Introduction
Emerging evidence links exposure to ambient air pollution during the prenatal period to increased respiratory morbidity later in childhood.1, 2 Additionally, increased prenatal maternal stress and stress correlates (e.g., maternal anxiety) have been independently associated with early asthma phenotypes in a number of prospective epidemiological studies.3–8 Other data suggests that lower socio-economic status populations are more susceptible to air pollution related health effects, potentially due to concomitant increased psychosocial stress.9, 10
Epidemiological studies have begun to document synergistic effects between stress and ambient air pollution exposure in early childhood on incidence and exacerbation of asthma and decrements in lung function.11–16 For example, in a 3-year follow-up study of children enrolled at age 5–9 years in Southern California, annual residential exposure to NOx was associated with greater asthma incidence in children whose parents also reported higher perceived stress.12 In this same study, exposure to nitric oxide (NO), nitrogen dioxide (NO2), and total oxides of nitrogen (NOx) estimated at children’s residences and schools was also associated with greater decrements in lung function among children whose parents reported high perceived stress when compared to parents who reported low stress.11 Our group previously reported that higher lifetime exposure to ambient NO2, estimated using land use regression (LUR), in urban children followed to approximately age 7 years was associated with increased risk of asthma diagnosis only among children also exposed to higher levels of community violence in the neighborhood.13 Our team has also reported that prenatal exposure to black carbon (BC) and particulate matter≤ 2.5 microns in diameter (PM2.5) was associated with increased odds of repeated wheeze at age 2 years with an additive effect of concurrent prenatal community violence exposure.15 Data on the potential synergistic effects of increased ambient particulate air pollution exposure and psychosocial stress starting in utero on children’s respiratory health remain sparse.
Moreover, fetal lung development occurs through a complex orchestration of sequential biologic events and there may be periods of time in which the fetus is more sensitive to pro- oxidant exposures. Recent epidemiological evidence demonstrates that exposure to ambient air pollution at different periods in pregnancy is associated with respiratory outcomes in childhood.2, 17, 18 Our group showed that PM2.5 exposure during the second trimester was associated with asthma at age 6 in children in Boston.2 A study in China reported varying periods of importance for outdoor prenatal exposure to NO2 and allergic or respiratory outcomes, with first trimester exposure associated with eczema, exposure in the second trimester associated with asthma, and third trimester exposure linked with allergic rhinitis.18 In a study in Spain, second trimester NO2 exposure estimated using LUR was associated with reduced lung function at age 4.5 years and increased risk of low lung function (<80% of predicted forced expiratory volume in 1 second [FEV1]).17 Therefore, there is reason to expect that the particular timing of exposure during the prenatal period may be of importance. We leveraged existing data from an established population-based prenatally enrolled longitudinal cohort in Mexico City to examine the association between prenatal PM2.5 averaged over trimesters and report of wheeze in children followed to 48 months of age. We examined whether these associations were modified by prenatal psychosocial stress.
Methods
Study population
Pregnant women who were receiving health service and prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social –IMSS) were recruited into the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study between July 2007 and February 2011. Women were eligible to participate if they were less than 20 weeks gestation, at least 18 years old, planned to stay in Mexico City for the next 3 years, had telephone access, reported no medical history of heart or kidney disease, no daily alcohol consumption, and of no use any steroid or anti-epilepsy medications. Following birth, 815 mother-child dyads had at least one follow-up visit and 552 had completed the 48 months of age visit and had all the necessary covariates for these analyses. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. Women provided written informed consent.
Prenatal PM2.5 Levels
Our group has developed a hybrid satellite-based method to estimate daily PM2.5 levels across Mexico City for the years 2004–2014. Daily exposures to PM2.5 were then estimated for each participant during pregnancy using a novel spatio-temporal model that incorporates Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements from the Multiangle Implementation of Atmospheric Correction (MAIAC) at a 1× 1 km spatial resolution.19 These remote sensing data are calibrated with data from 12 municipal ground level monitors of PM2.5, roadway density and meteorological data (temperature, relative humidity, planetary boundary layer and daily precipitation) to yield estimates of daily residential PM2.5 levels for each participant. Mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5-AOD relationship. For days without AOD data, the model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring. Model performance was assessed using monitor-level leave one-out cross-validation with an R2 of 0.724. For more in depth model details please refer to Just et al, 2015.19 Each woman's individual daily exposure to PM2.5 across the pregnancy was estimated based on their gestational age and residential address during the pregnancy. Because ultrasounds were not routinely performed as standard of care, gestational age at birth was estimated based on mother’s report of her last menstrual period (LMP) and by a standardized physical examination.20 If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam was used instead of the gestational age determined by LMP. We calculated the average PM2.5 over clinically defined trimesters (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks-delivery).
Measures of psychosocial stress
The validated21 Spanish version of the Crisis in Family Systems-Revised (CRISYS) survey was administered by a trained psychologist during the second or third trimester of pregnancy and during the 48-month follow-up postnatal visit. The CRISYS questionnaire assesses life events across 11 domains: financial, legal, career, relationship, home safety, neighborhood safety, medical issues (self and others), home, prejudice and authority. Participants rated life events occurring in the past 6 months as positive, negative or neutral. Previous research has shown increased vulnerability across multiple domains3, 8, 22 therefore domains with one or more negative life event were summed into a negative life event (NLE) domain score, with higher scores indicating greater stress. The range of NLE score in our sample was 0 to 11 for prenatal NLE and 0 to 9 for 48-month NLE. Prenatal stress was dichotomized as low stress (NLE scores=0–3) and high stress (NLE scores>3) based on the median value.
Outcome measures
The validated Spanish version of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire was administered at the 48-month visit. Ever wheeze was defined as caregiver report of “Has your child ever had wheezing or whistling in the chest at any time in the past?” and current wheeze was defined based on response to “Has your child had wheezing or whistling of the chest in the past 12 months?”
Covariates
Child’s sex was obtained from mother’s report and delivery records. Mother’s age at delivery was calculated by subtracting child’s birthdate from mother’s birthdate collected at enrollment. Mother’s report of ever having asthma was collected through questionnaires at enrollment (second or third trimester). Exposure to environmental tobacco smoke was ascertained during pregnancy (either during the second or third trimester) and during the 48-month visit through report of any smoker in the home during these time periods. Thirteen variables derived from prenatal questionnaire results were used to classify study participant families into six levels based on the socioeconomic status (SES) index created by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (AMAI).23 These levels were then collapsed into low, medium, and high socioeconomic status.
Statistical Analyses
In order to obtain risk ratios for our prospectively measured dichotomous outcomes, data were analyzed using a modified Poisson regression approach that allows us to estimate risk ratios instead of odds ratios.24 Models were adjusted for child's sex, report of any smoker in the home during pregnancy and at 48 months, mother's age at delivery and maternal ever asthma. Co-exposures for every trimester and postnatal year 1 exposures were included in final models. Low stress (NLE scores=0–3) and high stress (NLE scores>3) groups were based on the median split. SES and NLE scores at 48 months of age were also examined as potential confounders, but were not independently associated with the outcome nor did they significantly affect the estimate, thus they were excluded from final analyses. Statistical interactions between prenatal stress and PM2.5 levels were determined by including a product term between average PM2.5 levels and the dichotomized NLE score to test for a departure in multiplicativity. Models were then stratified by low and high stress with adjustment for other covariates. Generalized additive models (GAMs) were used to model NLE scores as numeric measures. Because research links prenatal air pollution to preterm birth25 and in turn, preterm birth is linked to childhood respiratory disease26 we also carried out sensitivity analyses by restricting the analyses to only full term children (gestational age ≥37 weeks). Analyses were performed in R version 3.2.3 (Vienna, Austria) 27 and SPSS version 23 (Chicago, IL).
Results
Table 1 shows the distribution of covariates, exposure and outcomes across the sample as a whole and then stratified by low and high stress. Sample characteristics did not differ significantly by stress except for prenatal ETS, with a higher percentage of participants reporting a smoker in the home in the high stress group when compared to the low stress group (43% vs. 33%, respectively). Overall 25% of the children had ever wheezed and 12% had report of wheeze in the past 12 months. Supplemental Table 1 shows there were no significant differences between participants who had all necessary covariates when compared to those who did not by mother’s age at delivery, maternal asthma, child’s sex, NLE score or prenatal ETS exposure. Participants in the remainder of cohort did not vary by trimester exposure to PM2.5, except for slightly higher exposure during the first trimester with borderline significance.
Table 1.
Sample characteristics
| Characteristic | Overall n=552 | Low Stress (≤3) n=349 | High Stress (>3) n=203 | p-valuea |
|---|---|---|---|---|
| Male sex, n (%) | 279 (51) | 168 (48) | 111 (55) | 0.14 |
| Maternal asthma, n (%) | 7 (1.2) | 5 (1.4) | 2 (1.0) | 0.65 |
| Prenatal ETS, n (%) | 202 (37) | 114 (33) | 88 (43) | |
| Postnatal ETS, n (%) | 79 (14) | 48 (14) | 31 (15) | 0.62 |
| Mother’s age at delivery, median (IQR) | 27.6 (23.6,31.8) | 27.5 (23.5, 31.6) | 27.7 (24.2, 32.0) | 0.65 |
| Average 1st trimester PM2.5μg/m3, median (IQR) | 22.0 (18,9, 25.7) | 22.2 (18.9, 25.6) | 21.6 (18.9, 25.9) | 0.99 |
| Average2nd trimester PM2.5μg/m3, median (IQR) | 21.1 (18.8, 25.6) | 21.0 (18.6, 25.4) | 21.2 (19.2, 25.7) | 0.23 |
| Average 3rd trimester PM2.5μg/m3, median (IQR) | 22.5 (19.0, 27.3) | 22.7 (18.8, 27.3) | 22.2 (19.1, 27.2) | 0.68 |
| Ever wheeze, n (%) | 136 (25) | 77 (22) | 59 (29) | 0.07 |
| Current wheeze, n (%) | 66 (12) | 35 (10) | 31 (15) | 0.07 |
Abbreviations: ETS, Environmental tobacco smoke; IQR, interquartile range; PM2.5, particulate matter 2.5 microns and less in diameter.
Differences in categorical variables tested using Fisher’s Exact test or Pearson Chi-Square test. Differences in continuous variables tested using Mann Whitney U test.
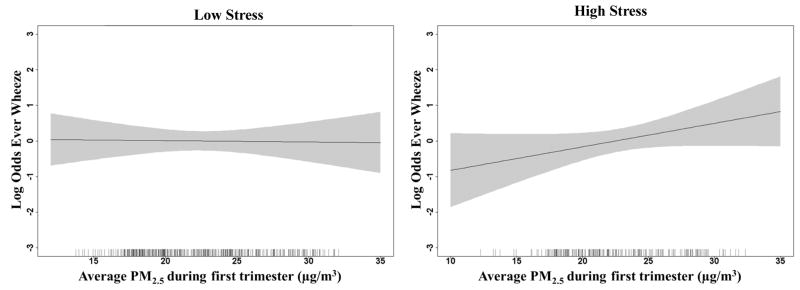
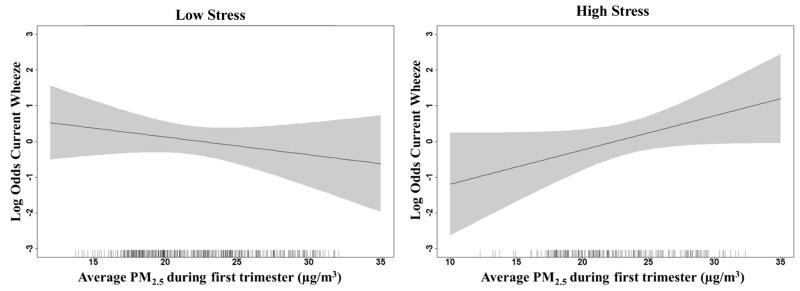
We did not see any significant associations between any trimester PM2.5 levels and wheeze outcomes in adjusted models prior to stratification. Table 2 shows results of adjusted models for the association between trimester PM2.5 concentrations and ever wheeze and current wheeze, stratified by low and high stress with p-values for interaction models when including a product term for PM2.5 and NLE score in the combined sample. The interaction p-value represents the formal test for interaction between PM2.5 and NLE scores independent of the stratum specific RRs shown. RRs are shown for an IQR increase in PM2.5 levels during each trimester. For the current wheeze outcome, we found a significant interaction between first trimester PM2.5 and prenatal stress on current wheeze. There was a positive association between first trimester PM2.5 concentrations and current wheeze only among children born to mothers who also reported experiencing higher stress during pregnancy. We did not find significant interactions for the ever wheeze outcome, although we saw a similar pattern for first trimester PM2.5 concentrations and higher risk of ever wheeze only in the children in the high stress stratum. The dose response relationships between first trimester PM2.5 and each wheeze outcome are also depicted using generalized additive models (GAM) in Figures 1 and 2, stratified by low/high stress. As shown in Figures 1 and 2 we see increasing odds of ever wheeze and current wheeze with increasing PM2.5 concentrations during the first trimester only in the children whose mothers reported higher stress during pregnancy.
Table 2.
Interaction models and stress-stratified associations between prenatal PM2.5 concentrations and wheeze outcomes and RRs shown for an average pregnancy IQR (3.8 μg/m3) increase in PM2.5. Models adjusted for child’s sex, maternal asthma, maternal age at delivery, prenatal ETS exposure, ETS exposure at 48 months of age, other trimester PM2.5 concentrations and postnatal year 1 average PM2.5.
| RR (95% CI) | |||
|---|---|---|---|
| Low Stress (≤3) Stratum N=349 | High Stress (>3) Stratum N=203 | Combined data N=552 | |
|
| |||
| p-value for interaction between PM2.5 and stress | |||
| Ever Wheeze | |||
| 1st trimester PM2.5 | 0.99 (0.83, 1.18) | 1.18 (0.97, 1.43) | 0.19 |
| 2nd trimester PM2.5 | 0.92 (0.76, 1.12) | 1.06 (0.85, 1.32) | 0.32 |
| 3rd trimester PM2.5 | 0.96 (0.82, 1.13) | 0.94 (0.78, 1.15) | 0.66 |
| Current wheeze | |||
| 1st trimester PM2.5 | 0.84 (0.61, 1.16) | 1.35 (1.00, 1.83) | 0.04 |
| 2nd trimester PM2.5 | 0.74 (0.54, 1.04) | 0.99 (0.71, 1.38) | 0.22 |
| 3rd trimester PM2.5 | 0.96 (0.74, 1.26) | 0.83 (0.61, 1.13) | 0.28 |
Abbreviations: CI, confidence interval; RR, relative risk.
Figure 1.
Figure 2.
In sensitivity analyses adjusting for NLE scores collected at 48 months, effect estimates remained virtually unchanged. Restricting the analyses to participants who were considered full-term (≥37 weeks gestation) also did not alter the results.
Discussion
This is the first study to prospectively demonstrate associations between prenatal PM2.5 concentrations, maternal stress in pregnancy, and wheeze outcomes among 4 year old children in an urban population in Mexico City. In general, we saw that increasing ambient PM2.5 exposure during the first trimester was associated with increased risk of wheeze outcomes in children whose mothers reported higher prenatal stress compared to children whose mothers reported low stress in pregnancy. Higher PM2.5 exposure during the first trimester was associated with increased risk of wheeze in the past 12 months at age 4, specifically in participants concurrently exposed to higher prenatal stress.
Evidence from both animal and human studies suggests that pregnancy may be a particular vulnerable period for ambient air pollution exposure for the developing fetus. Increased maternal systemic oxidative stress and production of pro-inflammatory cytokines due to air pollution exposure may lead to placental and endothelial dysfunction, and increased fetal oxidative stress affecting fetal immune and lung development. Pregnant mice challenged with airborne and inert particles show greater airway inflammation and lung hyperresponsiveness when compared to non-pregnant controls.28 Mouse pups exposed to PM2.5 perinatally had decreased alveolarization and reduced lung volumes when compared to unexposed controls.29 In humans, Janssen and colleagues reported that prenatal PM10 was associated with significantly lower mitochondrial DNA content in placenta, an indicator of enhanced placental oxidative stress.30 Prenatal PM2.5 levels have also been associated with significantly lower mitochondrial DNA content in cord blood.31 Placental 3-nitrotyrosine (3-NTp), another biomarker of oxidative stress was positively associated with prenatal PM2.5 concentrations, particularly during the first and third trimesters.32 In the Generation R study, increased PM10 prior to delivery has been associated with elevated fetal C-reactive protein, a marker of systemic inflammation.33 Psychosocial stress during pregnancy may also disrupt maternal systems like the hypothalamic-pituitary-adrenocortical (HPA) axis, the sympathetic-adrenal-medullary system and immunomodulation which may lead to a potentiation of the fetal immune system through the upregulation of maternal and fetoplacental cytokine or IgE production.34, 35
Previous research has demonstrated variability in downstream effects like eczema, allergic rhinitis and asthma in offspring depending on the trimester during which exposure to air pollution occurs.18, 36 In our study, we report associations only during the first trimester of pregnancy, which corresponds to the pseudo-glandular stage of fetal lung development. During this period budding and branching of the tracheobronchial tree is completed and further structural formation of the conducting airways such as vascular, smooth muscle and mucosal glands formation occurs. We can hypothesize that early toxicant exposure may impact the global transcriptional program of progenitor cells meant to differentiate into bronchial epithelium and other crucial components of future airway structure and defense.37 However, further work is needed in order to better elucidate windows of susceptibility to these exposures.
Strengths of our study include the prospective design and a validated spatio-temporal satellite method of high spatial and temporal resolution to assign PM2.5 exposure during pregnancy and postnatal year 1 allowing us to co-adjust for all trimester and postnatal year 1 PM2.5 concentrations. Another strength of our study is the assessment of stress in pregnancy, a previously identified vulnerable developmental window for asthma risk and stress programming using a well-validated measure of negative life events. Our study also adds to the growing area of study of environmental risk factors, by examining ambient air pollution and stress, for the development of childhood wheezing and asthma in Latin America by focusing in understudied population in Mexico City. We were also able to adjust for important covariates like ETS exposure during the prenatal period, which was higher in the mothers who reported high stress, and concurrent with the administration of the ISAAC questionnaire. We also acknowledge some limitations. Children’s wheeze was reported by mothers; however, caregiver-reported wheeze is commonly used in moderate- to larger-sized epidemiological studies38, 39 and this was assessed using a standardized measure validated for Spanish-speaking populations.39 Future studies should aim to examine more definitive outcomes including physician diagnosed asthma or lung function as these children continue to be followed.
Our prospective study adds to a growing literature underscoring the need to consider both chemical and non-chemical stressors together in order to more comprehensively characterize children’s environmental risk and is the first study to examine these associations in a Latin American sample. These data show that children born to mothers exposed to higher levels of particulate air pollution during pregnancy are at greater risk of wheeze at age 4 years, particularly if their mothers also experienced more toxic levels of stress prenatally. Knowledge of interactions between chemical and non-chemical environmental toxins can better inform prevention and intervention strategies that may preclude persistence of symptoms or lung function deficits in later childhood. For example, these findings suggest that interventions aimed at reducing prenatal stress could mitigate the effects of chemical toxins such as air pollution which may be harder to eliminate in the shorter term. A first step is to train clinicians to counsel pregnant women and young mothers on the important role of psychological stress in this context, validating for their patients that stress has medical implications for the mother as well as her developing child. Notably, the modifying effect of stress considered women who were above the median as high stress compared to those below the median. This underscores that interventions do not need to eliminate stress altogether but rather they should be designed to reduce stress to more normative or manageable levels for pregnant women and young families. This is more practical and achievable when designing stress reduction strategies in the clinical setting.
Acknowledgments
Funding: The (PROGRESS) project has been funded by grants R01 ES021357 and R01 ES013744 (Wright RO, PI). Phenotyping and biostatistical support was funded by P30 ES023515. During preparation of this manuscript, MJR was supported by T32 HD049311-09; AL was supported by K23 HL135349; ACJ was supported by R00ES023450. This study was supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico and we thank the ABC (American British Cowdray) Hospital in Mexico for providing research facilities.
Abbreviations
- CRISYS
Crisis in Family Systems-Revised questionnaire
- HPA
hypothalamic-pituitary-adrenocortical
- ISAAC
International Study of Asthma and Allergies in Childhood
- NLE
Negative Life Events
- PM2.5
Particulate matter <2.5 microns in diameter
Footnotes
Authors’contributions: MJR generated data, performed analyses, interpreted data and drafted the manuscript. ACJ, IK, IP, LS, BC and JS generated data, assisted in analysis and interpretation of data and revised the manuscript. AL, SB, YHMC, HHLH and SC assisted in analysis and interpretation of data and revised the manuscript. ROW, MMTR and RJW conceived and designed original study, assisted in analysis and interpretation of the data and manuscript preparation and revisions.
Trial registration: Not applicable
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark NA, Demers PA, Karr CJ, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu HHL, Chiu YHM, Coull BA, et al. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children Identifying Sensitive Windows and Sex Differences. Am J Resp Crit Care. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu YHM, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and Postnatal Maternal Stress and Wheeze in Urban Children Effect of Maternal Sensitization. Am J Resp Crit Care. 2012;186:147–154. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartwig IR, Sly PD, Schmidt LA, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. 2014;134(1):160–169. doi: 10.1016/j.jaci.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123(4):847–853. doi: 10.1016/j.jaci.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes M, Perzanowski MS, Whyatt RM, et al. Relationship between maternal demoralization, wheeze, and immunoglobulin E among inner-city children. Ann Allerg Asthma Immunol. 2011;107(1):42–49. doi: 10.1016/j.anai.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guxens M, Sonnenschein-van der Voort AMM, Tiemeier H, et al. Parental psychological distress during pregnancy and wheezing in preschool children: The Generation R Study. J Allergy Clin Immunol. 2014;133(1):59. doi: 10.1016/j.jaci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Rosa MJ, Just AC, Tamayo YOM, et al. Prenatal and postnatal stress and wheeze in Mexican children: Sex-specific differences. Ann Allergy Asthm Immunol. 2016 Apr;116(4):306–312.e1. doi: 10.1016/j.anai.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajat A, Hsia C, O'Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep. 2015;2(4):440–450. doi: 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright RJ. Moving towards making social toxins mainstream in children's environmental health. Curr Opin Pediatr. 2009;21(2):222–229. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam T, Urman R, Gauderman WJ, et al. Parental stress increases the detrimental effect of traffic exposure on children's lung function. Am J Respir Crit Care Med. 2011;184(7):822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. P Natl Acad Sci USA. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clougherty JE, Levy JI, Kubzansky LD, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson B, Kubzansky LD, Cohen S, Weiss S, Wright RJ. A matter of life and breath: childhood socioeconomic status is related to young adult pulmonary function in the CARDIA study. Int J Epidemiol. 2004;33(2):271–278. doi: 10.1093/ije/dyh003. [DOI] [PubMed] [Google Scholar]
- 15.Chiu YHM, Coull BA, Sternthal MJ, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133(3):713. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Loo KF, van Gelder MM, Roukema J, Roeleveld N, Merkus PJ, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing: a meta- analysis. Eur Respir J. 2016;47:133–146. doi: 10.1183/13993003.00299-2015. [DOI] [PubMed] [Google Scholar]
- 17.Morales E, Garcia-Esteban R, de la Cruz OA, et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax. 2015;70:64–73. doi: 10.1136/thoraxjnl-2014-205413. [DOI] [PubMed] [Google Scholar]
- 18.Deng Q, Lu C, Li Y, Sundell J, Dan N. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–127. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Just AC, Wright RO, Schwartz J, et al. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environ Sci Technol. 2015;49:8576–8584. doi: 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- 21.Berry CA, Quinn KA, Portillo N, Shalowitz MU. Reliability and validity of the Spanish version of the crisis in family systems-revised. Psychol Rep. 2006;98:123–132. doi: 10.2466/pr0.98.1.123-132. [DOI] [PubMed] [Google Scholar]
- 22.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009;32:9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco AV. The AMAI system of classifying households by socio-economic level: ESOMAR. 2002 [Google Scholar]
- 24.Zou GY. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Been JV, Lugtenberg MJ, Smets E, et al. Preterm Birth and Childhood Wheezing Disorders: A Systematic Review and Meta-Analysis. Plos Med. 2014:11. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparrini A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J Stat Softw. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- 28.Fedulov AV, Leme A, Yang Z, et al. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mauad T, Rivero DH, de Oliveira RC, et al. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen BG, Munters E, Pieters N, et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect. 2012;120:1346–1352. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosa MJ, Just AC, Guerra MS, et al. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int. 2017;98:198–203. doi: 10.1016/j.envint.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saenen ND, Vrijens K, Janssen BG, et al. Placental Nitrosative Stress and Exposure to Ambient Air Pollution During Gestation: A Population Study. Am J Epidemiol. 2016;184:442–449. doi: 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- 33.van den Hooven EH, de Kluizenaar Y, Pierik FH, et al. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: the Generation R Study. Environ Health Perspect. 2012;120:746–751. doi: 10.1289/ehp.1104345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright RJ, Visness CM, Calatroni A, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TAE, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy. 2012;67:545–551. doi: 10.1111/j.1398-9995.2012.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. 2017;85:85–95. doi: 10.1016/j.jdermsci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Carsin A, Mazenq J, Ilstad A, Dubus JC, Chanez P, Gras D. Bronchial epithelium in children: a key player in asthma. Eur Respir Rev. 2016;25:158–169. doi: 10.1183/16000617.0101-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of Childhood Asthma in the United States, 1980–2007. Pediatrics. 2009;123:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 39.Mallol J, Sole D, Baeza-Bacab M, et al. Regional Variation in Asthma Symptom Prevalence in Latin American Children. J Asthma. 2010;47:644–650. doi: 10.3109/02770901003686480. [DOI] [PubMed] [Google Scholar]