Abstract
Stress and anxiety during pregnancy are associated with a range of adverse health outcomes, thus there is an unmet need for low-barrier treatments that target stress and anxiety. One such treatment approach, attention bias modification training (ABMT), reduces the anxiety-related attentional threat bias, which is also associated with disrupted neural processing of threat. It remains unclear, however, whether reducing treatment barriers via mobile delivery of ABMT is effective and whether ABMT efficacy varies depending on individual differences in neural processing of threat. The present study tested whether mobile, gamified ABMT reduced prenatal threat bias, anxiety and stress, and whether ABMT efficacy varied with individual differences in neural responses to threat. Participants were 29 women in their 19th – 29th week of pregnancy, randomized to four weeks of ABMT versus placebo training (PT) versions of the mobile app using a double-blind design. Self-report of anxiety, depression, and stress were obtained, and salivary cortisol was collected at home and in lab in response to stressors to index biological stress reactivity. Threat bias was measured using a computerized attention assay during which EEG was recorded to generate event-related potentials (ERPs) to threat cues. Results showed lower levels of threat bias (1-tailed) and lab cortisol following ABMT versus PT. Although the main effect of ABMT on subjective anxiety was not significant, the magnitude of cortisol reduction was correlated with lower levels of subjective anxiety and threat bias. Those receiving ABMT also reported less anxiety when showing smaller ERPs to threat (P1, P2) prior to training, but, conversely reported more anxiety when showing larger ERPs to threat. Use of gamified, mobile ABMT reduced biobehavioral indices of prenatal stress and anxiety, but effects on anxiety varied with individual differences in cortisol response and neurocognitive indices of early attention to threat.
High levels of antenatal anxiety and stress, which occur in as many as 20% of pregnant women (Austin et al., 2010; Dunkel Schetter & Tanner, 2012), have been associated with a range of adverse outcomes, including increased obstetric complications and preterm delivery (Mulder et al., 2002). In offspring, a range of adverse physical and mental health outcomes have been documented, from low birth weight, alterations in brain morphology (Weinstock, 2001) and attention deficit hyperactivity disorder (Monk, 2001; Van den Bergh, Mulder, Mennes, & Glover, 2005), even after controlling for obstetric risk factors (Dole et al., 2003; Paarlberg, Vingerhoets, Passchier, Dekker, & Van Geijn, 1995; Williamson, LeFevre, & Hector, 1989). Indeed, more chronic and intense antenatal stress may result in greater general susceptibility to psychopathology in offspring (Huizink, Mulder, & Buitelaar, 2004).
Given the significant negative health impact of stress and anxiety on pregnant women and their offspring, access to effective anxiety- and stress-reduction treatments via easily-accessible and cost-effective therapies are crucial public health goals and are essential for improving the health and well-being of pregnant women and their children (Adler, Fink, Urech, Hösli, & Bitzer, 2011; Evans, Spiby, & Morrell, 2015). Although current best practices include the use of medication and long-term cognitive behavior therapy, alternatives to pharmaceutical treatments or resource-heavy psychological interventions are highly desirable to patients and health professionals alike and will increase the frequency and acceptability of such treatments during pregnancy (Bledsoe & Grote, 2006; Yonkers et al., 2009). The healthcare and psychology fields also have highlighted the need to develop mobile, computerized interventions in order to optimize treatment acceptability, overcome barriers related to accessibility, cost, and stigma (Harwood & L’Abate, 2010; Kazdin & Blase, 2011; Kazdin & Rabbitt, 2013; L’Abate, 2007; Mosa, Yoo, & Sheets, 2012; Rotheram-Borus, Swendeman, & Chorpita, 2012), and to more effectively target discrete pathological mechanisms (Kazdin & Rabbitt, 2013; Mosa et al., 2012).
One targeted cognitive mechanism in anxiety and stress-related pathology is the threat bias, or selective and exaggerated attention to threat (Hakamata et al., 2010; Hallion & Ruscio, 2011; MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002). Attention bias modification training (ABMT; MacLeod et al., 2002; Van Bockstaele et al., 2014) uses simple, brief, computerized techniques to systematically train attention away from threat in order to directly reduce threat bias (Hakamata et al., 2010; Hallion & Ruscio, 2011; MacLeod et al., 2002). A meta-analysis of randomized clinical trials showed that four to six weeks of ABMT significantly reduced anxiety and stress reactivity compared to placebo training (Hakamata et al., 2010) and comparable to the effect size of a typical 12-session cognitive behavioral therapy (Hazen, Vasey, & Schmidt, 2009). More recently, additional meta-analyses suggest that efficacy of ABMT is mixed, and may depend on a range of individual differences and characteristics of the intervention design (Clarke, Notebaert, & MacLeod, 2014; MacLeod & Clarke, 2015; Price et al., 2016). Thus, although ABMT, which is brief, accessible, cost-effective, and low-toxicity, may represent an optimal anxiety- and stress-reduction intervention for pregnant women, relatively little is understood about how to optimize the efficacy of ABMT.
In pursuit of this goal, we have created a mobile ABMT application or “app” (for iOS devices like iPhones), which takes the core components of the most-commonly used ABMT protocol (the dot probe) and puts them in the context of an appealing game, incorporating video game-like features such as animated characters and sound effects. Like traditional ABMT, attention is still systematically redirected away from threat-relevant stimuli (angry faces), but in a more appealing and engaging format.
We have recently demonstrated in two placebo-controlled studies with moderately anxious college students that the app reduces anxiety, stress reactivity, and threat bias in a single, lab-based session (Dennis-Tiwary, Egan, Babkirk, & Denefrio, 2016; Dennis & O’Toole, 2014). However, in the most recent study (Dennis-Tiwary et al., 2016), ABMT versus placebo resulted in improved behavioral performance during a stressor for females but not males. Given these early indications that the app is an effective delivery system for ABMT, but may be more effective for females, we expected that the app would be effective during the prenatal period. It is unknown, however, whether extended, non-lab based use of the app will result in similar positive stress and anxiety-reduction effects. In the present study, we tested whether a month of using the ABMT app outside the lab reduced stress and anxiety in a group of pregnant women.
Research on stress during pregnancy has targeted the stress hormone cortisol in relation to perinatal outcomes in mothers and their offspring (Austin & Leader, 2000; Sandman, Wadhwa, Hetrick, Porto, & Peeke, 1997). The human stress response is modulated by several complementary systems, with the core components being the autonomic nervous system and the hypothalamic-pituitary adrenal (HPA) axis. The glucocorticoid, cortisol, is an end-product of the stress response system and can be measured in saliva. Elevated cortisol responses have been directly linked to a range of outcomes including pre- and post-mature births (McCool, Dorn, & Susman, 1994; Ponirakis, Susman, & Stifter, 1998) and higher incidence of post-partum depression (Bloch, Daly, & Rubinow, 2003; Hendrick, Altshuler, & Suri, 1998; Alder, Fink, Bitzer, Hösli, Holgreve, 2007). Thus, in testing the efficacy of prenatal stress-reduction interventions, it is informative to measure the impact of interventions on cortisol response.
While ABMT holds great promise as both a clinic-based and mobile treatment strategy, researchers have recently identified a range of individual differences that may impact the efficacy of ABMT (Clarke, Browning, Hammond, Notebaert, & MacLeod, 2014; Mogoase, David, & Koster, 2014; O’Toole & Dennis, 2012). For example, anxious adults evidencing a pre-treatment bias towards (compared to away from) threat showed greater symptom reduction (Kuckertz, Gildebrant, et al., 2014), although in a study of adults with post-traumatic stress disorder, those evidencing a pre-treatment bias away from threat showed greater symptom reduction (Kuckertz, Amir, et al., 2014). These findings highlight the need to improve personalization of ABMT and increase the ability to identify those for whom ABMT may be most effective (Kapur, Phillips, & Insel, 2012).
Scalp-recorded event-related potentials (ERPs) are particularly well-suited for measuring individual differences that may influence the efficacy of ABMT, as well as treatment-related changes in neural processing of threat. ERPs in response to visual information can be used to quantify distinct components of exaggerated attention to threat due to their high functional sensitivity and excellent temporal resolution (Banaschewski & Brandeis, 2007). For example, The P1, P2, and N2 reflect distinct stages of attentional processing. The P1, peaking around 100 ms in occipital-parietal electrodes indexes very early activity of the extra-striate visual cortex and thus reflects relatively rapid and automatic shifts in attention (Hillyard & Anllo-Vento, 1998; Luck, Heinze, Mangun, & Hillyard, 1990; Smith, Cacioppo, Larsen, & Chartrand, 2003). The P2, peaking slightly later around 200 ms in posterior scalp electrodes, reflects an early stage of affectively-charged attention (Carretié, Martín-Loeches, Hinojosa, & Mercado, 2001). Finally, the N2, peaking around 250–350 in frontal scalp electrodes has been linked to the maturation and recruitment of cognitive control capacities (Ladouceur, Dahl, & Carter, 2007; van Veen & Carter, 2002). A small number of prior studies have shown that ABMT directly modfies ERP measures of attention and procesing of threat. For example, Eldar and Bar-Haim (2010) reported both reductions in P2 amplitudes and enhanced N2 amplitudes in anxious participants trained away from threat. More recently, evidence from our lab showed decreases in P1 amplitudes following ABMT (O’Toole & Dennis, 2012).
In the proposed study, we will examine whether ABMT directly modifies ERP responses to threat stimuli, but will focus most directly on testing whether individual differences in ERPs prior to ABMT predict training response, including threat bias and cortisol response. Specifically, we will use ERPs generated during a threat bias assay to test whether those women showing reduced attention allocation (P1) and affective processing (P2) of threat, but enhanced cognitive control of threat (N2) prior to ABMT may be most amenable to and benefit most from ABMT. For example, using the ABMT app in a recent study (Dennis-Tiwary et al., 2016), ABMT versus placebo resulted in reduced stress reactivity (measured as improved performance during a social stressor) when participants also showed smaller P1 amplitudes to threat cues prior to ABMT. This suggests that the P1 signals the ability to minimize attention capture by threat prior to ABMT, thus facilitating the positive effects of attention training.
The present study was a pilot double-blind, randomized, placebo-controlled trial of the ABMT mobile app in pregnant women. We tested whether extended use of the app outside the lab reduced three key outcomes during pregnancy: stress reactivity measured via cortisol response, anxiety, and threat bias. We further examined whether ERP responses during the threat bias assay at baseline predicted improved training response, specifically: (a) smaller P1 and P2 amplitudes (reflecting reduced attention allocation to threat); and (b) larger N2 amplitude (reflecting enhanced recruitment of cognitive control resources).
METHOD
Participants
One hundred-and-two women receiving prenatal treatment from a large urban hospital, who were between their 19th and 29th week of pregnancy according to medical records, were approached by study recruiters in the ultrasound and clinic waiting rooms. They were told that the study “is testing whether a mobile application for iOs devices reduces stress in pregnant women.” Of these, 33 women aged 23–45 (M = 33.12, SD = 5.78) agreed to participate.
Of these 33 women, 29 completed both the Time 1 (pre-intervention) assessment and the T2 (post-intervention) assessment. Of the four who were not able to complete the full study, one withdrew due to technical difficulties in completing the mobile intervention, one withdrew due to health problems and admission into the hospital, one did not respond to efforts to reschedule, and one refused EEG administration and decided to discontinue participation in the study.
The final sample consisted of 29 women aged 23 to 45 (M = 32.97, SD = 5.52; average weeks gestational M = 22.44, SD = 2.43) who were randomly assigned to either the ABMT (n=15) or placebo (PT; n=14) group. The average annual household income in US dollars was M = 209,180, SD = 232,990, ranging from 17,000 to 1,000,000. Mean years of education was 17.97 (SD = 2.21). Self-reported race/ethnicity was: 15 White, 7 Asian, 1 African American, 1 Native American/Native Alaskan, 2 more than one race, and 3 self-reported “other” race/ethnicity. Of these, 6 identified as Hispanic/Latino.
Questionnaires and Assessments
All questionnaires were administered at Visits 1 and 2.
The Depression, Anxiety, and Stress Scale
The Depression, Anxiety, and Stress Scale (DASS-21; Henry & Crawford, 2005). The DASS-21 is a 21-item questionnaire that measures the severity of symptoms across three domains: depression, anxiety, and stress. Each subscale contains 7 items, scored on a 0 to 3 scale, and with scores ranging from 0 to 21 for each subscale. A score of 4–5 indicates mild anxiety, a score of 5–6 indicates mild depression, and a score of 8–9 indicates mild stress. Participants’ anxiety scores ranged from 0 to 17 and stress scores ranged from 0 to 11, with most (82%) reporting normal levels of anxiety and stress.
The Hamilton Anxiety Scale
The Hamilton Anxiety Scale (HAM-A; Hamilton, 1959) was used to assess severity of anxiety symptoms with scores ranging from 0 to 56. Higher scores indicate increased severity, with scores greater than 17 indicating mild severity, 18–24 indicating mild-moderate severity, and 25 to 30 moderate to severe. Participants’ scores ranged from 0 to 32, with most (93%) reporting normal levels of anxiety.
Lab-based Stressor
During Visit 2 only, an anagrams task followed by the Trier Social Stress Test (TSST) were administered (Kirschbaum, Pirke, & Hellhammer, 1993). Neither the anagrams task nor the TSST were administered prior to attention training because acute stress may induce shifts in threat-related attention (Bar-Haim, 2010) thus distorting the measurement of pre-training bias. The anagrams task (MacLeod et al., 2002) includes 40 medium to difficult anagrams. Fourteen of the mixed letter words were not resolvable as real words (unsolvable). Mixed letter words were presented on the computer and participants were asked to write down the words on a sheet of paper. Participants received the following instructions, “For the following task you will be asked to solve forty anagrams. Each string of letters creates a word. When you have figured out what that word is please write it down on the answer sheet in front of you. You will have three minutes to complete this task. Please do this task as quickly as you can.”
The TSST included both a social-evaluative threat (giving a speech for three minutes) and a lack of control task (three minute arithmetic task). Both tasks were video-recorded and completed in front of two research assistants described as judges. Participants were told that their performance would be compared to others in the study and that an analysis of voice-frequency and behavior would be conducted.
During the lab-based stressor period, self-report of mood was obtained at three time points using three adapted visual analog mood scales as a manipulation check (AMS; See also MacLeod et al., 2002): baseline (ten minutes prior to stress tasks), after anagrams, and after the TSST. Each scale consisted of a series of horizontal lines divided into 30 equal sized partitions followed by the following questions (How anxious are you?; How sad are you?; How happy are you?). Participants are asked to identify a number on a scale of 1 to 30 that best represents their mood at the present time with higher scores indicating more intensity.
The dot probe
The dot probe task (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & Van Ijzendoorn, 2007; Mathews & Mackintosh, 1998) followed parameters of the Tel-Aviv University/National Institute of Mental Health protocol. Stimuli for the dot probe task are pictures of 20 different individuals (10 males, 10 females) from the NimStim stimulus set (Tottenham et al., 2009) with one female taken from the Matsumoto and Ekman (1989) set. Stimuli were programmed using E-Prime version 2.0 (Schneider, Eschman, & Zuccolotto, 2002).
During each trial, two pictures were presented, either angry-neutral face pairs or neutral-neutral face pairs (depicting the same individual). The pictures were shown above and below a fixation cross, with 14 mm between them. The task included 120 trials (80 threat [angry faces] and neutral faces [TN] and 40 non-threat both neutral faces [NN]). Each trial comprised: (a) 500 ms fixation, (b) 500 ms face-pair cue, which then disappears, (c) probe (target) in the former location of one of the faces until a response is made via the left or right mouse button to indicate the direction in which the arrow is pointing, and (d) 500 ms inter-trial interval. Participants were asked to respond as quickly and as accurately as possible whether the arrow was pointing to the left or the right. Probes were equally likely to appear on the top or bottom, in the location of the angry or neutral face cues, and pointing to the left or the right.
Quantifying behavioral threat bias
Three measures of threat bias were derived from the Visit 1 baseline dot probe and from the dot probe administered at the beginning of Visit 2, which followed the four-week training period. Dot probe trials with incorrect responses were excluded from further processing and analyses. Responses faster than −3SD from an individual’s mean and slower than +3SD from an individual’s mean were removed. The average response time was 508.60 (SD = 59.37) and the overall accuracy rate prior to training was 0.98 (SD = .02). Three threat bias scores were generated. Attention bias was calculated as the average RTs for neutral probes in TN trials minus RTs for angry probes in TN trials. Because attention bias scores can be elevated due to facilitated detection of threat (vigilance) or difficulty disengaging from threat, both vigilance and disengagement scores were also calculated. Vigilance was calculated as the average RTs for neutral probes in NN trials minus RTs for angry probes in TN trials. Higher scores indicate more facilitated detection of threat relative to a true neutral baseline. Disengagement was calculated as the average RTs for neutral probes on TN trials minus RTs for neutral probes on NN trials. Higher scores indicate greater difficulty disengaging from threat.
Saliva collection and cortisol measurement
Cortisol was measured from saliva samples using color-coded Salimetrics® tubes and analyzed using a competitive immunoassay technique specifically designed and validated for quantitative measurement of salivary cortisol. Saliva was collected both in the lab and at home over a four-week period. In the lab, saliva was collected once at Visit 1 for a baseline cortisol value. At Visit 2, saliva was collected at three time points (arrival, pre-stressor, post-stressor) and stored in a freezer at −80°C. The arrival sample was taken immediately after consent procedures. Participants were instructed to wash their mouth with water approximately 15 minutes prior to collecting the first sample. The time between arrival and the pre-stressor was approximately 59 minutes (SD = 0:12) and included the consent and questionnaire period, EEG set-up, and 10 minutes of computerized assessment including the dot probe. The time between the pre- and post-stressor sample was 20 minutes (SD = 0:05) and included pre-stressor mood assessments, anagrams task, TSST, and post-stressor mood assessment.
At home saliva was collected on four days [days 1 and 2 immediately following Visit 1; days 3 and 4 immediately prior to Visit 2 (four weeks later)] at three time points each day (waking, 30 minutes post waking and just before going to sleep). Individuals were instructed to abstain from eating or drinking prior to both morning samples on each home collection day. Home saliva was stored in the individual’s freezer immediately after each collection until returning to the lab for Visit 2.
A square root transformation was used on raw cortisol concentrations to approximate a normal distribution. Two measures were derived. First, for both home and Visit 2 lab cortisol area under the curve with respect to increase (AUCI) was quantified. AUCI indicates change in concentration across a specific period of time providing the magnitude and direction of change. Thus, a negative value reflects a decrease over time whereas a positive value reflects an increase over time (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Second, from Visit 2 lab cortisol only, cortisol reactivity was calculated as the concentration of the third sample (post-stressor) minus the first sample (pre-stressor). This provided a measure of cortisol response to a discrete stressor.
Electrophysiological recording and data reduction
A Biosemi system (BioSemi; Amsterdam, NL), was used to record EEG activity continuously during the pre- and post-training dot probe tasks using 64 Ag/AgCl scalp electrodes. Electrodes were fixed into an elasticized nylon cap and arranged according to the international 10/20 system. Eye movements were monitored by electro-oculogram (EOG) signals from electrodes placed 1 cm above and below the left eye (to measure vertical eye movements) and 1 cm on the outer edge of each eye (to measure horizontal eye movements). Preamplification of the EEG signal occurred at each electrode which improves the signal-to-noise ratio. EEG was recorded at a sampling rate of 512 Hz. During EEG acquisition, the voltage from each of the 64 electrodes from which data was collected was referenced online with respect to the common mode sense active electrode and driven right leg electrode, which produces a monopolar (nondifferential) channel. Brain Vision Analyzer (Version 2.2, GmbH; Munich, DE) was used to prepare the data. Offline, all data were re-referenced to the average of the scalp and filtered with a high pass frequency of 0.1 Hz and a low pass frequency of 30 Hz. Data were then segmented 200 ms prior to face-pair cue onset (during the fixation period, used for baseline correction) and continued for 500 ms until face-pair cue offset. Trials with incorrect responses were excluded from further processing and analyses. Standard ocular and artifact identification and removal were used.
The Gratton, Coles, and Donchin (1983) ocular correction method was used to identify and remove blinks. Artifacts were identified using the following criteria and removed from analyses: voltage steps greater than 50 μV, changes within a given segment greater than 300 μV, and activity lower than .5 μV per 100 ms. In addition to this method of artifact identification, trials were visually inspected for artifacts, which were removed on a trial-by-trial-basis. Examples include high frequency muscle movement and partial blinks that may not be detected by the applied filter. This additional step accounted for minimal data removal.
Electrodes were chosen via visual inspection of the topographical distribution of the pre-training dot probe task data, grand averaged across all stimulus conditions and participants (see Figure 1). ERPs were quantified as the mean amplitude for each cue condition: the P1 was generated from 80–130 ms over P5/P7/PO7 and P6/P8/PO8; the N170 was generated from 130–180 ms over CP5/P7/P9/PO7 and CP6/P6/P8/P10/PO8; the P2 was generated from 180–280 ms over O1/Oz/O2; the N2 was generated from 290–350 ms over FCz/Fz.
Figure 1.
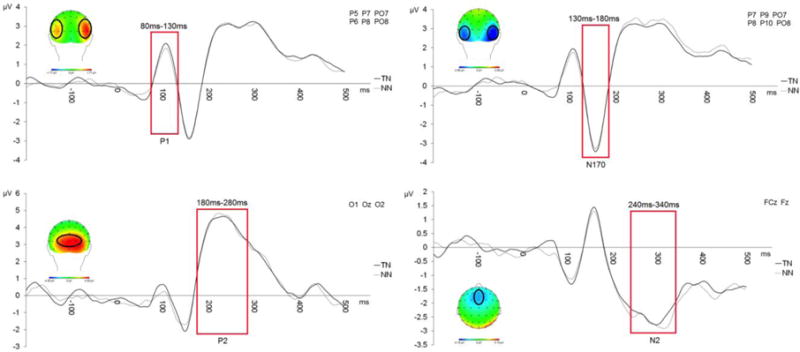
Grand averaged scalp topographies and waveforms for ERP components (P1, N170, P2, N2) generated to the face pair cues during the pre-training dot probe task.
For each component, difference scores were generated to threat cues using TN trials versus non-threat cues using NN trials (TN-NN). These difference scores were used in all ERP analyses reported below. Trial counts were grand averaged across stimulus conditions and participants. The average trial count for P1 was 39.15 (SD = .84), for N170 was 39.10 (SD = .90), for P2 was 39.13 (SD = .92) and for N2 was 39.32 (SD = .67). There were no significant difference in the average trial counts between ABMT and PT groups, all t’s < 0.83, p’s > 0.42.
ABMT and PT versions of the app
Participants were told that there was an ABMT and PT version of the app, and then were randomly assigned to either one or the other version of the app. The app was downloaded by the experimenter from on the App Store under the name Personal Zen onto either the participant’s iOS device or an iPod Touch provided to them by the lab. Participants did not view the app or the App Store during the downloading procedure. Participants’ devices were remotely “switched” into ABMT or placebo mode by a different experimenter using the heroku.com cloud application platform. Participants were debriefed about the app at the end of Visit 2 and asked about their experience using it. Both experimenters and participants were blind to group assignment. Participants reported no previous familiarity with Personal Zen.
Following the app download, participants sat comfortably at a table, and were given an iPod Touch or used their iOS device (e.g., iPhone) to practice the app to insure understanding (see Dennis-Tiwary et al., 2016). The following instructions were provided: “In this game, two animated characters will appear on the screen. Shortly after, they will burrow into a hole. One of them will cause a path of grass to rustle behind it. With your fingers, trace the path of the rustling grass, beginning from the burrow. Try to complete this task as quickly and as accurately as possible.” Then, they were allowed to complete one practice round under the guidance of the experimenter who answered any questions about the app. For every trial, two cartoon characters (sprites), one showing an angry expression and one showing a neutral/mildly pleasant expression, appeared simultaneously on the screen for 500 ms. Next, both sprites simultaneously “burrowed” into the grass field (See Dennis & O’Toole and Dennis-Tiwary et al, 2016 for images of the app). In the ABMT version, a trail of grass appeared in the location of the non-threat character for every trial, whereas in the PT version, a trail was equally likely to appear in the location of the angry or neutral sprite. The grass remained until participants responded by correctly tracing the grass path starting from the point at which the sprite burrowed out of sight. Points were accrued based on speed and accuracy (see Dennis & O’Toole, 2014 for scoring and feedback details).
Participants were instructed to complete 10 rounds of the app (25 trials per round or ~ 10 minutes) each day for four days/week over a period of four weeks, for a total of 40 rounds (~40 minutes of app play) per week and 160 rounds for the duration of the study. Number of training trials were consistent with previous ABMT studies (Eldar & Bar-Haim, 2010; Klumpp & Amir, 2009) and studies with the app (Dennis-Tiwary et al., 2016; Dennis & O’Toole, 2014).
App use fidelity check
Play was tracked via self-report (a log) but could also be tracked through the mobile analytics platform, Mixpanel. Mixpanel data were incomplete for six (20.69 %) of the women due to iOS updates, changes in device use, and unavailability of data from Mixpanel. Four (13.79 %) of the women did not submit a self-reported log of their play. Nineteen (65.52 %) women had complete usage data from both mix panel and self-report. Average self-report of rounds completed during the four-week long study period was 151 rounds for the placebo group (SD = 18.12) and 153 rounds for the ABMT group (SD = 16.36). Average use reflected by Mixpanel was 120.75 rounds (SD = 79.34) for the placebo group and 120.07 rounds (SD = 55.23) for the ABMT group. The high standard deviations for Mixpanel data were due to excessively low count of rounds for some participants, which Mixpanel reported reflected missing data rather than lack of use. Self-report and Mixpanel usage did not significantly differ between groups. Of the women having both self-report and Mixpanel data, the correlation between self-report and Mixpanel data was r(28) = 0.375, p = 0.065.
Procedure
Visit 1
Participants spent approximately 2 hours in the laboratory for the Visit 1 assessment. After consent, demographic questions and self-reports of mood and anxiety were completed electronically. Following the brief questionnaire period, a saliva sample was collected, after which baseline threat bias was assessed using the dot probe. Then, EEG electrodes were applied and participants were seated in an EEG recording booth 65 cm from a 17 in monitor to record neurocognitive responses to threat, measured during a second dot probe task. At the completion of the visit, Personal Zen was downloaded to the participant’s personal iOs device,1 practice was completed, and the usage-tracking log was explained.
Home
During the four-week period between visits, participants were asked to follow the scheduled app play and keep a record of usage. In addition, each participant was asked to collect three saliva samples on four different days [days 1 and 2 immediately following Visit 1; days 3 and 4 immediately prior to Visit 2 (four weeks later)].
Visit 2
Participants returned to the lab approximately four weeks later for a 2.5-hour visit. The session began with EEG application and administration of the post-training dot probe, followed by Visit 1 questionnaires, and two stressor tasks, during which mood was also assessed three times as a manipulation check. Saliva was collected at three time points (arrival, pre-stressor, and post-stressor).
Results
Baseline Analyses and Stressor Manipulation Check
All statistical analyses were conducted in SPSS (Version 21). Demographics and self-report of anxiety, stress, and depression are presented in Table 1 and threat bias scores, ERP, and cortisol measures are presented in Table 2. There were no training group differences in any demographic or self-report measures (all p’s > 0.14). We confirmed that the difficult anagrams task and TSST significantly changed mood and anxiety using paired-samples t-tests. Individuals reported significantly higher levels of anxiety post-stressor (M = 15.23, SD = 8.55) compared to pre-stressor (M = 7.00, SD = 7.08), t(27) = −5.96, p < .001, as well as lower levels of positive mood post-stressor (M = 17.73, SD = 8.57) compared to pre-stressor (M = 23.55, SD = 5.10), t(27) = 4.31, p < .001.
Table 1.
Descriptive Statistics, including Baseline and Post-Assessment Anxiety and Depression
| Variable | ABMT
|
PT
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (years) | 34.67 | 4.39 | 31.14 | 6.16 |
| Ethnicity (count) | ||||
| American Indian/Alaskan Native | 0 | 1 | ||
| Asian | 5 | 2 | ||
| Black or African American | 0 | 1 | ||
| White | 9 | 6 | ||
| More than one race | 0 | 2 | ||
| Unreported | 1 | 2 | ||
| Pre-Assessment ABMT |
PT
|
Post-Assessment ABMT |
PT
|
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| DASS Anxiety | 2.87 | 1.96 | 2.15 | 2.67 | 3.20 | 3.00 | 2.07 | 3.60 |
| DASS Stress | 5.20 | 2.86 | 4.54 | 3.78 | 6.00 | 2.83 | 4.36 | 4.18 |
| DASS Depression | 1.20 | 1.37 | 2.31 | 3.09 | 2.07 | 2.63 | 2.29 | 3.20 |
| HAM-A | 7.73 | 7.92 | 7.92 | 6.20 | 9.20 | 6.71 | 6.93 | 9.10 |
Table 2.
Descriptive Statistics for Threat Bias, ERP Amplitudes (μV), and Cortisol
| Pre-Assessment ABMT |
PT
|
Post-Assessment ABMT |
PT
|
|||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Attention Bias | 0.87 | 25.98 | 2.71 | 25.23 | −3.40 | 19.27 | 6.79 | 12.30 |
| Vigilance | −3.93 | 25.68 | 2.86 | −13.57 | −3.07 | 15.35 | 2.64 | 9.51 |
| Disengagement | 4.80 | 24.62 | 0.14 | 20.26 | −0.33 | 16.54 | 4.14 | −14.11 |
| P1 | 0.20 | 0.64 | 0.15 | 0.92 | 0.00 | 0.77 | 0.08 | 0.99 |
| N170 | 0.07 | 1.00 | −0.05 | 1.34 | 0.07 | 1.04 | −0.04 | 1.21 |
| P2 | −0.14 | 1.12 | −0.17 | 1.37 | 0.23 | 0.94 | −0.25 | 1.43 |
| N2 | 0.06 | 0.90 | 0.29 | 1.23 | 0.31 | 0.95 | −0.22 | 1.07 |
| AUCI [home Day 4; min × (μg/dl) | −1.79 | 2.20 | −1.15 | 2.50 | ||||
| Cortisol Reactivity (Lab; μg/dl) | −0.05 | 0.09 | 0.01 | 0.09 | ||||
| AUCI [Lab; min × (μg/dl)] | −0.03 | 0.05 | 0.02 | 0.09 | ||||
ABMT = Attention bias modification training; PT = placebo training. ERP amplitudes are the difference between the Threat-Neutral and Neutral-Neutral condition. AUCI = area under the curve with respect to increase.
If participants did not own a personal iOS device, one was provided.
Correlations among Study Variables at Baseline
We conducted a series of bivariate correlations between self-report measures, measures of threat bias, and ERPs at baseline. Self-report DASS subscales (depression, anxiety, and stress) were highly positively inter-correlated (ranging between 0.41 – 0.53, all p’s < 05), except DASS-depression was not significantly correlated with DASS anxiety, p = 0.18. In addition, anxiety severity (HAM-A) was also highly positively correlated with DASS subscales (ranging between 0.39 – 0.61, all p’s < 0.05). Correlations between ERPs and self-report measures show that greater stress (r = 0.40, p = 0.033), and depression (r = 0.57, p = 0.002) were associated with reduced N2 amplitudes to threat versus neutral cues. Attention bias scores were not significantly correlated with ERPs, and no other correlations reached significance.
Effects of Training on Target Outcomes
Next, we tested the hypotheses that ABMT versus PT would reduce threat bias (attention bias, vigilance, disengagement), self-reported anxiety (DASS-anxiety, HAM-A), and salivary cortisol (AUCI home and lab, cortisol reactivity lab only). Hypotheses were tested using a series of 8 ANCOVAs with Training Group (ABMT or PT) as a between-subjects factor, post-training outcomes as the dependent variable, and the corresponding pre-training measure as a covariate. For cortisol analyses, weeks pregnant upon entry into the study was used as a covariate given the link between gestational period and cortisol levels (Allolio et al., 1990).
There was a trend-level main effect of Training Group on attention bias, F(1, 26) = 3.03, p = 0.047, 1-tailed, partial η2 = 0.10. Attention towards threat was reduced for the ABMT versus PT group (see Figure 2, top panel).
Figure 2.
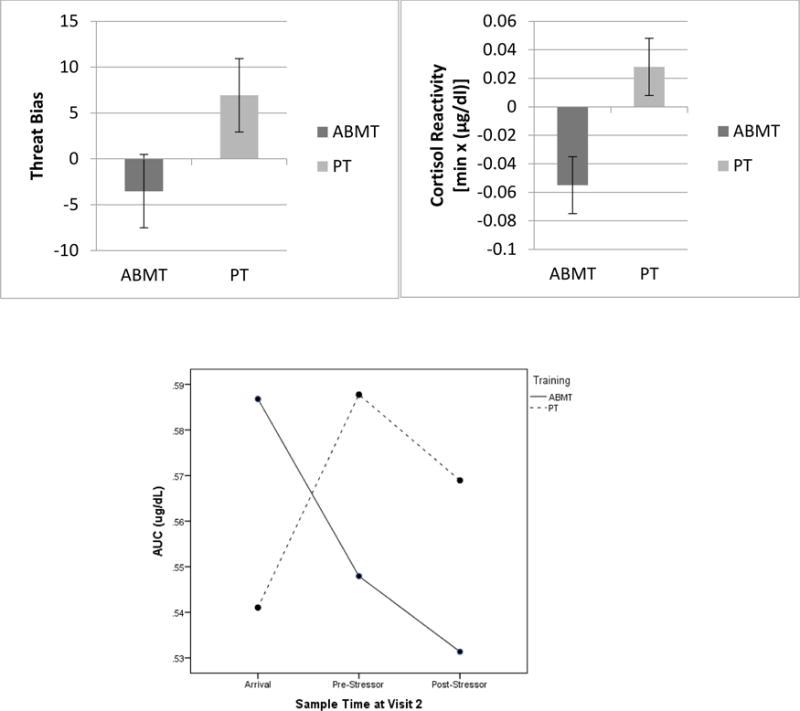
Post-treatment threat bias (top left), salivary cortisol reactivity (top right), and salivary AUCI (bottom) were reduced in the ABMT versus PT Group.
There was a significant main effect of Training Group on lab cortisol reactivity, F(1, 22) = 4.96, p = 0.037, partial η2 = 0.18, and on lab AUCI, F(1, 22) = 4.68, p = 0.042, partial η2 = 0.18. Cortisol secretion over the course of lab-based stressors was reduced in the ABMT versus PT group (see Figure 2, top and bottom panel, respectively).
No other main effects of Training Group reached significance.
Correlations between Cortisol Measures and Measures of Threat Bias and Anxiety
To examine the functional implications of reductions in cortisol following training, we conducted a series of bivariate correlations between the three cortisol metrics and post-training threat bias and self-report measures. For the sample as a whole, reduced lab AUCI was associated with lower levels of vigilance toward threat, r(29) = 0.435, p = 0.018. In addition, reduced home AUCI (from the first morning sample to the evening sample) was associated with less post-training anxiety (HAM-A), r(23) = 0.463, p = 0.026. No other correlations reached significance.
Moderators of Training Effects
Next, we used a series of hierarchical regressions to test the hypothesis that individual differences in ERP responses to threat prior to training would moderate ABMT effects on anxiety and stress. Each of the post-training measures were entered separately as the dependent variable with the following variables entered in separate steps: 1) the corresponding pre-training measure; 2) Training Group; 3) ERPs to threat versus non-threat (P1, N170, P2, or N2); 4) interaction between Training and ERP (e.g., ABMT × N2). There were a total of 8 regressions: two dependent variables (DASS-Anxiety and HAM-A) × four moderators (P1, N170, P2, N2). Given recommendations concerning probing interaction effects (Aiken & West, 1991; Finney, Mitchell, Cronkite, & Moos, 1984), if interaction terms’ contributions to R2 approached significance (p = 0.10), the Interactions were followed up with the PROCESS macro for SPSS (Hayes, 2013) by using simple regression equations. Regression lines were generated as the mean value and +/− one standard deviation from the mean.
Two Training Group × ERP interaction effects emerged. First, for self-reported anxiety (DASS), effects of ABMT varied with P1 magnitude: anxiety was reduced when participants showed smaller P1 amplitudes, but was increased when participants showed larger P1 amplitudes [Full model: F(4, 23) = 17.28, p < 0.001, R2 = 0.75; interaction step change statistics: F(1, 23) = 3.60, p = 0.06, R2 = 0.04; see Figure 3, left panel]. The same effect emerged for P2 (Training Condition × P2) such that anxiety was reduced when participants showed smaller P2 amplitudes, but was increased when participants showed larger P2 amplitudes [Full model: F(4, 23) = 19.81, p < 0.001, R2 = 0.78; interaction step change statistics: F(1, 23) = 5.93, p = 0.02, R2 = 0.06; see Figure 3, right panel].
Figure 3.
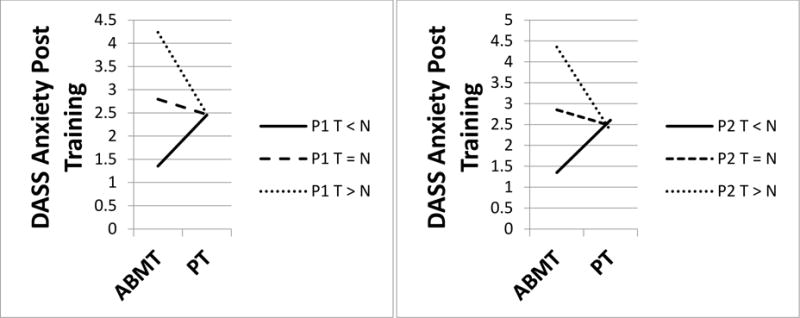
Self-reported anxiety was reduced when participants showed smaller P1 (left panel) and smaller P2 (right panel) amplitudes to threat versus non-threat, but was increased when participants showed larger amplitudes. ABMT = attention bias modification training. PT = placebo training.
Discussion
Given the significant impact of stress and anxiety on health during the pre- and post-natal periods–for mothers and their newborns – low-barrier interventions that can effectively reduce stress and anxiety are a pressing public health priority. Results of the present study showed that a key biological measure of stress, cortisol response, was reduced following the lab-based stressor in the ABMT versus PT group. Furthermore, the magnitude of decrease in cortisol both at home and in the lab was correlated with less post-training anxiety, depression, and vigilance for threat. In addition, threat bias was reduced in the ABMT versus PT group, and self-reported anxiety was reduced when those in the ABMT group showed less early visual processing of threat (smaller P1 and P2 amplitudes), thus highlighting the importance of considering neurocognitive individual differences prior to ABMT in order to better refine delivery of ABMT, and to identify those for whom ABMT might be most effective.
In addition to these promising early findings, it is important to note the sizeable number of null findings, including failure of ABMT to change subjective emotional reactivity to a stressor, two of the three possible behavioral measures of TB (with the third being significant 1-tailed), and cortisol response measured at home. Thus, results should be interpreted cautiously, and the need for additional research to replicate findings in a larger sample is a crucial next step.
A month of using Personal Zen reduced one of the three threat bias metrics generated by an untrained measure of threat bias (the dot probe), although at the level of a trend. Two previous studies using the app documented that a single 25-minute session of using the app in the lab was insufficient to reduce threat bias measured via the dot probe (Dennis-Tiwary et al., 2016), but that a longer exposure time (45 minutes) effectively reduced threat bias (Dennis & O’Toole, 2014). Yet, a critique of this longer exposure time is that it does not mirror real-world app use, with apps typically played in shorter bursts (Duggan, 2013). Thus, the design of the present study, which was to use the app for 10 minutes a day, 4 days a week, with breaks allowed between 1-min blocks of trials, more closely resembled likely patterns of gameplay while administering an adequate “dosage” over an extended period of time. Further attempts to encourage and measure “dosages” of mobile ABMT delivery modes are a crucial future research goal, as dosages in the current study were on the whole less than the planned amount. The current study did not include a longitudinal component, and so did not generate data on the sustainability of the positive benefits of mobile, gamified ABMT, nor whether “booster sessions” or regular use is necessary for gains to be maintained.
Cortisol secretion was reduced following the lab-based stressor in the ABMT versus PT group (although not home-based measures of cortisol), and the magnitude of decrease both at home and in the lab was correlated with less post-training anxiety and vigilance, showing the functional implications of this decrease. The specific link between cortisol and vigilance suggests that broader changes in the stress response system may directly influence mechanisms associated with exaggerated detection and vigilance for threat rather than more controlled attentional disengagement from threat. These findings are among the first to document that a gamified mobile intervention can alter a neuroendocrine index of stress reactivity and speak to the potential for brief, mobile intervention approaches to treat and prevent disease in at-risk health groups. Although effects of the current study were not significant for waking cortisol, effects support the recent finding that ABMT significantly decreased waking cortisol among individuals at risk for depression (Browning, Holmes, Charles, Cowen, & Harmer, 2012). In particular, pregnancy may be one optimal group for mobile stress- and anxiety-focused interventions given the link between cortisol and stress during pregnancy and maternal and fetal outcomes, and given the importance of brief, low-barrier, non-medication based treatments during pregnancy. Future research should further focus on the perinatal period and attention bias modification techniques to target depression and other affective psychopathology, as well as expanding measurement of stress-related outcomes, including alternative measures of cortisol secretion (e.g., cortisol levels derived from blood or hair samples) and other biobehavioral measures of stress reactivity (e.g., cardiac and other peripheral physiological measures).
Individual differences in very early-emerging ERP response to threat predicted ABMT effects. Those in the ABMT versus PT condition showed reduced self-report of anxiety severity when they also showed smaller P1 and P2 amplitudes. The effect for P1 is consistent with a previous study with the app, which showed that adults showing smaller P1 amplitudes at baseline showed improved performance during an anxiety-related stressor following a single session of app use (25 minutes; Dennis-Tiwary et al., 2016). One way to interpret this finding is that when the “cost” of rapid attention allocation (P1) is low, the cognitive flexibility required for attention training may be optimized and subjective anxiety will be most effectively reduced. This is further consistent with research showing that the earliest stages of attention allocation to threat, indexed by P1 and P2, are elevated in anxiety (Mueller et al., 2009; Rossignol, Philippot, Bissot, Rigoulot, & Campanella, 2012) and thus may divert resources away from task-focused attention (Mathews & Mackintosh, 1998; Mogg & Bradley, 1998) such as that happening during attention training. Counter to previous research focusing on role of cognitive control in ABMT (Eldar & Bar-Haim, 2010), this finding highlights the importance of very early-emerging and relatively automatic stages of attention to threat. It is also important to note that those who showed larger P1 and P2 amplitudes prior to training showed greater subjective anxiety after ABMT training. Although these small increases in anxiety were not likely to be clinically meaningful given the relatively low levels of anxiety severity in the study sample, this finding highlights the need to investigate in future research whether ABMT could increase anxiety in a subset of individuals.
Some important methodological issues should be noted when interpreting results. First, participants in the present study were not clinically anxious, reporting primarily normal or mild levels of anxiety. Given these promising early findings, future research should target women experiencing significant levels of anxiety severity, and who therefore may be at significant risk for negative effects of stress both prenatally and in the postnatal period.
Second, in the ABMT condition, there was 100% likelihood that participants would be required to respond to the trail made by the neutral/mildly pleasant sprite. In contrast, in many ABMT designs, baseline trials (i.e., trials in which only two neutral stimuli are present) are randomly interspersed on up to 20% of trials (e.g., Amir et al., 2009; Heeren, Reese, McNally, & Philippot, 2012) given research on variable contingency reinforcement schedules (Ferster & Skinner, 1957). This design element was chosen assuming that in real-world use, individuals might play the app in unpredictable intervals and durations. Thus, it could be most advantageous to administer only the active training condition. Comparing variable to 100% contingency is an important goal of future studies, as is potential differences between using pleasant rather than neutral non-threat stimuli during training, the latter of which is more usual for ABMT for stress and anxiety, and the former for ABMT for depression (Beard, Sawyer, & Hofmann, 2012; Hallion & Ruscio, 2011).
Taken together, results suggest the ABMT-based digital mental health tools such as Personal Zen for stress and anxiety reduction in this important health group, particularly as pregnancy is a time of risk and sensitivity to stress and anxiety. But given the pattern of mixed findings, including null effects, more research is needed to examine the conditions under which such tools may be most effective, individual differences in who best responds to them, and whether ABMT has a larger clinically-relevant impact on stress and anxiety during the perinatal period. The present study leveraged the sensitivity and specificity of ERPs to identify treatment-relevant individual differences predicting ABMT anxiety-reduction effects, which may be useful in future studies with the goal of improving the personalization of ABMT for a range of health groups. Findings also add to the growing body of research demonstrating that evidence-based treatment mechanisms can be embedded into highly accessible mobile and gamified formats, particularly those that target cognitive biases (Dennis-Tiwary et al., 2016; Dennis & O’Toole, 2014; Enock & McNally, 2013; Holmes, Lang, & Shah, 2009).
Highlights.
Stress reduction effects of a mobile app were examined in pregnant women.
Compared to placebo, the app reduced threat bias and cortisol reactivity.
Effects on anxiety symptoms differed depending on neural responses to threat.
Acknowledgments
Financial support: This research was made possible by grant TR000457 of the National Center for Advancing Translational Sciences of the National Institutes of Health. This publication was also made possible by a Research Centers in Minority Institutions Program grant from the National Institute on Minority Health and Health Disparities (MD007599) of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIMHD or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Ethical standards: “The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.”
References
- Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome?critical review of the literature. Journal of Maternal-Fetal & Neonatal Medicine. 2007;20(3):189. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- Adler J, Fink N, Urech C, Hösli I, Bitzer J. Identification of antenatal depression in obstetric care. Archives of Gynecology and Obstetrics. 2011;284(6):1403–1409. doi: 10.1007/s00404-011-1872-3. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Los Angeles, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Allolio B, Hoffmann J, Linton EA, Winkelmann W, Kusche M, Schulte HM. Diurnal salivary cortisol patterns during pregnancy and after delivery: relationship to plasma corticotrophin-releasing-hormone. Clin Endocrinol (Oxf) 1990;33(2):279–289. doi: 10.1111/j.1365-2265.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of consulting and clinical psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Priest SR, Reilly N, Wilhelm K, Saint K, Parker G. Depressive and anxiety disorders in the postpartum period: how prevalent are they and can we improve their detection? Arch Womens Ment Health. 2010;13(5):395–401. doi: 10.1007/s00737-010-0153-7. [DOI] [PubMed] [Google Scholar]
- Austin MP, Leader L. Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Australian and New Zealand journal of obstetrics and gynaecology. 2000;40(3):331–337. doi: 10.1111/j.1479-828X.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D. Annotation: What electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. Journal of Child Psychology and Psychiatry. 2007;45(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y. Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. The Journal of Child Psychology and Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43:724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SE, Grote NK. Treating depression during pregnancy and the postpartum: A preliminary meta-analysis. Research on Social Work Practice. 2006;16(2):109–120. doi: 10.1177/1049731505282202. [DOI] [Google Scholar]
- Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using attentional bias modification as a cognitive vaccine against depression. Biological psychiatry. 2012;72(7):572–579. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, Martín-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001;13(8):1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortext in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biological psychiatry. 2014;76(12):946–952. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Notebaert L, MacLeod C. Absence of evidence or evidence of absence: Reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry. 2014;14(8) doi: 10.1186/1471-244X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Egan LJ, Babkirk S, Denefrio S. For whom the bell tolls: Neurocognitive individual differences in the acute stress-reduction effects of an attention bias modification game for anxiety. Behav Res Ther. 2016;77:105–117. doi: 10.1016/j.brat.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, O’Toole LJ. Mental Health on the Go: Effects of a Gamified Attention-Bias Modification Mobile Application in Trait-Anxious Adults. Clinical Psychological Science. 2014 doi: 10.1177/2167702614522228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. American journal of epidemiology. 2003;157(1):14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Duggan M. Cell phone activities 2013. Cell 2013 [Google Scholar]
- Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40:667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Enock PM, McNally RJ. How mobile apps and other Web-based interventions can transform psychological treatment and the treatment development cycle. The Behavior Therapist. 2013;36(3):56–66. [Google Scholar]
- Evans K, Spiby H, Morrell CJ. A psychometric systematic review of self-report instruments to identify anxiety in pregnancy. Journal of Advanced Nursing. 2015 doi: 10.1111/jan.12649. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. East Norwalk, CT: Appleton-Century-Crofts; 1957. [Google Scholar]
- Finney JW, Mitchell RE, Cronkite RC, Moos RH. Methodological issues in estimating main and interactive effects: Examples from coping/social support and stress field. Journal of Health and Social Behavior. 1984;25:85–98. [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Lieibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on depression and anxiety. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Harwood TM, L’Abate L. Self-help in mental health: A critical review. New York, NY: Springer Science + Business Media; 2010. [Google Scholar]
- Hayes AF. Introductionto mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- Hazen RA, Vasey MW, Schmidt NB. Attentional retraining: A randomized clinical trial for pathological worry. Journal of psychiatric research. 2009;43(6):627–633. doi: 10.1016/j.jpsychires.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Heeren A, Reese HE, McNally RJ, Philippot P. Attention training toward and away from threat in social phobia: Effects on subjective, behavioral, and physiological measures of anxiety. Behavior Research and Therapy. 2012;50:30–39. doi: 10.1016/j.brat.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. The 21-item version of the Depression Anxiety Stress Scales (DASS-21): Normative data and psychometric evaluation in a large non-clinical sample. British Journal of Clinical Psychology. 2005;44:227–239. doi: 10.1177/0163278711424282. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes EA, Lang TJ, Shah DM. Developing interpretation bias modification as a “cognitive vaccine” for depressed mood: Imagining positive events makes you feel better than thinking about them verbally. Journal of Abnormal Psychology. 2009;118(1):76–88. doi: 10.1037/a0012590. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Blase SL. Interventions and models of their delivery to reduce the burden of mental illness: Reply to commentaries. Perspectives on Psychological Science. 2011;6:507–510. doi: 10.1177/1745691611418241. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Rabbitt S. Novel models for delivering mental health services and reducing the burdens of mental illness. Clinical Psychological Science. 2013:170–191. doi: 10.1177/2167702612463566. [DOI] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The ‘Trier Social Stress Test’: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Amir N. Examination of vigilance and disengagement of threat in social anxiety with a probe detection task. Anxiety, Stress & Coping. 2009;22(3):283–296. doi: 10.1080/10615800802449602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Amir N, Boffa JW, Warren CK, Rindt SEM, Norman S, McLay R. The effectiveness of an attention bias modification program as an adjunctive treatment for Post-Traumatic Stress Disorder. Behaviour Research and Therapy. 2014;63:25–35. doi: 10.1016/j.brat.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckertz JM, Gildebrant E, Liliequist B, Karlstrom P, Vappling C, Bodlund O, Carlbring P. Moderation and mediation of the effect of attention training in social anxiety disorder. Behaviour Research and Therapy. 2014;53:30–40. doi: 10.1016/j.brat.2013.12.003. [DOI] [PubMed] [Google Scholar]
- L’Abate L. Low-cost approaches to promote physical and mental health. In: L’Abate L, editor. Low-cost approaches to promote physical and mental health: Theory, research, and practice. New York, NY: Springer Science + Business Media; 2007. [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental science. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays: II. Functional dissociation of P1 and N1 components. Electroencephalography & Clinical Neurophysiology. 1990;75(6):528–542. doi: 10.1016/0013-4694(90)90139-B. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Clarke PJF. The attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3(1):58–78. doi: 10.1177/2167702614560749. [DOI] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107–123. doi: 10.1037//0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22(6):539–560. doi: 10.1023/A:1018738019346. [DOI] [Google Scholar]
- Matsumoto D, Ekman P. American-Japanese cultural differences in intensity ratings of facial expressions of emotion. Motivation and Emotion. 1989;13(2):143–157. doi: 10.1007/BF00992959. [DOI] [Google Scholar]
- McCool WF, Dorn LD, Susman EJ. The relation to cortisol reactivity and anxiety to perinatal outcome in primiparous adolescents. Research in Nursing & Health. 1994;17(6):411–420. doi: 10.1002/nur.4770170604. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogoase C, David D, Koster EHW. Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. Journal of Clinical Psychology. 2014 doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- Monk C. Stress and Mood Disorders During Pregnancy: Implications for Child Development. Psychiatric Quarterly. 2001;72(4):347–357. doi: 10.1023/a:1010393316106. [DOI] [PubMed] [Google Scholar]
- Mosa ASM, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Medical Informatics and Decision Making. 2012;12(1):67. doi: 10.1186/1472-6947-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2009;39(7):1141–1152. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Robles de Medina PG, Huizink AC, Van den Bergh BR, Buitelaar JK, Visser GH. Prenatal maternal stress: effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70(1–2):3–14. doi: 10.1016/s0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- O’Toole LJ, Dennis TA. Attention training and the threat bias: An ERP study. Brain and Cognition. 2012;78:63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole L, Dennis TA. Attention training and the threat bias: An ERP study. Brain and Cognition. 2012;78(1):63–73. doi: 10.1016/j.bandc.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paarlberg KM, Vingerhoets AJJM, Passchier J, Dekker GA, Van Geijn HP. Psychosocial factors and pregnancy outcome: A review with emphasis on methodological issues. Journal of Psychosomatic Research. 1995;39(5):563–595. doi: 10.1016/0022-3999(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Ponirakis A, Susman EJ, Stifter CA. Negative emotionality and cortisol during adolescent pregnancy and its effects on infant health and autonomic nervous system reactivity. Developmental psychobiology. 1998;33(2):163–174. [PubMed] [Google Scholar]
- Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, Bar-Haim Y. Pooled patient-level meta-analysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clinical Psychology Review. 2016;50:37–49. doi: 10.1016/j.cpr.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Philippot P, Bissot C, Rigoulot S, Campanella S. Electrophysiological correlates of enhanced perceptual processes and attentional capture by emotional faces in social anxiety. Brain Research. 2012;1460:50–62. doi: 10.1016/j.brainres.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus M, Swendeman D, Chorpita BF. Disruptive innovations for designing and diffusing evidence-based interventions. American Psychologist. 2012;67(6):463–476. doi: 10.1037/a0028180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa P, Hetrick W, Porto M, Peeke HVS. Human fetal heart rate dishabituation between thirty and thirty-two weeks gestation. Child Development. 1997;68(6):1031–1040. doi: 10.2307/1132289. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41(2):171–183. doi: 10.1016/S0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EHW. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140(3):682–721. doi: 10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29(2):237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77:477–482. doi: 10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65(5):427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Williamson HA, LeFevre M, Hector M. Association between life stress and serious perinatal complications. The Journal of Family Practice. 1989;29(5):489–494. [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. General hospital psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


