Abstract
An individual’s age at first substance use may be associated with their risk for progression toward heavier substance involvement. To our knowledge, however, no studies within nationally-representative samples have examined the relation between the timing of initiation and progression in use of multiple substances. The present study employed a sample of 9,421 participants from the National Longitudinal Study of Adolescent to Adult Health who reported on their ages of tobacco, alcohol, and cannabis initiation; frequency of tobacco, alcohol, and cannabis use; and quantity of tobacco and alcohol use across four waves. We fit latent growth models to examine (a) associations between the age of initiation and initial status and rate of change in substance involvement, and (b) the degree to which the timing of first substance use accounted for differences in trajectories. There were significant relations between all ages of initiation and rates of change in tobacco (βs=−0.21 to −0.31, ps<.01) and alcohol use frequency (βs=0.14 to 0.31, ps<.001), age of cannabis initiation and rate of change in tobacco use quantity (β=0.23, p<.01), and age of tobacco initiation and rate of change in cannabis use frequency (β= −0.14, p<.01). After adjusting for age of initiation, significant associations were observed between trajectories for tobacco and alcohol (r=0.43, p<.0001) and alcohol and cannabis (r=0.20, p<.05). Results highlight differences in within- and cross-substance relations between the age of initiation and rate of change in use across substances. They suggest that differences in substance use trajectories are partly accounted for by age at first use.
Keywords: Age of initiation, substance use frequency, substance use quantity, progression, latent growth modeling
Early first substance use is robustly associated with increased risk for heavier substance use and substance use disorder (SUD; e.g., Dawson, Goldstein, Chou, Ruan, & Grant, 2008; Jackson & Sartor, 2016; SAMHSA, 2016). These associations do not, however, necessarily imply causality. Much of the relation between early initiation and problems may be explained by a shared liability (e.g., Sartor et al., 2009), and some studies find that after accounting for socioeconomic and psychiatric covariates, the relation is markedly attenuated (e.g., Lopez-Quintero et al., 2011; Rossow & Kuntsche, 2013; see also Maimaris & McCambridge, 2014 for a systematic review of prospective studies of age at first drink). By contrast, some analyses support a potentially causal impact of early initiation on problems. These include longitudinal studies which observe associations after controlling for confounding variables (e.g., Liang & Chikritzhs, 2015; van Leeuwen et al., 2014) and genetically-informed analyses which document a relation after accounting for familial overlap (e.g., Lynskey et al., 2003; Richmond-Rakerd et al., 2016).
Risk for SUD may arise through pharmacological and neurobiological processes. Exposure to substances in adolescence may confer neurocognitive alterations that increase vulnerability for disorder (e.g., Bava & Tapert, 2010; Casey & Jones, 2010; Hurd et al., 2014; Spear, 2016). In addition, the timing of exposure within adolescence (e.g., alcohol exposure in early vs. late periods) may confer differential effects on the developing brain (Spear, 2015).
Several recent studies have used prospective data to examine the relation between early substance use initiation and later progression patterns. Analyses have identified escalated alcohol use frequency among early initiators (Bolland et al., 2016), as well as gender (Tomek et al., 2016) and racial/ethnic (Malone, Northrup, Masyn, Lamis, & Lamont, 2012) differences in alcohol use trajectories following initiation. Behrendt and colleagues (2009) found that later ages of substance use onset were associated with faster transition to SUD, with the exception of DSM-IV cannabis dependence. They later found that after adjusting for externalizing behavior and parental SUD, early first tobacco and alcohol use did not predict rate of progression to cannabis use disorder (Behrendt et al., 2012).
These studies have provided important insights into the relation between the timing of first substance use and progression. However, with the exception of Behrendt and colleagues’ (2009, 2012) analyses, they did not consider progression in use of multiple substances. Further, only one study (Malone et al., 2012) utilized a nationally-representative sample. Analyses of substance use patterns and trajectories within nationally-representative samples (e.g., Brooks-Russell et al., 2015; Chen & Jacobson, 2012; Jackson et al., 2002; Keyes et al., 2015; Moss, Chen, & Yi, 2014; Mulia et al., 2017; Muthén & Muthén, 2000; Tucker, Ellickson, Orlando, Martino, & Klein, 2005) have not examined relations between age at initiation and later polysubstance use. The present study aimed to identify substance-specific and cross-substance associations between early initiation and later trajectories, to (a) inform more parsimonious models of substance use and (b) promote the development of appropriately-targeted intervention strategies to reduce involvement with multiple drugs.
The current study used data from a nationally-representative, prospective study of adolescents and young adults to conduct a multivariate latent growth curve analysis of tobacco, alcohol, and cannabis involvement. We had two primary aims. First, we aimed to determine the extent to which initial status and rate of change in substance use frequency and quantity were related to the age of substance use initiation. Second, we aimed to identify the degree to which the age of substance use initiation moderated the magnitude of associations across tobacco, alcohol, and cannabis use trajectories.
Method
Participants
Participants were from the National Longitudinal Study of Adolescent to Adult Health (Add Health; Harris et al., 2009). A random sample of schools was selected from all high schools in the U.S., which were stratified by region, school size and type, and racial composition. A random subsample of students in grades 7–12 completed an in-home interview in 1994–1995 (Wave I; n=20,745, M age=16 years, response rate (RR)=79.0%). The second panel of interviews was conducted in 1996 with a sample drawn primarily from Wave I respondents (Wave II; n=14,738, age=11–23 years, RR = 88.6%), who were surveyed again in 2001–2002 (Wave III; n=15,197, age=18–26 years, RR=77.4%) and 2008–2009 (Wave IV; n=15,701, age=24–32 years (with a small number of participants aged 33 or 34), RR=80.3%).
Data come from all four waves. Add Health incorporates sampling weights that account for the unequal probability of selection; for proper weighting within longitudinal analyses, weights from the last wave are used and individuals with missing weights must be removed (Chen & Chantala, 2014). After removing individuals with missing Wave IV weight variables, we were left with a sample of 9,421 individuals.
Measures
Frequency of alcohol use
At all waves, participants were asked how many days in the past 12 months they drank alcohol: (1) every day or almost every day, (2) 3 to 5 days a week, (3) 1 or 2 days a week, (4) 2 or 3 days a month, (5) once a month or less (3–12 times in the past 12 months), (6) 1 or 2 days in the past 12 months, or (7) never. At Waves III and IV, responses were coded in the opposite direction. To remain consistent (and reflect an order of increasing frequency of use), response options for Waves I and II were recoded to 0 to 6, with 0 indicating no use. To facilitate sufficient covariance coverage, this ordinal variable was recoded into four categories: (0) none, (1) monthly or less, (2) 2 or 3 days a month, and (3) weekly to daily.
Quantity of alcohol use
At all waves, participants were asked, “Think of all the times you have had a drink during the past 12 months. How many drinks did you usually have each time?” Respondents were allowed to report any number of drinks at Waves I and II (range at Wave I=1 to 90, range at Wave II=1 to 95), whereas at Waves III and IV the upper limit of responses was restricted to age 18. To maintain consistency across waves and reduce the influence of extreme values, individuals who reported a number of drinks greater than 18 at Wave I (4.6% of the sample) and Wave II (5.8% of the sample) were recoded to 18.
Frequency of tobacco use
At all waves, participants were asked, “During the past 30 days, on how many days did you smoke cigarettes?” Responses ranged from 0 to 30 days. We recoded the variable to an ordinal scale as (a) it was not normally distributed, and (b) we wished to remain consistent with the ordinal coding scheme for alcohol and cannabis. At Wave III, frequency of use was only queried among regular smokers (individuals who had ever smoked at least one cigarette per day for 30 days). Because of this high threshold of use and its inconsistency with the assessments at other waves, the Wave III assessment was dropped from analyses. We used an ordinal coding scheme consistent with that employed for cannabis: (0) none, (1) 1 to 3 days, (2) 1 to 2 days a week, and (3) 3 days a week to daily. Individuals who reported smoking between 9 and 11 days in the past month were grouped into the category “3 days a week to daily.”
Quantity of tobacco use
At all waves, participants were asked, “During the past 30 days, on the days you smoked, how many cigarettes did you smoke each day?” Responses ranged from 0 to 95 at Wave I, 0 to 95 at Wave II, 1 to 100 at Wave III, and 1 to 100 at Wave IV. To reduce the influence of improbably high values, data were winsorized such that individuals reporting values above the 95th percentile (20 at Waves I and II and 25 at Waves III and IV) were recoded to the 95th percentile. Quantity of use at Wave III was only queried among regular smokers; therefore, the Wave III assessment was dropped from analyses.
Frequency of cannabis use
At Waves I, II, and III, participants were asked how many times they had used marijuana in the past 30 days. Responses were coded on a continuous scale. At Wave IV, respondents were asked how many days they had smoked marijuana in the past 30 days, and responses were coded on an ordinal scale: (0) none, (1) one day, (2) 2 or 3 days, (3) 1 day a week, (4) 2 days a week, (5) 3 to 5 days a week, or (6) every day or almost every day. The response distributions at Waves I–III were highly skewed. Further, we wished to maintain consistency with the ordinal coding scheme employed at Wave IV. Therefore, we recoded the marijuana frequency variable at Waves I–III on the same scale. Due to low frequencies in some response categories, we subsequently recoded the variable into four ordinal categories: (0) none, (1) 1 to 3 times/days, (2) 1 to 2 times/days a week, and (3) 3 times/days a week to daily.
Note that quantity of cannabis use was not assessed in Add Health.
Age of alcohol initiation
At Waves I and II, participants were asked if they had had a drink of beer, wine, or liquor – not just a taste of someone else’s drink – more than two or three times, and if they ever drank alcohol when they were not with their parents or other adults in their family. If they responded yes to both, they were asked to report the age at which they first had a drink of beer, wine, or liquor when they were not with their parents or other adults in their family. At Wave IV, participants were asked if they had had a drink of beer, wine, or liquor more than two or three times. If yes, respondents were asked to report the age at which they first had an alcoholic drink. To limit risk of retrospective reporting bias, respondents’ age of onset was taken from the earliest wave at which they reported having tried alcohol. Age of onset data were available for 8,101 respondents. As of Wave IV, 20.1% of individuals had not tried alcohol.
Age of tobacco initiation
At Waves I and III, participants were asked whether they had ever tried smoking, and if so, the age at which they first smoked a whole cigarette. At Wave IV, they were asked if they had ever smoked an entire cigarette, and if so, the age at which they first smoked a whole cigarette. Respondents’ age of onset was taken from the earliest wave at which they reported having tried smoking. Age of onset data were available for 6,649 individuals. As of Wave IV, 37.0% of participants had not yet tried cigarettes.
Age of cannabis initiation
At Waves I and IV, participants were asked to report the age at which they first used cannabis. Their age of onset was taken from the earliest wave at which they reported having tried cannabis. Age of initiation data were available for 5,548 respondents. As of Wave IV, 46.4% of participants had not tried cannabis.
Statistical Analysis
Age-based restructuring
Latent growth models (LGMs) were fit to evaluate changes in substance use frequency and quantity across age. There is variability in chronological age at each wave in Add Health. With regard to progression in substance use, age is a more meaningful time metric than wave. We thus restructured the data to provide age-based measurement for analyses. Some individuals (n=964) were interviewed at the same age at Waves I and II. If they provided substance use data at only one wave, their score was taken from that wave. If they provided data at both waves, their score was taken from Wave II. To provide sufficient coverage, we collapsed measurements into age groups: early adolescence (11–15 years), mid-to-late adolescence (16–20 years), early adulthood (21–27 years), and “later” early adulthood (28–34 years). When individuals were assessed more than once in a given age group, their substance use score was taken from the later age. The overlapping nature of the data and missing data analyses permitted estimation of growth parameters for all respondents who provided at least one measurement. Table 1 displays the number of individuals providing data within each age group.
Table 1.
Number of Individuals Providing Data Within Each Age Group
| Age Group
|
||||
|---|---|---|---|---|
| 11 → 15
|
16 → 20
|
21 → 27
|
28 → 34
|
|
| Tobacco Use Frequency | 2,286 | 3,338 | 3,375a | 5,974 |
| Tobacco Use Quantity | 1,465 | 2,465 | 1,837a | 2,035 |
| Alcohol Use Frequency | 2,661 | 5,903 | 7,525 | 4,702 |
| Alcohol Use Quantity | 2,188 | 5,426 | 7,036 | 4,232 |
| Cannabis Use Frequency | 1,196 | 3,195 | 2,768 | 1,249 |
Notes.
Because the Wave III assessments of tobacco use frequency and quantity were dropped, these groupings exhibited extremely low covariance coverage with the 28 → 34 year age groupings for all phenotypes. Thus, the 21 → 27 year age groupings for tobacco use were excluded from analyses.
Latent growth models
To examine changes in substance use frequency and quantity across age, we fit univariate growth models. LGMs measure the development within individuals of constructs over time using random effects, conceptualized as latent growth factors (Grimm, Ram & Estabrook, 2017; Newsom, 2015). Intercept and slope factors represent initial levels of and change in the outcome variables, respectively. To determine best-fitting models, three unconditional models were fit for each construct: (1) intercept and linear slope, (2) intercept and partly nonlinear slope, and (3) intercept and fully nonlinear slope. Best-fitting models were carried forward into multivariate model-fitting.
Our first aim was to test whether there was a significant relation between the age at first substance use and initial status and (of paramount interest) rate of change in substance involvement. We therefore regressed the slope and intercept factors for each substance onto the ages of tobacco, alcohol, and cannabis initiation. Three sets of regressions, with six regressions total, were run within each model (the slope and intercept factors for each substance use outcome were regressed onto the age of initiation for each substance). Thus, a Bonferroni correction at α=.05 (critical value=.008) was used to determine significance for these analyses.
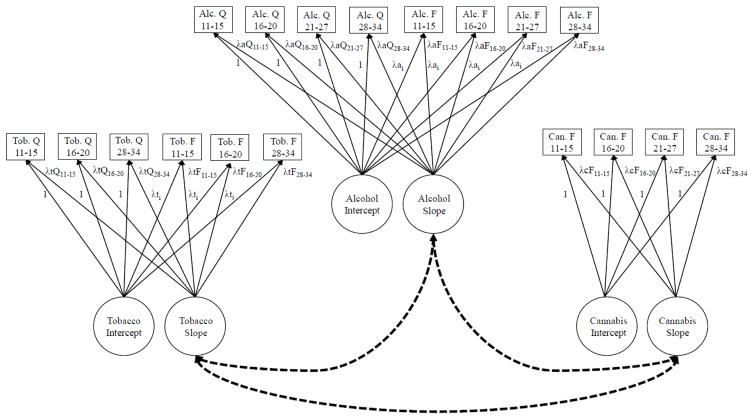
Our second aim was to determine the extent to which differences in the ages of tobacco, alcohol, and cannabis initiation accounted for differences in trajectories across substances. To accomplish this, we fit a multivariate LGM. Figure 1 displays the proposed model. Only the associations of primary interest (correlations between slope factors) are displayed. In the final model, however, the intercept and slope factors across substances were correlated. A significant association between slope factors would indicate correlated change in use of tobacco, alcohol, and/or cannabis. For example, a significant positive correlation between the slopes for alcohol and tobacco use would indicate that individuals who increased in their alcohol use frequency and quantity were more likely to also increase in their tobacco use frequency and quantity. After establishing baseline correlations across trajectories, we assessed the magnitude of associations after controlling for the age of initiation.
Figure 1.
Multivariate latent growth model. This is the parameterization for a free curve slope intercept model. In this model, (a) the intercept factor loadings are fixed to unity and the slope factor loadings are freed, (b) the intercept and slope factor means are freed, (c) the intercept factor variance is freed and the slope factor variance is fixed to 1.0, and (d) the intercept and slope are uncorrelated within each construct. To account for scaling differences across the frequency and quantity variables, (a) the paths from the intercept factor to the quantity outcomes were fixed to unity and (b) the paths to the frequency variables were specified to be tau-equivalent. The dotted lines between the slope factors indicate correlated change in tobacco, alcohol, and cannabis use. For simplicity, only the associations of primary interest (correlations between slope factors, indicated by the dashed lines) are depicted. Within the actual model, however, the intercept and slope factors across constructs were also correlated. Tob.=tobacco, Alc.=alcohol, Can.=cannabis, Q=quantity, F=frequency.
Mplus removes individuals who are missing on covariates from analyses. Thus, the adjusted multivariate model included only individuals who reported valid ages of initiation for tobacco, alcohol, and cannabis (n=4,609). We therefore ran the baseline multivariate model within this sample. For comparison, we also report results for the baseline model run within the full sample.
Figure 1 depicts a fully nonlinear or “free curve slope intercept” (FCSI) model (Meredith & Tisak, 1990; Wood, Steinley, & Jackson, 2015). This is the least restrictive of available models and imposes no constraints on the shape of change (Wood, 2011; Wood et al., 2015). In this parameterization, the intercept factor loadings are fixed to unity and the slope factor loadings are freed; the intercept and slope factor means are freed; the intercept factor variance is freed and the slope factor variance is fixed to 1.0; and the intercept and slope are uncorrelated.
For tobacco and alcohol, where both frequency and quantity outcomes were available, we modeled a single random intercept and slope factor for each substance. To enable incorporating all indicators while accounting for scaling differences across the frequency and quantity variables, (a) the paths from the intercept factor to the quantity outcomes were fixed to unity and (b) the paths to the frequency variables were specified to be tau-equivalent (Figure 1).
We present the FCSI model in Figure 1 to outline its parameterization. Prior to fitting the multivariate LGM, the fit of the FCSI model against that of alternative models was tested. All LGMs were fit within Mplus version 7 (Muthén & Muthén, 1998–2015). Univariate LGMs for quantity outcomes were fit using a robust maximum likelihood estimator (MLR). Univariate LGMs for frequency outcomes and multivariate LGMs employing both quantity and frequency outcomes were fit using a robust weighted least squares estimator (WLSMV). Nested models fit using the MLR estimator were compared using Satorra-Bentler scaled chi-square difference test. Nested models fit using the WLSMV estimator were compared using the DIFFTEST command.
An orthogonal model of growth in which intercept and slope are uncorrelated has several advantages. First, rather than scaling the factor solution relative to the initial time of measurement, this model is scaled relative to the “aperture,” or beginning, of the growth process. When models are scaled to the initial time point, solutions will not replicate across studies employing different measurement occasions, even if they capture the same growth pattern. The orthogonal solutions, however, will replicate. Second, it is likely that the starting point of growth differs across substances. The orthogonal model enables estimation of random intercept and slope factors specific to each substance, capturing this variability. Third, estimating the covariance between intercept and slope reduces statistical power. Lastly, it is possible to conduct nested comparisons between the FCSI model and more parsimonious models of growth.
Latent growth modeling with ordinal outcomes assumes threshold invariance; that is, that thresholds are held equal across time (Mehta, Neale, & Flay, 2004). In other words, it is assumed that the thresholds defining the relation between the latent response variable and the observed ordered categories remain the same across assessment periods. This assumption can be relaxed and tested; however, at least one threshold must be held invariant in order to properly identify the structural portion of the model (Masyn, Petras, & Liu, 2014; Newsom, 2015). As part of our modeling sequence, we tested whether the assumption of threshold invariance was appropriate for our ordinal frequency outcomes. This was done by comparing the relative fits of models in which thresholds were freed across age.
Results
Response Distributions for the Age of Substance Use Initiation
Responses ranged from 1–32 years, 1–31 years, and 1–30 years for the ages of alcohol, tobacco, and cannabis initiation, respectively. To reduce the influence of low values, reported ages of first use below 5 years were equated to 5 years. For alcohol initiation, reported ages were 15.3 years ((standard deviation (SD)=3.4) in men and 15.6 years (SD=3.3) in women. For tobacco initiation, reported ages were 14.7 years (SD=3.6) in men and 14.7 years (SD=3.5) in women. For cannabis initiation, reported ages were 15.8 years (SD=3.4) for men and 16.3 years (SD=3.2) for women. Ages of initiation of all substances were moderately correlated with each other and modestly to moderately inversely correlated with frequency and quantity of use (Table 2).
Table 2.
Correlations Between Study Variables
| Tobacco use F | Tobacco use Q | Alcohol use F | Alcohol use Q | Cannabis use F | Age of initiation | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||||||
| 11–15 | 16–20 | 28–34 | 11–15 | 16–20 | 28–34 | 11–15 | 16–20 | 21–27 | 28–34 | 11–15 | 16–20 | 21–27 | 28–34 | 11–15 | 16–20 | 21–27 | 28–34 | Tobacco | Alcohol | Cannabis | |
|
|
|||||||||||||||||||||
| Tobacco use F | |||||||||||||||||||||
| 11–15 | -- | ||||||||||||||||||||
| 16–20 | 0.63 | -- | |||||||||||||||||||
| 28–34 | 0.46 | 0.51 | -- | ||||||||||||||||||
| Tobacco use Q | |||||||||||||||||||||
| 11–15 | 0.63 | 0.47 | 0.29 | -- | |||||||||||||||||
| 16–20 | 0.53 | 0.65 | 0.37 | 0.61 | -- | ||||||||||||||||
| 28–34 | 0.22 | 0.28 | 0.54 | 0.36 | 0.42 | -- | |||||||||||||||
| Alcohol use F | |||||||||||||||||||||
| 11–15 | 0.36 | 0.26 | 0.24 | 0.18 | 0.26 | 0.10 | -- | ||||||||||||||
| 16–20 | 0.08 | 0.27 | 0.18 | 0.03 | 0.15 | 0.02 | 0.22 | -- | |||||||||||||
| 21–27 | 0.01 | 0.08 | 0.18 | −0.09 | −0.03 | −0.05 | 0.06 | 0.27 | -- | ||||||||||||
| 28–34 | 0.01 | 0.002 | 0.17 | −0.07 | −0.04 | −0.11 | 0.10 | 0.16 | 0.49 | -- | |||||||||||
| Alcohol use Q | |||||||||||||||||||||
| 11–15 | 0.29 | 0.21 | 0.18 | 0.24 | 0.25 | 0.06 | 0.30 | 0.13 | 0.005 | 0.002 | -- | ||||||||||
| 16–20 | 0.11 | 0.21 | 0.17 | 0.14 | 0.16 | 0.06 | 0.14 | 0.36 | 0.15 | 0.09 | 0.22 | -- | |||||||||
| 21–27 | 0.13 | 0.14 | 0.21 | 0.16 | 0.11 | 0.07 | 0.09 | 0.18 | 0.31 | 0.13 | 0.14 | 0.27 | -- | ||||||||
| 28–34 | 0.12 | 0.16 | 0.29 | 0.04 | 0.14 | 0.14 | 0.08 | 0.17 | 0.19 | 0.24 | 0.19 | 0.19 | 0.34 | -- | |||||||
| Cannabis use F | |||||||||||||||||||||
| 11–15 | 0.41 | 0.33 | 0.12 | 0.25 | 0.20 | 0.001 | 0.33 | 0.18 | −0.05 | 0.07 | 0.22 | 0.03 | 0.08 | 0.02 | -- | ||||||
| 16–20 | 0.13 | 0.30 | 0.22 | 0.15 | 0.18 | 0.07 | 0.09 | 0.27 | 0.14 | 0.14 | 0.11 | 0.14 | 0.11 | 0.13 | 0.30 | -- | |||||
| 21–27 | 0.12 | 0.20 | 0.28 | 0.07 | 0.14 | 0.08 | 0.09 | 0.07 | 0.02 | 0.11 | 0.15 | 0.11 | 0.11 | 0.12 | 0.20 | 0.34 | -- | ||||
| 28–34 | −0.03 | 0.03 | 0.25 | 0.06 | 0.09 | 0.13 | 0.14 | 0.01 | 0.02 | −0.07 | 0.11 | 0.09 | 0.04 | 0.08 | 0.02 | 0.12 | 0.45 | -- | |||
| Age of initiation | |||||||||||||||||||||
| Tobacco | −0.18 | −0.20 | −0.17 | −0.25 | −0.28 | −0.21 | −0.22 | −0.07 | 0.02 | 0.05 | −0.19 | −0.13 | −0.10 | −0.11 | −0.11 | −0.11 | −0.11 | −0.07 | -- | ||
| Alcohol | −0.21 | −0.18 | −0.20 | −0.14 | −0.13 | −0.08 | −0.33 | −0.20 | −0.16 | −0.11 | −0.20 | −0.17 | −0.14 | −0.15 | −0.16 | −0.11 | −0.12 | −0.05 | 0.42 | -- | |
| Cannabis | −0.30 | −0.24 | −0.18 | −0.23 | −0.22 | −0.08 | −0.27 | −0.13 | −0.01 | 0.04 | −0.24 | −0.14 | −0.10 | −0.11 | −0.11 | −0.18 | −0.21 | −0.15 | 0.42 | 0.44 | -- |
Notes. Correlations among initiation, among quantity, and between initiation and quantity variables are Pearson coefficients. Correlations between frequency variables are polychoric coefficients. Correlations between initiation and frequency and between frequency and quantity variables are Spearman coefficients. F=frequency, Q=quantity, 11–15=11–15 years, 16–20=16–20 years, 21–27=21–27 years, 28–34=28–34 years.
Response Distributions for Substance Use Frequency
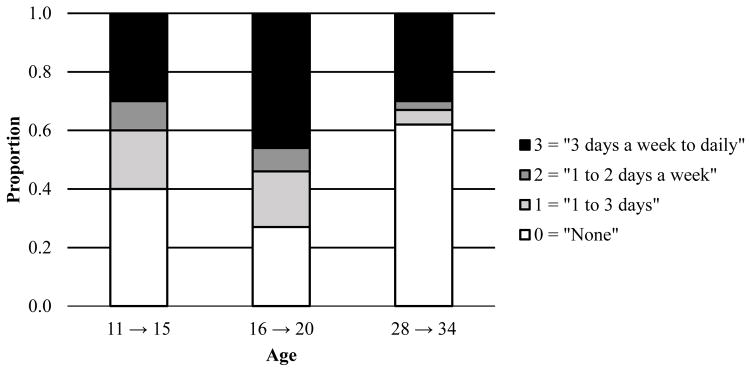
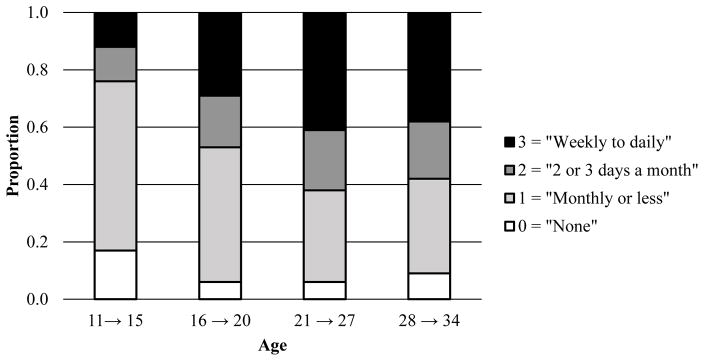
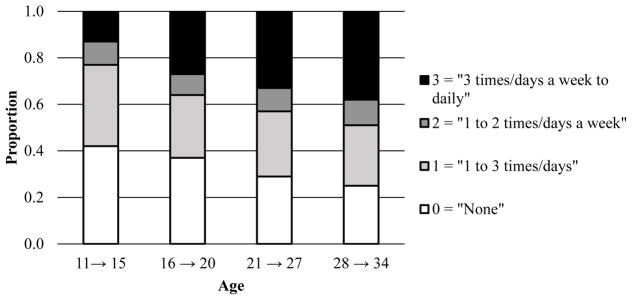
Figures 2–4 display the response proportions for the ordinal tobacco, alcohol, and cannabis use frequencies across age. Because the Wave III assessment for tobacco use was excluded, low covariance coverage with the “age 28 to age 34” age groupings for all phenotypes necessitated dropping the “age 21 to age 27” tobacco use age group. Thus, response proportions and correlations (Table 2) for only three age groups are presented for tobacco. The data suggest a quadratic shape of change for tobacco, such that individuals increased in their frequency of use across the first two time points and decreased at the last point. For alcohol, the data suggest an increase in use across the first three time points and a leveling off at the last point. For cannabis, the data indicate a relatively steady increase in use across all time points.
Figure 2.
Proportion of individuals (by age in years) responding to the ordinal tobacco use frequency item.
Figure 3.
Proportion of individuals (by age in years) responding to the ordinal alcohol use frequency item.
Figure 4.
Proportion of individuals (by age in years) responding to the ordinal cannabis use frequency item.
Response Distributions for Substance Use Quantity
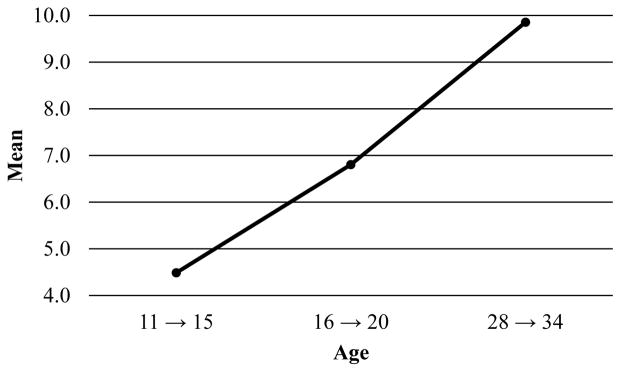
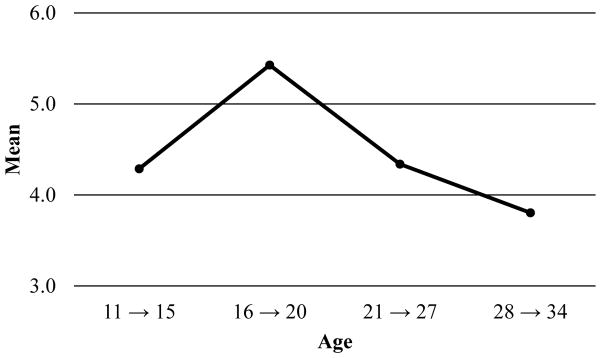
Figures 5 and 6 depict the mean reported tobacco and alcohol use quantities for each age grouping. As for tobacco use frequency, the “age 21 to age 27” group for tobacco use quantity was dropped. The pattern of means suggests an increase in tobacco use quantity across age. The data suggest a quadratic shape of change for alcohol: individuals increased in their quantity of use between the first two time points and decreased across the subsequent time points.
Figure 5.
Observed mean-level changes in tobacco use quantity across age.
Figure 6.
Observed mean-level changes in alcohol use quantity across age.
Univariate Latent Growth Model-Fitting
Table 3 displays the univariate model-fitting results. In the form considered here, the FCSI model is not nested within the partly nonlinear model. However, the partly nonlinear model can be compared by fitting a transformation of the FCSI model which is covariance nested with the partly nonlinear model (see Wood et al., 2015). This rotation can be accomplished by the following constraints: the first slope loading is fixed to 0.0, the last slope loading is fixed to 1.0, and the intermediary loadings are freely estimated; the intercept and slope means are freely estimated; the intercept and slope variances are freely estimated; and the covariance between the intercept and slope factors is freed. For alcohol use frequency and quantity and cannabis use frequency, the FCSI model provided the best fit. For tobacco use frequency, the partly nonlinear model fit significantly better than the linear model. It was not possible to compare the partly linear and FCSI models for tobacco use frequency as there were only three assessment occasions (making the models identical). Further, because the partly nonlinear and FCSI models for tobacco use quantity were just identified, no chi-square fit statistics were available, prohibiting nested model comparisons. However, we fit FCSI models to the data for both outcomes, as (a) we wished to remain consistent with the approach for alcohol and cannabis, and (b) the response proportions for tobacco use frequency indicated the shape of change was highly nonlinear.
Table 3.
Model-Fitting Results for Univariate Latent Growth Models
| Δχ2
|
Δdf
|
p-value
|
|
|---|---|---|---|
| Tobacco Use Frequency | |||
| Linear | -- | ||
| Partly nonlineara | 559.13 | 1 | <.0001 |
| Freeb,c | -- | -- | -- |
| Alcohol Use Frequency | |||
| Linear | -- | ||
| Partly nonlineara | 180.53 | 1 | <.0001 |
| Freeb | 9.88 | 1 | .002 |
| Alcohol Use Quantity | |||
| Linear | -- | ||
| Partly nonlineara | 5.16 | 1 | .023 |
| Freeb | 83.34 | 1 | <.0001 |
| Cannabis Use Frequency | |||
| Linear | -- | ||
| Partly nonlineara | 7.45 | 1 | .006 |
| Freeb | 4.68 | 1 | .030 |
Notes. The partly nonlinear models for alcohol and cannabis use frequency freed the slope loadings for the last time point.
Comparison for chi-square test is between linear and partly nonlinear models.
Comparison for chi-square test is between partly nonlinear and free models.
Because the univariate model for tobacco use frequency contained only three times of measurement, the partly nonlinear and free models were not nested and could not be formally compared. No nested model comparisons were conducted for tobacco use quantity, as the partly nonlinear and FCSI models were just identified and therefore chi-square estimates of model fit were not available. Model comparisons for categorical outcomes were conducted using the DIFFTEST option. Model comparisons for continuous outcomes were conducted using the Satorra-Bentler scaled chi-square difference test.
Threshold Invariance
We tested for threshold invariance across age for the substance use frequency outcomes. Freeing the third threshold resulted in a significant improvement in fit for all models (ps<.0001). We therefore fit models in which the first two thresholds were constrained to equality and the third was allowed to be free across time.
Age of Substance Use Initiation and Univariate Substance Use Trajectories
As shown in Table 4, all constructs exhibited significant variability in the intercept, indicating meaningful individual differences at the initial level. In addition, the mean slope estimates were significant, indicating meaningful rates of change in tobacco, alcohol, and cannabis use across age.
Table 4.
Standardized Means (and Unstandardized Variances) of Slope and Intercept Factors Within the Univariate Latent Growth Models
| Substance Use Outcome
|
Intercept mean
|
Slope mean
|
Intercept variance
|
|---|---|---|---|
| Tobacco Use Frequency | 0.87*** | 0.73*** | 1.42** |
| Tobacco Use Quantity | 1.56*** | 1.21*** | 22.35*** |
| Alcohol Use Frequency | 2.68*** | 0.99*** | 0.22*** |
| Alcohol Use Quantity | 2.27*** | 0.78*** | 3.23*** |
| Cannabis Use Frequency | 0.63*** | 0.72*** | 0.39*** |
Notes. Slope variances are not presented as they were fixed to 1.0 for model identification. Note that although the tobacco use frequency and alcohol use quantity slope means are positive, the overall shape of change for these outcomes was quadratic, with individuals increasing and then decreasing in their use across age.
p<.01,
p<.001.
Substance use frequency
Within the univariate LGMs, we regressed the intercept and slope factors for substance use frequency and quantity onto the ages of tobacco, alcohol, and cannabis initiation. Table 5 displays the regression coefficients. With regard to tobacco, there was evidence that individuals with later ages of initiation of all substances tended to decline in their frequency of use at steeper rates (have more negative slopes) than individuals with an earlier age of initiation. Respondents with a later age of cannabis initiation exhibited lower initial frequency. Concerning alcohol, respondents with later ages of initiation of all substances had a lower initial level of use compared with respondents with early ages of initiation; however, they increased in their frequency of use at steeper rates. Regarding cannabis use, the age of alcohol initiation was negatively associated with the intercept factor. Respondents with a later age of tobacco initiation tended to decline their frequency of use across the last age period of assessment.
Table 5.
Regression Coefficients from Latent Growth Models Predicting Intercept and Slope Factors for the Frequency and Quantity of Substance Use from the Age of Substance Use Initiation
| Phenotype
|
Intercept
|
Slope
|
|---|---|---|
| Age of Tobacco Initiation
|
||
| Tobacco Use Frequency | −0.16 | −0.31*** |
| Tobacco Use Quantity | −0.33*** | 0.16 |
| Alcohol Use Frequency | −0.23*** | 0.25*** |
| Alcohol Use Quantity | −0.19*** | −0.15 |
| Cannabis Use Frequency | −0.38 | −0.14** |
|
| ||
| Age of Alcohol Initiation
|
||
| Tobacco Use Frequency | −0.38 | −0.21** |
| Tobacco Use Quantity | −0.17*** | 0.06 |
| Alcohol Use Frequency | −0.52*** | 0.14*** |
| Alcohol Use Quantity | −0.28*** | −0.13 |
| Cannabis Use Frequency | −0.25*** | 0.01 |
|
| ||
| Age of Cannabis Initiation
|
||
| Tobacco Use Frequency | −0.31** | −0.29** |
| Tobacco Use Quantity | −0.24*** | 0.23** |
| Alcohol Use Frequency | −0.38*** | 0.31*** |
| Alcohol Use Quantity | −0.22*** | −0.07 |
| Cannabis Use Frequency | −0.05 | −0.36 |
Notes. An alpha level of .008 was used to determine significance for these analyses.
p<.01,
p<.001.
Substance use quantity
There were fewer associations observed between age of initiation and the slope factors for alcohol and tobacco use quantity, compared with alcohol and tobacco use frequency (Table 5). For tobacco use quantity, individuals with a later age of cannabis initiation increased in their use at steeper rates than individuals with an earlier age of initiation. No associations were observed between age of initiation and the slope factor for alcohol use quantity. Respondents with earlier ages of onset of all substances had higher initial levels of alcohol and tobacco use than respondents with later ages of onset.1
Sensitivity analyses
To determine the extent to which the ordering of substance use onset impacted results, we re-ran all regression analyses within sub-samples in which (a) use of the initiation substance occurred prior to or in the same year as the outcome substance, and (b) use of the initiation substance occurred prior to the outcome substance. A trend was observed in which the direction of associations with the slope and intercept factors remained the same; however, estimates tended to become non-significant across more restrictive models. This was likely due to reduced statistical power. There were two exceptions to this trend: (1) There were minimal changes in the significance of regression coefficients for the slope factor for alcohol use frequency, and (2) the relation between the age of cannabis initiation and the slope factor for tobacco use frequency was positive and significant in both restricted models (βs=0.16–0.20, ps<.01, not tabled (results available upon request)).
Age of Substance Use Initiation and Multivariate Substance Use Trajectories
Table 6 displays the correlations observed before and after the intercept and slope factors for each substance were regressed onto the age of initiation of that substance. Within the baseline multivariate model, no significant associations were observed between the slope factors for any of the substances. However, after adjusting for the ages of tobacco, alcohol, and cannabis initiation, correlations between the slope factors for tobacco and alcohol (r=.43, p<.0001) and alcohol and cannabis (r=.20, p<.05) were significant.2
Table 6.
Correlations Between Slope and Intercept Factors Within the Multivariate Latent Growth Model, Prior to and After Adjusting for the Age of Substance Use Initiation
| Substances
|
Baseline
|
Adjusted
|
|---|---|---|
| Slopes
|
||
| Tobacco and alcohol | −0.13 | 0.43*** |
| Tobacco and cannabis | 0.12 | −0.11 |
| Alcohol and cannabis | −0.08 | 0.20* |
|
| ||
| Intercepts
|
||
| Tobacco and alcohol | 0.38*** | −0.27* |
| Tobacco and cannabis | 0.46*** | −0.29* |
| Alcohol and cannabis | 0.58*** | 0.30*** |
Notes. The baseline and adjusted models were run using only individuals who reported a valid age of initiation for tobacco, alcohol, and cannabis (n=4,609). In the adjusted multivariate model, the intercept and slope factor for each substance were adjusted for the age of initiation of that substance.
p<.05,
p<.0001.
Discussion
The present study consisted of a multivariate latent growth curve analysis of tobacco, alcohol, and cannabis involvement. Using data from a nationally-representative, longitudinal sample, we examined (a) the extent to which initial status and rate of change in substance use frequency and quantity were related to the age of substance use initiation, and (b) the degree to which age of initiation moderated the magnitude of associations across substance use trajectories.
Latent Growth Model-Fitting
For alcohol use frequency and quantity and cannabis use frequency, the FCSI model provided the best fit to the data. Although the relative fit of the FCSI model could not be evaluated for tobacco use frequency, the shape of change in this outcome was nonlinear, suggesting that an unspecified form of growth was appropriate. Tobacco use quantity was the only outcome for which the pattern of means appeared potentially consistent with a linear form. These findings highlight the importance of employing flexible statistical methods to account for the dynamic changes in substance use that occur across development. Polynomial models may fail to capture developmentally-limited nonlinear changes (Grimm et al., 2017; Ram & Grimm, 2007). Free curve models may more accurately account for short-term, nonlinear behavioral change. Unconstrained growth models may most appropriately capture changes in the use of tobacco, alcohol, and cannabis across adolescence and young adulthood.
Age of Initiation and Univariate Substance Use Trajectories
Consistent with prior developmental research (e.g., Brodbeck, Bachmann, Croudace, & Brown, 2013), trajectories of alcohol, tobacco, and cannabis involvement exhibited different patterning across age. We observed significant cross-substance associations between age at first use and both initial level and rate of change in substance use trajectories. Results indicated potentially stronger associations with frequency compared with quantity, as limited relations were observed between age at first use and the slope factors for quantity trajectories.
The slope factor for cannabis use frequency was related only to the age of tobacco initiation, and the slope factor for tobacco use quantity was associated only with the age of cannabis initiation. Mounting research suggests that unique mechanisms (e.g., common familial influences, shared route of administration, and co-administration (Agrawal, Budney, & Lynskey, 2012; Agrawal & Lynskey, 2009)) may explain progression between and co-use of tobacco and cannabis. Early tobacco smoking may increase risk for later cannabis involvement (Mathers et al., 2006). Increased attention has also been paid to “reverse gateways,” in which cannabis use precedes tobacco initiation (e.g., Agrawal et al., 2011; Patton, Coffey, Carlin, Sawyer & Lynskey, 2005; Richmond-Rakerd et al., 2015). Present analyses suggest a specificity and particular relevance of the timing of tobacco initiation for trajectories of cannabis use, and of the age of cannabis initation for quantity of cigarettes smoked.
Age of Initiation and Associations Between Substance Use Trajectories
Before controlling for the age of substance use initiation, no significant associations were observed across trajectories. There are limited prior studies for comparison, as investigations of progression within nationally-representative, prospective samples have examined use of only one or two substances, modeled trajectories separately, or compared associations across constructs within assessment periods rather than across age (e.g., Chen & Jacobson, 2012; Jackson et al., 2002; Keyes et al., 2015; ; Moss et al., 2014; Mulia et al., 2017; Muthén & Muthén, 2000; Tucker et al., 2005). Nevertheless, comparison with studies of samples that are not nationally-representative may prove informative. A previous analysis of correlated change in cigarette, alcohol, and marijuana use in a sample of adolescents from the Pacific Northwest (Duncan, Duncan, Biglan, & Ary, 1998) found that change in use was significantly correlated across substances and could be modeled by a higher-order construct. This may reflect differences in sampling procedures, operationalization of variables, and sample ages (14–17 years in Duncan and colleagues’ (1998) analysis, compared with 11–34 years in the present study).
It should be noted that failing to obtain significant associations across slope factors does not suggest a lack of common risk mechanisms across substances. Use of tobacco, alcohol, and cannabis is influenced by shared genetic and environmental risk factors (e.g., Kendler et al., 2008; Palmer et al., 2012; Richmond-Rakerd et al., 2016; Sartor et al., 2009; Sartor et al., 2010). Further, analyses of patterns of contemporaneous substance use indicate that a notable proportion of adolescents engage in early polysubstance involvement (e.g., Moss et al., 2014) and exhibit increasing multiple-substance use across age (e.g., Brooks-Russell et al., 2015).
After adjusting for age at first use, correlations between the slope factors for alcohol and cannabis and tobacco and alcohol were significant, with small and moderate associations observed, respectively. This indicates that some of the differences in tobacco, alcohol, and cannabis use trajectories result from individuals’ initiation of these substances at different ages. Initiation of multiple substances at the same age is less common than a sequence in which individuals progress from use of tobacco and alcohol onto use of cannabis and other illicit drugs (Degenhardt et al., 2009; Kandel, Yamaguchi, & Chen, 1992). Although some people may wait to commence using substances until later in life, it is more likely that these respondents are experimenting with multiple substances at an early age. A prior analysis by our group (Richmond-Rakerd, Fleming, & Slutske, 2016) found that risk of concurrent substance use initiation (particularly tobacco and alcohol initiation) within the Add Health sample was greater for respondents who started using substances during the middle and high school years than respondents who initiated substance use in early adulthood. Present findings suggest that among individuals who start using tobacco, alcohol, and cannabis at the same age (particularly an early age), their subsequent trajectories of use are likely to be more strongly associated than among individuals who exhibit a more normative sequence of initiation.
Limitations
Results should be interpreted with consideration of several limitations. The first concerns the generalizability of results for cannabis, as there have been changes in legalization since data were collected. Second, at Waves I and II, participants were asked to report the age at which they first consumed alcohol when not with parents or other adults in their family. Although this question was intended to capture parental supervision, it may have excluded individuals who first consumed alcohol with other adult family members. Further, although early alcohol use outside of the presence of a family member may be considered more “deviant,” there may be risk associated with early use that exists regardless of familial context. Third, we had to exclude the Wave III tobacco assessment (and the 21–27 year age bin) from analyses as it was conducted among regular smokers. Thus, the trajectories of change identified for tobacco may not capture use in young adulthood. Fourth, cannabis use frequency was assessed as a continuous measure across the first three waves and a polytomous measure at the last wave. We recoded the variables for the first three waves to approximate the ordinal categories at Wave IV; however, this may not have fully accounted for differences in response patterns. In addition, low frequencies within some ordinal response categories necessitated combining categories. Fifth, to provide sufficient coverage, we collapsed age-based measurements into groups. More intensive assessments of substance use across age will enable a more fine-grained analysis. Nevertheless, age-based measurement is preferable to wave-based assessment in capturing developmental changes in use. Sixth, within regression models, some respondents may have first used the outcome substance prior to the predictor substance. Results of analyses that placed restrictions on temporal ordering were largely consistent, and differences in statistical significance likely resulted primarily from a reduction in statistical power. Restricted models did, however, indicate a different direction of association (positive) for the relation between the age of cannabis initiation and the slope factor for tobacco use frequency. Lastly, the assessment periods for substances differed, such that individuals were queried about their use of alcohol in the past year and their use of tobacco and cannabis in the past 30 days. In comparison to alcohol, the shorter time span employed for tobacco and cannabis may have provided less opportunity to capture use of these substances that occurred between waves.
Conclusions
Notwithstanding these limitations, the present findings offer a notable advance in our understanding of the association between the age of substance use initiation and later substance use trajectories. Results indicate unique pathways of progression in tobacco, alcohol, and cannabis use across adolescence and young adulthood. Further, within- and cross-substance relations between the age of initiation and rate of change in substance use differ across tobacco, alcohol, and cannabis. Differences in substance use trajectories are partly accounted for by variability in the ages at which individuals initiate their use of these substances. Future investigations aiming to build on these findings will benefit from incorporating additional measures of early-onset substance involvement and substance-related problems.
Acknowledgments
This work was supported by NIAAA grant AA023419 to Leah S. Richmond-Rakerd. This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
In a series of supplemental regression analyses, we tested for sex differences in the associations between the ages of tobacco, alcohol, and cannabis initiation and the intercept and slope factors for all frequency and quantity outcomes. None of the interactions with sex were significant after correction for multiple testing, indicating that the associations were comparable across men and women.
We re-ran the baseline multivariate model within the full sample, including all individuals who provided at least one measurement (n=9,240). Within this model, there was a significant negative association between the slope factors for tobacco and alcohol (r=−0.22, p=.001). Significant positive correlations were observed between all intercept factors (tobacco and alcohol: r=0.45 (p<.0001), tobacco and cannabis: r=0.51 (p<.0001), alcohol and cannabis: r=0.61 (p<.0001)).
There are no conflicts of interest to declare.
References
- Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: A review. Addiction. 2012;107:1221–1233. doi: 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Tobacco and cannabis co-occurrence: Does route of administration matter? Drug and Alcohol Dependence. 2009;99:240–247. doi: 10.1016/j.drugalcdep.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Scherrer JF, Lynskey MT, Sartor CE, Grant JD, Haber JR, … Xian H. Patterns of use, sequence of onsets and correlates of tobacco and cannabis. Addictive Behaviors. 2011;36:1141–1147. doi: 10.1016/j.addbeh.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychological Review. 2010;29:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt S, Beesdo-Baum K, Höfler M, Perkonigg A, Bühringer G, Lieb R, Wittchen HU. The relevance of age at first alcohol and nicotine use for initiation of cannabis use and progression to cannabis use disorders. Drug and Alcohol Dependence. 2012;123:48–56. doi: 10.1016/j.drugalcdep.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen HU, Höfler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: Is early onset associated with a rapid escalation? Drug and Alcohol Dependence. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Bolland KA, Bolland JM, Tomek S, Devereaux RS, Mrug S, Wimberly JC. Trajectories of adolescent alcohol use by gender and early initiation status. Youth and Society. 2016;48:3–32. doi: 10.1177/0044118X13475639. [DOI] [Google Scholar]
- Brooks-Russell A, Conway KP, Liu D, Xie Y, Vullo GC, Li K, … Simons-Morton B. Dynamic patterns of adolescent substance use: Results from a nationally representative sample of high school students. Journal of Studies on Alcohol and Drugs. 2015;76(6):962–970. doi: 10.15288/jsad.2015.76.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck J, Bachmann MS, Croudace TJ, Brown A. Comparing growth trajectories of risk behaviors from late adolescence through young adulthood: An accelerated design. Developmental Psychology. 2013;49:1732–1738. doi: 10.1037/a0030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1189–1285. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chantala K. Guidelines for analyzing Add Health data. 2014 Retrieved from http://www.cpc.unc.edu/projects/addhealth/data/guides/wt-guidelines.pdf.
- Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. Journal of Adolescent Health. 2012;50:154–163. doi: 10.1016/j.jadohealth.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clincial and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Conway K, Dierker L, Glantz M, Kalaydjian A, … Kessler RC. Does the “gateway” matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychological Medicine. 2009;39:157–167. doi: 10.1017/S0033291708003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Biglan A, Ary D. Contributions of the social context to the development of adolescent substance use: A multivariate latent growth modeling approach. Drug and Alcohol Dependence. 1998;50:57–71. doi: 10.1016/S0376-8716(98)00006-4. [DOI] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Estabrook E. Growth Modeling: Structural Equation and Multilevel Modeling Approaches. New York: Guilford; 2017. [Google Scholar]
- Harris KM, Halpern CT, Whitsel E, Hussey J, Tabor J, Entzel P, Udry JR. The National Longitudinal Study of Adolescent Health: Research design. 2009 [WWW document]. Retrieved from http://www.cpc.unc.edu/projects/addhealth/design.
- Hurd Y, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76:416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sartor CE. The natural course of substance use and dependence. In: Sher KJ, editor. The Oxford Handbook of Substance Use and Substance Use Disorders. New York, NY: Oxford University Press; 2016. pp. 67–134. [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: Onset, persistence and trajectories of use across two samples. Addiction. 2002;97:517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Vo T, Wall M, Caetano R, Suglia SF, Martins SS, Galea S, Hasin D. Racial/ethnic differences in use of alcohol, tobacco, and marijuana: is there a cross-over from adolescence to adulthood? Social Science and Medicine. 2015;124:132–141. doi: 10.1016/j.socscimed.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Chikritzhs T. Age at first use of alcohol predicts the risk of heavy alcohol use in early adulthood: A longitudinal study in the United States. International Journal of Drug Policy. 2015;26:131–134. doi: 10.1016/j.drugpo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PAF, Nelson EC, … Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Maimaris W, McCambridge J. Age of first drinking and adult alcohol problems: Systematic review of prospective cohort studies. Journal of Epidemiology and Community Health. 2014;68:268–274. doi: 10.1136/jech-2013-203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone PS, Northrup TF, Masyn KE, Lamis DA, Lamont AE. Initiation and persistence of alcohol use in United States Black, Hispanic, and White male and female youth. Addictive Behaviors. 2012;37:299–305. doi: 10.1016/j.addbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyn KE, Petras H, Liu W. Growth curve models with categorical outcomes. In: Bruinsma G, Weisburd D, editors. Encyclopedia of Criminology and Criminal Justice. 1. New York, NY: Springer; 2014. pp. 2013–2025. [DOI] [Google Scholar]
- Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: A review of prospective behavioural studies. Addiction. 2006;101:948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Neale MC, Flay BR. Squeezing interval change from ordinal panel data: Latent growth curves with ordinal outcomes. Psychological Methods. 2004;9:301–333. doi: 10.1037/1082-989X.9.3.301. [DOI] [PubMed] [Google Scholar]
- Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55:107–122. doi: 10.1007/BF02294746. [DOI] [Google Scholar]
- Moss HB, Chen CM, Yi H. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence. 2014;135:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Mulia N, Karriker-Jaffe KJ, Witbrodt J, Bond J, Williams E, Zemore SE. Racial/ethnic differences in 30-year trajectories of heavy drinking in a nationally representative U.S. sample. Drug and Alcohol Dependence. 2017;170:133–141. doi: 10.1016/j.drugalcdep.2016.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- Muthén BO, Muthén LK. The development of heavy drinking and alcohol-related problems from ages 18 to 37 in a U.S. national sample. Journal of Studies on Alcohol. 2000;61:290–300. doi: 10.15288/jsa.2000.61.290. [DOI] [PubMed] [Google Scholar]
- Newsom JT. Longitudinal Structural Equation Modeling: A Comprehensive Introduction. New York: Routledge; 2015. [Google Scholar]
- Palmer RHC, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, Hewitt JK. Genetic etiology of the common liability to drug dependence: Evidence of common and specific mechanisms for DSM–IV dependence symptoms. Drug and Alcohol Dependence. 2012;123(Suppl 1):S23–S32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Ram N, Grimm K. Using simple and complex growth models to articulate change: Matching theory to method. International Journal of Behavioral Development. 2007;31:303–316. doi: 10.1177/0165025407077751. [DOI] [Google Scholar]
- Richmond-Rakerd LS, Fleming KA, Slutske WS. Investigating progression in substance use initiation using a discrete-time multiple event process survival mixture (MEPSUM) approach. Clinical Psychological Science. 2016;4:167–182. doi: 10.1177/2167702615587457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Deutsch AR, Lynskey MT, Agrawal A, Madden PAF, Bucholz KK, Heath AC, Martin NG. Progression in substance use initiation: A multilevel discordant monozygotic twin design. Journal of Abnormal Psychology. 2015;124:596–605. doi: 10.1037/abn0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Lynskey MT, Agrawal A, Madden PAF, Bucholz KK, Heath AC, Statham DJ, Martin NG. Age at first use and later substance use disorder: Shared genetic and environmental pathways for nicotine, alcohol, and cannabis. Journal of Abnormal Psychology. 2016;125:946–959. doi: 10.1037/abn0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow I, Kuntsche E. Early onset of drinking and risk of heavy drinking in young adulthood – a 13-year prospective study. Alcoholism: Clinical and Experimental Research. 2013;37:297–304. doi: 10.1111/j.1530-0277.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Agrawal A, Lynskey MT, Bucholz KK, Madden PAF, Heath AC. Common genetic influences on the timing of first use for alcohol, cigarettes, and cannabis in young African-American women. Drug and Alcohol Dependence. 2009;102:49–55. doi: 10.1016/j.drugalcdep.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, Madden PAF, Heath AC, Agrawal A, … Lynskey MT. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcoholism: Clinical and Experimental Research. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: Evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiology & Behavior. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Alcohol consumption in adolescence: A translational perspective. Current Addiction Reports. 2016;3:50–61. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Detailed Tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2016. [Google Scholar]
- Tomek S, Bolland KA, Bolland JM, Hooper LM, Church WT, II, Bolland AC. Age of alcohol initiation matters: examining gender differences in the recency and frequency of alcohol use across adolescence using a sample of impoverished minority adolescents. Youth & Society. 2016:1–26. doi: 10.1177/0044118X16662749. [DOI] [Google Scholar]
- Tucker JS, Ellickson PL, Orlando M, Martino SC, Klein DJ. Substance use trajectories from early adolescence to emerging adulthood: A comparison of smoking, binge drinking, and marijuana use. Journal of Drug Issues. 2006:307–332. [Google Scholar]
- Wood PK. Developmental models for children’s temperament: Alternatives to chronometric polynomial curves. Infant and Child Development. 2011;20:194–212. doi: 10.1002/icd.692. [DOI] [Google Scholar]
- Wood PK, Steinley D, Jackson KM. Right-sizing statistical models for longitudinal data. Psychological Methods. 2015;20:470–488. doi: 10.1037/met0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen AP, Creemers HE, Verhulst FC, Vollebergh WAM, Ormel J, van Oort F, Huzink AC. Legal substance use and the development of a DSM-IV cannabis use disorder during adolescence: the TRAILS study. Addiction. 2014;109:303–311. doi: 10.1111/add.12346. [DOI] [PubMed] [Google Scholar]