Abstract
Objectives
It is unknown if preoperative tracheostomy for persistent/recurrent laryngeal squamous cell carcinoma(LSCC) plays a role in unrecognized local disease spread and disease recurrence after salvage laryngectomy. The goals of this study were to determine the effect of preoperative tracheostomy on disease-free survival(DFS) in patients with recurrent/persistent LSCC undergoing salvage laryngectomy.
Study Design
Retrospective case series derived from prospectively maintained database.
Setting
Tertiary care academic center.
Subjects
Patients with recurrent/persistent LSCC after radiation/chemoradiation (RT/CRT) who underwent salvage laryngectomy at the University of Michigan from 1997–2015.
Methods
Demographic, clinical, pathologic, and survival data were collected. Kaplan-Meier survival estimates were performed.
Results
DFS was worse for patients with tracheostomy prior to laryngectomy than patients without a tracheostomy(5 year: 39% vs. 67%;p<0.001). Patients with tracheostomy prior to RT/CRT compared to patients with tracheostomy after RT/CRT or patients without a tracheostomy had worse DFS(5-year: 25%, 49% and 67% respectively;p < 0.001). In bivariable analyses controlling for T classification, N classification or overall stage, preoperative tracheostomy was associated with worse DFS. In multivariable analysis, presence of a preoperative tracheostomy had a worse DFS(HR: 1.63;95% CI 1.00–2.67; p=0.048).
Conclusion
Preoperative tracheostomy is associated with disease recurrence in patients with persistent/recurrent LSCC undergoing salvage laryngectomy, particularly in patients who had tracheostomy prior to completion of initial RT/CRT. Notably, preoperative tracheostomy as a causal factor versus marker for disease recurrence is difficult to ascertain. Nevertheless, clinicians should be aware of the increased risk of locoregional recurrence in patients with preoperative tracheostomy when counseling on surgical salvage, and when considering the role of additional therapy.
Keywords: laryngeal squamous cell carcinoma, salvage surgery, laryngectomy, tracheostomy, survival
Background
Laryngeal squamous cell carcinoma (LSCC) continues to pose a challenge to head and neck oncologists, with over 13,000 new cases of LSCC diagnosed annually in the United State alone1. Primary treatment has increasingly shifted toward organ-preservation therapies, namely radiation (RT) and chemoradiation (CRT)2. However, a notable subset of these patients develop recurrences3; for these patients, laryngectomy is often the only curative treatment option. For patients undergoing laryngectomy after RT or CRT, predictors of subsequent disease recurrence are limited and poorly understood.
Identifying predictors of disease-free survival (DFS) in patients undergoing salvage laryngectomy is crucial in order to risk-stratify patients. With better understanding of risk factors for disease recurrence, we can better counsel patients, adjust screening regimens, and provide adjuvant therapy to those at incrementally higher risk for recurrence.
Preoperative tracheostomy for LSCC has been hypothesized to predispose patients to stomal recurrence and local disease spread4–7. Although unable to be verified, theoretically patients may have unrecognized tumor violation and surreptitious local spread of disease. However, this concept has been difficult to prove or separate from potential confounders. Moreover, the effect of preoperative tracheostomy on locoregional recurrence has not been studied in patients with recurrent LSCC after RT/CRT. In this cohort, timing of tracheostomy in relation to RT/CRT treatment may have implications on survival and disease recurrence.
Herein, we investigate the effects of preoperative tracheostomy in a large recurrent/persistent LSCC cohort undergoing total laryngectomy. Our aim was to investigate the association of preoperative tracheostomy status on DFS.
Methods
Patient Identification and Data Collection
A prospectively-maintained single-institution epidemiology database of patients with head and neck cancer was generated as previously described3. Adult patients were included if they had pathologically confirmed laryngeal squamous cell carcinoma initially treated with RT/CRT, who underwent salvage total laryngectomy for recurrent/persistent disease at the primary site (n=244) between 1998–2015. This study was approved by the institutional review board at the University of Michigan (HUM00081554).
Statistical Analysis
The primary outcome measure was DFS (time from salvage laryngectomy to LSCC recurrence). Secondary measures included locoregional DFS, and distant DFS. Death was verified via medical records and the social security death index. Kaplan Meier survival curves were generated for univariable and bivariable survival analysis using SPSS version 22.0 (IBM, Armonk, NY) for DFS. Bivariable and Multivariable cox regression were performed for DFS. Demographic, clinical and pathologic factors were included in multivariable modeling (Table 1). Due to the large number of predictors, we used forward selection to identify the relevant covariates. Multivariable modeling was performed using R version 3.3.0 (Vienna, Austria).
Table 1.
Clinical Characteristics of Patients with Recurrent/Persistent LSCC after RT/CRT
| No Tracheostomy (n = 168) N (%) or mean (SD) |
Preoperative Tracheostomy (n = 76) N (%) or mean (SD) |
p-value* | |
|---|---|---|---|
|
| |||
| Patient Characteristic | |||
| Gender | |||
| Male | 143 (85) | 65 (86) | 0.94 |
| Female | 25 (15) | 11 (15) | |
| Ethnicity | |||
| Caucasian | 153 (91) | 70 (92) | 0.79 |
| Black/Other/Unk | 15 (9) | 6 (8) | |
| Tobacco | |||
| Current | 66 (39) | 26 (34) | 0.51 |
| Former | 97 (58) | 49 (64) | |
| Never | 5 (3) | 1 (1) | |
| Initial cStage | |||
| cI | 54 (32) | 9 (12) | <0.001 |
| cII | 53 (32) | 9 (12) | |
| cIII | 33 (20) | 30 (39) | |
| cIV | 16 (10) | 23 (30) | |
| Unk | 12 (7) | 5 (7) | |
| Time to Recur (mo) | 23 (32) | 19 (26) | 0.39 |
|
| |||
| Recurrent Clinical | |||
| Recurrent ycT class | |||
| ycT1 | 13 (8) | 1 (1) | <0.001 |
| ycT2 | 78 (46) | 19 (25) | |
| ycT3 | 46 (27) | 19 (25) | |
| ycT4 | 31 (18) | 37 (49) | |
| Recurrent cN class | |||
| ycN0 | 154 (92) | 61 (80) | 0.01 |
| ycN+ | 14 (8) | 15 (20) | |
| Recurrent cStage | |||
| ycI | 12 (8) | 1 (1) | <0.001 |
| ycII | 75 (45) | 18 (24) | |
| ycIII | 44 (26) | 18 (24) | |
| ycIV | 37 (22) | 39 (51) | |
|
| |||
| Recurrent Pathologic | |||
| Recurrent pT class | |||
| ypT1 | 10 (6) | 1 (1) | <0.001 |
| ypT2 | 64 (39) | 11 (14) | |
| ypT3 | 47 (28) | 22 (29) | |
| ypT4 | 47 (28) | 42 (57) | |
| Recurrent pN class | |||
| ypN0 | 141 (84) | 50 (66) | 0.001 |
| ypN+ | 27 (16) | 26 (34) | |
| Recurrent pStage | |||
| ypI | 10 (6) | 1 (1) | <0.001 |
| ypII | 58 (35) | 10 (13) | |
| ypIII | 45 (27) | 20 (26) | |
| ypIV | 55 (33) | 45 (59) | |
| Margins | |||
| Negative | 160 (95) | 69 (91) | 0.18 |
| Positive | 8 (5) | 7 (9) | |
| Extracapsular Spread | |||
| No | 13 (48) | 14 (54) | 0.68 |
| Yes | 14 (52) | 12 (46) | |
mo = months. ycT = recurrent clinical T stage. ycN = recurrent clinical nodal status. ypT = recurrent pathologic T stage. ypN = recurrent pathologic nodal status.
Comparison of demographic and clinical factors between patients with preoperative tracheostomy and those without was performed with chi-square or student’s t-test where applicable.
Results
Cohort Characteristics
Demographic, clinical, pathologic and survival data were collected (Table 1). The cohort was predominantly male (85%) and Caucasian (91%). There were 76 (31%) patients who had tracheostomy placement prior to their salvage surgery, 33 prior to completion of initial RT/CRT and 43 after completion of initial RT/CRT, but prior to salvage laryngectomy. Patients with preoperative tracheostomy tended to have higher initial overall stage, recurrent T classification, N classification and overall stage, both clinically and on final pathologic diagnosis.
Univariable Analysis of Preoperative Tracheostomy on Survival
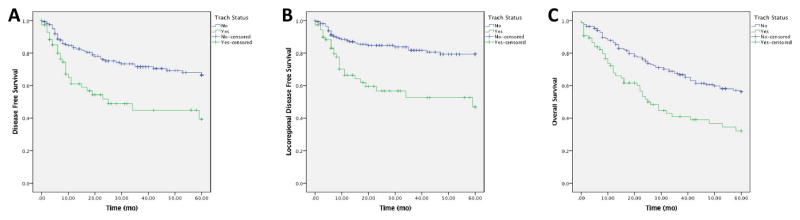
We first analyzed the effect of presence of a preoperative tracheostomy in our cohort. In univariable analysis, DFS was significantly worse (p < 0.001) for patients with tracheostomy prior to laryngectomy than patients without a tracheostomy. 5-year DFS estimates were 39% for patients with tracheostomy and 67% for patients without tracheostomy (Figure 1A). In addition, locoregional DFS was worse for patients with tracheostomy before laryngectomy (p < 0.001), with 5-year estimates of 47% with tracheostomy versus 79% for no tracheostomy (Figure 1B). Interestingly, there was no significant survival difference in distant DFS between the groups (p = 0.14).
Figure 1. Survival Stratified by Preoperative Tracheostomy.
DFS (A) and locoregional DFS (B) were significantly worse in patients who had a tracheostomy prior to laryngectomy.
Univariable Analysis of the timing of Preoperative Tracheostomy on Survival
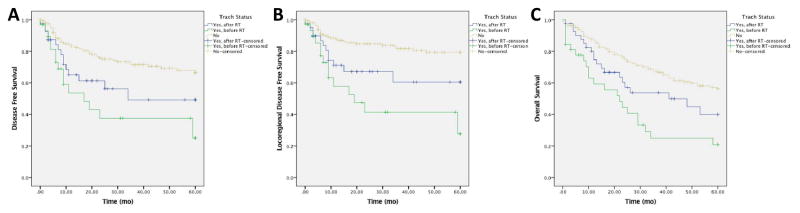
The timing of tracheostomy was then dichotomized (before RT/CRT; after RT/CRT) to examine the effect on survival. There were 33 patients who underwent tracheostomy prior to completion of initial RT/CRT and 43 after completion of initial RT/CRT, but prior to salvage laryngectomy. In univariable analysis, DFS was worse for patients with tracheostomy prior to RT/CRT in comparison to patients with tracheostomy after RT/CRT or patients without a tracheostomy, with 5-year DFS estimates of 25%, 49% and 67% respectively (p < 0.001; Figure 2A). In addition, locoregional DFS was worse for patients with tracheostomy before RT/CRT, with a 5-year DFS estimate of 28% compared to 61% for tracheostomy after RT/CRT and 79% for no tracheostomy (p<0.001; Figure 2B). There was no significant survival difference in distant DFS between the groups (p=0.316).
Figure 2. Survival Stratified by Timing of Preoperative Tracheostomy.
DFS (A), locoregional DFS (B) were worse in patients who had a tracheostomy prior to RT/CRT in comparison to tracheostomy after RT/CRT and no tracheostomy.
Bivariable Analysis of Preoperative Tracheostomy on Survival
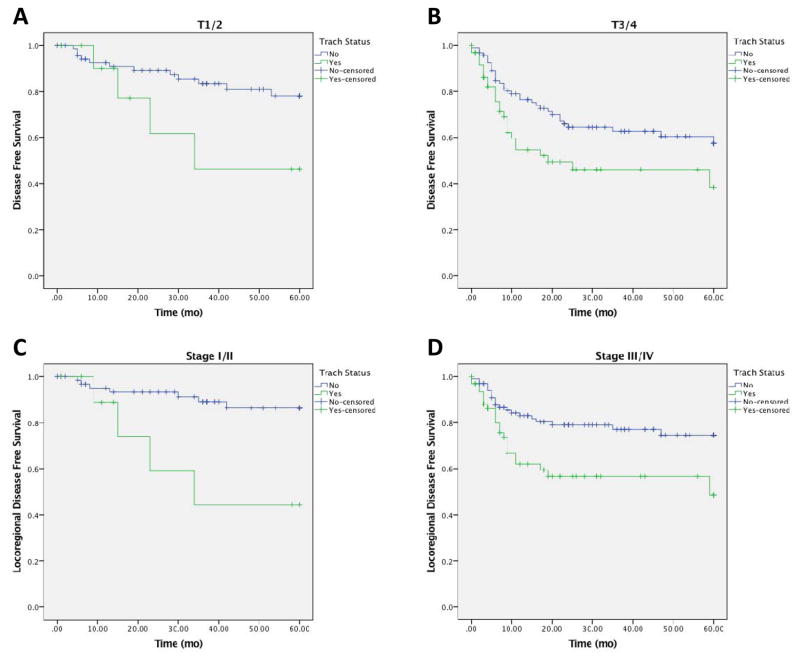
Given the difference in initial stage, recurrent T classification, N classification and overall stage between the cohort of patients with and without tracheostomy, we sought to control for these potential confounding variables. We used clinical staging for the initial staging and pathologic staging for the recurrence staging for this analysis. In bivariable analysis, preoperative tracheostomy was associated with worse DFS in comparison to no tracheostomy in patients with early (ypT1/T2) and advanced (ypT3/T4) T classifications, (p = 0.005; Figure 3A,B). When controlling for nodal status (ypN0 vs ypN+), patients who underwent preoperative tracheostomy had a worse DFS (p = 0.001). Similarly, when controlling for overall recurrent stage (Stage ypI/II vs ypIII/IV), patients who underwent preoperative tracheostomy had a worse DFS (p = 0.003). When controlling for initial stage (Stage cI/II vs cIII/IV), patients with preoperative tracheostomy had a worse DFS (p = 0.004).
Figure 3. Bivariable Analysis of Preoperative Tracheostomy and Survival.
Controlling for ypT classification, DFS was worse with tracheostomy(ypT1/T2; 3A; ypT3/T4; 3B). Controlling for overall stage, patients with tracheostomy had worse DFS and locoregional DFS(3C, 3D).
We next sought to analyze for any significance in recurrence patterns (locoregional or distant) given preoperative tracheostomy status in bivariable analysis. In a similar fashion, when controlling for pathologic T classification, patients with tracheostomy before laryngectomy had a worse locoregional DFS (p < 0.001). When controlling for pathologic N classification, preoperative tracheostomy had worse locoregional DFS (p = 0.001). When controlling for recurrent overall stage, preoperative tracheostomy had worse locoregional DFS (p < 0.001, Figure 3C,D).
Multivariable Analysis of Preoperative Tracheostomy on Survival
We next performed a multivariable Cox proportional hazard regression to control for additional potential confounders for DFS. We used forward selection to identify the relevant covariates within the demographic, clinical and pathologic variables (Table 1). With this model, presence of a preoperative tracheostomy had a DFS hazard ratio of 1.63 (95% CI 1.00 – 2.67; p = 0.048; Table 2). Other significant factors for DFS included positive margin status (HR 4.74; 95% CI 2.21 – 10.2) clinical node positive status (HR 2.17; 95% CI 1.13 – 4.18), and pathologic node positive status (HR 2.29; 95% CI 1.21 – 4.35 for no extracapsular spread and HR 1.36; 95% CI 0.64 – 2.91 for extracapsular spread). Advanced recurrent clinical T stage (ycT3 or ycT4) did not achieve significance (HR 1.70; 95% CI 0.91 – 3.16). Younger age demonstrated improved disease free survival (HR 0.96; 95% CI 0.94 – 0.99).
Table 2. Multivariable Analysis of Disease Free Survival.
Presence of a tracheostomy had an DFS hazard ratio of 1.63 (95% CI 1.00 – 2.67; p = 0.048). Other significant factors for DFS included positive margin status and node positive status.
| Characteristic | DFS HR | 95% CI |
|---|---|---|
|
| ||
| Margin status | ||
| Positive | 4.74 | 2.21 – 10.2 |
| Negative (ref) | --- | --- |
|
| ||
| Pathologic node status | ||
| ypN+, no ECS | 2.29 | 1.21 – 4.35 |
| ypN+, ECS | 1.36 | 0.64 – 2.91 |
| ypN0 (ref) | --- | --- |
|
| ||
| Clinical node status | ||
| ycN+ | 2.17 | 1.13 – 4.18 |
| ycN0 (ref) | --- | --- |
|
| ||
| Preoperative Trach | ||
| Yes | 1.63 | 1.00 – 2.67 |
| No (ref) | --- | --- |
|
| ||
| Clinical T stage | ||
| ycT3-4 | 1.70 | 0.91 – 3.16 |
| ycT1-2 (ref) | --- | --- |
|
| ||
| Age Recurrence | 0.96 | 0.94 – 0.99 |
Discussion
Recurrent/persistent LSCC after RT or CRT remains an aggressive and difficult to treat disease. Investigation into the association of preoperative tracheostomy for these patients has been limited to date.
Our results suggest the presence of a tracheostomy at time of salvage laryngectomy is an independent prognostic factor for worse disease recurrence, and specifically worse locoregional disease recurrence. This is particularly the case in patients who had tracheostomies placed prior to completion of RT/CRT. This finding may have clinical importance in guiding individualized care for patients. Notably, we can only demonstrate an association for preoperative tracheostomy and disease recurrence, not any causality. In a retrospective cohort, we cannot establish whether the tracheostomy itself is a direct contributor to disease recurrence, or an indicator for other unidentified factors.
Nevertheless, presence of a preoperative tracheostomy may have clinical utility in guiding patient discussions and care for recurrent LSCC. Caregivers should consider the potential increased risk of recurrence in these patients. In such patients, there may be increased scrutiny when contemplating a heroic attempt at surgical salvage, consideration of more aggressive salvage surgery, or additional postoperative adjuvant measures. In the era of reirradiation, precision medicine and targeted therapies for recurrent/metastatic head and neck cancers, this cohort may benefit from consideration of postoperative adjuvant therapy given their high risk for disease recurrence8–11.
Some have historically proposed that preoperative tracheostomy for LSCC may lead to microscopic spread of disease as the tumor may be violated during the tracheostomy process. Indeed, there have been case reports of tumor seeding from tracheostomies through or adjacent to LSCC. There have been limited investigations into this concept in patients undergoing primary laryngectomies4–7. Esteban et al.4 examined a cohort of 209 patients undergoing total laryngectomy, and found prior tracheostomy to be an independent predictor of stomal recurrence, although this was in a primary surgery cohort. Carrillo et al.5 studied T3 transglottic LSCC specifically undergoing primary total laryngectomy, and found a strong association with recurrence and poor survival in patients who had preoperative tracheostomy. Imauchi et al.6 studied 69 patients undergoing laryngectomy for LSCC in a mixed cohort of patients having had no or preoperative radiation. They found a trend to increased recurrence in patients with preoperative tracheostomy, but were limited in power and did not specifically study a recurrent/persistent cohort. Finally, Basheeth et al.7 analyzed 75 patients undergoing total laryngectomy, with 50 having had initial RT/CRT, and 25 with primary disease. They found significant stomal recurrence for patients who had undergone preoperative tracheostomy in univariable analysis. However, this was a mixed cohort, and bivariable analysis was not performed to control for potential confounders. To date, there has not been a cohort focused specifically on recurrent LSCC after RT/CRT.
While our robust cohort attempts to address such questions, our retrospective series cannot be presumed to demonstrate causality, and it is entirely possible that preoperative tracheotomy is simply a surrogate rather than representative of the specific cause of worse outcomes.
Timing of preoperative tracheostomy in relation to RT/CRT appeared to have significant effects on DFS. Patients who had a tracheostomy prior to or during initial RT/CRT had the worst DFS. Patients who had a tracheostomy after RT/CRT had slightly better DFS, but still worse than those who did not have preoperative tracheostomy.
Additional predictors of DFS in our cohort are well-established, including positive margin status, and positive pathologic nodal disease. Interestingly, in multivariable analysis, presence of a preoperative tracheostomy is the third strongest effector of DFS, after positive margin status and positive nodal status.
While it may be difficult to ascertain a causative role of preoperative tracheostomy for disease recurrence, presence of a preoperative tracheostomy nevertheless is an independent predictor of DFS in a recurrent/persistent LSCC cohort undergoing salvage laryngectomy. As such, further treatment options, including closer clinical monitoring, wider excision margins, postoperative reirradiation or cytotoxic chemotherapy, and newer adjuvant therapies may be entertained12. For clinicians, discussions with patients of the potential risk of pre-salvage tracheostomy should include potential implications for worse disease-free survival. Further investigation into indications for preoperative tracheostomy, and evaluation of prospective cohorts, may give additional insight into effects on post-salvage surgery disease recurrence for LSCC.
Acknowledgments
Grant support: Dr. Birkeland was funded with an NIH T32 DC005356 and a University of Michigan Otolaryngology Resident Research Grant. Ms. Beesley was funded with an NIH T32 CA-83654 grant.
Footnotes
Financial disclosure: None
Conflict of interest: None
Presentation at meetings: AAO-HNS Annual Meeting, Oral Presentation, September 20, 2016, San Diego, CA
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:855–860. doi: 10.1001/jamaoto.2014.1671. [DOI] [PubMed] [Google Scholar]
- 3.Birkeland AC, Rosko AJ, Issa MR, et al. Occult nodal disease prevalence and distribution in recurrent laryngeal cancer requiring salvage laryngectomy. Otolaryngol Head Neck Surg. 2016;154:473–479. doi: 10.1177/0194599815627811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteban F, Moreno JA, Delgado-Rodriguez M, Mochon A. Risk factors involved in stomal recurrence following laryngectomy. J Laryngol Otol. 1993;107:527–531. doi: 10.1017/s0022215100123618. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo JF, Frias-Mendivil M, Lopez-Graniel C, Beitia AI, Ochoa-Carrillo FJ. The impact of preoperative tracheotomy on T3 transglottic carcinomas of the larynx. Eur Arch Otorhinolaryngol. 1999;256:78–82. doi: 10.1007/s004050050120. [DOI] [PubMed] [Google Scholar]
- 6.Imauchi Y, Ito K, Takasago E, Nibu K, Sugasawa M, Ichimura K. Stomal recurrence after total laryngectomy for squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg. 2002;126:63–66. doi: 10.1067/mhn.2002.121515. [DOI] [PubMed] [Google Scholar]
- 7.Basheeth N, O’Leary G, Khan H, Sheahan P. Oncologic outcomes of total laryngectomy: impact of margins and preoperative tracheostomy. Head Neck. 2015;37:862–869. doi: 10.1002/hed.23681. [DOI] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602252. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris LG, Chandramohan R, West L, et al. Molecular landscape of recurrent and metastatic head and neck cancers: Insights from a precision oncology sequencing platform. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1790. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedberg ML, Goh G, Chiosea SI, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126:1606. doi: 10.1172/JCI86862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig ML, Birkeland AC, Hoesli R, Swiecicki P, Spector ME, Brenner JC. Changing the paradigm: The potential for targeted therapy in laryngeal squamous cell carcinoma. Cancer Biol Med. 2016;13:87–100. doi: 10.28092/j.issn.2095-3941.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist. 2005;10:215–224. doi: 10.1634/theoncologist.10-3-215. [DOI] [PubMed] [Google Scholar]