Abstract
Zebrafish (Danio rerio) are a popular model organism used in a growing number of research fields. Maintaining healthy, disease-free laboratory fish is important for the integrity of many of these studies. Mycobacteriosis is a chronic bacterial infection caused by several Mycobacterium spp. and is the second most common disease found in laboratory zebrafish. Current mycobacteriosis control measures recommend the removal of infected fish and in severe outbreaks, depopulation. These measures can be effective, but less disruptive measures should be assessed for controlling mycobacteriosis, particularly when valuable and rare lines of fish are affected. Here, the in vivo efficacy two drug candidates, tigecycline (1 µg/g) and clarithromycin (4 µg/g), was tested in adult zebrafish experimentally infected with Mycobacterium chelonae. We assessed both short (14 day) and long-term (30 day) treatments and evaluated fecundity and pathological endpoints. Fecundity and histology results show that zebrafish tolerated antibiotics. Antibiotic treatments did not significantly impact the prevalence of acid-fast granulomas; however, the severity of infections (acid fast granuloma intensity) was significantly decreased following treatments.
Keywords: zebrafish, mycobacteriosis, antibiotic treatment, clarithromycin, tigecycline, Mycobacterium chelonae
Introduction
The Zebrafish (Danio rerio) is a popular vertebrate model organism used in a wide range of research fields (Dahm & Geisler 2006; Phillips & Westerfield 2014). Zebrafish are currently considered rising stars of model-organism research with almost a 60% increase in National Institutes of Health R01 awards from 2008–2015 (Gaind 2016). Zebrafish are used in an ever-increasing range of disciplines including, but not limited to, toxicology (Truong, Harper & Tanguay 2011), aging (Gerhard 2003), oncology (Feitsma & Cuppen 2008), and behavior (Wong, Elegante, Bartels, Elkhayat, Tien, Roy, Goodspeed, Suciu, Tan, Grimes, Chung, Rosenberg, Gaikwad, Denmark, Jackson, Kadri, Chung, Stewart, Gilder, Beeson, Zapolsky, Wu, Cachat & Kalueff 2010). Mycobacteriosis is the second most common disease in laboratory zebrafish (Kent, Spitsbergen, Matthews, Fournie, Murray & Westerfield 2012), caused by several Mycobacterium spp. (Astrofsky, Schrenzel, Bullis, Smolowitz & Fox 2000; Kent, Whipps, Matthews, Florio, Watral, Bishop-Stewart, Poort & Bermudez 2004; Kent 2012; Whipps, Lieggi & Wagner 2012). Several species and strains of Mycobacterium have been implicated in zebrafish mycobacteriosis including both rapid-growing species (e.g. Mycobacterium chelonae, Mycobacterium abscessus, Mycobacterium peregrinum, Mycobacterium fortuitum) and slow-growing species (e.g. Mycobacterium marinum and Mycobacterium haemophilum) (Astrofsky et al. 2000; Kent et al. 2004; Whipps, Matthews & Kent 2008). The severity of mycobacteriosis in zebrafish varies among species and can range between high levels of mortality with M. marinum and M. haemophilum to little observed mortality with M. abscessus and M. chelonae (Watral & Kent 2007; Whipps, Dougan & Kent 2007; Whipps et al. 2012). Infection related morbidity is also variable and includes external signs such as skin lesions, emaciation, raised scales, swollen abdomen, and irregular/lethargic swimming behavior, as well as internal signs like granulomas, especially on haematopoeitic organs (Astrofsky et al. 2000; Kent 2012; Whipps et al. 2012). Additionally, signs of disease may not be present in the case of subclinical infections (Kent et al. 2004; Whipps et al. 2012). Mycobacterial infections in zebrafish are detrimental to research both when severe outbreaks result in high levels of mortality and also when subclinical infections persist undetected in populations as a source uncontrolled experimental variance (Kent et al. 2004; Whipps et al. 2012).
Control recommendations for zebrafish mycobacteriosis focus on disease prevention through quarantine, disinfection, UV disinfection, and sentinel programs for health monitoring (Kent, Feist, Harper, Hoogstraten-Miller, Mac Law, Sanchez-Morgado, Tanguay, Sanders, Spitsbergen and Whipps 2009; Whipps et al. 2012; Chang, Colicino, DiPaola, Al-Hasnawi & Whipps 2015). Mycobacteria are facultative pathogens and persist environmentally in surface biofilms (Falkinham 2009); Falkinham, Norton & LeChevallier 2001). Thus, established mycobacterial infections in zebrafish facilities substantially complicate disease control and management and involve invasive management steps such as depopulation, facility sterilization, and rederivation of zebrafish populations (Whipps et al. 2012). These measures have been demonstrated to be effective at controlling zebrafish mycobacteriosis outbreaks, but such extreme measures may not always be feasible for research facilities (Whipps et al. 2012). For example, if an outbreak occurs in during an ongoing experiment or in a valuable mutant line depopulation and rederivation may not be an option (Whipps et al. 2012). This challenge is also true in the case of mycobacteriosis infections in other fish species such as valuable zoo collections (Strike, Feltrer, Flach, Macgregor & Guillaume 2016). There is a need to explore alternative methods for disease management, such as antibiotic treatment.
The treatment of non-tuberculosis mycobacteriosis in humans is routine (Griffith, Aksamit, Brown-Elliott, Catanzaro, Dalet, Gordin, Holland, Horsburgh, Huitt, Iademarco, Iseman, Olivier, Ruossvon, Reyn, Wallace Jr & Winthrop 2007; Wu, Chiu, Yang, Leu, Huang, Chen, Wu, Chang, Su, Kuo, Chia, Lu & Lai 2012), but similar treatments in fish have yet to be thoroughly investigated (Chang & Whipps 2015). We previously evaluated the in vitro susceptibility of several rapid- and slow-growing strains of Mycobacterium spp. isolated from infected zebrafish from different facilities in the United States (Chang & Whipps 2015). We observed differential susceptibility to the antibiotic treatments across species and drugs that coincided to phylogenetic groupings of Mycobacterium spp. and were also similar to those observed in human isolates (Chang & Whipps 2015). We were able to highlight key antibiotic candidates that demonstrated in vitro effectiveness against Mycobacterium species that commonly cause disease in zebrafish, as well as determine the minimum inhibitory concentration (MIC) for these antibiotics (Chang & Whipps 2015). Two candidates identified in this study were tigecycline and clarithromycin, which were effective at very low concentrations against several species of Mycobacterium (Chang & Whipps 2015).
In this study we evaluate the in vivo efficacy of tigecycline and clarithromycin treatments of M. chelonae infection in adult zebrafish, the most common Mycobacterium species found in laboratory zebrafish. We evaluate treatment using these drugs at the MIC determined in our previous study (Chang & Whipps 2015), which also corresponds to the dosages recommended for treatment of NTM infections in humans (Muralidharan, Micalizzi, Speth, Raible & Troy 2005; Kim, Chi, Oh, Kim, Kim, Lim, Kim & Kwon 2011). Both 14-day and 30-day treatments will be evaluated for both drugs. Our first study goal is to evaluate the safety of these treatments and evaluate effects on fish health and fecundity. We hypothesize that because treatments are at a dose similar to what is currently used for human treatments, there will be no effects on morbidity, mortality or fecundity for both tigecycline and clarithromycin treatments. Our second study goal was to evaluate the effectiveness of these treatments in eliminating M. chelonae from infected zebrafish. We hypothesize that these treatments will result in a significant decrease in mycobacteria, with longer-term (30-d) treatments being more effective than short-term (14-d) treatments.
Methods
Fish
All fish used in this study were bred and maintained in the zebrafish facility at the SUNY-ESF Center for Integrated Teaching and Research in Aquatic Science. Adult AB wild type line zebrafish (n=576; 288 male and 288 female; age = 6 months), originally obtained from the SARL at Oregon State University (Corvallis, OR) and bred for two generations at SUNY-ESF, were utilized in this study. Animals were housed at a density of 6–10 fish/liter in either 1.8 L (tolerance experiment) or 2.6 L (efficacy study) tanks on a timed, flow-through housing system (Aquaneering, San Diego, CA). The housing system included ultraviolet disinfection of dechlorinated (carbon filter) municipal tap water maintained to pH 7.6, a conductivity of 600–700 us/cm2, and a temperature of 28.5°C, and ammonia levels ranging from 0–0.25 ppm. The zebrafish facility maintained a 14:10 light:dark photoperiod. Fish were fed a commercial feed for zebrafish (Gemma, Skretting) twice daily on weekdays and once daily on weekends during periods when they were not being fed a treatment gelatin feed. Prior to the experiment, routine cleaning of all equipment (e.g. tanks, lids, baffles, nets, and tubing) consisted of bi-weekly washing and scrubbing in warm water with a new soft sponge and bleaching in 1000 ppm chlorine bleach for 30 minutes, followed by rinsing three times in dechlorinated water and drying. All tanks, lids, and baffles were also autoclaved using a program specified by the tank manufacturer that reaches a temperature of 105–110°C for 15 minutes. During experiments this tank cleaning procedure occurred when the placement of fish in a new tank is mentioned in the methods below. All animal work was approved by the SUNY-ESF Institutional Animal Care and Use Committee, protocol #151001.
Medicated Feed Preparation
Antibiotics were delivered orally through a commercially developed gelatin feed (Gelly Belly, Florida Aqua Farms). This gelatin feed has previously been shown to be comparable to other zebrafish diets (Sciarra, Tyler & Kolb 2014). Prior to preparing medicated feeds, concentrated stock solutions of each antibiotic were prepared as followed. Tigecycline (Sigma Aldrich) was initially dissolved in DMSO to reach a concentration of 3 mg/mL as per manufacturer’s recommendation. This dilution was then further diluted further to 1.5 mg/mL in Hanks Buffered Salt Solution as recommended in (Adekambi & Drancourt 2004) and stored at −20°C to minimize drug loss through storage. Clarithromycin (Sigma Aldrich) was dissolved in DMSO to reach a final concentration of 1 mg/ml as per manufacturer recommendations, and stored at −20°C. Medicated Gelly Belly feed was prepared with the addition of clam juice as in (Sciarra, et al. 2014). Medicated Gelly Belly was weighed and minced following this method as well. Food aliquots for each tank were stored in sterile culture tubes at −20°C until immediately prior to feeding. Tigecycline feed was prepared so that fish would receive a target dosage of 1 µg/g of body weight in each feeding. Clarithromycin feed was prepared so fish received a target of 4 µg/g of body weight in each feeding. The average body weight was 600 mg/fish. Control feeds were prepared with the addition of no antibiotics; sterile water was used in the place of any medication. Once medicated and control feeds were prepared they were re-labelled A, B, or C by a third party so that further observations were blinded.
Tolerance Study Design
Experimental groups (n=36/group) were organized as follow into feed treatment: untreated 14-d (no antibiotic feed), untreated 30-d (no antibiotic feed), treated tigecycline treatment 14-d (1 µg/g tigecycline/day), treated tigecycline treatment 30-d (1 µg/g tigecycline/day), treated clarithromycin treatment 14-d (4 µg/g clarithromycin/day), treated clarithromycin treatment 30-d (4 µg/g clarithromycin/day). For each experimental group fish were divided evenly into12 fish/tank replicated three times. Each tank had an equal number of male and female fish.
Fish were measured prior to the start of the study so that average growth could be evaluated. For measurements, fish fasted for 12-h were anaesthetized in a bath treatment of 0.15 g/L MS222 buffered to a pH of 7.5. Once anesthetized, the sex and the standard length (snout to the end of the caudal peduncle) of each fish were measured to the nearest millimeter. Once measured, fish recovered in fresh zebrafish system water and were returned to the appropriate study tank. This measurement procedure was also carried out for all groups post-treatment (14-d and 30-d) as well as 11-weeks post-treatment (14-d or 30-d).
Two weeks prior to the start of treatment feeding, background breeding was conducted weekly to determine a baseline for fecundity values. Each tank was bred overnight in its own breeding chamber (Aquaneering). The following morning fish were given 3 hours to breed following the beginning on the light photoperiod. Following breeding, fish were returned to new clean (autoclaved) tanks. The number of embryos collected as well as the 24-hour embryo mortality for each tank was determined. Following 24-hour mortality data collection, all embryos were euthanized in 0.3g/L MS222 buffered to a pH of 7.5. Following euthanasia, all embryo waste was bleached at an estimated concentration of 1000 ppm prior to disposal. This same breeding procedure was repeated weekly from 2–11 weeks post-treatment.
Antibiotic feed treatments were administered daily from 9:30–10:30 am for either 14 days or 30 days. Prior to feeding all tanks were cleaned to remove any feces and/or detritus from tanks. At feeding, pre-prepared (described above) gelatin-based food was distributed to each tank. Fish were allowed 20 minutes of eating time, following that excess food was removed by siphoning. Observations (changes in skin color, irregular swimming, lethargy, mortality) of fish in all tanks were made at 5 min, 30 min, 1 hour, and 2 hours post-feeding. Following tigecycline, clarithromycin, and control treatments (14-d and 30-d) standard length measurements were collected again using the method described above. There were no timed water exchanges during the pre-feeding cleaning and feeding periods. Following the removal of excess food after the feeding period, a timed water exchange was carried out replacing approximately 20 percent of the tank water.
At 11 weeks post-treatment all fish were measured as described above and euthanized in 0.3 g/L MS222 buffered to a pH of 7.5. Euthanized fish were fixed in Davidson’s solution for 48h, decalcified in 0.5M EDTA for 5 days, rinsed, and dehydrated to 70% EtOH for histology through a dehydration series (25% EtOH for 30 min, 50% EtOH for 30 min, 70% EtOH to store). Three representative fish from each replicate tank were sectioned and stained with hematoxylin and eosin (H&E).
Statistics - Tolerance
The analysis described below was used to compare differences between treated tigecycline, treated clarithromycin, and untreated treatments for the same duration (14-d or 30-d) and also to compare differences between durations (14-d and 30-d) for the same treatment (tigecycline, clarithromycin, or untreated controls). The same statistical method was used for all analyses and was carried out in R 3.1.0 (R Core Team 2013) and R Studio (R Studio 2012). Length data, embryo count data, and 24-hour embryo mortality data were organized in a spreadsheet. Growth was calculated for each replicate tank by subtracting average standard length measured before treatment, from post-treatment and 11 weeks post-treatment length (e.g. Growth=average standard length at 11 weeks post-treatment – average standard length at pre-treatment). Percent embryo survival was calculated as follows {Percent Survival = [1 - (24-hour embryo mortality/Total Number of Embryos Collected)]*100}. Data were sorted into separate spreadsheets by time-point and treatment and saved as individual text files for analyses. Prior to each statistical analysis, data were checked for normality and equal variances using the “stats” package (R Core Team 2013) and “car” package (Fox and Weisberg 2011) respectively. Residuals were also plotted using the “stats” package to further evaluate data normality. If data had a normal distribution (p>0.05) and equal variances (p>0.05), parametric ANOVA test was performed using the “stats” package (R Core Team 2013). Post-hoc analyses pair-wise analyses were conducted using t-tests using the “foreign” and “car” packages. Non-normally distributed data and data with unequal variances (p<0.05) were evaluated with the Kruskal-Wallis rank sum test using the “stats” package (R Core Team 2013). Post-hoc testing used the Nemenyi-tests for multiple comparisons of rank sums using the “PMCMR” package (Pohlert 2015). Descriptive statistics were determined using the “psych” package. Results were organized into bar plots using the “sciplot” package.
Efficacy Study Design
Experimental groups (N=60) were organized as follows: untreated (no antibiotic feed) sham injected, untreated (no antibiotic feed) M. chelonae injected, treated tigecycline (1 µg/g) sham injected, treated tigecycline (1 µg/g) M. chelonae injected, treated clarithromycin (4 µg /g) sham injected, treated clarithromycin (4 µg /g) M. chelonae injected. For each experimental group, fish were divided evenly into two tanks of 30 fish. Each tank had an equal number of male and female fish.
Prior to the set-up of experimental groups, mycobacterial infections or sham injections were established in the fish. Mycobacterium chelonae injected fish (N=180 fish) were injected intraperitonally (IP) with Mycobacterium chelonae (H1E2), and sham injected fish (N=180) injected with sterile saline, following the method described in Watral et al. (2007). The concentration of the bacterial inoculum was determined using a nephelometer (Sensititre) and confirmed with colony counts following culture on Middlebrook 7H10 agar plates. After IP injection, fish were placed in new tanks and maintained on the flow-through system for 8 weeks in order to allow for the development of infections in bacterium-injected fish. During this 8 week period fish were fed a commercial feed (Gemma, Skretting) twice daily on weekdays and once daily on weekends. Tanks were siphoned daily following feeding during this period.
Following the 8-week incubation period, fish were arranged in their respective experimental group in new tanks. Mycobacterium chelonae injected fish (N=180) were randomly divided into six tanks of N=30 (15 males, 15 females). Sham injected fish (N=180) were also divided into six tanks of N=30 (15 males, 15 females). At this point, each tank was fed either untreated Gelly Belly feed (prepared as described previously) or treated tigecycline or clarithromycin feed (prepared as described previously). Prepared tubes of thawed and minced gelatin feed were emptied into each tank using the same method described above for the tolerance study. Timed water exchanges were carried out similar to the tolerance study above. Fish were fed daily for 14 days. On the 15th day, following a 12-h fasting period, half of the fish from each tank were euthanized in 0.3g/L MS222 buffered to a pH of 7.5. Once euthanized, fish were prepared for histology as described above. The remaining fish in each tank continued experimental feeds daily up until day 30. Following the 30th feed, fish were fasted overnight for 12-h, then euthanized, fixed, decalcified and prepared for histology as described above. Sections from each specimen were stained with both hematoxylin and eosin (H&E) or Kinyoun’s acid fast stain.
Following staining, slides were examined, recording the number and location of granulomas in H&E sections. For acid fast-stained sections, the presence of granulomas containing acid fast bacilli (AFB) was recorded. The number and location of granulomas containing AFB was recorded, and the location of AFB not contained in granulomas was also recorded. AFB not within granulomas were considered to be internally located if they were located inside the epithelial boundary (e.g. not in the gut lumen or on the outside of the skin/scales). The prevalence of granulomas, granulomas containing AFB, and free AFB was calculated. The intensity of granulomas containing AFB was also calculated (Intensity = number of granulomas containing AFB/number of fish with the presence of granulomas containing AFB).
Statistics - Efficacy
For each experimental group, a Chi-squared analysis with a Monte Carlo simulation method using 20,000 replications was used to compare replicate tanks to determine whether data from different tanks can be pooled for further group comparisons. Results from this test showed no significant differences between replicate groups. Replicate data for each experimental group was pooled. Wilson’s 95% confidence intervals and prevalence values were determined using the “epitools” package (Aragon, Fay & Wollschlaeger 2012). There was no occurrence of acid-fast granulomas in sham injection fish and subsequent statistical analyses excluded these sham groups. Fisher’s exact test for count data was used to compare prevalence between treatment groups using the “stats” package (R Core Team 2013). Data was analyses comparing experimental groups for both sexes, with males or females only, and between sexes for each group. Results were organized into bar plots using the “sciplot” package (Morales 2012).
Results
Tolerance Study - Behavior
The prepared gelatin-based feed was eaten by all the fish without hesitation and as noted in Sciarra et al. (2014), a feeding frenzy occurred. The fish generally consumed most of their food within the 20-minute period; however, any remaining food was removed as it deteriorated water quality quickly. Observations at 5 min, 30 min, 1 hour and 2 hours post-feeding included no changes in skin color, no irregular swimming, no lethargy or mortality. The only observation noted, is that female fish would become very full and sometimes have a distended belly from consuming the gelatin feed. There was no resulting morbidity or mortality.
Tolerance Study - Embryos
Generally, all experimental groups spawned on every breeding occasion throughout this study and 24-hour embryo mortality ranged from 0–42%. When embryo counts and 24-hour embryo mortality were compared (Table 1), there were only two instances of significant differences between groups. First, for average embryo count, there was a significantly higher average embryo count for both 14- and 30-d tigecycline treatments at 4 weeks post-treatment compared to 14-d clarithromycin, untreated, and 30-d clarithromycin treatment [F(5)=7.627, p=0.002] (Table 1). Second, there was significantly higher percent mortality at both 2 and 3 weeks post-treatment for the 14-d tigecycline treatment group compared to 30-d tigecycline and both 14- and 30-d untreated groups [week 2: F(5)=4.162, p=0.02; week 3: F(5)=4.509, p=0.0152] (Table 1). When comparisons were made for the same experimental group over time, there was only one instance where embryo count differed significantly between weeks. For the 14-d tigecycline treatment, the average embryo count at 7-weeks post-treatment (61) was significantly lower than counts at weeks two and three (226) [F(1)=5.37, p=0.03] (Table 1).
Table 1.
Average total embryo count and 24-hour percent mortality (brackets) per treatment group per week before and after 14-d and 30-d antibiotic treatment with tigecycline (1 µg/g), clarithromycin (4 µg/g), or control feed. Bolded values indicate those that differ significantly between treatment groups for the same week. Bolded and italicized values indicate those that differ for between weeks for the same treatment.
| Week |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Duration | −2 | −1 | Tx | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 2–10 Avg |
| Control | 14 Days | 76(5) | 44(6) | N/A | 113(3) | 113(1) | 50(42) | 81(4) | 68(6) | 44(4) | 55(2) | 55(0) | 81(3) | 84(3) | 74(7) |
| Control | 30 Days | 44(13) | 49(9) | 153(3) | 153(3) | 106(5) | 209(2) | 74(3) | 25(1) | 80(3) | 45(16) | 8(0) | 48(2) | 90(4) | |
| Clarithromycin | 14 Days | 93(4) | 55(15) | 198(7) | 198(7) | 92(5) | 85(5) | 71(4) | 53(8) | 41(1) | 128(3) | 106(5) | 79(2) | 105(5) | |
| Clarithromycin | 30 Days | 39(0) | 76(7) | 171(8) | 171(8) | 96(2) | 73(8) | 85(7) | 93(2) | 95(4) | 133(5) | 105(3) | 59(4) | 108(5) | |
| Tigecycline | 14 Days | 8(12) | 72(3) | 226(20) | 226(20) | 248(6) | 130(3) | 111(3) | 61(11) | 162(5) | 107(3) | 127(6) | 105(3) | 150(8) | |
| Tigecycline | 30 Days | 106(3) | 56(3) | 202(2) | 202(2) | 255(2) | 131(9) | 143(6) | 38(0) | 104(5) | 120(5) | 110(5) | 74(3) | 138(4) | |
Tolerance Study - Growth
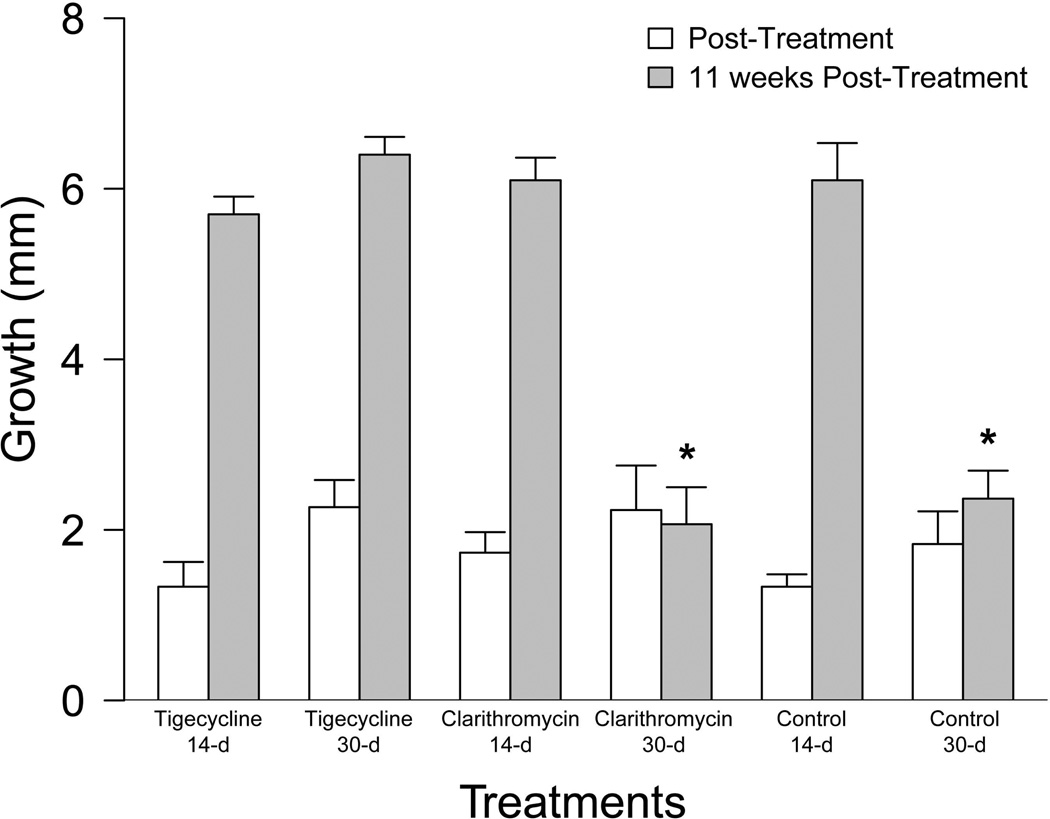
When post-treatment growth between experimental groups was compared immediately following treatment, there was no significant difference between treated and untreated groups (χ2(5)=6.05, p=0.30)(Figure 1). However; when comparing growth 11 weeks post treatment, there was a significant difference between groups (χ2 (5)=13.17, p=0.02)(Figure 1). For this time-point, the 30-d clarithromycin and untreated groups resulted in significantly less growth than the other groups.
Figure 1.
Tolerance Study Growth Results. Growth (mm) in standard length (SL) immediately following 14-d and 30-d antibiotic treatment with tigecycline (1 µg/g), clarithromycin (4 µg/g), or control feed (white bars) and at 11-weeks post-treatment (grey bars). Significant differences are indicated by an asterisk (*).
Treatment Efficacy – H&E Granuloma Prevalence
Granulomas were mainly located in the reproductive tissues of both male and female fish. For the sham injected groups, granulomas were restricted to the ovaries, testes, and swim bladder. For the M. chelonae-injected tigecycline and clarithromycin treated groups the granulomas were located in additional types of tissues in addition to the ovaries, testes, and swim bladder, including: intestine, liver, pancreas, and adipose. The M. chelonae injected untreated group had granulomas in more tissue types (ovaries, testes, swim bladder, intestine, liver, pancreas, adipose, and kidney) compared to the M. chelonae injected tigecycline and clarithromycin treated groups.
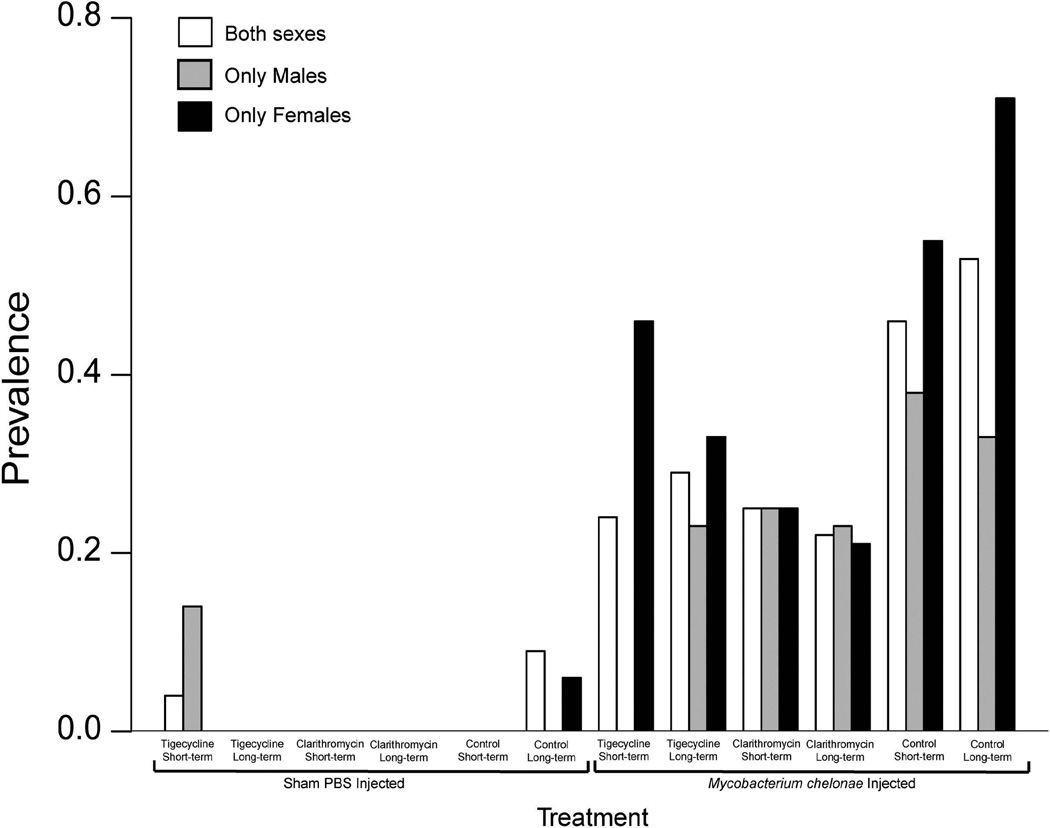
The prevalence of granulomas in H&E stained slides varied between experimental groups and sex (Supplemental Table 1). Sham-injected groups displayed prevalence ranging from 0–0.13 (Figure 2). Mycobacterium chelonae-injected groups display a granuloma prevalence ranging from 0.22–0.71(Figure 2). There was no significant difference between the prevalence of H&E stained granulomas between experimental groups when comparing groups including both sexes, only males, and only females (Figure 2). However, when granuloma prevalence for males and females were compared for each group the M. chelonae injected 14-d tigecycline treated females had a significantly higher prevalence compared to males (p=0.01718) (Figure 2).
Figure 2.
Barplot displaying prevalence of H&E granulomas for both sexes (white bars), males only (grey bars), and females only (black bars) for both sham-injected and M. chelonae–injected zebrafish treated with 14-d or 30-d tigecycline (1 µg/g), clarithromycin (4 µg/g), or control feeds. A significantly higher prevalence is observed for females compared to males for the 14-d tigecycline treatment (*).
Treatment Efficacy - AF Granuloma Prevalence
When comparing the prevalence of acid-fast granulomas to granulomas observed in H&E stained sections, there was generally a lower prevalence of acid-fast positive granulomas compared to H&E stained counts. Two exceptions to this were observed for female granuloma prevalence of the M. chelonae tigecycline 14-d treated group and the M. chelonae 30-d clarithromycin treated group where a higher prevalence for granulomas in the ovaries were observed in acid-fast stained sections compared to H&E (Compare Figure 2 to Figure 3A).
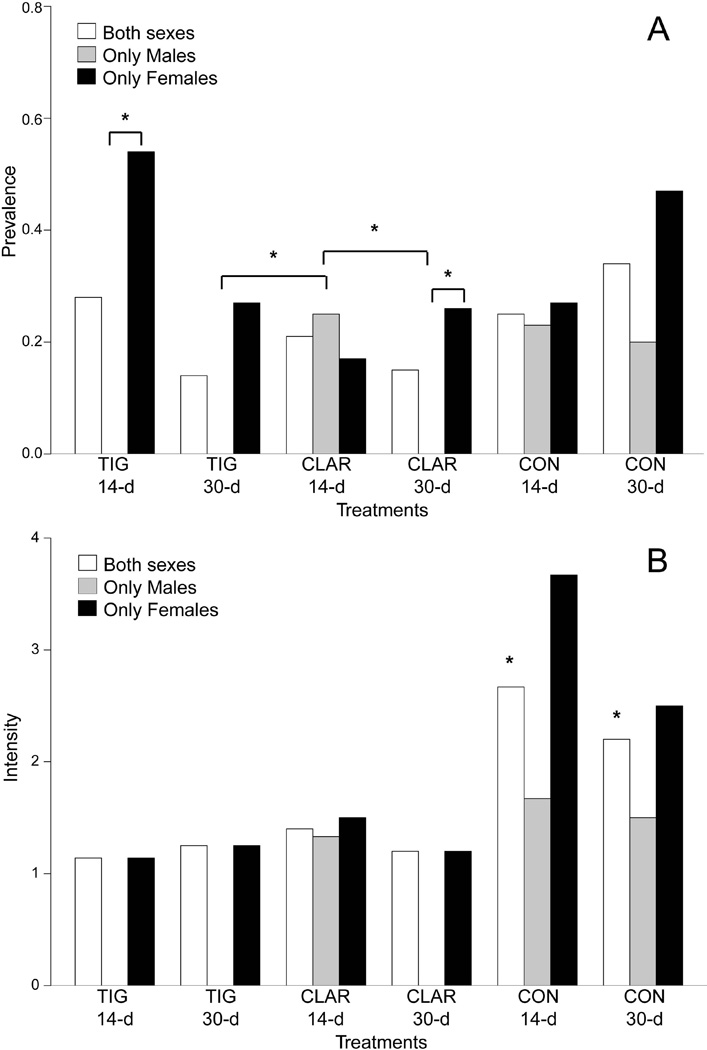
Figure 3.
Barplots displaying the (A) prevalence and (B) intensity for both sexes (white bars), males only (grey bars), and females only (black bars) of acid fast positive granulomas for M. chelonae–injected zebrafish treated with 14-d or 30-d tigecycline (1 µg/g), clarithromycin (4 µg/g), or control feeds. Significant differences in prevalences are indicated by asterisks (*). For males only a significantly higher prevalence of AFG is observed for 30-d tigecycline and clarithromycin treatments compared to 14-d clarithromycin treatment. Higher prevalence was observed for females compared to males from the same treatment for 14-d tigecycline and 30-clarithromycin treatments. A significant difference between intensity (B) for treatment groups of both sexes was also observed with control groups having the highest intensities.
The prevalence of acid-fast positive granulomas (Supplemental Table 1). differed between experimental groups and sexes (Figure 3A). There were no acid-fast positive granulomas in sham-injected groups. When comparing the prevalence of acid-fast positive granulomas between M. chelonae injected experimental groups, there was no significant difference between treatments when considering groups including both sexes. However, significant differences were observed when single-sex groups were compared. A significantly higher prevalence was observed for males in the 14-d clarithromycin treated group compared to both the 30-d tigecycline and 30-d clarithromycin treated groups (p=0.04634) (Figure 3A). Males had a significantly lower acid-fast granuloma prevalence compared to females for the 14-d tigecycline treated group (p=0.004577) as well as 30-d clarithromycin treated group (p=0.06413) (Figure 3A).
Treatment Efficacy - AF Granuloma Intensity
For individual fish with acid-fast positive granulomas, the intensity of acid-fast positive granulomas was determined and varied between experimental groups and sexes (Figure 3B). When intensity was compared between experimental groups there was significant difference (p<0.1) between groups [F(5)=3.631, p=0.0739], with the untreated groups having a significantly higher number of granulomas (*) (Figure 3B).
Treatment Efficacy - AFB Prevalence
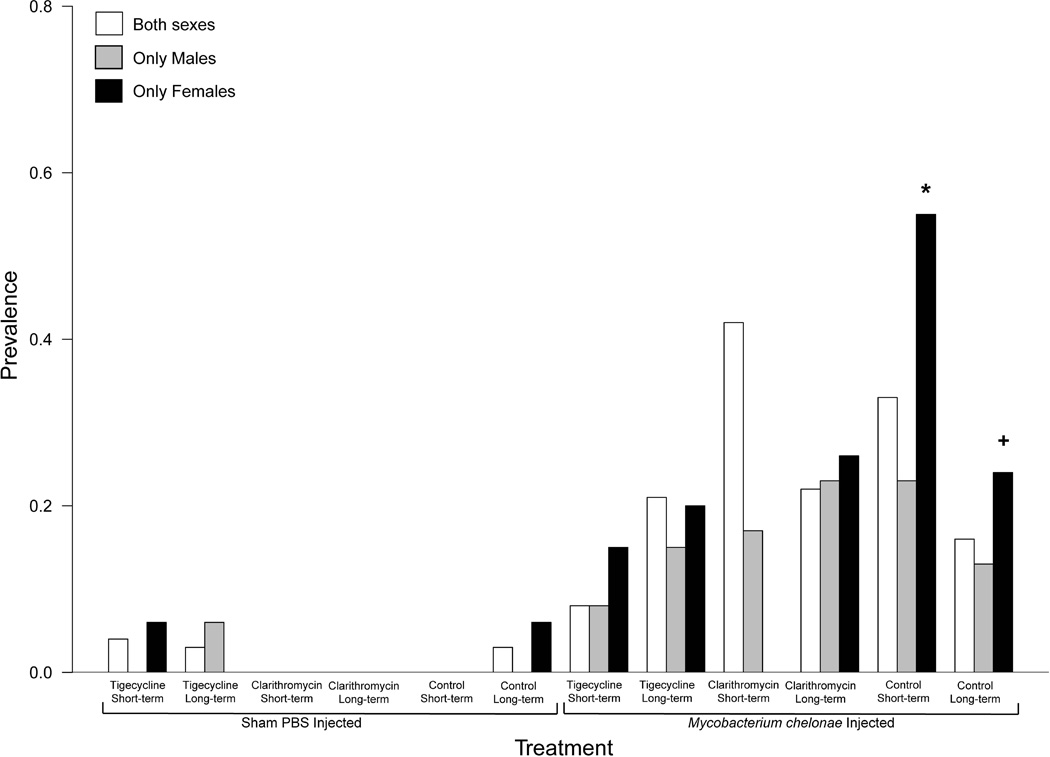
The prevalence of acid-fast bacilli (Supplemental Table 1). varied between experimental groups and sexes (Figure 4). The prevalence of internally-located acid-fast bacilli was also compared between experimental groups. For females, there was a significant difference in the prevalence of AFB (p=0.0536). Tigecycline and clarithromycin treated groups for 14-d, resulted in significantly lower prevalence compared to the 14-d untreated group (*). AFB in the 14-d clarithromycin treated group was also significantly lower than 30-d untreated females (+) (Figure 4).
Figure 4.
Barplot displaying prevalence of acid fast positive bacilli (AFB) for both sexes (white bars), males only (grey bars), and females only (black bars) for both sham-injected and M. chelonae–injected zebrafish treated with 14-d or 30-d tigecycline (1 µg/g), clarithromycin (4 µg/g), or control feeds. A significantly higher prevalence is observed (*) for 14-d control females compared to 14-d tigecycline and clarithromycin treated females. Significantly higher AFB prevalence is also observed for 30-d control females compared to 30-d clarithromycin females (*).
Treatment Efficacy - Granuloma Descriptive
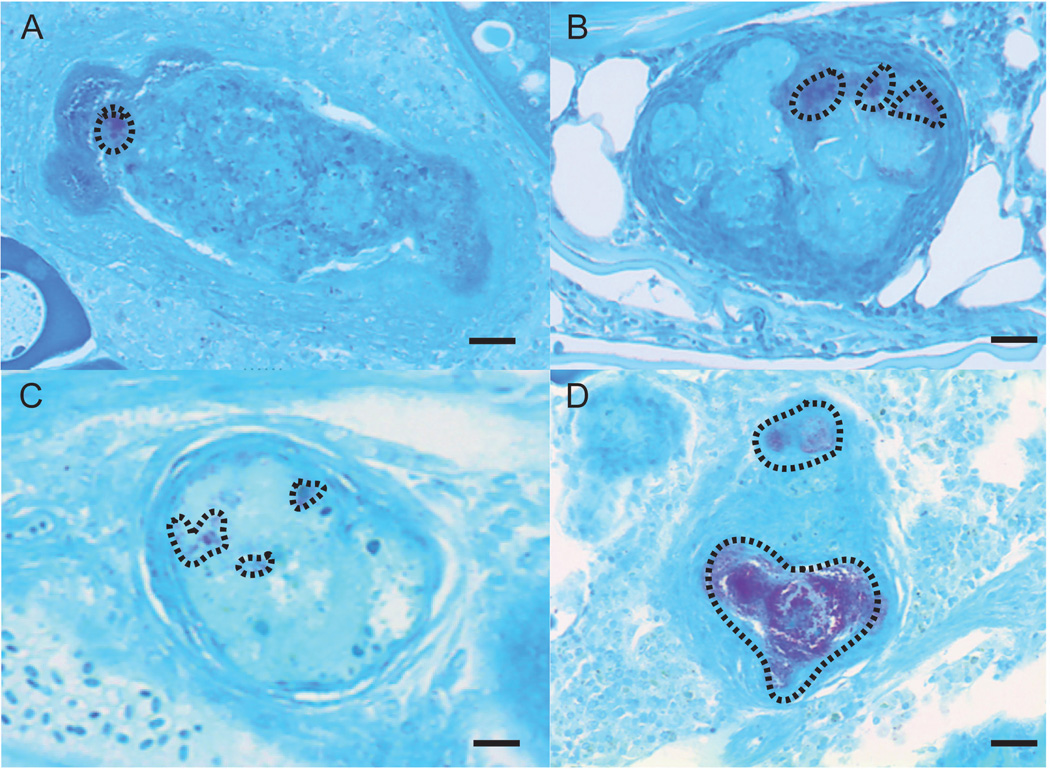
Through visual observations of acid-fast positive granulomas there was a general trend in the amount of AFB located within positive granulomas of untreated and treated groups. This is most clear in representative images of AFB positive granulomas from tigecycline, clarithromycin and untreated fish (Figure 5). Untreated M. chelonae injected fish had granulomas containing visually more acid-fast bacilli relative to tigecycline and clarithromycin treated fish.
Figure 5.
Representative images of acid fast stained granulomas with acid fast positive bacilli (enclosed in dashed line trace) for (A) 14-d clarithromycin treatment located in the ovaries, (B) 30-d clarithromycin treatment located ventral to the liver in a male fish , (C) 14-d tigecycline treatment located in the ovaries, and (D) 30-d control treatment located in the ovaries.
Discussion
Treatment Tolerance
In the tolerance study there were no differences in behavioral observations, morbidity or mortality between zebrafish experimental groups. This suggests that the tigecycline and clarithromycin treatments were tolerated well by the zebrafish and that there is potential for higher dosages to be further considered. To our knowledge there are no previous reports of treatment of zebrafish with these two particular drugs; however, fish have been treated with antibiotics for mycobacterial infections at much higher dosages than those used in this study (Bernut, Le Moigne, Lesne, Lutfalla, Herrmann & Kremer 2014; Strike et al. 2016). The dosages we used were in the ranges used for the treatment of human mycobacterial infections (Muralidharan et al. 2005; Kim et al. 2011) which suggests this might be safe for other vertebrates. It should be noted that these dosages were targeted and may not equal the exact amount of antibiotic available to the fish as oral delivery is subject to loss of drug during digestion (discussed in Chang et al., 2015). There were no observed detrimental effects on zebrafish growth as there was no significant difference in growth post-treatment or 11 weeks post-treatment that differed significantly from untreated groups. Regarding effects on fecundity, there was no breeding cessation; however, treatment with tigecycline did significantly increase average embryo count and increase 24-hour percent morality compared to other treatment groups. Altogether, all experimental groups were able to reproduce following treatment allowing for the potential for treated fish population to be re-derived from embryos in the case of an outbreak in a zebrafish facility. Although this difference did not result in severely low embryo counts or percent mortality, impacts of tigecycline treatment on fecundity may be more severe over a longer treatment period or higher dosage.
Treatment Efficacy
Observation of H&E stained fish revealed that granulomas were predominately located within the reproductive tissues and the swim bladder which is consistent with observations from other studies using the intraperitoneal injection mode of infection (Watral et al. 2007).
The highest prevalence of granulomas was observed in untreated M. chelonae–injected groups followed by M. chelonae-injected fish treated with clarithromycin, M. chelonae-injected fish treated with tigecycline, and lastly sham injected fish. No difference between experimental groups was observed when sexes were pooled; however, when groups of single sex were analysed separately a significant difference is observed between sexes for the 14-d tigecycline treatment. This finding indicated that further analyses should look at differences between sexes. Further analyses using acid fast staining techniques to identify AFB in granulomas was necessary in order to differentiate naturally occurring granulomas [e.g. in the case of egg degeneration (Kent et al., 2004)].
The locations of acid fast positive granulomas were similar to that observed for the H&E stained granulomas. None of the sham injected fish were observed to have any acid fast positive granulomas, which was expected as these fish were not exposed to M. chelonae. Generally the prevalence of acid fast positive granulomas was lower than H&E identified granulomas with the exception of two female fish where acid fast staining allowed for the detection of a positive granuloma in the ovaries where previous H&E stained fish could not be differentiated from the appearance of a degenerating egg. This difference provides further support of using acid fast staining to correctly diagnose a mycobacterial infection (Noga, 2010). When groups of pooled sexes were analysed there was no significant differences between experimental groups. Further analyses of single sex groups indicate that for males, 14-d clarithromycin treatment resulted in a higher prevalence of acid fast positive granulomas than 14-d tigecycline, 30-d tigecycline or 30-d clarithromycin treatments. Treatments with either 14-d tigecycline or 30-d clarithromycin were less effective for female fish compared to males as females in these groups had a higher prevalence of acid fast positive granulomas for these treatments.
Importantly, we also evaluated the intensity of acid fast positive granulomas in order to better understand the severity of infections following different antibiotic treatments. There was a significant difference in acid fast positive granuloma intensity between the different experimental groups with the untreated groups having the highest intensity, followed by the 14-d clarithromycin treated group, then the 30-d tigecycline treated group, then the 30-d clarithromycin treated group, and the lowest intensity was observed for the 14-d tigecycline treated group. This trend is also observed for females. Males had a similar trend as well, however the 14- and 30-d tigecycline and the 30-d clarithromycin treated groups shared an intensity of zero. Increased infection severity in untreated fish compared to tigecycline and clarithromycin treated fish was also observed visually in the amount of AFB seen within granulomas. It appears as though the antibiotic treatments help to reduce the amount of observed acid fast bacteria present within granulomas; however, there are still some remaining bacilli that have not been eradicated. Thus, while tigecycline and clarithromycin treatments may not have resulted in a reduction in the prevalence of acid fast positive granulomas, these treatments were effective at decreasing infection severity.
Conclusions and Recommendations
This study used a conservative dosage of tigecycline and clarithromycin based on in vitro minimum inhibitory concentration (MIC) values for the strain of M. chelonae used in this study and commonly found in zebrafish. Because there were only minimal effects on fecundity and no morbidity or mortality, alternative doses are likely to be tolerated by zebrafish. Also, because a complete eradication of AFB and granulomas was not observed following treatment, higher dosages and/or longer treatment durations should be investigated to determine if the effect on infection correlates positively with these factors. If a higher dose or longer treatment duration is observed to be effective eliminating the infection, additional effects on growth and fecundity should be examined as we did observe some effect on embryo count and 24-hour mortality following treatment with tigecycline. Further differentiation of these two treatments effectiveness could then be evaluated following these additional studies. Additionally, evaluation of a combined tigecycline/clarithromycin treatment should be examined. Future studies should also investigate the effect of these treatments on zebrafish infected with more severe M. marinum or M. haemophilum infections in order to evaluate if these treatments are effective at decreasing the severity of these infections.
We found that tigecycline and clarithromycin treatments of 14- and 30-d are effective at decreasing the severity of infections but not eliminating infections. Generally, we do not advocate for treatment of whole colonies of zebrafish, and treatment should only be reserved for preserving valuable fish lines. As latency and reinfection could occur, treated fish should be used to re-derive valuable lines using surface disinfected embryos (Whipps et al., 2012). We recommend treated fish not be placed back on a main system as they may still be an infection risk factor for other fish. Also, treatment alone in order to control an outbreak is not enough, system disinfection is also required or else reinfection can occur from mycobacteria in surface biofilms (Whipps et al. 2012). Disease prevention and detection should still be primary mechanisms for controlling mycobacteriosis in zebrafish populations.
Supplementary Material
Acknowledgments
This research was funded in part by the Office of Research Infrastructure Programs of the National Institutes of Health (NIH) under a subcontract of the award number R24OD010998 to CMW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Adekambi T, Drancourt M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. International Journal of Systematic and Evolutionary Microbiology. 2004;54:2095–2105. doi: 10.1099/ijs.0.63094-0. [DOI] [PubMed] [Google Scholar]
- Aragon T, Fay M, Wollschlaeger D. Epitools: R Package for Epidemiologic Data and Graphics. R Package version 0.5–7. 2012 https://cran.r-project.org/web/packages/epitools/epitools.pdf.
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comparative Medicine. 2000;50:666–672. [PubMed] [Google Scholar]
- Bernut A, Le Moigne V, Lesne T, Lutfalla G, Herrmann JL, Kremer L. In vivo assessment of drug efficacy against Mycobacterium abscessus using the embryonic zebrafish test system. Antimicrobial Agents Chemotherapy. 2014;58:4054–4063. doi: 10.1128/AAC.00142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Colicino EG, Dipaola EJ, Al-Hasnawi HJ, Whipps CM. Evaluating the effectiveness of common disinfectants at preventing the propagation of Mycobacterium spp. isolated from zebrafish. Comparative Biochemistry and Physiology Part C. 2015;178:45–50. doi: 10.1016/j.cbpc.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CT, Whipps CM. Activity of Antibiotics against Mycobacterium Species Commonly Found in Laboratory Zebrafish. Journal of Aquatic Animal Health. 2015;27:88–95. doi: 10.1080/08997659.2015.1007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm R, Geisler R. Learning from small fry: the zebrafish as a genetic model organism for aquaculture fish species. Marine Biotechnology. 2006;8:329–345. doi: 10.1007/s10126-006-5139-0. [DOI] [PubMed] [Google Scholar]
- Falkinham JO., 3rd Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. Journal of Applied Microbiology. 2009;107:356–367. doi: 10.1111/j.1365-2672.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- Falkinham JO, 3rd, Norton CD, Lechevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare and other Mycobacteria in drinking water distribution systems. Applied Environmental Microbiology. 2001;67:1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitsma H, Cuppen E. Zebrafish as a cancer model. Molecular Cancer Research. 2008;6:685–694. doi: 10.1158/1541-7786.MCR-07-2167. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. Thousand Oaks, CA, USA: Sage; 2011. [Google Scholar]
- Gaind N. This week in science: Trendwatch. Nature. 2016;536:131. [Google Scholar]
- Gerhard GS. Comparative aspects of zebrafish (Danio rerio) as a model for aging research. Experimental Gerontology. 2003;38:1333–1341. doi: 10.1016/j.exger.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Dalet C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, Von Reyn CF, Wallace RJ, Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculosis mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Mac Law J, Sanchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comparative Biochemistry and Physiology Part C. 2009;149:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Murray KN, Westerfield M. Diseases of Zebrafish in research facilities. Bulf Breeze, Fl: Zebrafish International Resource Center; 2012. Available: https://zebrafish.org/wiki/health/disease_manual/start (November 2016) [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comparative Biochemistry and Physiology Part C. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kim EY, Chi SY, Oh IJ, Kim KS, Kim YI, Lim SC, Kim YC, Kwon YS. Treatment outcome of combination therapy including clarithromycin for Mycobacterium avium complex pulmonary disease. The Korean Journal of Internal Medicine. 2011;26:54–59. doi: 10.3904/kjim.2011.26.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M With Code Developed by the R Development Core Team & With General Advice from the R-Help Listserv Community and Especially Duncan Murdoch. sciplot: Scientific Graphing Functions for Factorial Designs. R package version 1.1-0 ed. 2012 https://cran.r-project.org/web/packages/sciplot/sciplot.pdf. [Google Scholar]
- Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrobial Agents and Chemotherapy. 2005;49:220–229. doi: 10.1128/AAC.49.1.220-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga E. Fish disease: diagnosis and treatment. 2nd. Ames, Iowa: Wiley-Blackwell Scientific; 2010. [Google Scholar]
- Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Disease Models and Mechanisms. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlert T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums. R Package version 1.1. 2015 https://cran.r-project.org/web/packages/PMCMR/vignettes/PMCMR.pdf.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- R Studio. R Studio: Integrated development environment for R. Boston, MA, USA: R Studio; 2012. [Google Scholar]
- Sciarra JB, Tyler AT, Kolb A. A gelatin-based diet for oral dosing juvenile to adult zebrafish (Danio rerio. Laboratory Animal Science Professional. 2014 Jun;:32–35. [Google Scholar]
- Strike TB, Feltrer Y, Flach E, Macgregor SK, Guillaume S. Investigation and management of an outbreak of multispecies mycobacteriosis in Australian lungfish (Neoceratodus fosteri) including the use of triple antibiotic treatment. Journal of Fish Diseases. 2016 doi: 10.1111/jfd.12535. [DOI] [PubMed] [Google Scholar]
- Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods in Molecular Biology. 2011;691:271–279. doi: 10.1007/978-1-60761-849-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Com Comparative Biochemistry and Physiology Part C. 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiology Letters. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in Zebrafish Colonies. Institute for Laboratory Animal Research. 2012;53:95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Diseases of Aquatic Organisms. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, Goodspeed J, Suciu C, Tan JL, Grimes C, Chung A, Rosenberg M, Gaikwad S, Denmark A, Jackson A, Kadri F, Chung KM, Stewart A, Gilder T, Beeson E, Zapolsky I, Wu ND, Cachat J, Kalueff AV. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behavioural Brain Research. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Wu TS, Chiu CH, Yang CH, Leu HS, Huang CT, Chen YC, Wu TL, Chang PY, Su LH, Kuo AJ, Chia JH, Lu CC, Lai HC. Fish tank granuloma caused by Mycobacterium marinum. Plos One. 2012;7:e41296. doi: 10.1371/journal.pone.0041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.