Abstract
Objective
Major depressive disorder (MDD) is common and associated with impaired functioning after traumatic brain injury (TBI). Few placebo-controlled antidepressant trials exist in this population. We evaluated the efficacy and tolerability of sertraline for MDD within one year of sustaining a TBI.
Setting
Level I trauma center.
Participants
Adults with MDD within one year of hospitalization for complicated mild to severe TBI.
Design
Randomized, double-blind, placebo-controlled trial.
Main Measures
12-week treatment response on 17-item Hamilton Depression Rating Scale. We also assessed symptom improvement and remission.
Results
We randomized 62 participants: 32% sustained a severe TBI, 68% had significant anxiety, 63% had history of prior MDD, 69% had history of alcohol or drug dependence. Depression significantly improved from baseline to 12 weeks in both treatment groups (P<.001). There were no significant differences between the sertraline and placebo groups over 12 weeks on depression severity, response or remission. The sertraline group had significant improvement on speed of information processing compared to the placebo group (P<.006).
Conclusion
Sertraline monotherapy was not superior to placebo for MDD in people with post-acute complicated mild to severe TBI. Research is needed on the effectiveness of interventions that also address the significant psychosocial needs of this population.
Keywords: traumatic brain injury, head injury, depression, antidepressant, placebo, treatment, clinical trial, quality of life
Introduction
Major depressive disorder (MDD) is prevalent following traumatic brain injury (TBI). The highest risk for MDD occurs within the first year following injury, where over half of individuals who sustain a complicated mild to severe TBI experience MDD.1 MDD is significantly associated with comorbid anxiety2 and substance abuse1 and decreased quality of life.1,3 Despite the high rate of MDD and associated adverse outcomes during this post-acute period, less than half of those with MDD receive any medication or psychotherapeutic treatment for their depression.1
Few randomized controlled trials (RCTs) have examined the efficacy of antidepressants for MDD following TBI, despite the frequent use of these medications in this population.4 Systematic reviews of depression treatment studies reveal only one evidence class I5 and one evidence class II6 pharmacotherapy study.7–10 Based on this preliminary data on efficacy and tolerability, the strongest evidence supports the use of selective serotonin reuptake inhibitors (SSRIs), particularly sertraline and citalopram.
Two preliminary studies by Fann et al.11 and Turner-Stokes et al.12 provided evidence for the potential efficacy and tolerability of sertraline in persons with TBI and MDD. Findings from our group’s initial pilot study suggested that sertraline was not only well-tolerated and potentially efficacious for treating MDD in persons with mild TBI, but also associated with improvements in anger and aggression, functional status, postconcussive symptoms, and domains of subjective and objective cognitive functioning.11,13
In the only published evidence class I pharmacotherapy study, Ashman and colleagues5 randomized 52 patients with mild, moderate or severe TBI to sertraline or placebo (41 patients completed the study). The mean time since injury was 17.7 (SD 13.7) years, making it difficult to ascertain the relative contribution of the TBI to their depressive episode. Although there were no statistically significant group differences in response rates or decrease in Hamilton Depression Rating Scale (HAM-D) scores over 10 weeks, among the completers 59% of the sertraline group were responders (≥50% decrease in baseline HAM-D), while 32% of the placebo group responded. The authors did not report final dosage ranges or specifics about adverse effects, though they report that only one subject withdrew due to adverse effects.
The aims of the current trial were to: (1) compare the efficacy of sertraline and placebo for MDD during the first year following complicated mild to severe TBI, (2) determine tolerability of treatment with sertraline compared with placebo, and (3) evaluate secondary outcomes, including quality of life, disability, speed of information processing, postconcussive symptoms, pain, anger and aggression, and anxiety.
Methods
Procedures and Participants
Eligibility criteria for the study are described elsewhere1 and included admission to Harborview Medical Center (a level I trauma center in Seattle, Washington) with TBI and radiological evidence of acute, traumatically induced brain abnormality or Glasgow Coma Scale (GCS) score lower than 13 (based on the lowest score within 24 hours after admission or the first after paralytic agents were withdrawn). Participants were at least 18 years old and English speaking. We obtained a waiver of consent to determine eligibility; otherwise, participation required written consent. Study procedures were approved by the University of Washington institutional review board and followed guidelines from the Health Insurance Portability and Accountability Act.
During the Surveillance Phase of the study, cases with probable MDD were identified using a structured interview based on the Patient Health Questionnaire 9-item depression scale (PHQ-9), which has excellent inter-rater reliability (0.99) and diagnostic sensitivity (0.93) and specificity (0.89) in individuals with TBI.14,15 At screening, history of prior psychiatric disorders and involvement in litigation were assessed.
Subjects with probable MDD on the PHQ-9 were asked to undergo an in-person diagnostic interview using the Structured Clinical Interview for DSM-IV (SCID).16 To be included, patients had to meet criteria for MDD on the SCID and score at least 15 on the Hamilton Depression Rating Scale 17-item version (HAM-D).17 We excluded those who met SCID criteria for bipolar disorder, a psychotic disorder, or bereavement as well as those with suicide intent or plan. We also excluded people who had a prior adverse reaction or nonresponse to sertraline, had an unstable medical condition that would preclude treatment with sertraline, anticipated major surgery within the next three months, were already taking an antidepressant, or were pregnant or not using reliable birth control if a woman of childbearing age.
Randomization, which was conducted by the study biostatisticians (NT, JB), following baseline assessment, was 1:1 to sertraline or placebo and stratified by past history of MDD and history of alcohol or other substance dependence based on the SCID using permuted blocks of 2. Participants who consented to randomization were asked to refrain from engaging in non-study related pharmacotherapy or psychotherapy for depression during the course of the study.
Materials
Demographic, medical, radiologic, and International Classification of Diseases, Ninth Revision (ICD-9) diagnostic data were obtained via participant interviews, medical record reviews, and the Harborview Trauma Registry. The SCID was used to diagnose the presence of a current anxiety disorder. Race was obtained via self-report and record review. Other system injury severity was based on the Injury Severity Score excluding head injury.18 Serum blood alcohol level and toxicology screening results (cocaine and amphetamine) were collected when available.
Study assessments and dosage titrations occurred in person at baseline and 1, 3, 6, 8, 10 and 12 weeks. The primary depression outcome was the HAM-D 17-item version.17 As a secondary outcome, we also included the 6-item Maier subscale of the HAM-D19 because it is a unidimensional scale that excludes somatic items and therefore may have superior sensitivity to change in individuals with medical comorbidity.20–22 We used a structured version of the HAM-D23 for improved inter-rater-reliability. All research staff conducting screening, intervention and outcome procedures were blinded to randomization status.
Other secondary depression outcomes, assessed at each visit, were self-rated depression severity on the Symptom Checklist-2024 and the clinician-rated Clinical Global Impression scale (CGI).25 At baseline and 12 weeks, we also assessed quality of life (SF-36),26 subjective functional impairment (Sheehan Disability Scale),27 performance based assessment of speed of information processing (Trail Making Test B),28 bothersomeness of postconcussive symptom rated from 0–5 (Head Injury Symptom Checklist),29 anger and aggression (Brief Anger and Aggression Questionnaire),30 pain intensity (Brief Pain Inventory),31 and anxiety (Hamilton Anxiety Rating Scale, HAM-A).32
Medication titration and tolerability
Study medication (identical-appearing sertraline or inert placebo) was administered per a flexible-dose algorithm. Medication was started at 25 mg every morning. After 1 week, the dose was adjusted up to 50 mg per day, depending on tolerability. Further adjustment was made in week 3 up to 100 mg/day and up to 150 mg/day during week 6, as tolerated. At weeks 8 and 10 the dose was adjusted up to 200 mg/day, depending on clinical response on HAM-D and tolerability. Staff counted pills at each in-person visit to monitor adherence to study drug. Tolerability, defined as new or worsening side effects, was assessed at each follow-up using a standard side effect assessment that captured the onset, severity (none, mild, moderate and severe) and resolution of 25 medication-related symptoms.
Statistical Analysis
Exact logistic regression was used to compare treatment groups on rates of response (primary outcome) and remission. Mixed-effects linear regression models were used to compare treatment efficacy of sertraline with placebo.33 Analyses included observations at baseline, 1, 3, 6, 8, 10, and 12 weeks analyzed according to the intent-to-treat principle. Random effects were included for participants’ intercepts. Fixed effects were time (linear), treatment (sertraline vs. placebo), interaction of time with treatment, stratification factors, and potential confounders. The indicator of treatment effect was the interaction of time by treatment. Secondary outcomes were assessed using a similar strategy. Variables examined as potential confounders included: stratification variables, age and sex, time since injury (≤3 months vs. >3 months), medical comorbidity, history of depression, and current anxiety disorder. All analyses were adjusted for multiple comparisons where appropriate using the Holm-Bonferroni method.34 All analyses were performed using SAS (version 9.3).
Results
We identified 408 participants who met screening criteria, 234 completed baseline assessments (common reasons for exclusion were being bipolar, n=15, already being on an antidepressant, n=13, and not wanting to take an antidepressant, n=12), and 62 participants were randomized (Figure 1). Of those, 21 (68%) participants assigned to the sertraline group completed the medication protocol, compared to 28 (90%) in the placebo group (P=.059). Characteristics of the study sample are presented in Tables 1 and 2. The mean age of participants in the study was 37.5 (SD 12.5) years (range 18.9–66.6). Fifty (81%) were non-Hispanic White, 20 (32%) had a severe TBI, and 36 (67%) reported unstable employment status at the time of randomization. The mean time from TBI to randomization was 4.6 (SD 2.8) months (range 1.1–10.9). Thirty-eight (63%) reported a history of pre-injury depression, 43 (69%) reported a history of alcohol or drug dependence, and 42 (68%) had a current anxiety disorder.
Figure 1.
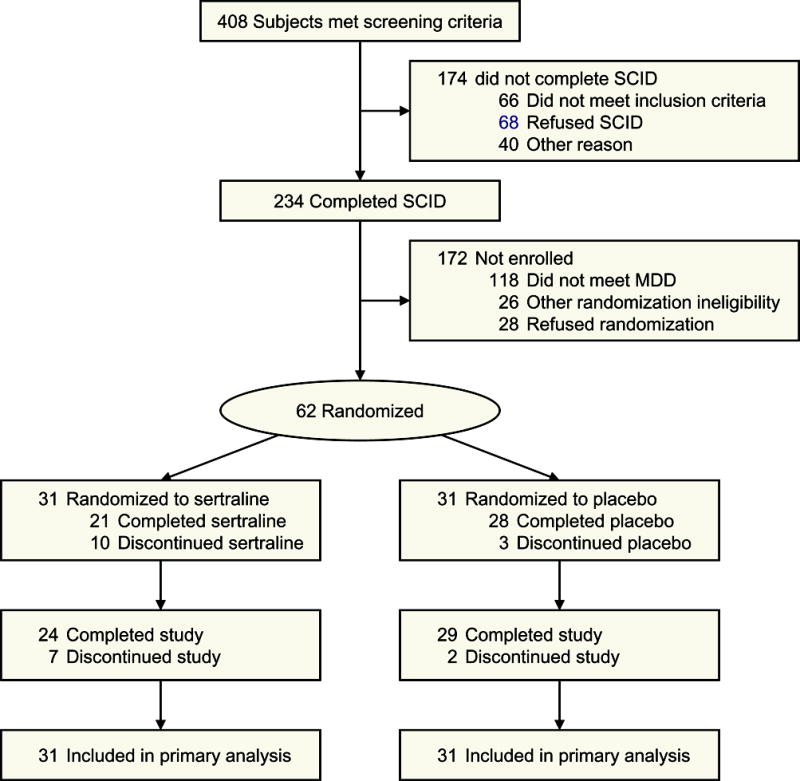
Flow of Participants in the Trial
Table 1.
Demographic and Injury Characteristics
| Variable | Sertraline n=31 |
Placebo n=31 |
P-value a |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 38.0 (12.3) | 36.9 (12.9) | .69 |
| 18–29 | 8 (26%) | 10 (32%) | .88 |
| 30–44 | 15 (48%) | 13 (42%) | |
| 45–59 | 6 (19%) | 7 (23%) | |
| ≥ 60 | 2 (6%) | 1 (3%) | |
| Sex | |||
| Female | 8 (26%) | 7 (23%) | 1.0 |
| Male | 23 (74%) | 24 (77%) | |
| Race | |||
| Non-Hispanic White | 25 (81%) | 25 (81%) | 1.0 |
| Hispanic or Latino | 2 (6%) | 2 (6%) | |
| Non-Hispanic Black | 2 (6%) | 3 (10%) | |
| Asian/Pacific Islander | 1 (3%) | 0 (0%) | |
| Other | 1 (3%) | 1 (3%) | |
| Education | |||
| Did Not Complete High School | 7 (23%) | 8 (26%) | 1.0 |
| Completed High School | 24 (77%) | 23 (74%) | |
| Marital status | |||
| Never Married | 11 (35%) | 14 (45%) | .39 |
| Married | 11 (35%) | 6 (19%) | |
| Divorced/Separated/Widowed | 9 (29%) | 11 (35%) | |
| Insurance | |||
| Commercial/Private | 10 (37%) | 15 (50%) | .71 |
| Medicare | 2 (7%) | 2 (7%) | |
| Medicaid | 15 (56%) | 13 (43%) | |
| Unknown | 4 | 1 | |
| Cause of injury | |||
| Fall | 8 (26%) | 10 (32%) | .97 |
| Vehicular | 11 (35%) | 10 (32%) | |
| Violence (gunshot or assault) | 6 (19%) | 6 (19%) | |
| Other | 6 (19%) | 5 (16%) | |
| Glasgow Coma Scale Score | |||
| Mean (SD) | 10.7 (4.4) | 10.4 (4.0) | .74 |
| Complicated Mild (13–15) b | 16 (52%) | 13 (42%) | .80 |
| Moderate (9–12) | 6 (19%) | 7 (23%) | |
| Severe (3–8) | 9 (29%) | 11 (35%) | |
| Cortical contusions | |||
| No | 20 (74%) | 21 (70%) | .78 |
| Yes | 7 (26%) | 9 (30%) | |
| Unknown | 4 | 1 | |
| Intracerebral contusions | |||
| No | 10 (37%) | 8 (27%) | .57 |
| Yes | 17 (63%) | 22 (73%) | |
| Unknown | 4 | 1 | |
| Injury score, excluding head | |||
| Mean (SD) | 1.5 (1.3) | 2.0 (1.5) | .14 |
| 0 | 11 (37%) | 8 (26%) | .23 |
| 1–2 | 10 (33%) | 7 (23%) | |
| 3–4 | 9 (30%) | 16 (52%) | |
| Unknown | 1 | 0 | |
| Length of hospital stay (days) | |||
| Mean (SD) | 8.5 (7.7) | 9.9 (9.2) | .81 |
| 0–3 | 10 (33%) | 11 (35%) | .88 |
| 4–7 | 7 (23%) | 5 (16%) | |
| 8–14 | 8 (27%) | 8 (26%) | |
| ≥ 15 | 5 (17%) | 7 (23%) | |
| Unknown | 1 | 0 | |
| Months from injury to randomization | |||
| Mean (SD) | 4.3 (2.7) | 4.9 (2.9) | .34 |
| Median (range) | 3.6 (1.1–10.9) | 3.7 (1.3–10.7) | |
| ≤ 3 months post-TBI | 13 (42%) | 10 (32%) | .60 |
| > 3 months post-TBI | 18 (58%) | 21 (68%) | |
| Litigation related to TBI | |||
| No | 15 (71%) | 18 (75%) | 1.0 |
| Yes | 6 (29%) | 6 (25%) | |
| Unknown | 10 | 7 | |
| Cocaine positive at injury | |||
| No | 19 (83%) | 18 (86%) | 1.0 |
| Yes | 4 (17%) | 3 (14%) | |
| Unknown | 8 | 10 | |
| Amphetamine positive at injury | |||
| No | 21 (91%) | 19 (90%) | 1.0 |
| Yes | 2 (9%) | 2 (10%) | |
| Unknown | 8 | 10 | |
| Blood Alcohol Level at injury (mg/dl) | |||
| 0 | 15 (58%) | 14 (64%) | .33 |
| 1–79 | 1 (4%) | 3 (14%) | |
| ≥ 80 | 10 (38%) | 5 (23%) | |
| Unknown | 5 | 9 | |
| Charlson Medical Comorbidity Score | |||
| 0 | 24 (77%) | 28 (90%) | .30 |
| 1 | 7 (23%) | 3 (10%) | |
| Employment status | |||
| Stable (working part/full time, on leave, student, retired) | 7 (26%) | 11 (41%) | .39 |
| Unstable (unemployed, homemaker, disabled, other) | 20 (74%) | 16 (59%) | |
| Unknown | 4 | 4 |
Statistical significance by Fisher’s exact test or Mann-Whitney U test
Has radiological evidence of acute, traumatically induced brain abnormality
Table 2.
Clinical Characteristics
| Variable | Sertraline n=31 |
Placebo n=31 |
P-valuea |
|---|---|---|---|
| History of depression | |||
| No history of depression | 11 (37%) | 11 (37%) | .092 |
| Pre-injury history of depression, not at injury | 17 (57%) | 11 (37%) | |
| Pre-injury history of depression, depressed at injury | 2 (7%) | 8 (27%) | |
| Unknown | 1 | 1 | |
| History of posttraumatic stress disorder | |||
| No | 27 (90%) | 28 (90%) | 1.0 |
| Yes | 3 (10%) | 3 (10%) | |
| Unknown | 1 | 0 | |
| History of other mental health diagnosis b | |||
| No | 27 (87%) | 28 (90%) | 1.0 |
| Yes | 4 (13%) | 3 (10%) | |
| History of alcohol or drug dependence | |||
| No | 10 (32%) | 9 (29%) | 1.0 |
| Yes | 21 (68%) | 22 (71%) | |
| Current anxiety disorder | |||
| No | 9 (29%) | 11 (35%) | .79 |
| Yes | 22 (71%) | 20 (65%) | |
| Concurrent counseling during year following TBI | |||
| No | 18 (75%) | 24 (83%) | .52 |
| Yes | 6 (25%) | 5 (17%) | |
| Unknown | 7 | 2 |
Statistical significance by Fisher’s exact test
Other mental health diagnoses included bipolar disorder or manic depression, generalized anxiety disorder, panic disorder, obsessive-compulsive disorder, any phobia, schizophrenia, schizoaffective disorder, or any psychotic disorder.
Treatment Efficacy
The HAM-D 17-item and Maier subscale scores over time are shown in Figure 2. Within both treatment groups, HAM-D 17-item, Maier, and SCL-20 depression scores improved from baseline to 12 weeks (all P<.001). However, intent-to-treat adjusted models revealed no significant differences between the sertraline and placebo groups in percentage of responders or remitters or improvement on the HAM-D 17-item, Maier subscale, SCL-20, or clinician ratings on the Clinical Global Impression scale from baseline to 12 weeks (Table 3). Restricting analyses to participants who completed the 12-week assessment, remained on medications throughout the study, or achieved 100 mg of study drug did not alter the results (data not shown).
Figure 2.
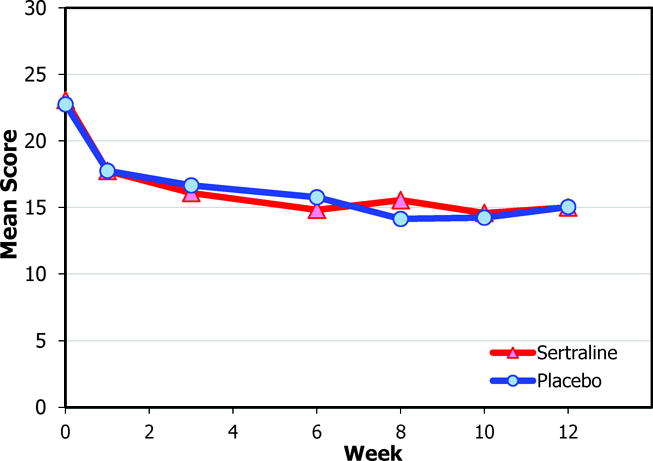
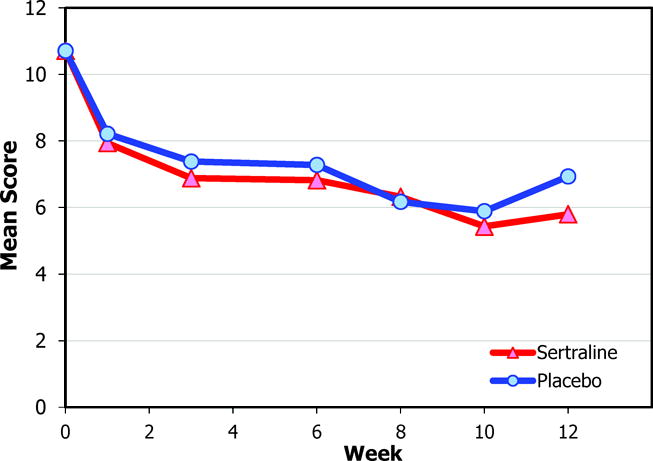
Mean Scores for the Sertraline and Placebo Groups
a. Hamilton Depression Rating Scale 17-item version
b. Hamilton Depression Rating Scale Maier subscale
Table 3.
Intent-to-Treat Depression Outcomes
| Variable | Sertraline n=31 |
Placebo n=31 |
P-value a |
|---|---|---|---|
| Completed 12 week assessment, n (%) | 24 (77%) | 29 (94%) | .15 |
| HAM-D 17-item score | |||
| Baseline, mean (SD) | 23.1 (5.3) | 22.7 (4.8) | .96 |
| Week 12, mean (SD) | 16.2 (8.4) | 14.8 (8.2) | |
| Week 12 Response, ≥50% reduction from baseline, n (%) b |
9 (30%) | 13 (43%) | .44 |
| Week 12 Remission, Score ≤7, n (%)b |
2 (7%) | 7 (23%) | .27 |
| HAM-D Maier subscale score | |||
| Baseline, mean (SD) | 10.7 (2.8) | 10.7 (1.9) | .46 |
| Week 12, mean (SD) | 6.5 (3.8) | 6.9 (3.9) | |
| Week 12 Response, ≥50% reduction from baseline, n (%) b |
11 (37%) | 16 (53%) | .32 |
| Week 12 Remission, Score ≤4, n (%)b |
9 (30%) | 10 (33%) | 1.0 |
| SCL-20 | |||
| Baseline, mean (SD) | 1.98 (0.64) | 1.95 (0.65) | .63 |
| Week 12, mean (SD) | 1.52 (0.89) | 1.18 (0.77) | |
| Week 12 Response, ≥50% reduction from baseline, n (%)b |
9 (30%) | 12 (40%) | .78 |
| Week 12 Remission, Score <0.5, n (%)b |
3 (10%) | 5 (17%) | .49 |
| CGI | |||
| mildly ill or betterb | 14 (58%) | 14 (48%) | .42 |
| much or very much improvedb | 10 (42%) | 13 (45%) | 1.0 |
CGI=Clinical Global Impression; HAM-D=Hamilton Depression Rating Scale; SCL-20=Symptom Checklist-20
Mixed effects models adjusted for time, stratification, age, and current anxiety. Reported means and response and remission models use last observation carried forward if there is no 12-week value. Response, remission, and CGI exact logistic regression models adjust only for stratification due to insufficient sample size.
One subject in each of the sertraline and placebo groups dropped out after baseline assessment and were not included in the response and remission models.
Participants in the sertraline group had significantly better performance on the Trail Making Test B, a measure of information processing speed, improving from 92.3 (SD 56.5) seconds at baseline to 76.3 (SD 55.0) seconds at 12 weeks, compared to the placebo group, which worsened from 83.0 (SD 38.6) seconds at baseline to 97.9 (SD 52.3) seconds at 12 weeks (P<.006) (Table 4). There were no other significant group differences on secondary outcomes. When comparing the treatment responders (≥50% drop in HAM-D 17-item from baseline) from both treatment groups (n=22) to all non-responders from both groups, responders had significantly improved mental health on the SF-36 (P=.004), anger and aggression (P<.001), and anxiety (P=.005).
Table 4.
Secondary Outcomes
| Outcome Measure | Sertraline n=31 |
Placebo n=31 |
P-valuea | ||
|---|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | ||
| Health-related Quality of Life (SF-36) | |||||
| Physical Component Score | 47.8 (30.0) | 54.2 (28.1) | 40.0 (21.6) | 46.0 (22.4) | .917 |
| Mental Component Score | 28.2 (25.0) | 36.9 (27.7) | 29.0 (16.2) | 40.0 (22.5) | .816 |
| Functional impairment (SDS) | 15.0 (8.6) | 12.7 (10.0) | 13.8 (8.3) | 12.8 (9.3) | .48 |
| Information processing speed (Trail Making Test B) | 92.3 (56.5) | 76.3 (55.0) | 83.0 (38.6) | 97.9 (52.3) | .006 |
| Postconcussive Symptoms (HISC) | 37.3 (14.7) | 35.5 (19.3) | 39.9 (15.8) | 32.8 (16.4) | .29 |
| Pain Intensity (BPI) | 3.5 (3.0) | 2.9 (2.6) | 4.0 (2.8) | 3.5 (2.7) | .84 |
| Anger & Aggression (BAAQ) | 8.3 (5.9) | 7.9 (5.3) | 10.2 (5.6) | 9.9 (6.2) | .85 |
| Anxiety (HAM-A) | 24.1 (8.7) | 19.0 (10.9) | 24.2 (8.9) | 16.5 (9.0) | .33 |
BAAQ=Brief Anger and Aggression Scale; BPI=Brief Pain Inventory; HAM-A=Hamilton Anxiety Rating Scale; HISC=Head Injury Symptom Checklist; SDS=Sheehan Disability Scale; SF-36=Medical Outcome Study Short-form 36
Mixed effects models adjusted for time, stratification, age, and current anxiety.
Adherence and Tolerability
Final study medication doses are presented in Table 5. Only 55% of the sertraline group reached at least 100 mg/day of study medication, compared to 74% in the placebo group. Participants in the sertraline group took the study drug for nearly two weeks less than those in the placebo group.
Table 5.
Medication Dosage & Adherence
| Variable | Sertraline n=31 |
Placebo n=31 |
P-value a |
|---|---|---|---|
| Maximum dosage, mean (SD), mg/d | 116 (66) | 127 (62) | .48 |
| Dosage reached, n (%) | |||
| ≥100 mg | 17 (55%) | 23 (74%) | .18 |
| ≥150 mg | 15 (48%) | 15 (48%) | 1.0 |
| 200 mg | 9 (29%) | 10 (32%) | 1.0 |
| Prescribed ≥100 mg/d of study drug for at least 6 weeks | 14 (45%) | 19 (61%) | .31 |
| Duration taking study drug (weeks) | 9.6 (3.7) | 11.4 (2.0) | .20 |
Statistical significance by Fisher’s exact test or Mann-Whitney U test
Reported adverse effects are presented in Table 6. There were no significant group differences in mean number of new or worsened adverse effects. The only new or worsened adverse effects ≥10% more prevalent in the sertraline group compared to the placebo group were flatulence/gas, agitation/restlessness, and decreased libido/sexual interest. Irritability was 13% more common in the placebo group than in the sertraline group. There were no serious adverse events.
Table 6.
Adverse Effects
| Variable | Sertraline n=30 |
Placebo n=30 |
P-valueb |
|---|---|---|---|
| Number of adverse effects, new or worsened, mean (SD) a | 2.2 (1.9) | 1.8 (1.8) | .48 |
| System adverse effects, n (%) | |||
| Autonomic Nervous System | 5 (17%) | 5 (17%) | 1.0 |
| Dry Mouth | 3 (10%) | 3 (10%) | 1.0 |
| Increased Sweating | 3 (10%) | 2 (7%) | 1.0 |
| Central and Peripheral Nervous System | 8 (27%) | 10 (33%) | .78 |
| Dizziness, lightheadedness | 2 (7%) | 3 (10%) | 1.0 |
| Headache | 1 (3%) | 3 (10%) | .61 |
| Paresthesia, numbness, tingling | 1 (3%) | 1 (3%) | 1.0 |
| Sedation, somnolence, drowsiness | 4 (13%) | 4 (13%) | 1.0 |
| Tremor, shakiness | 2 (7%) | 0 (0%) | .49 |
| Gastrointestinal | 10 (33%) | 6 (20%) | .38 |
| Constipation | 0 (0%) | 1 (3%) | 1.0 |
| Diarrhea, loose stools | 4 (13%) | 3 (10%) | 1.0 |
| Dyspepsia, abdominal discomfort, heartburn | 2 (7%) | 3 (10%) | 1.0 |
| Flatulence, gas | 5 (17%) | 0 (0%) | .052 |
| Nausea | 3 (10%) | 1 (3%) | .61 |
| Vomiting | 0 (0%) | 1 (3%) | 1.0 |
| General | 6 (20%) | 6 (20%) | 1.0 |
| Anorexia, decreased appetite | 0 (0%) | 1 (3%) | 1.0 |
| Fatigue, lack of energy | 5 (17%) | 5 (17%) | 1.0 |
| Hot flashes | 1 (3%) | 0 (0%) | 1.0 |
| Rash, itch, irritation | 1 (3%) | 0 (0%) | 1.0 |
| Psychiatric | 13 (43%) | 11 (37%) | .79 |
| Agitation, restlessness | 5 (17%) | 2 (7%) | .42 |
| Anxiety, nervousness | 4 (13%) | 3 (10%) | 1.0 |
| Irritability | 2 (7%) | 6 (20%) | .25 |
| Insomnia | 2 (7%) | 3 (10%) | 1.0 |
| Decreased libido or sexual interest | 4 (13%) | 1 (3%) | .35 |
| Difficulty with erection | 0 (0%) | 1 (3%) | 1.0 |
| Ejaculation failure or delay | 2 (7%) | 0 (0%) | .49 |
| Special Senses | 2 (7%) | 0 (0%) | .49 |
| Blurred vision | 2 (7%) | 0 (0%) | .49 |
| Other | 5 (17%) | 6 (20%) | 1.0 |
An adverse effect is considered “New or Worsened” if it is reported at any time during the 12 week intervention period and not reported at baseline or if the severity (larger of self-report and cued) had worsened since baseline.
Fisher exact test was used for all analyses except number of side effects (Mann-Whitney test)
Discussion
To our knowledge, this is the largest randomized placebo-controlled trial of antidepressant treatment for major depression after TBI and only the second evidence class I antidepressant study in this population. Although participants in both treatment groups had significant pre-post improvement in depression severity, we did not find a significant difference in efficacy on HAM-D between sertraline and placebo over 12 weeks. Moreover, no differences were found on secondary outcomes of depression (Maier subscale, SCL-20 self-report), anxiety, postconcussive symptoms, pain, functioning, or health-related quality of life. Participants in the sertraline group showed improvement on a measure of information processing speed, compared to those in the placebo group. Although not statistically significant, detailed tolerability data showed that more participants in the sertraline group reported medication adverse effects and dropped out of treatment, and fewer achieved a therapeutic medication dose and duration prior to completing the study.
The lack of significant difference in efficacy between sertraline and placebo is consistent with the findings by Ashman et al., the only other evidence class I study of pharmacotherapy for depression following TBI.5,7 Ashman and colleagues also found no significant depression treatment differences between sertraline and placebo. However, compared to the current study, the Ashman study had a higher HAM-D response rate for the sertraline group (30% vs. 59%) and a lower placebo response rate (43% vs. 32%). The response rate for sertraline in the current study is 37% using the criteria used in the Ashman study. The Ashman et al. study enrolled 52 TBI patients (41 completed the study, 36% had mild TBI) an average of 17.7 (SD 13.7) years post-injury. In contrast, the current study enrolled 62 complicated mild to severe TBI patients (53 completed the study) within one year of injury who had high rates of recurrent or chronic depression, current anxiety, and history of substance dependence. The Ashman et al. study did not report detailed dosage and adverse effects data, so it is difficult to compare medication tolerability across the studies.
Several possibilities exist for the lack of difference in efficacy between sertraline and placebo. First, more side effects and lower adherence to sertraline compared to placebo may have contributed to the low response to sertraline. However, restricting the analyses to those who completed the intervention or achieved ≥100 mg/d did not change the results. Second, response to placebo was high and may have been boosted by frequent in-person contact and support from the study staff, particularly in patients who are often socially isolated following TBI.35 Previous antidepressant trials have shown placebo response rates similar to those found in this study and higher antidepressant-placebo differences with greater baseline depression severity.36 Although we did require a minimum baseline HAM-D score of 15, a higher cutoff may have resulted in different findings Third, our study sample exhibited high rates of psychiatric comorbidity, including current anxiety and history of depression and substance dependence, which can contribute to depression treatment resistance.37 Fourth, there is evidence that the HAM-D may have suboptimal psychometric properties (e.g., responsiveness to change) in persons with TBI.38 Finally, the significant psychosocial challenges, exemplified by the large proportion of patients in our study with unstable employment, unmarried and on Medicaid, faced by depressed individuals in the year following TBI has been well-documented.35 Antidepressants alone are unlikely to adequately treat MDD for many of these individuals.
Antidepressants have been shown to improve depression-related cognitive impairment.39,40 Our finding that sertraline, perhaps due to its strong dopaminergic activity,41 was associated with significantly better performance on a measure of information processing speed compared with placebo is consistent with prior findings in persons with TBI and depression.13,42 Although antidepressant administration in patients with TBI without depression has not been shown to improve cognitive functioning,43 the use of antidepressants to improve cognitive function in patients with TBI and comorbid depression warrants further study.
The 22 study participants whose depression responded to treatment intervention also had significant improvement in anxiety, anger and aggression, and mental health-related quality of life, compared to those who did not respond to treatment, after correcting for multiple comparisons. This finding suggests that decreasing depression is associated with improvement in other common comorbid psychiatric conditions, although the directionality of these improvements cannot be determined from these data.
Several study limitations warrant mention. The sample size was limited to 62 participants from a single site. Despite the high rate of MDD in our study population,1 only a small proportion were eligible and consented to this highly controlled efficacy study. Participants had limited sociodemographic diversity and high depression chronicity and psychiatric comorbidity, which may limit generalizability of findings to other TBI populations. We did not enroll persons with uncomplicated mild TBI, a group that also experiences high rates of MDD.44 There was a differentially higher rate of study drug discontinuation in the sertraline group, which may have biased the results. Prior non-response to antidepressants and the high chronicity of depression may have negatively influenced response rates. Finally, as noted above, the choice of the HAM-D as the primary outcome measure in this TBI population may have biased the results toward the null.
Despite these limitations, our findings have relevance for clinical practice. It is important to educate TBI patients about the potential adverse effects of antidepressants and closely monitor adherence, since many of the adverse effects (e.g., gastrointestinal, psychiatric) may be treatable. It is possible that patients with TBI may be less able to tolerate medication adverse effects than other patient groups, particularly during the first year after injury. Our prior research documented that patients with TBI often have strong depression treatment preferences, which should be considered before embarking on a specific treatment approach.45 Our results are suggestive of potential beneficial effects of sertraline on cognition in the post-acute phase for patients with MDD, an area that deserves further study. Furthermore, multimodal treatment approaches, such as those that include cognitive behavioral strategies,46,47 that also address psychosocial stressors may be needed to maximize depression treatment effectiveness.
Given the high prevalence of depression following TBI, strategies to decrease the burden of depression in this population are needed. Studies that administered sertraline to prevent depression following TBI show promise for decreasing early onset of depression while taking the antidepressant.48,49 However, from a population-based standpoint, more research is needed to determine whether administration of prophylactic antidepressant is a cost-effective strategy for the prevention of depression and its associated sequelae.
Future pharmacotherapy studies using other medications and with larger sample sizes are needed to determine which patient, injury, clinical, pharmacologic, and biomarker variables may predict treatment response. Due to the many potential biological, psychological and social contributors to depression following TBI, we need studies of combined pharmacotherapy and psychotherapy, as well as patient-centered, collaborative, stepped care treatment approaches that also address comorbid conditions. To maximize generalizability, more ‘effectiveness’ trials in real-world settings are needed, including those that test innovative delivery systems, such as telehealth models, which have the potential of overcoming treatment barriers faced by patients with TBI.46,50
Acknowledgments
We thank Erika Pelzer, BS, University of Washington School of Medicine, who provided expert assistance and oversight in data collection efforts.
Sources of Funding:
Dr Fann reported ownership of Pfizer stock and consultancy with Quartet Health. Dr Bombardier reported ownership of Pfizer and Amgen stock. Dr Temkin reported ownership of stock in Kimberly-Clark and consultancies with Novartis, Celgene, TauRx, Sage, University of Pittsburgh. Dr Esselman reported ownership of Eli Lilly stock.
This work was supported by the National Center for Medical Rehabilitation Research, the National Institute of Child Health and Human Development, and National Institutes of Health grant R01HD39415. Pfizer supplied masked sertraline and placebo for the controlled trial. The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest:
No other disclosures were reported.
References
- 1.Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. Jama. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart T, Fann JR, Chervoneva I, et al. Prevalence, Risk Factors, and Correlates of Anxiety at 1 Year After Moderate to Severe Traumatic Brain Injury. Arch Phys Med Rehabil. 2016;97(5):701–707. doi: 10.1016/j.apmr.2015.08.436. [DOI] [PubMed] [Google Scholar]
- 3.Fann JR, Katon WJ, Uomoto JM, Esselman PC. Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. Am J Psychiatry. 1995;152(10):1493–1499. doi: 10.1176/ajp.152.10.1493. [DOI] [PubMed] [Google Scholar]
- 4.Hammond FM, Barrett RS, Shea T, et al. Psychotropic Medication Use During Inpatient Rehabilitation for Traumatic Brain Injury. Arch Phys Med Rehabil. 2015;96(8 Suppl):S256–253 e214. doi: 10.1016/j.apmr.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashman TA, Cantor JB, Gordon WA, et al. A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90(5):733–740. doi: 10.1016/j.apmr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Kim SW, Kim JM, Shin IS, Yang SJ, Yoon JS. Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum Psychopharmacol. 2005;20(2):97–104. doi: 10.1002/hup.668. [DOI] [PubMed] [Google Scholar]
- 7.Fann JR, Hart T, Schomer KG. Treatment for depression after traumatic brain injury: a systematic review. Journal of neurotrauma. 2009;26(12):2383–2402. doi: 10.1089/neu.2009.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. Journal of neurotrauma. 2006;23(10):1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 9.Barker-Collo S, Starkey N, Theadom A. Treatment for depression following mild traumatic brain injury in adults: a meta-analysis. Brain Inj. 2013;27(10):1124–1133. doi: 10.3109/02699052.2013.801513. [DOI] [PubMed] [Google Scholar]
- 10.Guillamondegui OD, Montgomery SA, Phibbs FT, et al. Comparative Effectiveness Review No 25. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Traumatic Brain Injury and Depression. [PubMed] [Google Scholar]
- 11.Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12(2):226–232. doi: 10.1176/jnp.12.2.226. [DOI] [PubMed] [Google Scholar]
- 12.Turner-Stokes L, Hassan N, Pierce K, Clegg F. Managing depression in brain injury rehabilitation: the use of an integrated care pathway and preliminary report of response to sertraline. Clin Rehabil. 2002;16(3):261–268. doi: 10.1191/0269215502cr489oa. [DOI] [PubMed] [Google Scholar]
- 13.Fann JR, Uomoto JM, Katon WJ. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics. 2001;42(1):48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 14.Fann JR, Bombardier CH, Dikmen S, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. The Journal of head trauma rehabilitation. 2005;20(6):501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First M, Gibbon M, Spitzer R, Williams B. User’s Guide for the Structured Clinical Interview for DSM IV TR Axis I Disorders–Research Version. New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 17.Hamilton MA. A rating scale for depression. J Neuro Neurosurg Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 19.Maier W, Philipp M. Improving the assessment of severity of depressive states: a reduction of the Hamilton Depression Scale. Pharmacopsychiatry. 1985;18:114–115. [Google Scholar]
- 20.Ruhe HG, Dekker JJ, Peen J, Holman R, de Jonghe F. Clinical use of the Hamilton Depression Rating Scale: Is increased efficiency possible? A post hoc comparison of Hamilton Depression Rating Scale, Maier and Bech Subscales, Clinical Global Impression, and Symptom Chicklist-90 scores. Comprehensive Psychiatry. 2005;46(6):417–427. doi: 10.1016/j.comppsych.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Faries D, Herrera J, Rayamajhi J, DeBrota D, Demitrack M, Potter WZ. The responsiveness of the Hamilton Depression Rating Scale. Journal of Psychiatric Research. 2000;34(1):3–10. doi: 10.1016/s0022-3956(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 22.Entsuah R, Shaffer M, Zhang J. A critical examination of the sensitivity of unidimensional subscales derived from the Hamilton Depression Rating Scale to antidepressant drug effects. Journal of Psychiatric Research. 2002;36(6):437–448. doi: 10.1016/s0022-3956(02)00024-9. [DOI] [PubMed] [Google Scholar]
- 23.Williams JBW. A structured interview guide for the Hamilton Depression Rating Scale. Archives of general psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 24.Derogatis L, Rickels K, Uhlenhuth E, Covi L. The Hopkins Symptom Checklist: a measure of primary symptom dimensions. In: Pichot P, Olivier-Martin R, editors. Psychological Measurements in Psychopharmacology. Basel, Switzerland: Karger; 1974. pp. 79–110. [Google Scholar]
- 25.Guy W. In: ECDEU Assessment Manual for Psychopharmacology —Revised. Department of Health Education and Welfare Public Health Service Alcohol Drug Abuse and Mental Health Administration NIMH Psychopharmacology Research Branch Division of Extramural Research Programs, editor. Rockville, MD: 1976. pp. 218–222. [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 27.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 28.Ricker JH, Axelrod BN. Analysis of an Oral Paradigm for the Trail Making Test. Assessment. 1994;1(1):47–52. doi: 10.1177/1073191194001001007. [DOI] [PubMed] [Google Scholar]
- 29.McLean A, Jr, Dikmen SS, Temkin NR. Psychosocial recovery after head injury. Arch Phys Med Rehabil. 1993;74(10):1041–1046. doi: 10.1016/0003-9993(93)90059-j. [DOI] [PubMed] [Google Scholar]
- 30.Maiuro R, Vitaliano P, Cahn T. A brief measure for the assessment of anger and aggression. J Interpersonal Violence. 1987;2:166–178. [Google Scholar]
- 31.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 32.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Archives of general psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 34.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 35.Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. Atlanta, GA: National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention; 2015. [Google Scholar]
- 36.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. Jama. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iosifescu DV. Treating depression in the medically ill. Psychiatr Clin North Am. 2007;30(1):77–90. doi: 10.1016/j.psc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Dyer JR, Williams R, Bombardier CH, Vannoy S, Fann JR. Evaluating the Psychometric Properties of 3 Depression Measures in a Sample of Persons With Traumatic Brain Injury and Major Depressive Disorder. The Journal of head trauma rehabilitation. 2016;31(3):225–232. doi: 10.1097/HTR.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 39.Amado-Boccara I, Gougoulis N, Poirier Littre MF, Galinowski A, Loo H. Effects of antidepressants on cognitive functions: a review. Neurosci Biobehav Rev. 1995;19(3):479–493. doi: 10.1016/0149-7634(94)00068-c. [DOI] [PubMed] [Google Scholar]
- 40.Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression–a systematic review. Psychiatry Res. 2014;219(1):25–50. doi: 10.1016/j.psychres.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Previc FH. Dopamine and the origins of human intelligence. Brain Cogn. 1999;41(3):299–350. doi: 10.1006/brcg.1999.1129. [DOI] [PubMed] [Google Scholar]
- 42.Yengo-Kahn AM, Solomon G. Are psychotropic medications associated with differences in baseline neurocognitive assessment scores for young athletes? A pilot study. Phys Sportsmed. 2015;43(3):227–235. doi: 10.1080/00913847.2015.1071638. [DOI] [PubMed] [Google Scholar]
- 43.Banos JH, Novack TA, Brunner R, Renfroe S, Lin HY, Meythaler J. Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. The Journal of head trauma rehabilitation. 2010;25(5):357–361. doi: 10.1097/HTR.0b013e3181d6c715. [DOI] [PubMed] [Google Scholar]
- 44.Fann JR, Burington B, Leonetti A, Jaffe K, Katon WJ, Thompson RS. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Archives of general psychiatry. 2004;61(1):53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- 45.Fann JR, Jones AL, Dikmen SS, Temkin NR, Esselman PC, Bombardier CH. Depression treatment preferences after traumatic brain injury. The Journal of head trauma rehabilitation. 2009;24(4):272–278. doi: 10.1097/HTR.0b013e3181a66342. [DOI] [PubMed] [Google Scholar]
- 46.Fann JR, Bombardier CH, Vannoy S, et al. Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. Journal of neurotrauma. 2015;32(1):45–57. doi: 10.1089/neu.2014.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponsford J, Lee NK, Wong D, et al. Efficacy of motivational interviewing and cognitive behavioral therapy for anxiety and depression symptoms following traumatic brain injury. Psychol Med. 2016;46(5):1079–1090. doi: 10.1017/S0033291715002640. [DOI] [PubMed] [Google Scholar]
- 48.Novack TA, Banos JH, Brunner R, Renfroe S, Meythaler JM. Impact of early administration of sertraline on depressive symptoms in the first year after traumatic brain injury. Journal of neurotrauma. 2009;26(11):1921–1928. doi: 10.1089/neu.2009.0895. [DOI] [PubMed] [Google Scholar]
- 49.Jorge RE, Acion L, Burin DI, Robinson RG. Sertraline for Preventing Mood Disorders Following Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Psychiatry. 2016;73(10):1041–1047. doi: 10.1001/jamapsychiatry.2016.2189. [DOI] [PubMed] [Google Scholar]
- 50.Bombardier CH, Bell KR, Temkin NR, Fann JR, Hoffman J, Dikmen S. The efficacy of a scheduled telephone intervention for ameliorating depressive symptoms during the first year after traumatic brain injury. The Journal of head trauma rehabilitation. 2009;24(4):230–238. doi: 10.1097/HTR.0b013e3181ad65f0. [DOI] [PubMed] [Google Scholar]


