespaul P, Myin-Germeys I. Temporal dynamics of visual and auditory hallucinations in psychosis. Schizophrenia Research. 2012;140(1):77–82. doi: 10.1016/j.schres.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Mulle J, Nestadt G, Swartz KL, Blouin JL, Dombroski B, … Lasseter VK. Genetic heterogeneity in schizophrenia: stratification of genome scan data using co-segregating related phenotypes. Molecular Psychiatry. 2000;5(6):650–653. doi: 10.1038/sj.mp.4000814. [DOI] [PubMed] [Google Scholar]
- Roe D. Recovering from severe mental illness: mutual influences of self and illness. Journal of Psychosocial Nursing and Mental Health Services. 2005;43(12):34–40. doi: 10.3928/02793695-20051201-05. [DOI] [PubMed] [Google Scholar]
- Saeb S, Zhang M, Karr CJ, Schueller SM, Corden ME, Kording KP, Mohr DC. Mobile Phone Sensor Correlates of Depressive Symptom Severity in Daily-Life Behavior: An Exploratory Study. Journal of Medical Internet Research. 2015;17(7) doi: 10.2196/jmir.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniel F, Bakstein E, Anyz J, Hlinka J, Sieger T, Hrdlicka J, … Höschl C. Relapse in schizophrenia: Definitively not a bolt from the blue. Neuroscience Letters. 2016 doi: 10.1016/j.neulet.2016.04.044. [DOI] [PubMed] [Google Scholar]
- Sun SX, Liu GG, Christensen DB, Fu AZ. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Current Medical Research and Opinion. 2007;23(10):2305–2312. doi: 10.1185/030079907X226050. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Harrow M, Grossman LS, Rosen C. Periods of recovery in deficit syndrome schizophrenia: A 20-year multi-follow-up longitudinal study. Schizophrenia Bulletin. 2010;36:788–799. doi: 10.1093/schbul/sbn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Ben-Zeev D, Granholm E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. American Journal of Psychiatry. 2011;168(2):202–209. doi: 10.1176/appi.ajp.2010.10030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torous J, Kiang MV, Lorme J, Onnela JP. New Tools for New Research in Psychiatry: A Scalable and Customizable Platform to Empower Data Driven Smartphone Research. JMIR mental health. 2015;3(2):e16–e16. doi: 10.2196/mental.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annual Review of Clinical Psychology. 2013;9:151–176. doi: 10.1146/annurev-clinpsy-050212-185510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV. The case for heterogeneity in the etiology of schizophrenia. Schizophrenia Research. 1995;17(2):161–175. doi: 10.1016/0920-9964(95)00057-s. [DOI] [PubMed] [Google Scholar]